Abstract
Nanomaterials, because of their tunable properties and performances, have been utilized extensively in everyday life related consumable products and technology. On exposure, beyond physiological range, nanomaterials cause health risks via affecting the function of organisms, genomic systems, and even central nervous system. Thus, new analytical approaches for nanotoxicity assessment to verify the feasibility of nanomaterials for future use are in demand. The conventional analytical techniques, such as spectrophotometric assay-based techniques usually require a lengthy and time-consuming process and many times produces false positives, and many times cannot be implemented at a single cell level measurement for studying cell behavior without interference of their surrounding environment. Hence, there is demand of a precise, accurate, sensitive assessment for toxicity using single cell. Recently, due to advantages of automation of fluids and minimization of human error, the integration of cell-on-a-chip (CoC) with microfluidic system is in practice for nanotoxicity assessments. This review explains nanotoxicity and assessment approaches with advantages/limitations and new approaches to overcome the confines of traditional techniques. Recent advances in nanotoxicity assessment using CoC integrated with microfluidic system are also discussed in this review, which may be of use for nanotoxicity assessment and diagnostics.
Keywords: Nanotoxicity, Nanobiotchnolgy, Electrochemical techniques, Single cell-on-a-chip
1 Nanotoxicity
Nanotechnology has been one of the hot topics in not only research but also the consumer market in last 20 years. The ongoing trend and success suggest an ever-increasing use of the nanotechnology in every sector. At the nano-scale, nanomaterials/nanoparticles (NP ~1–50 nm) demonstrate unique physico-chemical properties in comparison to their bulk form. The unique features, such as size, shape, surface properties, etc., have inspired the production of nanomaterials not only at the research level, but also at the industrial scale for various applications. NPs are increasingly exploited in cosmetics, food, electronics, paint, material science, medicine, biotechnology, and energy technologies. The Nanotechnology Consumer Products Inventory list currently contains 1628 consumer products (not comprehensive), a 24% increase from the 2010 update 1 and maybe thousands of nanomaterials are handled at research level without knowing proper details about them. With increased production and use of nanomaterials lies the risk of increased intentional/unintentional exposure to these particles 2–4.
Despite the NPs positive properties, they do have adverse effects on human, animals and environment living due to their own toxicity. NP toxicity has been studied and assessed for years; however it lacks any gold standard techniques due to several limitations. First and foremost, even the toxicity of nanoparticles does not have a common agreement 6. Some limitations are due to the lack of understating of the NPs potential interaction with biological systems 7, 8 and there are no specific tools which can provide rapid nanotoxicity assessment.
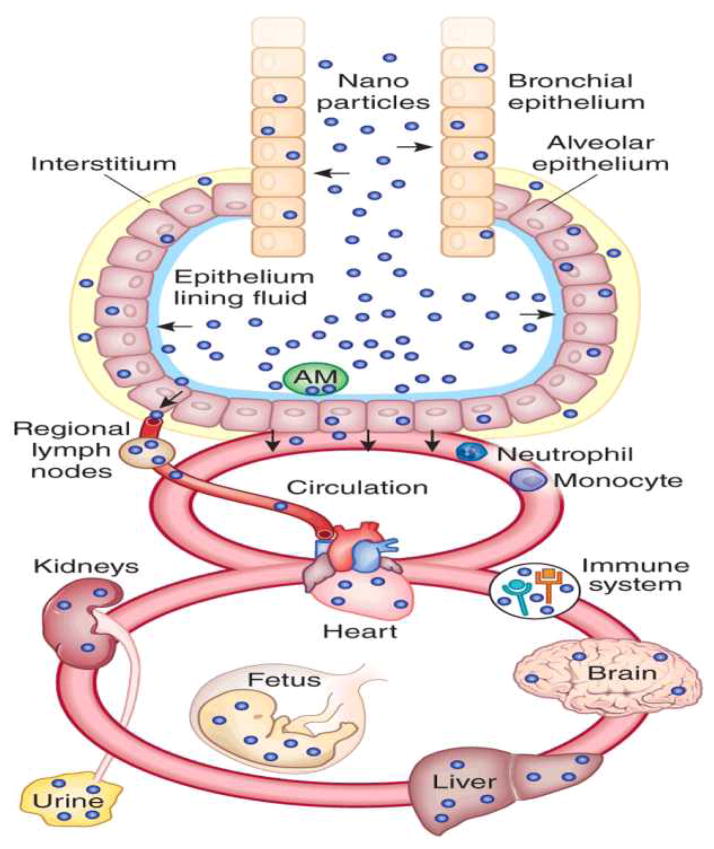
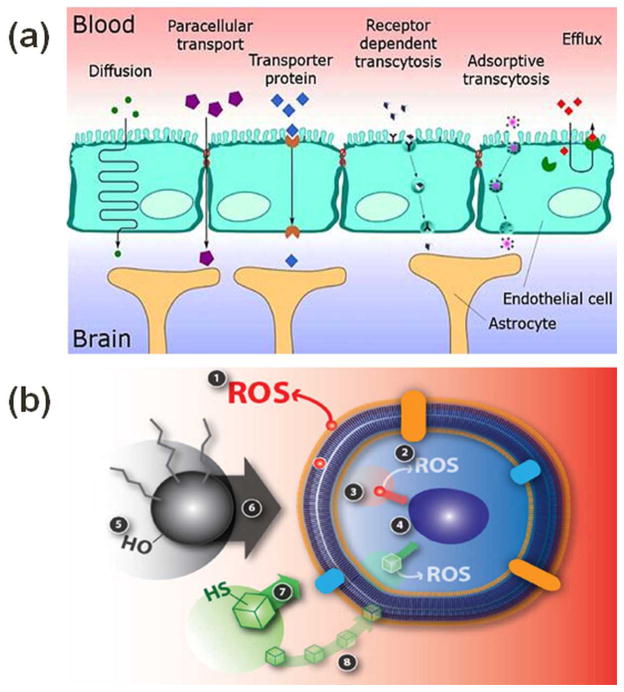
NPs with many novel properties are used in various applications and come in interaction with complex and dynamic biological systems. It is challenging to characterize NPs throughout their biological interaction and to quantify the uptake rate and localization inside the body parts and cells. Cells under nanotoxicity may undergo necrosis or repairable oxidative DNA damage and recover from it eventually, or may result in apoptosis. Nanotoxicity may alter cell differentiation, proliferation, morphology, or cell-cell communication. Upon exposure, NPs can enter into human body by either inhalation, ingestion, injection or through skin contact. NPs can localize into different organ and tissues and further induce organ targeted toxicity/disease. Figure 1 highlights the possible accumulation of NPs in various organs. Some NPs can cross blood-brain barrier (BBB) 9 (Figure 2-a 10) and hence have been proposed for diagnostic and therapeutic applications 11, 12. Their smaller size and larger surface area provides NPs’ unique properties in their translocation to the systemic circulation and CNS. Once inhaled, nanoparticles can pass through epithelia of respiratory track and access the bloodstream directly or via lymphatic pathways 13, 14. NPs also can be translocated in the CNS by nerve endings embedded in airway epithelia and nerve endings of the olfactory bulb 13, 15. NPs can damage cells by reactive oxygen species (ROS) formation, mechanical damage of intracellular organelles, and an imbalance in cytosolic Ca2+ concentration 16–18 (Figure 2-b). NPs’ hostility on ion channels and synapse, important components in neuron communication, cannot be denied. Usually, neurodegenerative diseases such as Alzheimer, Parkinson, Huntington’s disease, etc., are diagnosed at a much matured stage and very narrow success has been achieved to improve the patients’ conditions. To understand the role of the NPs interaction with neurons is critical to sense any dis-functionality in cell behavior.
