Abstract
Objective
There are limited data on treatment-related anemia in Asian HIV-infected children.
Methods
Data from Asian HIV-infected children aged <18 years on first-line highly active antiretroviral therapy (HAART) were used. Children who had preexisting severe anemia at baseline were excluded. Anemia was graded by using the DAIDS 2004 table. Potential risk factors of severe anemia were assessed by logistic regression.
Results
Data from 1,648 children (51.9% female, 62.8% WHO stage 3/4) were analyzed. Median (IQR) age was 6.8 (3.7–9.6) years, CD4% was 9 (3–16)% and plasma HIV-RNA was 5.2 (4.7–5.6) log10 copies/ml at HAART initiation in those with available testing. The most common regimens were stavudine/lamivudine/nevirapine (42%) and zidovudine/lamivudine/nevirapine (25%). Severe anemia was identified in 47 (2.9%) children after a median time of 6 months after HAART initiation with an incidence rate of 5.4 per 100 child-years. Mild anemia or moderate anemia at baseline (p=0.024 and p=0.005, respectively), previous or current use of zidovudine (p<0.0001 and p=0.013, respectively), and male sex (p=0.008) were associated with severe anemia. Higher weight-for-age z-score (p=0.004) was protective.
Conclusions
The incidence of severe anemia in Asian HIV-infected children after HAART initiation was low and mainly occurred during the first few months after HAART initiation. Mild to moderate anemia at baseline and using AZT were independent risk factors of developing severe anemia.
Keywords: pediatric HIV, Asia, antiretroviral therapy, anemia
INTRODUCTION
Increasing access to potent antiretroviral therapy (ART) in resource-limited settings has transformed the prognosis of HIV infection, but adverse events may still occur after therapy initiation. Anemia is commonly seen after ART initiation, whether due to pre-existing HIV-related bone marrow suppression or as a side effect of antiretrovirals like zidovudine (AZT) (1, 2). Anemia in HIV-infected patients has been associated with worse quality of life, disease progression and increased risk of death (1, 3–6). However, there is a lack of data on post-ART anemia in HIV-infected children in Asia (1). We aimed to describe the incidence of severe anemia after the first 24 weeks of HAART initiation in a regional observational cohort of Asian children, and identify associated factors.
METHODS
Patients
The TREAT Asia Pediatric HIV Observational Database (TApHOD) is a longitudinal, multicenter, cohort study of HIV-infected children in Asia. TApHOD is a member cohort of the US National Institutes of Health (NIH) International Epidemiology Databases to Evaluate AIDS (IeDEA) program. Detailed information on the overall study design and description of the cohort has been published elsewhere (7). In brief, children eligible for inclusion in TApHOD must be ≤18 years and have been conclusively diagnosed with HIV, by age-appropriate testing or a presumptive clinical diagnosis of HIV infection defined as meeting World Health Organization criteria for initiating HAART (8).
Data collection
The study protocol was approved by the local ethics committees or institutional review boards of each participating clinical site, the data management and analysis center (Kirby Institute for Infection and Immunity in Society, University of New South Wales, Australia), and the coordinating center (TREAT Asia/amfAR, Thailand). For this analysis, we included children from 18 clinics in six countries; Cambodia (n=3), India (n=1), Indonesia (n=2), Malaysia (n=4), Vietnam (n=3), and Thailand (n=5), that routinely provide pediatric HIV clinical care and treatment. The database includes information about demographic characteristics, and laboratory and treatment information. This information is collected from medical records and at clinic visits, and entered into computerized databases by trained staff. Data are transferred to the Kirby Institute twice yearly for cleaning and analysis.
Eligibility criteria and definitions
The present study includes information on children (≤18 years) who received HAART between November 1997 and March 2012, and had hemoglobin (Hgb) measurements at HAART initiation and at any time during the first six months of HAART. Children who had severe anemia at baseline were excluded. We defined severe anemia according to the US NIH Division of AIDS 2004 toxicity grading table (9) as; Hgb <10 g/dL for children <21 days; Hgb <8 g/dL for children between 22 and 35 days; Hgb <7 g/dL for children between 36 and 56 days; Hgb <7.5 g/dL for children ≥57 days. Baseline values at HAART initiation for laboratory and clinical measurements were defined as the values closest to HAART initiation that fell into a window of six months prior to and seven days after HAART initiation. Children were considered lost to follow-up if the time between their last visit and the date of last data transfer was more than one year.
