Abstract
Background
Children and adolescents with chronic kidney disease (CKD) are inactive relative to their peers.
Methods
Forty-four children and adolescents aged 7-20 years with CKD, end-stage renal disease (ESRD) on dialysis or a kidney transplant participated in a12-week pedometer-based intervention to increase physical activity. Patients recorded daily step counts and reported them weekly. Pediatric Quality of Life Inventory (PedsQL) and six-minute walk (6MW) were administered at baseline and after 12 weeks.
Results
Age was 15.1±3.4 years; 27% had CKD, 16% were receiving dialysis, and 57% had received a kidney transplant. Mean daily step count did not change significantly (+48, 95% CI −48 to +145 steps/day per week). Transplant recipients and patients with CKD increased their activity by 100 steps/day (95% CI −14 to 208) and 73 steps/day (95% CI −115 to 262) each week, respectively, and patients on dialysis decreased by 133 steps/day (95% CI −325 to 58; p-value for interaction 0.03) in multivariable analysis. Change in physical activity was associated with change in 6MW distance (r=0.74, p<0.001) and change in physical functioning (r=0.53, p=0.001).
Conclusions
Youths with CKD did not significantly increase their activity over 12 weeks of a pedometer-based intervention. However, changes in physical activity were associated with changes in physical functioning and performance.
Keywords: Physical activity, children, adolescents, health-related quality of life, physical performance
Introduction
It is well known that adults with chronic kidney disease (CKD) are inactive compared to individuals without kidney disease [1,2], and lower levels of physical activity are associated with higher mortality [3,4]. However, it is difficult to determine among adults whether associations between physical inactivity and mortality are independent of comorbidity that might limit physical activity. Furthermore, if disease is limiting activity, it may be difficult to intervene to increase activity.
Preliminary data suggest that children and adolescents with CKD are inactive relative to their peers and that their activity is considerably less than recommended levels [5]. Although the relationship between physical activity and outcomes has not been studied in children with CKD, ample data from the general population links childhood inactivity to lifelong obesity and other adverse outcomes [6-8]. Among adults, inactivity has been associated with faster progression of CKD [9] and with worse outcomes after kidney transplantation [10], and these associations probably also apply to children. Thus, increasing physical activity among children and adolescents with CKD is potentially of great benefit.
We designed a 12-week pedometer-based intervention among children with CKD, including those with non-dialysis dependent CKD (CKD-ND), end-stage renal disease on dialysis, and those with a transplanted kidney. We sought to determine whether the intervention would lead to an increase in steps walked per day and whether any increase in physical activity would be associated with improvements in physical function or quality of life.
Materials and methods
Participants
Children and adolescents aged 7–20 years with CKD, ESRD on dialysis or with a kidney transplant were recruited from the UCSF Pediatric Nephrology Outpatient Clinic and the Children’s Outpatient Hemodialysis Center as previously reported [5]. We excluded patients who were hospitalized or had initiated peritoneal dialysis or hemodialysis within the prior month or had received a kidney transplant within the prior 3 months. Parents and patients ≥18 years provided written consent for study participation; younger participants also provided assent. The study was approved by the Committee on Human Research at UCSF and registered on ClinicalTrials.gov (NCT01270529).
Study procedures
Height and weight were recorded at a visit to the Pediatric Clinical Research Center (PCRC), and body mass index (BMI) was calculated as kg/m2. The medical record was reviewed and laboratory data from the prior month were abstracted. Glomerular filtration rate (GFR) was estimated using the modified Schwartz equation [11] for patients with CKD and transplant recipients. For patients on dialysis, creatinine clearance was calculated from a 24-hour urine collection and normalized to body surface area. If creatinine clearance data were not available in the participant’s medical record, a new collection was initiated.
Physical activity
Physical activity was measured using a Yamax Digi-walker SW-200 pedometer, which was given to each participant. Participants were instructed to attach the pedometer at the waistline each morning and to wear the pedometer throughout the day while doing usual activities, except swimming or bathing. They were asked to remove the pedometer before going to bed and to record the day’s step count each night before resetting the device to zero for the next day. Participants also recorded any additional activities such as cycling, swimming, etc, and these activities were converted to steps using an activity conversion chart included with the pedometer.