Figure 1.
Schematic of NP localization in various body organs (Figure is reused with permission from reference 5).
Figure 2.
(a) Illustration of possible pathways in which NPs can cross blood brain barrier. The pathway depends on the material and size of NPs. (b) Illustration of cell-NP interaction and nanotoxicity generation. Event 1 represents the extracellular ROS generation outside and inside the cell. Event 2 represents the damage to the cell membrane integrity. Event 3 is particle dissolution or ion leaching affecting the cell function. Event 4 signifies mechanical damage to intracellular organelles due to NP intrusion. Event 5 is indicating the role of NP surface properties (roughness, charge, and active groups) whereas event 6 is highlighting the role of NP size in toxic effects to a cell. Event 7 is representing the NP shape induced toxicity as different shapes of NP may interact with the cell differently. Event 8 is dissolution or leaching of nanoparticle outside the cell membrane and making it easier to penetrate the cell membrane for nanoparticle and affect the cell function. Figures 2-a and 2-b are reused with permission from references 10 and 16, respectively.
Research about the potential health risks of NPs exposure lags behind the rapid development of nanotechnology 19–22. Only the federal agency of United States alone has invested $750 million (from 2006–2014) in research to establish “risk assessment” of environment health and issues related to use of nanomaterials 23. The National Institute for Occupational Safety and Health (NIOSH) has identified risk assessment as one of the 10 critical areas that it wants to “guide in addressing knowledge gaps, developing strategies, and providing recommendations” 24. Strategic efforts of US and Europe are continuing to establish risk assessment of nanoparticle exposure. The silent features of a NP which cause toxicity are discussed on next section.
1.1 Causes of nanotoxicity
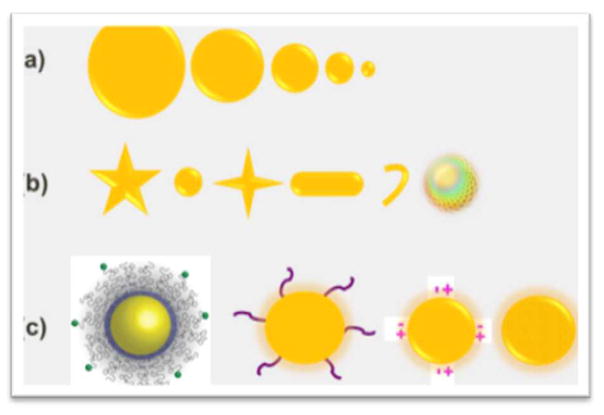
The physico-chemical properties of materials play an important role when NPs interact with biomolecules or biological systems. These properties not only define the uptake and excretion of NPs, but also explain the interference (toxicity) with the biological system 25. The most discussed properties of a NP causing toxicity are its chemical composition, shape, size, surface charge, surface functional group, reactivity, and ability of a material to be stable in the biological system (Figure 3). Also, the longevity of NP-cell interaction and the exposed dose are definitely deciding factors in the adverse effects of nanomaterials.
Figure 3.
Illustration of NPs basic properties responsible for toxicity. (a) Illustration of the size effect of NP, smaller NPs (<5 nm) not only can cross the cell membrane, but also can interfere and damage the intracellular organelles. (b) Illustration of different shape of nanomaterials; it is proven that a sphere shape NP is less toxic than a rod or star shape NP. (c) Illustration of NP surface functionalization; besides the intrinsic surface property i.e. surface roughness and surface charge, the functionalized group on the surface plays a major role in dictating the behavior of NP in biological system.
The intrinsic property (chemical composition) of a material defines the basic character of a material. For that reason, some nanomaterials (e.g., CdO, CuO) are toxic whereas some materials are biocompatible (less or non-toxic, e.g., Au, FeO3) 26. In an experiment performed by our group earlier, we found that gold NPs are nontoxic while cadmium oxide nanoparticles of the same size and dose are highly toxic, whereas Ag and CNT showed some sign of toxicity 27. Shape and size of a NP becomes an important factor to provide large surface to volume ratio to the nanoparticles. It is generally believed that the smaller the NP, more toxic it is 28. Due to larger surface to volume ratio, NPs have more molecules on the surface and possess higher reactivity which can enhance the intrinsic toxicity 29. In a recent study by Wang et al., it was found that longer single wall carbon nanotubes were more toxic than short single wall carbon nanotubes on PC12 cells 30. Another study conducted by Napierska et al. 31 showed that the mono-dispersed amorphous silica NPs exhibit size dependent toxicity on endothelial cells with smaller size of NPs of the same morphology being highly toxic compared to larger size of NPs at same concentration. The surface properties of a NP play a great role in defining its reactivity in biological systems. Sometimes, the toxicity of NPs can also be tuned by changing its surface property, such as by decorating a compatible functional group or by changing the surface charge (zeta potential). Huang et al. showed that the shape of the mesoporous silica NP affect the cellular functions 32. A study conducted by Marques et al. 33 to verify the surface charge effect on internalizations of noble NPs, Au (~26.5 nm) and Ag (~33.3 nm), showed that the positively charged NPs (Au+ and Ag+) were more susceptible to internalization by the mast cells compared to their negatively charged counterparts (Au− and Ag−).
1.2 Single cell nanotoxicity measurement
Traditional methods of analyzing cells are based on the averaging of results studied from multiple cells in an assay, also referred as bulk assay. The results are correlated and assumed to be equally contributed to by all cells of the population under study. However, recent advancements in single cell studies have shown that individual cells behave differently from the population even under identical environmental conditions.