Statistical analysis
Baseline categorical data are presented as frequencies (%) and continuous data as medians and interquartile ranges (IQR). The Wilcoxon signed-rank test was used to test the differences in medians and Chi-squared or Fisher’s exact tests to compare frequencies. Follow-up started at HAART initiation and ended at the date of diagnosis of first severe anemia, death or six months after starting HAART. The incidence rates of severe anemia in the six months after HAART were calculated per 100 child-years. If anemia occurred more than once in a single patient, the earliest event was analyzed for both overall incidence and risk factor analyses. Potential risk factors for development of severe anemia were explored by univariate and multivariate Cox proportional hazards models. The variables and possible confounders investigated included: age (<1.5, 1.5–4, 5–11, 12–14, and ≥15 years), sex, WHO clinical stage (stage 1, 2, 3 and 4), CD4% (<10, 10–14, and ≥15%), HIV- RNA log10copies/ml (<5 and ≥5 log10copies/ml), anemia (mild, moderate and no anemia), weight, height, and exposure to AZT and nevirapine (NVP). Weight and height measurements were converted into age- and sex-standardized z scores. For height-for-age z score (HAZ) the WHO 2006/2007 Child Growth Standards were used (10). WHO 1977 Standards were used for weight-for-age z scores (WAZ), to allow for scoring children >10 years of age (11). AZT and NVP use as part of a HAART regimen were included as time-dependent variables and categorized as: current use (currently receiving it or stopped ≤60 days before starting a new regimen), ever used (had used it but stopped >60 days before starting new regimen), and never used. Countries were categorized as upper middle-income (Thailand, Malaysia), lower middle-income (Indonesia, India and Vietnam) and low-income (Cambodia) (12). Variables were included in the full model if they were associated with severe anemia in the univariate analysis with p<0.10. A final model was then created, by using a forward step-wise with a p-value less <0.05 was considered statistically significant in the adjusted analysis. Analyses were done with STATA version 11.0 (Stata Corp, College Station, Texas).
RESULTS
By the end of March 2012, 3448 children who had been recruited into TApHOD received HAART when aged ≤18 years. We excluded 1099 (32%) children who did not have baseline Hgb values reported, 616 (18%) with no Hgb results in the first six months after HAART, and 85 (2.5%) who had severe anemia at baseline. The analysis was consequently based on 1648 children (Table 1), of whom 1123 (68.1%) had no anemia at HAART initiation, 407 (24.7%) had mild anemia and 118 (7.2%) had moderate anemia. About half (51.9%) of the children were girls. The median age was 6.8 (IQR: 3.7–9.6) years. The median pre-HAART CD4% was 9 (IQR: 3–16)%. Eleven percent of children had a history of prior mono- and/or dual-therapy with nucleoside reverse transcriptase inhibitors (NRTI). First-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based HAART was used in 91.7% and protease inhibitor (PI)-based HAART in 7.8%. The initial NNRTI-based HAART regimens were stavudine (d4T)/lamivudine (3TC)/NVP in 42%, AZT/3TC/NVP in 25%, AZT/3TC/efavirenz (EFV) in 17%, and d4T/3TC/EFV in 13% of children. The most common ritonavir-boosted PI-based HAART regimens were AZT/3TC/lopinavir/ritonavir (LPV/r) in 47%, d4T/3TC/LPV/r in 40%, and others (e.g., saquinavir/3TC/LPV/r, indinavir/3TC/LPV/r) in 14% of children.
Table 1.
