Abstract
The nematode worm Caenorhabditis elegans has been used to identify hundreds of genes that influence longevity and thereby demonstrate the strong influence of genetics on lifespan determination. In order to simplify lifespan studies in worms, many researchers have employed 5-fluoro-2′-deoxyuridine (FUdR) to inhibit the development of progeny. While FUdR has little impact on the lifespan of wild-type worms, we demonstrate that FUdR causes a dramatic, dose-dependent, two-fold increase in the lifespan of the mitochondrial mutant gas-1. Thus, the concentration of FUdR employed in a lifespan study can determine whether a particular strain is long-lived or short-lived compared to wild-type.
Keywords: Lifespan, Caenorhabditis elegans, FUdR, gas-1, genetics of aging
The first gene that has been shown to extend lifespan was identified in the roundworm C. elegans (Friedman and Johnson, 1988). Since that time, the worm has been used to identify at least 555 genes that are involved in aging (de Magalhaes et al., 2009) and this model organism has become a model of choice for studies of the genetic basis of aging. Importantly, genes and interventions that have been shown to increase the lifespan of the C. elegans have also been found to increase lifespan in other organisms. For example, following the observation that mutations in the insulin/IGF-1 receptor, daf-2, could more than double the lifespan of the worm (Kenyon et al., 1993), the insulin/IGF-1 signaling pathway has been shown to influence lifespan in yeast (Fabrizio et al., 2001), flies (Tatar et al., 2001), mice (Bluher et al., 2003; Holzenberger et al., 2003) and possibly humans (Guevara-Aguirre et al., 2011).
C. elegans offers several advantages as a genetic model organism that makes it an ideal model for aging studies. In particular, this animal develops to adulthood in 2 days and normally lives less than 3 weeks. Thus, lifespan experiments in the worm can be completed in less than a month, with little space requirements and minimal cost. However, one of the major limitations in completing lifespan experiments in the worm is the need to separate experimental animals from their offspring, which involves transferring the experimental animals onto fresh plates every 1–2 days. In addition, under normal growing conditions a proportion of the experimental animals are lost prematurely due to internal hatching of progeny.
To circumvent these limitations, lifespan studies are often completed on media supplemented with 5-fluoro-2′-deoxyuridine (FUdR) typically at a concentration of 50–100 μg/ml. This compound is thought to limit the production and development of progeny by inhibiting DNA synthesis. As all of the cells in an adult C. elegans are post-mitotic (and thus not undergoing cell division), it is generally assumed that this compound has little effect on their physiology and in fact multiple studies have demonstrated no effect of FUdR on the lifespan of wild-type N2 worms (Gandhi et al., 1980; Hosono, 1978; Hosono et al., 1982; Mitchell et al., 1979).
A recent study has demonstrated that lifespan of a specific mutant, tub-1, is increased by approximately 8% on plates containing 100 μg/ml FUdR (Aitlhadj and Sturzenbaum, 2010). Important questions arise from this result: Is the lifespan extension specific to the tub-1 mutant? Is the effect of FUdR on lifespan concentration dependent? What is the mechanism by which FUdR extends lifespan? Is there a concentration of FUdR that is sufficient to inhibit progeny production but does not affect lifespan? Should the use of FUdR be excluded from lifespan studies?
Is the lifespan extension specific to the tub-1 mutant? To address this question we compiled data for any strain in which we had completed at least one lifespan trial on both NGM (nematode growth medium) plates and plates containing FUdR. Among the approximately 30 strains superficially examined in this way, it was generally observed that strains lived as long or longer on plates containing 100 μM FUdR (data not shown). The most dramatic effect was observed for the mitochondrial mutant gas-1 (Kayser et al., 2004), which exhibits decreased lifespan on NGM plates but increased lifespan on plates containing 100 μM FUdR (Fig. S1A). Accordingly, we selected this strain for further studies described below. In contrast to gas-1, some mutants, such as the mitochondrial superoxide dismutase deletion mutant sod-2 (Van Raamsdonk and Hekimi, 2009), behaved as wild-type worms in showing identical lifespan on NGM and FUdR plates (Fig. S1B). The short-lived mitochondrial mutant mev-1 was found to be short-lived on 100 μM FUdR plates, indicating that FUdR does not extend the lifespan of all short-lived mitochondrial mutants (Fig. S2).