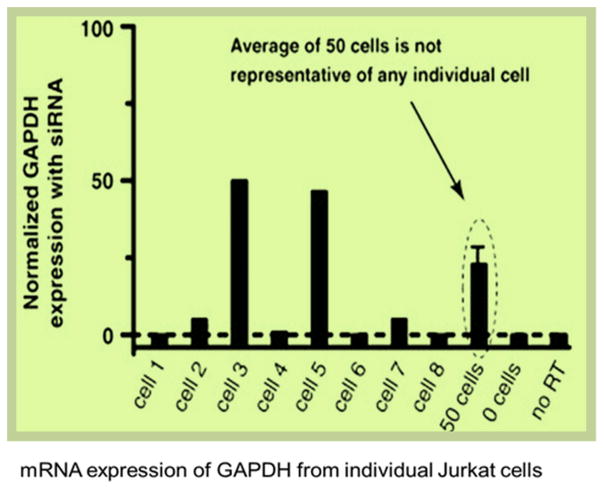
Cell heterogeneity has been studied by researchers recently, suggesting that the cells in a sub-population may have different behavior from the general population results 35–37. In a published study by Toriello et al. 34 (Figure 4), mRNA expression of GAPDH from individual Jurkat cells after siRNA knockdown, the levels fall into roughly two categories: 50% and 100% knockdowns (i.e. 50% and 0% expression remained). Importantly, the average GAPDH expression obtained from the measurement of 50 cells (21 ± 4%) was not representative of any individual cell. This means that the cell population study in aggregation may hinder some very important cell mechanisms which are only visible at the single cell level. To study the true functionality of a cell, it is really important to study a cell independently without interference from neighboring cells. Studying multiple cells in a single assay might obscure some important information that can only be understood by a single cell study. In bulk assays, the difficulties to distinguish the result from a smaller response of homogeneous population or a larger response from a small subpopulation of cells have been discussed earlier 38. Therefore, single cell analysis can be an equivalent and complementary strategy to existing approaches. Especially for neuronal cells, the physiological function can be monitored by recording the pattern of electrical activity and any disturbance in these patterns of electrical activity could serve as a highly sensitive way to measure the functional toxicity as interference of the functional activity can be observed before any other changes are monitored, and much before the cell death. Figure 5 illustrates the reasons of the selection of the single cell for nanotoxicity assessment.
Figure 4.
Gene expression of GAPDH for Jurkat cells treated with GAPDH siRNA relative to normal untreated cells. The levels of GAPDH expression roughly fall into two categories: 0% and 50% of normally untreated cells. However, the average GAPDH expression from bulk assay (50 cells) is 21±4 %, which is not representative of any individual cell. Figure adapted from 34.
Figure 5.
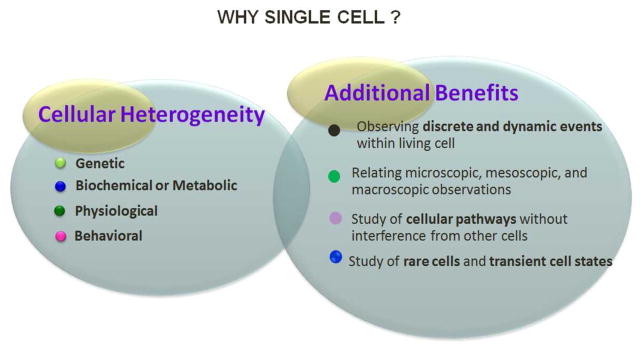
Highlights of the single cell study. Single cell behavior differs due to heterogeneity, which is a resultant of one of the genetic, biochemical/metabolic, physiological or behavioral heterogeneity. Additional benefits such as observation of discrete and dynamic events of cell during cell’s life-time, cellular pathways study without interference of neighbor cells, comparison or relating microscopic to macroscopic (single cell to a large population of cell) and study or rare and transient cell states can only be achieved by single cell analysis.
2. Assessment of nanotoxocity: state-of-the-art
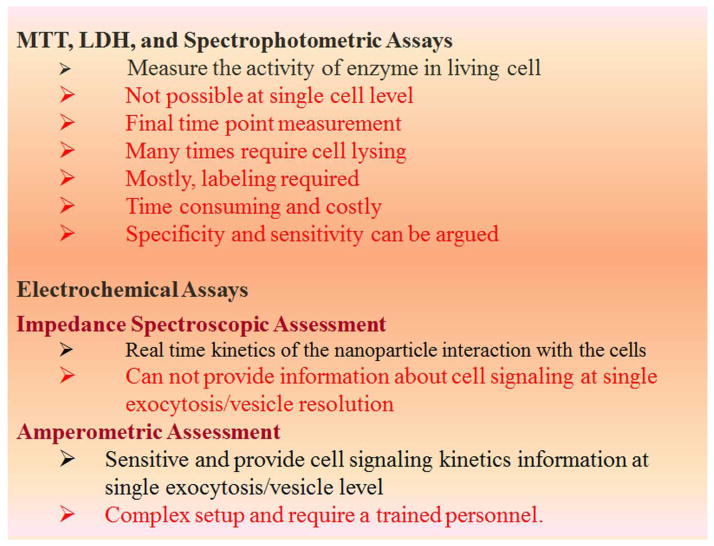
Since, various characteristics or properties of a NP can induce toxicity in a numerous ways; many toxicity assays from chemical toxicity assays are available for toxicity assessment. Principally, the toxicity of NPs is measured in terms of change in viability of cells or functional changes in the cells (i.e. DNA damage, gene alteration, ROS generation). The detailed explanation of conventional nanotoxicity assessment techniques have been explained in many reports 39, 40. Figure 6 illustrates the adopted strategies for nanotoxicity assessment. The brief introduction and state-of-the-art features of these techniques are described in the next section.
Figure 6.
Table highlighting the common limitations associated with dye/optical based assay and electrochemical approach as an alternative mechanism for nanotoxicity assessment.
2.1. Viability assays
Metabolic rate, cellular membrane integrity, apoptotic/necrotic cell death or the rate of proliferation properties of cells is utilized to evaluate cell viability. The parameters may overlap and is presented only as a classical way of cell viability demonstration. Dye based viability assays mostly work on the principle of inclusion, exclusion or conversion of an added dye in live versus dead cells which can further be identified by colorimetric or fluorescent assays 41. Trypan blue exclusion assay 41–44 is used to characterize the viability of cells. This assay is based on a diazo dye, which can only be taken up by dead cells and is excluded by live cells. Alamar Blue assay 41, 43, 45 is another common cell viability assay. The Alamar Blue reagent is a non-toxic, water-soluble resazurin dye that yields a fluorescent signal and a colorimetric change when incubated with metabolically active cells. There are many other dye based (fluorescent and non-fluorescent) live-dead assays, such as calcein AM and propedium iodide based assays 46, neutral red assay 47, and Live/Dead (Invitrogen, Carlsbad, CA) 48.
LDH (Lactate dehydrogenase) assay 31, 41, 49, 50 has been used to understand the integrity of cellular membrane. LDH is an indicator of lytic cell death, as soluble LDH is released into the extracellular medium through damaged cell membrane. Tetrazolium salt-based assays (MTT, MTS, WST) 49, 51, 52 are widely used for the proliferation rate measurement of cells under the influence of NPs. In this metabolic assay, MTT is reduced by cells into blue/purple color non soluble formazan dye. Formazan dye is then solubilized in DMSO to get the average idea of cell viability. Higher metabolic rate (more blue color) is an indication of more viable cells in the population. In a 3H Thymidin-based assay 53, uptake of 3H by newly synthesized DNA can be used as the detection of cell proliferation. Alamar Blue is comparatively simpler and more sensitive method for cell metabolism detection than MTT; however, possibilities of false positives or negatives cannot be nullified 54. A study conducted by Riviere and Zhang 55 showed that the obtained results of viability with different dyes for different materials were inconsistent and this inconsistency could be contributed by nanomaterial/dye interaction and nanomaterial adsorption of the dye. Thus, it is always recommended to use more than one type of dye-based assays before confirm the results. Casey et al. 56 found that the CNT interfering with MTT, Alamar Blue and neutral red dyes giving false measurements and so pressed the urgent need of developing alternative techniques to quantify nanotoxicity. These studies raised concern about widely used viability/cytotoxicity assays for nanomaterial toxicity screening and thoughtfully suggested the need of alternative techniques for nanomaterial and biological system interaction evaluation. This also highlights the basic limitation of the conventional and new dye-based assays in general.