Patient characteristics at HAART initiation
| Characteristics | All (N=1648) | No anemia (N=1123) | Mild anemia (N=407) | Moderate anemia (N=118) |
|---|---|---|---|---|
| Age in years – median (IQR) | 6.8 (3.7–9.6) | 7.3 (4.5–10.0) | 5.9 (2.9–8.6) | 5.1 (2.1–8.0) |
| Female sex – n (%) | 51.9 | 52.8 | 49.4 | 51.7 |
| Country by income level – n (%) | ||||
| Thailand/Malaysia | 1087 (66.0%) | 758 (67.5%) | 256 (62.9%) | 73 (61.9%) |
| Indonesia/India/Vietnam | 304 (18.5%) | 199 (17.7) | 75 (18.4%) | 30 (25.4%) |
| Cambodia | 257 (15.6%) | 166 (14.8%) | 76 (18.7%) | 15 (12.7%) |
| CD4% - median (IQR) | 9 (3–16) | 11 (5–17) | 6 (2–13) | 6 (2–11) |
| Missing data | 123 | 82 | 34 | 7 |
| CD4 count, cells/mm3 – median (IQR) | 228 (60–513) | 273 (89–547) | 115 (23–377) | 122 (24–400) |
| Missing data | 136 | 77 | 50 | 9 |
| HIV-RNA (log10copies/ml) – median (IQR) | 5.2 (4.7–5.6) | 5.0 (4.5–5.5) | 5.4 (5.0–5.8) | 5.5 (5.1–5.9) |
| Missing data | 853 | 553 | 227 | 73 |
| WHO clinical staging – n (%) | ||||
| Stage 1/2 | 612 (37.1) | 495 (44.1) | 152 (37.3) | 26 (9.3) |
| Stage 3 | 846 (51.3) | 499 (44.4) | 145 (35.6) | 42 (35.6) |
| Stage 4 | 190 (11.5) | 129 (11.5) | 110 (27.0) | 50 (42.4) |
| Weight for age z-score – median (IQR) | −2.3 (−3.6 to −1.2) | −1.9 (−3.1 to −1.0) | −3.1 (−4.2 to −2.0) | −3.5 (−4.9 to −2.3) |
| Missing data | 51 | 31 | 14 | 6 |
| Height for age z-score – median (IQR) | −2.2 (−3.2 to −1.3) | −2.0 (−2.9 to −1.1) | −2.7 (−3.6 to −1.8) | −3.2 (−3.9 to −2.4) |
| Missing data | 111 | 65 | 33 | 13 |
| Hemoglobin (g/dL) – median (IQR) | 10.6 (9.6–11.6) | 11.2 (10.5–12.0) | 9.3 (9.0–9.6) | 8.1 (7.8–8.3) |
HAART, highly active antiretroviral therapy.
Classification of anemia was based on the US NIH Division of AIDS 2004 toxicity grading table (9).
The 1800 children who were excluded from the study had similar median CD4 counts and CD4% from those included. However, they were younger (median age 5.4 vs. 6.8 years), and had lower median WAZ (−3.0 vs. −2.5; p<0.0001). Excluded children were more likely to be WHO stage 4 (21% vs. 12%; p<0.0001) and WHO stage 1–2 (49% vs. 37%; p<0.0001) at baseline.
Incidence rate and predictors of severe anemia
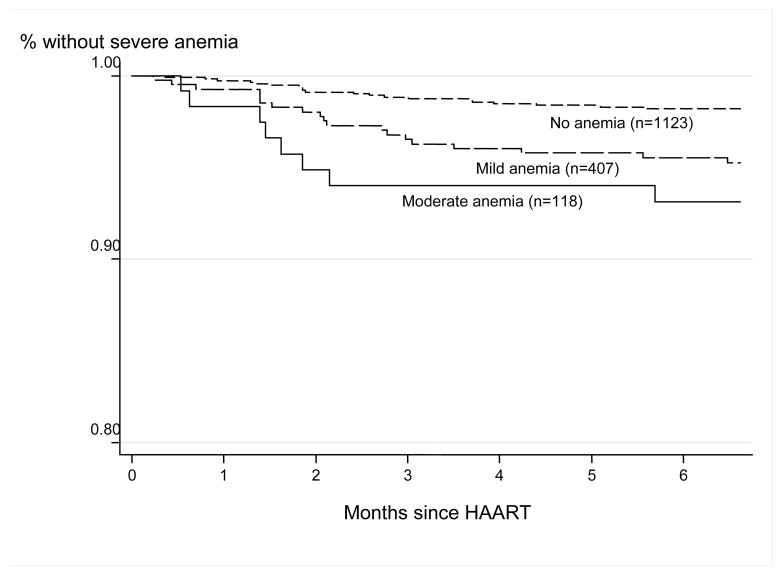
Forty-seven (2.9%) of 1648 children had severe anemia during the first six months of HAART start (incidence rate 5.4 per 100 child-years; 95% confidence interval [CI]: 4.1–7.2). The number (%) of children who developed severe anemia during month 0–2 was 26 (55.3%), during month 2–4 was 15 (31.9%), and during month 4–6 was 6 (12.8%). The incidence was 7.4 (5.2–10.6) per 100 child-years in boys and 3.5 (2.2–5.8) per 100 child-years in girls. Table 2 shows the incidence rate of severe anemia by baseline anemia status. A Kaplan–Meier analysis to estimate the probability of severe anemia categorized by baseline anemia status is shown in Figure 1. Results of the multivariate analyses showed increased risk of early severe anemia for children who had mild anemia or moderate anemia at baseline (p=0.024 and p=0.005, respectively), previous or current use of AZT (p<0.0001 and p=0.013, respectively), and male sex (p=0.008) (Table 3). However, higher WAZ (p=0.004) was protective.