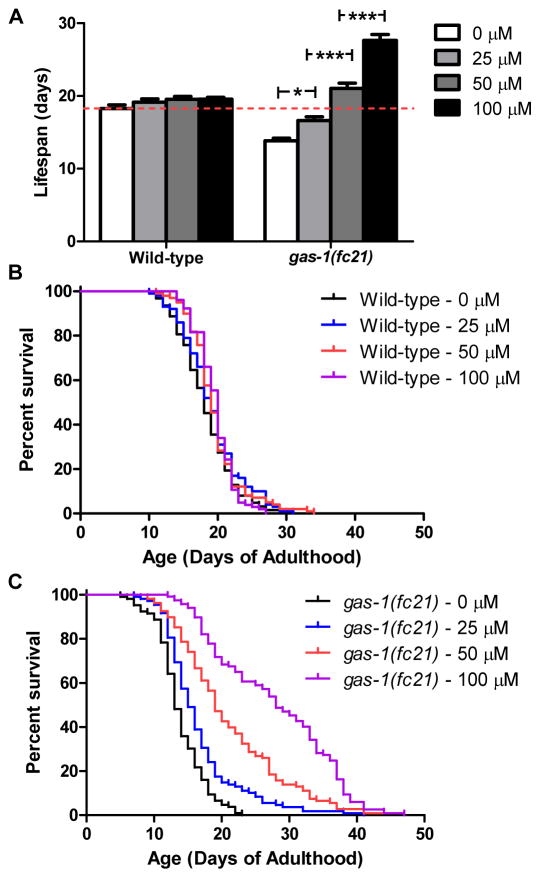
Is the effect of FUdR on lifespan concentration-dependent? To examine the effect of FUdR concentration on lifespan, we examined the lifespan of wild-type and gas-1 worms at multiple concentrations of FUdR. We found that there was no effect of FUdR on wild-type lifespan across a range of concentrations (Fig. 1A,B). In contrast, gas-1 exhibited an exquisitely dose-dependent increase in lifespan with increasing concentrations of FUdR (Fig. 1A,C). Compared to wild-type worms, gas-1 worms exhibited decreased lifespan at 0 and 25 μM FUdR, but increased lifespan at 50 and 100 μM FUdR. In fact, addition of 100 μM FUdR doubled the lifespan of gas-1 worms.
Fig. 1.
FUdR has a dose dependent effect on lifespan. The lifespan of wild-type and gas-1 worms was assessed on NGM plates containing four different concentrations of FUdR: 0 μM, 25 μM, 50 μM and 100 μM. Typically, lifespan experiments employing FUdR use 50–100 μM FUdR. (A,B) There was no significant effect of FUdR on the lifespan of wild-type worms across the entire range of FUdR concentrations. (A,C) In contrast, gas-1 worms exhibit a dose dependent increase in lifespan when grown in the presence of FUdR. At 0 μM and 25 μM, gas-1 worms have decreased lifespan compared to wild-type, while at 50 μM and 100 μM, gas-1 worms exhibit increased lifespan. The lifespan of gas-1 worms at 100 μM FUdR is double their lifespan at 0 μM FUdR. Error bars indicate SEM. * p<0.05, *** p<0.001.
What is the mechanism by which FUdR extends lifespan? We investigated three obvious mechanisms by which FUdR could increase lifespan. To test whether FUdR increases lifespan by reducing the number of times worms are transferred, we compared the lifespan of gas-1 worms on NGM and 100 μM FUdR plates in an experiment where worms under both conditions were always transferred on the same day. Under these conditions, we still observed a marked increase in gas-1 lifespan on plates containing FUdR (Fig. S3A). To test whether FUdR increases lifespan by inhibiting bacterial growth, which has been shown to affect lifespan (Klass, 1977), possibly through dietary restriction, we compared the lifespan of gas-1 worms on NGM and FUdR plates seeded with equal concentrations of heat-killed bacteria and found that gas-1 worms still lived longer on FUdR plates compared to NGM plates (Fig. S3B). Finally, we tested whether FUdR’s effect on inhibiting fertility could account for its lifespan-extending activity as the presence of the germline has been shown to influence lifespan (Hsin and Kenyon, 1999). We examined the lifespan of glp-1 worms on NGM and FUdR plates at a temperature where the worms are fertile and at a temperature at which they are sterile. In fact, we found that glp-1 worms show increased lifespan on FUdR plates at 25°C, when they are sterile, indicating that FUdR can still increase lifespan even under conditions in which the germline is missing (Fig. S3C).