The above-mentioned assays are bulk, require a huge population of cells to experiment and a consensus is built for every cell. Since, the role of the single cell study is found to be important, there are a few single-cell based viability dye-based cell viability assays which can further be incorporated with flow-cytometer for high throughput detection. Annexin V is a marker of externalization of phosphatidylserine on the outer surface of plasma membrane, is an early sign of apoptosis. Labeling of annexin V 57 with fluorescent or radioactive molecules makes it possible to identify the binding of annexin V on the surface of dead (apoptotic) cells. TUNEL assay 58 can be used to identify the cell death (apoptotic) by detecting fragmented DNA by labeling the terminal end of nucleic acids. Colony forming assay 59 does not require any additional tagging. Here, the ability of a single (or very few cells) to form a colony is measured as indication of being healthy over the period of few days.
2.2. Functional assays
Functional assay are more specific to detect nanotoxicity at the genomic level, change in gene formation, DNA damage, reactive oxygen species (ROS) generation, etc.
Oxidative stress is commonly observed due to the influence of NPs 41, 57. This phenomenon is linked with toxicity as un-repairable oxidative stress generates free intra-cellular radicals and damages the lipid, protein, and nucleic acids. Direct/indirect intracellular ROS measurement assays include the glutathione (GSH) assay 60, which is a luminescent-based assay and has been used for the detection and quantification of glutathione in cells. Lipid peroxidation measurement assay measures increasing concentrations of end products of lipid peroxidation indicating increased oxidative damage in the cells 39, 41, 42, 45, 61. For example, 2, 7-dichlorofluorescin (DCFH) assay which detects intracellular DCFH oxidation due to the presence of hydrogen peroxides 41, 43, 45, 47, 57, 60, 62 to measure free radicals in cells and tissue. Besides stress, cellular inflammation response also can be used as the measure of cytotoxicity by detecting specific biomarkers. Cytokines are particularly related with the cell proliferation and inflammation. Immunoassays (i.e. ELISA 63) are used to detect secreted cytokines such as detection of interleukin 6 (IL-6) 64, interleukin 8 (IL-8) 64, 65 or monocyte chemotactic protein-1 (MCP-1) 66. However, ELISA tests are usually time consuming and require multiple operations. Detection of DNA damage can be sensed at the single cell level by comet assay (single cell gel electrophoresis) 67. However, mitochondrial DNA damage and smaller DNA fragments (<50 Kb) are hard to be detected by comet assay, and some apoptotic cells also that can be washed during the lysis will not be detected by the assay. Mutation or the change in a particular gene can be identified by several assays for oxidized guanine bases. These modifications are often the resultant of oxidative stresses and traditionally are identified by immunohistochemistry or HPLC techniques. PCR/RT-PCR array 63 can be used to identify the panel of genes. Karyotype analysis 68 can provide the information about number and integrity of chromosome by detecting the micronucleis of the cell undergoing cell-division, under the NP influence.
Traditionally, we have seen dye based assays for the nanotoxicity assessment and it has been shown that NPs interfere with dye and many times give false positive results. Moreover, many times these techniques possess limitations of being end point measurements instead of dynamic and real-time measurements, requiring cell lysing and cannot be performed on single cell level without an additional labeling system. Recent advances in nanotoxicity assessment have tried to address some of the problems faced by these traditional techniques and are discussed in the following section.
3 Recent advances in nanotoxicity assessment
The growth of nanotechnology has offered not only the nanomaterials with unique properties, but also presented advanced analytical tools which exhibit highly sensitive sensing mechanisms. Efforts have been put together to develop either device based or new technique based approaches to evaluate the hidden parameters of NP-biological interaction (Figure 7).
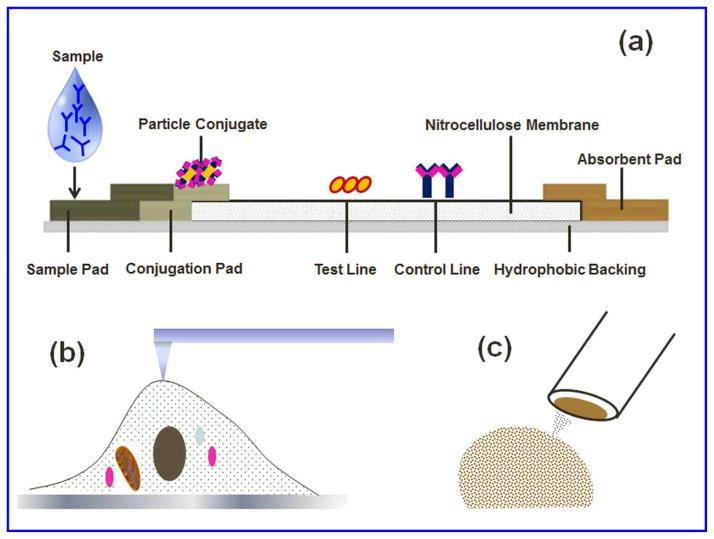
Figure 7.
Recent advancement for nanotoxicity assessment. (a) Lateral flow immune-strip (FLIS) is very common for pregnancy test and some other biomarker application. LFIS was first time used for evaluation of genotoxicity/DNA damage detection upon NP exposure in reference 70. (b) AFM is used to measure the adhesion force and stiffness of the cell membrane upon diesel NP exposure on human aortic endothelial cells 71. (c) Carbon fiber microelectrode is a very sensitive technique with very high temporal resolution and has been used recently to identify the change in exocytosis behavior of endocrine or immune cells upon NP exposure 72–74.
3.1 Lateral flow immunoassay
ROS induced oxidative DNA damage is a well-known trait of nanotoxicity. 8-hydroxyguanine and its nucleoside 8-hydroxy-2′-deoxyguanosine (8-OHdG) are the most studied oxidized guanine bases 69. Commonly used techniques for 8-OHdG analysis are high-performance liquid chromatography (HPLC) with electrochemical detection (ECD), gas chromatography-mass spectrometry (GCMS), HPLC tandem mass spectrometry, and Enzyme linked Immunosorbent Assay (ELISA). However, most of these techniques are time-consuming, expensive, and require special techniques and equipment. Our group has recently developed a novel lateral flow immunoassay (LFIA) 70 to overcome these challenges and to measure the concentration of 8-OHdG and thus reveal the nanotoxicity on the genomic level. The LFIA approach can be simple, scalable, and inexpensive analytical tool for nanotoxicity detection. However, cell lysing was a compulsion for this end point measurement technique.