Table 2.
Incidence of severe anemia in the first six months of HAART by baseline anemia status.
| Anemia at baseline | Children (N=1648) | Severe anemia | Incidence per 100 child years (95% CI) | Hgb at HAART, gd/L median (IQR) | Hgb at severe anemia, gd/L median (IQR) |
|---|---|---|---|---|---|
| No anemia | 1123 | 20 | 3.3 (2.2–5.2) | 10.9 (10.3–11.3) | 6.6 (5.4–7.1) |
| Mild anemia | 407 | 19 | 9.0 (5.7–14.0) | 9.4 (9.0–9.7) | 6.3 (4.5–6.9) |
| Moderate anemia | 118 | 8 | 13.3 (6.7–26.6) | 8.1 (7.7–8.3) | 6.4 (5.5–7.1) |
HAART, highly active antiretroviral therapy; Hgb, hemoglobin.
Classification of anemia was based on the US NIH Division of AIDS 2004 toxicity grading table (9).
Figure 1.
Kaplan–Meier estimates of the probability of severe anemia in 1648 children in the first six months of HAART; by anemia status at baseline.
HAART, highly active antiretroviral therapy
Table 3.
Factors associated with the incidence of severe anemia within the first six months of HAART initiation.
| Characteristics | Events | Person years | Univariate | Adjusted | ||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| Age, yearsa | ||||||
|
| ||||||
| <1.5 | 8 | 81 | 2.33 (0.94–5.78) | 0.069 | 1.10 (0.40–3.07) | 0.852 |
| 1.5–4.9 | 17 | 212 | 1.89 (0.89–4.03) | 1.00 | 1.56 (0.71–3.40) | 0.264 |
| 5–11.9 | 11 | 320 | 0.81 (0.35–1.87) | 0.626 | 0.78 (0.34–1.80) | 0.559 |
| >=12 | 11 | 259 | 1.0 | |||
|
| ||||||
| Sex | ||||||
|
| ||||||
| Female | 16 | 454 | 1.0 | - | 1.0 | |
| Male | 31 | 418 | 2.10 (1.15–3.84) | 0.016 | 2.36 (1.25–4.48) | 0.008 |
|
| ||||||
| CD4% a | ||||||
|
| ||||||
| 0–9% | 33 | 403 | 2.50 (1.15–5.40) | 0.02 | 1.89 (0.79–4.48) | 0.153 |
| 10–14% | 3 | 160 | 0.58 (0.15–2.17) | 0.415 | 0.68 (0.17–2.64) | 0.576 |
| ≥15% | 8 | 245 | 1.0 | 1.0 | ||
| Missing | 3 | 64 | ||||
|
| ||||||
| HIV-RNA at baselinea | ||||||
|
| ||||||
| <5 log10 | 3 | 158 | 1.0 | |||
| ≥5 log10 | 17 | 264 | 3.36 (0.98–11.4) | 0.053 | 2.43 (0.70–8.40) | 0.160 |
| Missing | 27 | 450 | ||||
|
| ||||||
| WHO stage a | ||||||
|
| ||||||
| 1–2 | 13 | 430 | 1.0 | 1.0 | ||
| 3 | 18 | 269 | 2.20 (1.08–4.49) | 0.030 | 2.03 (0.94–4.39) | 0.072 |
| 4 | 16 | 173 | 3.04 (1.46–6.31) | 0.003 | 2.38 (1.03–5.49) | 0.042 |
|
| ||||||
| Weight-for-age z-score | - | 0.77 (0.69–0.87) | <0.0001 | 0.80 (0.68–0.93) | 0.004 | |
| Height-for-age z-scorea | - | 0.82 (0.66–1.02) | 0.075 | 1.25 (0.90–1.73) | 0.185 | |