Is there a concentration of FUdR that is sufficient to inhibit progeny production but does not affect lifespan? To determine what concentration of FUdR is required to prevent the progeny of experimental animals from developing to adulthood, we transferred young adult wild-type worms to plates containing various concentrations of FUdR and monitored the development of their progeny. We found that a concentration of 50 μM FUdR is required to prevent progeny from reaching adulthood (Fig. S4A,B). As this concentration can have marked effects on lifespan, we sought to determine whether lower concentrations of FUdR could be used if the experimental worms are transferred to a new plate before their progeny reach adulthood. We found that at concentrations as low as 1 μM FUdR, experimental animals did not produce any more progeny on the second plate when transferred on day 3 of adulthood (Fig. S4C). At this concentration of 1 μM FUdR, the progeny of the experimental worms on the original plate do not develop to adulthood until day 4 or 5 and thus it is easy to distinguish these worms from their progeny on day 3 of adulthood (Fig. S4D).
Should the use of FUdR be excluded from lifespan studies? From these studies, we have demonstrated that the ability of FUdR to extend lifespan is not limited to tub-1 mutants and that FUdR can have a marked impact on lifespan. The doubling of gas-1 lifespan on plates containing FUdR demonstrates that the specific concentration of FUdR utilized can alter the interpretation of an experiment. This is consistent with the fact that any variation from the standard lifespan conditions of 20°C on NGM plates can affect the lifespan of strains to different extents (e.g. increasing temperature to 25°C, or growing on RNAi plates). Intuitively, this makes sense as the phenotype of an organism represents an interaction of its genotype and its environment. As most studies examine lifespan under a specific set of conditions, it is important to bear in mind that the differences observed, or not observed, may be specific to those particular conditions. In the case of FUdR, the effect on lifespan can be minimized by using a low concentration of 1 μM (compared to the standard 100 μM) and transferring worms to new plates after the first 3 days of adulthood.
Supplementary Material
Acknowledgments
We would like to thank the C. elegans knockout consortium, the National BioResource Project of Japan (Mitani laboratory) and the Caenorhabditis Genetics Center for providing strains used in this research. JVR is supported by the Canadian Institutes of Health Research, Parkinson Society Canada, the Hereditary Disease Foundation and the McGill Tomlinson Fellowships. SH is supported by grants from the Canadian Institutes of Health Research (#216377, #216376, and #218649), the Canadian Cancer Society (#89761) and McGill University. SH is Campbell Chair of Developmental Biology and Strathcona Chair of Zoology.
References
- Aitlhadj L, Sturzenbaum SR. The use of FUdR can cause prolonged longevity in mutant nematodes. Mechanisms of ageing and development. 2010;131:364–365. doi: 10.1016/j.mad.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- de Magalhaes JP, Budovsky A, Lehmann G, Costa J, Li Y, Fraifeld V, Church GM. The Human Ageing Genomic Resources: online databases and tools for biogerontologists. Aging Cell. 2009;8:65–72. doi: 10.1111/j.1474-9726.2008.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Santelli J, Mitchell DH, Stiles JW, Sanadi DR. A simple method for maintaining large, aging populations of Caenorhabditis elegans. Mechanisms of ageing and development. 1980;12:137–150. doi: 10.1016/0047-6374(80)90090-1. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le BY. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hosono R. Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine. Experimental gerontology. 1978;13:369–374. doi: 10.1016/0531-5565(78)90047-5. [DOI] [PubMed] [Google Scholar]
- Hosono R, Mitsui Y, Sato Y, Aizawa S, Miwa J. Life span of the wild and mutant nematode Caenorhabditis elegans. Effects of sex, sterilization, and temperature. Experimental gerontology. 1982;17:163–172. doi: 10.1016/0531-5565(82)90052-3. [DOI] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Kayser EB, Sedensky MM, Morgan PG. The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mech Ageing Dev. 2004;125:455–464. doi: 10.1016/j.mad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Mitchell DH, Stiles JW, Santelli J, Sanadi DR. Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol. 1979;34:28–36. doi: 10.1093/geronj/34.1.28. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.