3.2 Atomic force microscopy
Atomic force microscopy (AFM) is a powerful force sensitive technique and has been successfully applied in single cell studies to gather the information on cell structure, topography, membrane nanostructures, and mechanics (e.g., adhesion force, elasticity) of mammalian cells at a nano-scale resolution under physiological conditions or near physiological conditions 71. In a recent study by Blechinger et al. 75, the uptake and localization of SiO2 NPs were scanned by using AFM combined with fluorescence microscopy. An atomic force microscope can be used to study the mechanics of cell under the influence of nanoparticles. Recently, Wu et al. 71 used AFM to study the biophysical properties of vascular endothelial cells at a single cell level upon diesel exhaust particles exposure. By using AFM, Wu et al. measured the mechanical properties (young’s modulus and adhesion force) and the topography of the cell membrane.
3.3. Carbon fiber microelectrode
Carbon fiber microelectrodes (CFME) of tip size ~5–10 μm exhibit high sensitivity and low noise levels for single cell analysis. Their ability to detect diffusion limited current at very high scan rates allows better temporal resolution 76. CFME amperometry technique is used to explore biophysics of exocytosis, and has been proved as an important tool to understand cellular communication under the influence of NPs. Marquis et al. 77 used CFM amperometry to characterize serotonin exocytosis from murine peritoneal mast cells co-cultured with fibroblasts for 48 hours on interaction with Au nanoparticles (12–46 nm). A decrease in granule transport and fusion events along with increments in intracellular matrix expansion and higher number of serotonin exocytosis per granule was observed. The effect on cell viability when NP exposure was extended for 48 and 72 hours 74 was also studied. The effect of citrate reduced noble NPs, Au (28 nm) and Ag (61 nm), on neuroendocrine cells has been evaluated by Love and Haynes 72. In this work, the uptake quantification, the lysing of the cells, and the total metal content were measures of NP uptake and were measured using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The uptake rate for Ag and Au NPs (1 nM) was found different for each type after 24 hours of exposure, 3.4×104 versus 7.5×105 NPs per cell, respectively. This suggests higher internalization of the Au NPs. The observed different rate of NP internalization was dependent on factors like size, surface charge, and functionalization. CFME amperometry exposed the changed exocytosis behavior of chromaffin cells in this study.
Metal oxide NPs (MONP) such as nonporous SiO2, porous SiO2, and nonporous TiO2 are being used in consumer products of everyday life. CFME amperometry technique has explored the nanotoxicity effect of MONP on immune cells by Maurer-Jones et al. 73. The outcomes of their studies revealed functional changes in chemical messenger secretion from mast cell granules. The surface properties of NPs are known to play a major role to decide the nature of interaction with biological systems. Love et al. 78 further utilized this technique to evaluate the changes in cellular communication in neuroendocrine cells on exposure of size dependent Ag NPs and surface functionalized Au NP for 24 hours. Authors conclude that Ag NPs of 15 – 60 nm do not alter cell viability but exhibited size dependent cellular uptake and increase in the speed of exocytosis release kinetics. Beside this, PEG-functionalized Au NPs did not change the cell viability; however, they decreased the number of molecules released from each vesicle.
3.4. Fluidic based cell-on-chip (CoC) approach
Micro-chip-based biosensors show a promising future for monitoring cellular nanotoxicity as they allow rapid, real-time and multi-sample analysis creating a versatile, noninvasive tool that is able to provide quantitative information with respect to alteration in cellular function under various nanomaterials exposures. Most of the CoC-based approaches for nanotoxicity assessment at present are based on multiple cell screening. As the importance of single cell analysis besides the multiple cells screening already highlighted, some efforts have already been in the direction. In a recently published study by our group 79, we presented a chip-based biosensor capable of selective trapping of single cell dielectrophoretically in a micro-well and the same electrode can be used for further electrochemically study the captured cell. These electrodes are independently addressable for capturing a single cell in an individual microwell as well as study an independent cell on order. This CoC can be used for nanomaterial based toxicity assessment. Cell behavior can be monitored in terms of catacalomine release from cell vesicles.
Electrochemical and optical measurement based fluidic chip platform have been explored for cell analysis over last decade 80 which hold the potential to be used for nanotoxicity assessment. Zheng et al. 81 evaluated cytotoxicity of cadmium containing quantum dots on multicellular (HEK293 cells) events using microfluidic chip with fluorescent microscope. In another study by Hosokawa et al. 82, microfluidic chip based high throughput single cell array device was developed to study the gradient generated cytotoxic effect of toxin. Similar platform could be applied to study nanoparticle concentration dependent toxicity in single cell. However, optical assays are not label free and variation in batch to batch measurement due to die selection limits the application of these assays. These challenges are being addressed using electrochemical based nanotoxicity assessment.
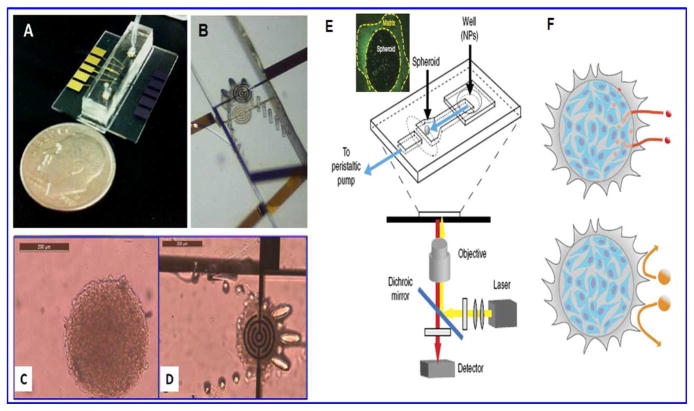
In a recent experiment by Kim et al. 83, a chip-based electrochemical approach was used for the assessment of nanotoxicity. On a lithographically patterned chip platform, gold electrode modified with RGD-MAP-C to enhance cell (SH-SY5Y) adhesion on the chip was used as the sensing electrode. Silica NPs of various sizes and surface chemistries were examined to understand the effects of induced nanotoxicity on SH-SY5Y cells by studying cell viability at different concentrations of NPs ranging from 50 μg/ml to 400 μg/ml at various time points. Electrochemical measurements of nanotoxicity were recorded using differential pulse voltammetry and were compared with absorption and fluorescence-based techniques to evaluate the benefits of electrochemical measurements to assess nanotoxicity. In another experiment to overcome the limitation of statics models, Kim et al. 84 studied the cytotoxicity of mesoporous silica NPs (< 50 nm) to human endothelial cells under microfluidic flow conditions to mimic more of a blood vessel environment. This study tried to cover the missing factor of the shear-stress component, missing in most of the in vitro nanotoxicity studies, to mimic the actual NP toxicity in blood vessels. In their study, it was found that unmodified mesoporous silica NPs induced larger loss of cell viability under shear stress conditions than static conditions whereas organo-modified NPs did not have a significant difference in toxicity of both conditions. The biggest advantage of the chip-based approach is that it provides the opportunity to measure the real-time kinetics (dynamic) of a cell rather than only providing the end point result (static) after a certain time, a disadvantage of major conventional nanotoxicity techniques. In a published study by our group, Hondroulis et al. 27, a whole cell based electrical impedance sensing (EIS) approach was developed for rapid and real-time assessment of nanomaterials (gold and silver NPs, single walled carbon nanotubes, and cadmium oxide) toxicity. This technique has huge advantages over the traditional nanotoxicity assessment techniques. EIS is cheaper, faster, and quantitative, and allows real time sensing of cell behavior. The study showed that gold nanoparticles to be nontoxic and cadmium oxide to be highly toxic, whereas, smaller size of silver nanoparticle (10 nm) were more toxic than larger (100 nm) and the length of SWCNT did not change the toxic effects. Recently, Alexander Jr. et al. 85 experimented silica nanowire toxicity on epithelial breast cancer cells and found dose dependent toxicity using an array based real-time impedance measurement chip. Impedance-based sensing approach is a highly sensitive method to monitor cell behavior (growth, health, motion, etc.) on top of the electrode in a very simple setup. The limitations of monolayer cell populations have also been highlighted recently in their limitation of not being able to mimic the 3D tissue culture and emphasis on in vitro 3D cell culture for nanotoxicity assessment. Luongo et al. 86 recently published a study on a microfluidic device fabrication for trapping and monitoring 3D multi-cell spheroid using real-time electrical impedance spectroscopy (figure 8). Also the proposed designs of 3D cellular/spheroid monitoring designs used for drug studies can be utilized for nanotoxicity evaluation 87–89. The role of NPs for drug delivery is highlighted many times, however, a recently published study by Albanese et al. 90 discussed NP transport kinetics and NP tissue accumulation in a tumor spheroid mounted on a microfluidic chip for different size, and surface modified NPs for drug delivery and diagnostic application. Similar designs in nanotoxicity evaluation will definitely curtail the gap of nanomaterial-biological interaction.