| Anemia at HAART | ||||||
|
| ||||||
| No anemia | 20 | 599 | 1.0 | 1.0 | ||
| Mild | 19 | 212 | 2.67 (1.43–5.00) | 0.002 | 2.16 (1.11–4.22) | 0.024 |
| Moderate | 8 | 60 | 3.98 (1.75–9.03) | 0.001 | 3.77 (1.49–9.54) | 0.005 |
|
| ||||||
| Zidovudine use | ||||||
|
| ||||||
| Never used | 13 | 498 | 1.0 | 1.0 | ||
| Ever used | 33 | 367 | 3.44 (1.80–6.52) | <0.0001 | 5.30 (2.67–10.53) | <0.0001 |
| Currently used | 1 | 7 | 11.90 (1.47–96.53) | <0.020 | 14.72 (1.78–121.97) | 0.013 |
|
| ||||||
| Nevirapinea | ||||||
|
| ||||||
| Never used | 23 | 320 | 1.0 | |||
| Ever used | 23 | 527 | 0.61 (0.34–1.08) | 0.089 | 0.93 (0.50–1.71) | 0.806 |
| Currently used | 1 | 25 | 0.84 (0.11–6.30) | 0.867 | 1.27 (0.17–9.79) | 0.816 |
These variables were only significant in the univariate analysis. The hazard ratio was obtained by adding each to the final model.
Long-term outcomes
The 407 of children who had baseline mild anemia were followed for a median (IQR) of 5.4 years (2.5–6.8) years. Among these, 134 (33%) used AZT in their initial regimen. At their last visit, the median (IQR) Hgb was 12.0 (11.1–12.8), CD4% was 27.0 (20.0–32.0), CD4 count was 737 (497–1032) cells/mm3, and of 344 with HIV-RNA testing, 80% had HIV-RNA <400 copies/ml.
The 118 children with baseline moderate anemia were followed for a median (IQR) of 4.7 (2.1–6.6) years, and had median (IQR) Hgb of 11.8 (10.7–12.7), CD4% of 27.3 (18.4–31.8), CD4 count of 780 (468–1219), and 76% of 84 tested had HIV-RNA <400 copies/ml. Twenty three (19%) of these children used AZT in their initial regimen.
The 47 children who developed severe anemia during the first six months of ART were followed for a median of 2.7 years (IQR: 1.0–5.1). At their last visit, the median (IQR) Hgb was 11.3 (7.2–12.7), CD4% was 23.5 (7.1–32.0), CD4 count was 509 (149–1000) cells/mm3, and of 37 with available test results, 76% had HIV-RNA <400 copies/ml.
DISCUSSION
Anemia is a common complication in HIV-infected children and is associated with disease progression and poor outcomes (1, 13). In our report, the incidence of severe anemia after ART initiation in children without severe anemia at baseline was low at 2.9%. This may reflect the historically predominant use of d4T in first-line therapy regimens in our cohort. The incidence of post-HAART severe anemia in our cohort was comparable to reports of HIV-infected adults in Asia (2), and Africa (14).
In our study, mild to moderate pre-HAART anemia, and the use of AZT were associated with post-HAART severe anemia, and higher weight-for-age z-score was protective. Although WHO guidelines recommend to avoid AZT in children with severe anemia (15), it may be appropriate to extend that to children with mild or moderate anemia in the initial phase of their treatment when other options are available, particularly in the malnourished or severely immunosuppressed. Age younger than six years, advanced HIV disease stage and presence of stunting (HAZ < −2) were associated with anemia in a study of HIV-infected children in India (13). However, we failed to identify these associations in this cohort. In addition, Shet et al. reported no association of sex and anemia in Indian HIV-infected children (13), but we found that boys had more than twice the risk of severe early anemia. The reasons for this finding are unclear. Additional studies of childhood nutrition, use of vitamin and mineral supplements, and hemoglobinopathy in the cohort may help explain our results.
The limitations of the study are primarily reflective of the observational nature of these data and the availability of lab-based monitoring of children in our cohort on HAART. Around half of the children in our cohort were excluded from analysis as they were missing baseline pre-HAART Hgb levels. We performed an additional subgroup analysis for occurrence of severe anemia among 445 children who did not have baseline Hgb levels, but had test results reported during the first six months of HAART. Twenty two (4.9%) of these children had severe anemia, with a median Hgb level of 6.5 (5.3–6.8) g/dL. This was higher than in our analysis sub-cohort, and their exclusion may have biased our results towards children who were more likely to have Hgb measurements, whether due to disease progression, malnutrition, or level of clinic resources. In addition, we did not have clinical information about other possible causes of anemia (e.g., history of hemoglobinopathy, iron deficiency anemia, concurrent bone marrow-suppressive medications), nor additional clinical management or outcomes data for the patients who developed severe anemia. Kosalaraksa et al. reported that mild to moderate anemia was common (around 50%) among 299 ART-naïve Thai and Cambodian children without advanced HIV (16). However, prevalence of iron deficiency anemia was low (2.7%) and the majority of cases were caused by carriage of a thalassemia trait (47%) and anemia of chronic disease (42%) (16). Therefore, iron supplementation in anemic HIV-infected Asian children without further assessment of red blood cell indices or iron levels may not necessarily be beneficial.
In conclusion, the incidence of severe anemia in Asian HIV-infected children was low (2.9%) during their initial period of HAART. However, mild to moderate anemia at baseline and use of AZT were independent risk factors for developing severe anemia. Monitoring of Hgb during the first few months of HIV treatment, either for children with baseline mild to moderate anemia or for children using AZT, is warranted and should be implemented more consistently in our region.
Acknowledgments
The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and the AIDS Life Association. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above. The authors wish to thank the children and the staff at the participating centers who have given their time so generously during the course of this project, and Dr. Gonzague Jourdain for his critical review of the manuscript.
The TREAT Asia Pediatric HIV Network
V Saphonn* and S Saramony, National Centre for HIV/AIDS Dermatology and STDs, Phnom Penh, Cambodia; U Vibol* and S Sophan, National Pediatric Hospital, Phnom Penh, Cambodia; J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia; FJ Zhang, Beijing Ditan Hospital, Capital Medical University, Beijing, China; N Kumarasamy* and S Saghayam, YR Gaitonde Centre for AIDS Research and Education, Chennai, India; DK Wati* and LPP Atmikasari, Sanglah Hospital, Udayana University, Bali, Indonesia; N Kurniati*,† and D Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; SM Fong* and M Thien, Hospital Likas, Kota Kinabalu, Malaysia; NK Nik Yusoff* and LC Hai, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia; KA Razali *, TJ Mohamed, and NF Abdul Rahman, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; R Nallusamy*,‡ and KC Chan, Penang Hospital, Penang, Malaysia; V Sirisanthana*, P Oberdorfer, and L Aurpibul, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; R Hansudewechakul* and P Taeprasert, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Lumbiganon*, P Kosalaraksa, and P Tharnprisan, Khon Kaen University, Khon Kaen, Thailand; G Jourdain, Program for HIV Prevention and Treatment, Chiang Mai, Thailand; J Ananworanich*, C Phasomsap, and T Suwanlerk, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; K Chokephaibulkit*, W Phongsamart and O Wittawatmongkol, Siriraj Hospital, Mahidol University, Bangkok, Thailand; HK Truong* and TQ Du, Children’s Hospital 1, Ho Chi Minh City, Vietnam; CV Do* and MT Ha, Children’s Hospital 2, Ho Chi Minh City, Vietnam; KTK Dung, NV Lam, PN An and NT Loan, National Hospital of Pediatrics, Hanoi, Vietnam; NO Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam; AH Sohn*, N Durier, and P Nipathakosol, TREAT Asia, amfAR -- The Foundation for AIDS Research, Bangkok, Thailand; DA Cooper, MG Law*, and A Kariminia, The Kirby Institute, University of New South Wales, Sydney, Australia;
Footnotes
TApHOD Steering Committee member
Current Steering Committee Chair;
co-Chair
Conflict of Interest Statement: All authors declare no conflict of interest and that members of their immediate families do not have a financial interest in or arrangement with any commercial organization that may have a direct interest in the subject matter of this article.