Figure 8.
A) is a microfluidic chip for spheroid capture and real-time impedimetric detection. B) is zoomed area of the microfluidic channel and the trap mechanism for spheroid. C) is the image of a multicellular spheroid before perfusion and D) is the entrapment of spheroid between two impedimetric electrodes. E) Another tumor on chip platform placed on an inverted microscope, the highlighted part is live spheroid and F) is schematic representation showing accumulation of smaller NPs (40 nm) in interstitial tissue whereas escape of bigger NPs from penetration in spheroid matrix. Images A–D are reused with permission from 86 and E–F from 90.
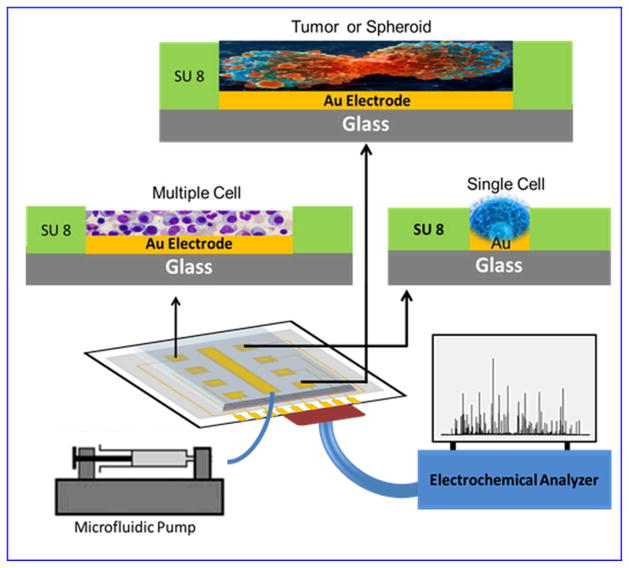
Looking at the recent advancements in nanotoxicity assessment, an ideal platform would look like the illustration in Figure 9 wherein, real-time cell analysis can be performed on a microfluidic chip platform. The new generation of microfluidic chips should include not only bulk assay, but also single cell analysis in order to evaluate and clearly identify the role of cell communication in spreading or deterring of nanotoxicity. The next section briefly discusses the associated challenges in nanotoxicity assessment with conclusion.
Figure 9.
Schematic of an ideal microfluidic CoC device for nanotoxicity assessment. The presented CoC can assess toxicity at single cell level and at group of cells. It can compare the microscopic and macroscopic effects and can present a clear role of NP interaction with single cell by blocking other stimulationg factors, such as cues from neighbour cells, and with the overall integrative effects produced by NP and the cell cell communicatopon in a population of cells.
4.0. Challenges and conclusion
Looking at the growth of nanotechnology, number of nanomaterials (metallic, metal oxide based, ceramic, carbon based, polymeric, polymer based, biomolecule based, magnetic and composites) are developed along with various sizes makes the matrix of nanomaterials to be tested very huge. Screening of the huge number of nanomaterials at in vivo level cannot provide the speed, cost and faces ethical concern. A large number of in vitro test are available, and we need to perform more than one type experiment to be sure about the results. In such a condition, we need very sensitive, rapid, cost effective and easy to operate analytical tools in labs. Single cell has been shown to be more sensitive towards a smaller change than population of cell in a shorter time 91.
To analyze single cells, sorting of a single cell from population of cell is necessary. One of the most frequently used method to quickly and efficiently sort, count and/or measure the characteristics of single cells in large volume (large throughput) is flow-cytometry (FCM). However, it requires cells to be tagged with fluorescent probes that may interfere with the natural behavior of the cell. A technique which measures cell behavior in its natural state would be ideal. Patch clamp and carbon fiber microelectrode based techniques are good for single cell analysis; however, require cumbersome and expensive equipment, as well as, a trained professional to conduct the experiments. Performing these techniques can be very time consuming and yield a low throughput. On the other hand, using a chip based approach to sort and analyze single cells out of a population seems to be more cost effective, simpler and can yield a higher throughput performance. Microelectrodes on chip integrated with microfluidic control and automation not only would make the initial nanotoxicity screening easy, but also would increase the participation from various authorities, and encourage the nanotoxicity assessment efforts throughout the world. CoC-based assays will provide more dynamic information of cell and particle interaction. Currently, not all the labs that work with nanomaterials have the facilities and equipment to perform traditional nanotoxicity assays. CoC-based assay can be an initial screening point for nanotoxicity.
Acknowledgments
This study was supported by the grant R15ES021079 from the National Institute of Health (U.S.A.) and the grant 21328501 from National Science Foundations of China to CZ Li and CX Zhang.
References
- 1. [Accessed 12/03/2013.];Consumer Products Inventory. http://www.nanotechproject.org/cpi.
- 2.Trout DB, Schulte PA. Toxicology. 2010;269:128–135. doi: 10.1016/j.tox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Simko M, Mattsson MO. Part Fibre Toxicol. 2010;7:42. doi: 10.1186/1743-8977-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzea C, Pacheco, Robbie K. Biointerphases. 2007;2:MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 5.Kreyling WG, Hirn S, Schleh C. Nat Biotechnol. 2010;28:1275–1276. doi: 10.1038/nbt.1735. [DOI] [PubMed] [Google Scholar]
- 6.Hartung T. ALTEX. 2010;27:87–95. doi: 10.14573/altex.2010.2.87. [DOI] [PubMed] [Google Scholar]
- 7.De Jong WH, Borm PJ. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu MS, Li J, Iwai H, Mei QS, Fujita D, Su HX, Chen HZ, Hanagata N. Sci Rep-Uk. 2012:2. doi: 10.1038/srep00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockman PR, Mumper RJ, Khan MA, Allen DD. Drug Dev Ind Pharm. 2002;28:1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 10.Nanoparticles for drug delivery to the brain. http://en.wikipedia.org/wiki/Nanoparticles_for_drug_delivery_to_the_brain.
- 11.Silva GA. Nat Rev Neurosci. 2006;7:65–74. doi: 10.1038/nrn1827. [DOI] [PubMed] [Google Scholar]
- 12.Jain KK. Clin Chem. 2007;53:2002–2009. doi: 10.1373/clinchem.2007.090795. [DOI] [PubMed] [Google Scholar]
- 13.Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Br J Pharmacol. 2007;150:552–558. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Rahman MF, Duhart HM, Newport GD, Patterson TA, Murdock RC, Hussain SM, Schlager JJ, Ali SF. Neurotoxicology. 2009;30:926–933. doi: 10.1016/j.neuro.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 16.Suh WH, Suslick KS, Stucky GD, Suh YH. Prog Neurobiol. 2009;87:133–170. doi: 10.1016/j.pneurobio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedorovich SV, Alekseenko AV, Waseem TV. Biochem Soc Trans. 2010;38:536–538. doi: 10.1042/BST0380536. [DOI] [PubMed] [Google Scholar]
- 18.George S, Pokhrel S, Xia T, Gilbert B, Ji ZX, Schowalter M, Rosenauer A, Damoiseaux R, Bradley KA, Madler L, Nel AE. ACS Nano. 2010;4:15–29. doi: 10.1021/nn901503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Win-Shwe TT, Fujimaki H. Int J Mol Sci. 2011;12:6267–6280. doi: 10.3390/ijms12096267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES. PLoS One. 2012;7:e47674. doi: 10.1371/journal.pone.0047674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shvedova AA, Kagan VE, Fadeel B. Annu Rev Pharmacol Toxicol. 2010;50:63–88. doi: 10.1146/annurev.pharmtox.010909.105819. [DOI] [PubMed] [Google Scholar]
- 22.Hubbs AF, Mercer RR, Benkovic SA, Harkema J, Sriram K, Schwegler-Berry D, Goravanahally MP, Nurkiewicz TR, Castranova V, Sargent LM. Toxicol Pathol. 2011;39:301–324. doi: 10.1177/0192623310390705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NNI Budget. http://nano.gov/about-nni/what/funding.
- 24.Howard J. The National Institute for Occupational Safety and Health (NIOSH) :10. http://www.cdc.gov/niosh/enews/enewsV10N3.html.
- 25.Chou LY, Ming K, Chan WC. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 26.Sohaebuddin S, Thevenot P, Baker D, Eaton J, Tang L. Particle and Fibre Toxicology. 2010;7:22. doi: 10.1186/1743-8977-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hondroulis E, Liu C, Li CZ. Nanotechnology. 2010;21:315103. doi: 10.1088/0957-4484/21/31/315103. [DOI] [PubMed] [Google Scholar]
- 28.Nel A, Xia T, Madler L, Li N. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ. Occup Environ Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Sun P, Bao Y, Liu J, An L. Toxicology in Vitro. 2011;25:242–250. doi: 10.1016/j.tiv.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Napierska D, Thomassen LC, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, Martens JA, Hoet PH. Small. 2009;5:846–853. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Teng X, Chen D, Tang F, He J. Biomaterials. 2010;31:438–448. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 33.Marquis BJ, Liu Z, Braun KL, Haynes CL. Analyst. 2011;136:3478–3486. doi: 10.1039/c0an00785d. [DOI] [PubMed] [Google Scholar]
- 34.Toriello NM, Douglas ES, Thaitrong N, Hsiao SC, Francis MB, Bertozzi CR, Mathies RA. Proc Natl Acad Sci U S A. 2008;105:20173–20178. doi: 10.1073/pnas.0806355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irish JM, Kotecha N, Nolan GP. Nat Rev Cancer. 2006;6:146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 36.Graf T, Stadtfeld M. Cell Stem Cell. 2008;3:480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Bodovitz S. Trends Biotechnol. 2010;28:281–290. doi: 10.1016/j.tibtech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trisha Eustaquio JFL. In: Nanotoxicity. Reineke J, editor. Vol. 5. Humana Press; 2012. pp. 69–85. [Google Scholar]
- 39.Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Fukagawa NK, Mossman BT. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:219–231. doi: 10.1002/wnan.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquis BJ, Love SA, Braun KL, Haynes CL. Analyst. 2009;134:425–439. doi: 10.1039/b818082b. [DOI] [PubMed] [Google Scholar]
- 41.Jones CF, Grainger DW. Adv Drug Deliv Rev. 2009;61:438–456. doi: 10.1016/j.addr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poonam Takhar SM. Archives of Applied Science Research. 2011;3:389–403. [Google Scholar]
- 43.Bhattacharya K, Davoren M, Boertz J, Schins RP, Hoffmann E, Dopp E. Part Fibre Toxicol. 2009;6:17. doi: 10.1186/1743-8977-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlsson HL, Cronholm P, Gustafsson J, Moller L. Chem Res Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 45.Fahmy B, Cormier SA. Toxicology in Vitro. 2009;23:1365–1371. doi: 10.1016/j.tiv.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders K, Degn LL, Mundy WR, Zucker RM, Dreher K, Zhao B, Roberts JE, Boyes WK. Toxicol Appl Pharmacol. 2012;258:226–236. doi: 10.1016/j.taap.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Pujalte I, Passagne I, Brouillaud B, Treguer M, Durand E, Ohayon-Courtes C, L’Azou B. Part Fibre Toxicol. 2011;8:10. doi: 10.1186/1743-8977-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JY, Lee SH, Na HB, An K, Hyeon T, Seo TS. Bioprocess Biosyst Eng. 2010;33:21–30. doi: 10.1007/s00449-009-0354-5. [DOI] [PubMed] [Google Scholar]
- 49.Ahamed M, Akhtar MJ, Siddiqui MA, Ahmad J, Musarrat J, Al-Khedhairy AA, AlSalhi MS, Alrokayan SA. Toxicology. 2011;283:101–108. doi: 10.1016/j.tox.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Soenen SJ, Himmelreich U, Nuytten N, De Cuyper M. Biomaterials. 2011;32:195–205. doi: 10.1016/j.biomaterials.2010.08.075. [DOI] [PubMed] [Google Scholar]
- 51.Huang CC, Aronstam RS, Chen DR, Huang YW. Toxicology in Vitro. 2010;24:45–55. doi: 10.1016/j.tiv.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Drescher D, Orts-Gil G, Laube G, Natte K, Veh RW, Osterle W, Kneipp J. Anal Bioanal Chem. 2011;400:1367–1373. doi: 10.1007/s00216-011-4893-7. [DOI] [PubMed] [Google Scholar]