This study was presented in part at the 2nd International Workshop on HIV Pediatrics, Vienna, Austria, 16 – 17 July 2010 (Abstract O_13).
References
- 1.Calis JC, van Hensbroek MB, de Haan RJ, Moons P, Brabin BJ, Bates I. HIV-associated anemia in children: a systematic review from a global perspective. AIDS (London, England) 2008 Jun 19;22(10):1099–112. doi: 10.1097/QAD.0b013e3282fa759f. [DOI] [PubMed] [Google Scholar]
- 2.Huffam SE, Srasuebkul P, Zhou J, Calmy A, Saphonn V, Kaldor JM, et al. Prior antiretroviral therapy experience protects against zidovudine-related anaemia. HIV medicine. 2007 Oct;8(7):465–71. doi: 10.1111/j.1468-1293.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 3.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004 May 15;38(10):1454–63. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 4.Semba RD, Martin BK, Kempen JH, Thorne JE, Wu AW. The impact of anemia on energy and physical functioning in individuals with AIDS. Arch Intern Med. 2005 Oct 24;165(19):2229–36. doi: 10.1001/archinte.165.19.2229. [DOI] [PubMed] [Google Scholar]
- 5.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. The American journal of medicine. 2004 Apr 5;116(Suppl 7A):27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe CG, van Dijk JH, Munsanje B, Hamangaba F, Siniwymaanzi P, Thuma PE, et al. Risk factors for pre-treatment mortality among HIV-infected children in rural Zambia: a cohort study. PloS one. 2012;6(12):e29294. doi: 10.1371/journal.pone.0029294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kariminia A, Chokephaibulkit K, Pang J, Lumbiganon P, Hansudewechakul R, Amin J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. International journal of epidemiology. 2010 Feb;40(1):15–24. doi: 10.1093/ije/dyp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Antiretroviral Therapy for Infants and Children. Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting; WHO Headquarters, Geneva, Switzerland. 10–11 April 2008.2008. [Google Scholar]
- 9.National Institute of Allergy and Infectious Disease. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. 2004 http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/PDF/Safety/DAIDSAEGradingTable.pdf. (Clarification August 2009)
- 10.Organisation WH. WHO growth chart. 2006. [Google Scholar]
- 11.Kuczmarski RJOC, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11(46):191. 2000. [PubMed] [Google Scholar]
- 12. [accessed 13 February 2013]; http://data.worldbank.org/income-level/LIC.
- 13.Shet A, Mehta S, Rajagopalan N, Dinakar C, Ramesh E, Samuel NM, et al. Anemia and growth failure among HIV-infected children in India: a retrospective analysis. BMC pediatrics. 2009;9:37. doi: 10.1186/1471-2431-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ssali F, Stohr W, Munderi P, Reid A, Walker AS, Gibb DM, et al. Prevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trial. Antiviral therapy. 2006;11(6):741–9. doi: 10.1177/135965350601100612. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access: recommendations for a public health approach 2010 revision. [PubMed] [Google Scholar]
- 16.Kosalaraksa P, Bunupuradah T, Saphonn V, Wiangnon S, Hansudewechakul R, Vibol U, et al. Prevalence of anemia and underlying iron status in naive antiretroviral therapy HIV-infected children with moderate immune suppression. AIDS research and human retroviruses. 2012 Jun 26; doi: 10.1089/aid.2011.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]