- 53.Vankoningsloo S, Piret JP, Saout C, Noel F, Mejia J, Zouboulis CC, Delhalle J, Lucas S, Toussaint O. Nanotoxicology. 2010;4:84–97. doi: 10.3109/17435390903428869. [DOI] [PubMed] [Google Scholar]
- 54.Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P. Toxicology in Vitro. 2004;18:703–710. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Monteiro-Riviere NA, Inman AO, Zhang LW. Toxicol Appl Pharmacol. 2009;234:222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 56.Casey A, Herzog E, Davoren M, Lyng FM, Byrne HJ, Chambers G. Carbon. 2007;45:1425–1432. [Google Scholar]
- 57.Chang Y, Yang ST, Liu JH, Dong E, Wang Y, Cao A, Liu Y, Wang H. Toxicol Lett. 2011;200:201–210. doi: 10.1016/j.toxlet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 58.Bae JE, Huh MI, Ryu BK, Do JY, Jin SU, Moon MJ, Jung JC, Chang Y, Kim E, Chi SG, Lee GH, Chae KS. Biomaterials. 2011;32:9401–9414. doi: 10.1016/j.biomaterials.2011.08.075. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Luanpitpong S, Castranova V, Tse W, Lu Y, Pongrakhananon V, Rojanasakul Y. Nano Lett. 2011;11:2796–2803. doi: 10.1021/nl2011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Brandau W, Simon U, Jahnen-Dechent W. Small. 2009;5:2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 61.Li SQ, Zhu RR, Zhu H, Xue M, Sun XY, Yao SD, Wang SL. Food Chem Toxicol. 2008;46:3626–3631. doi: 10.1016/j.fct.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Akhtar MJ, Ahamed M, Fareed M, Alrokayan SA, Kumar S. J Toxicol Sci. 2012;37:139–148. doi: 10.2131/jts.37.139. [DOI] [PubMed] [Google Scholar]
- 63.Ho CC, Luo YH, Chuang TH, Yang CS, Ling YC, Lin P. Toxicology. 2013;308:1–9. doi: 10.1016/j.tox.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Ekstrand-Hammarstrom B, Akfur CM, Andersson PO, Lejon C, Osterlund L, Bucht A. Nanotoxicology. 2012;6:623–634. doi: 10.3109/17435390.2011.598245. [DOI] [PubMed] [Google Scholar]
- 65.Cho WS, Duffin R, Poland CA, Duschl A, Oostingh GJ, MacNee W, Bradley M, Megson IL, Donaldson K. Nanotoxicology. 2012;6:22–35. doi: 10.3109/17435390.2011.552810. [DOI] [PubMed] [Google Scholar]
- 66.Morimoto Y, Ogami A, Todoroki M, Yamamoto M, Murakami M, Hirohashi M, Oyabu T, Myojo T, Nishi K, Kadoya C, Yamasaki S, Nagatomo H, Fujita K, Endoh S, Uchida K, Yamamoto K, Kobayashi N, Nakanishi J, Tanaka I. Nanotoxicology. 2010;4:161–176. doi: 10.3109/17435390903518479. [DOI] [PubMed] [Google Scholar]
- 67.Cancino J, Paino IM, Micocci KC, Selistre-de-Araujo HS, Zucolotto V. Toxicol Lett. 2013;219:18–25. doi: 10.1016/j.toxlet.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Diana V, Bossolasco P, Moscatelli D, Silani V, Cova L. PLoS One. 2013;8:e78435. doi: 10.1371/journal.pone.0078435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Fukagawa NK, Mossman BT. Wires Nanomed Nanobi. 2010;2:219–231. doi: 10.1002/wnan.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X, Hondroulis E, Liu W, Li CZ. Small. 2013;9:1821–1830. doi: 10.1002/smll.201201593. [DOI] [PubMed] [Google Scholar]
- 71.Wu Y, Yu T, Gilbertson TA, Zhou A, Xu H, Nguyen KT. PLoS One. 2012;7:e36885. doi: 10.1371/journal.pone.0036885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Love SA, Haynes CL. Anal Bioanal Chem. 2010;398:677–688. doi: 10.1007/s00216-010-3735-3. [DOI] [PubMed] [Google Scholar]
- 73.Maurer-Jones MA, Lin YS, Haynes CL. ACS Nano. 2010;4:3363–3373. doi: 10.1021/nn9018834. [DOI] [PubMed] [Google Scholar]
- 74.Marquis BJ, Maurer-Jones MA, Braun KL, Haynes CL. Analyst. 2009;134:2293–2300. doi: 10.1039/b913967b. [DOI] [PubMed] [Google Scholar]
- 75.Blechinger J, Bauer AT, Torrano AA, Gorzelanny C, Brauchle C, Schneider SW. Small. 2013;9:3970–3980. doi: 10.1002/smll.201301004. [DOI] [PubMed] [Google Scholar]
- 76.LRF, Bard AJ. Electrochemical Methods: Fundamentals and Applications. John Wiley & Sons; New York: 2001. [Google Scholar]
- 77.Marquis BJ, McFarland AD, Braun KL, Haynes CL. Anal Chem. 2008;80:3431–3437. doi: 10.1021/ac800006y. [DOI] [PubMed] [Google Scholar]
- 78.Love SA, Liu Z, Haynes CL. Analyst. 2012;137:3004–3010. doi: 10.1039/c2an00034b. [DOI] [PubMed] [Google Scholar]
- 79.Shah P, Zhu X, Chen C, Hu Y, Li C-Z. Biomedical Microdevices. 2013. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 80.Velve-Casquillas G, Le Berre M, Piel M, Tran PT. Nano Today. 2010;5:28–47. doi: 10.1016/j.nantod.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng X, Tian J, Weng L, Wu L, Jin Q, Zhao J, Wang L. Nanotechnology. 2012;23:055102. doi: 10.1088/0957-4484/23/5/055102. [DOI] [PubMed] [Google Scholar]
- 82.Hosokawa M, Hayashi T, Mori T, Yoshino T, Nakasono S, Matsunaga T. Anal Chem. 2011;83:3648–3654. doi: 10.1021/ac2000225. [DOI] [PubMed] [Google Scholar]
- 83.Kim TH, Kang SR, Oh BK, Choi JW. Sensor Lett. 2011;9:861–865. [Google Scholar]
- 84.Kim D, Lin YS, Haynes CL. Anal Chem. 2011;83:8377–8382. doi: 10.1021/ac202115a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alexander FA, Jr, Huey EG, Price DT, Bhansali S. Analyst. 2012;137:5823–5828. doi: 10.1039/c2an36341k. [DOI] [PubMed] [Google Scholar]
- 86.Luongo K, Holton A, Kaushik A, Spence P, Ng B, Deschenes R, Sundaram S, Bhansali S. Biomicrofluidics. 2013;7:034108. doi: 10.1063/1.4809590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tung YC, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. Analyst. 2011;136:473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poenick S, Jahnke HG, Eichler M, Frost S, Lilie H, Robitzki AA. Biosensors & Bioelectronics. 2014;53:370–376. doi: 10.1016/j.bios.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 89.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albanese A, Lam AK, Sykes EA, Rocheleau JV, Chan WCW. Nat Commun. 2013;4:3718. doi: 10.1038/ncomms3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Asphahani F, Wang K, Thein M, Veiseh O, Yung S, Xu J, Zhang M. Physiol. 2011;8:015006. doi: 10.1088/1478-3975/8/1/015006. [DOI] [PMC free article] [PubMed] [Google Scholar]