Physical performance
Physical performance was measured using the 6-minute walk test (6MWT), performed on a 60-foot track in a straight corridor. Participants were instructed to walk back and forth in the corridor, without jogging or running, covering as much distance as possible in 6 minutes. Participants were permitted to slow down, stop and rest as necessary but were instructed to resume walking as soon as they were able. After 6 min, the distance walked was recorded in meters.
Physical functioning
Physical functioning was measured using the PedsQL 4.0, a self-report instrument that measures HRQOL for children ages 2-4, 5-7, 8-12, and 13-18 years [12-15]. The multidimensional PedsQL Generic Core Scales measure 4 essential domains: 1) Physical Functioning (8 items), 2) Emotional Functioning (5 items), 3) Social Functioning (5 items), and 4) School Functioning (5 items). We report only the Physical Functioning score because we hypothesized that physical functioning is related to physical activity. This score ranges from 0 to 100, with higher scores indicating better functioning.
Intervention and follow-up
The goal of the intervention was to increase patients’ average daily step counts to the recommended targets for boys (15,000 steps/day) and girls (12,000 steps/day) over the 12-week study period [16]. Patients or parents were told these goals and given education about potential benefits of increasing physical activity to recommended levels. We then contacted participants weekly by telephone or email and asked to relay the daily step count recorded in their logs, and we calculated the mean number of steps taken per day over 7 days for each week. During each weekly participant contact, we provided feedback about whether they had met the weekly targeted step counts, solicited information about barriers to meeting target activity levels, and set a new goal for the upcoming week. Because we hypothesized that increasing activity gradually would be better tolerated and would result in better adherence, we set weekly activity targets that were no more than 1,000 steps/day above the previous week’s level.
Patients returned to the PCRC after 12 weeks, at which time measures of body composition, physical performance and functioning, and HRQOL were repeated.
Statistical analyses
Statistical analyses were performed using STATA 11. Descriptive statistics (means, standard deviations or median, 25th, 75th) were calculated for all continuous variables, and frequencies and percentages were generated for categorical variables. In order to include all participants’ available data in our analyses, we used linear mixed modeling to determine whether step counts increased over the time of the intervention using the average steps per day calculated for each week of study participation. We first performed unadjusted analyses, followed by multivariable analysis adjusting for age, sex, and CKD group (CKD-ND, dialysis, transplant), and baseline steps/day. We also used mixed models to assess changes in BMI, HRQOL, and 6-minute walk distance in the whole cohort and among patients who participated for more than 2 weeks and increased their physical activity by at least 1,000 steps/day. This threshold was set prior to data analysis as a potentially meaningful increase.
Results
Baseline data
We approached 54 potential participants, of whom 44 agreed to participate, and 40 returned for follow-up measurements (Figure 1). Age ranged from 7-20 years (mean 15.1±3.4 years), and twenty-two (50%) participants were boys. Twelve participants (27%) had CKD Stages 1-4, 7 were receiving dialysis (16%), and 25 (57%) had received a kidney transplant from 3 months to 15 years before study participation. Mean BMI was 24±5.6 kg/m2 (BMI-SD score median 0.92, interquartile range (0, 1.57)). Five participants were obese, 15 overweight, 23 normal weight, and one underweight. Median eGFR was 60 (12, 146) ml/min/m2 and mean hemoglobin concentration was 12.0±1.8 g/dl (Table 1). At baseline, patients were very inactive, with a median step count of 5,976 (IQR 3,309 to 8595) steps/day, and girls were substantially less active than boys (median steps/day 4,027 [IQR 2,682 to 5,667] vs 9,122 [IQR 6,547 to 11,951], p<0.001). The activity of both boys and girls was substantially below recommended targets (15,000 and 12,000 steps per day, respectively) [17]. Transplant recipients tended to be more active than CKD or ESRD patients (median 8,175 steps/day vs 4,978 for CKD and 4,566 for ESRD; p = 0.10). Self-reported physical function and six-minute walk distance were also below expected norms [5] (Table 1).
Figure 1.
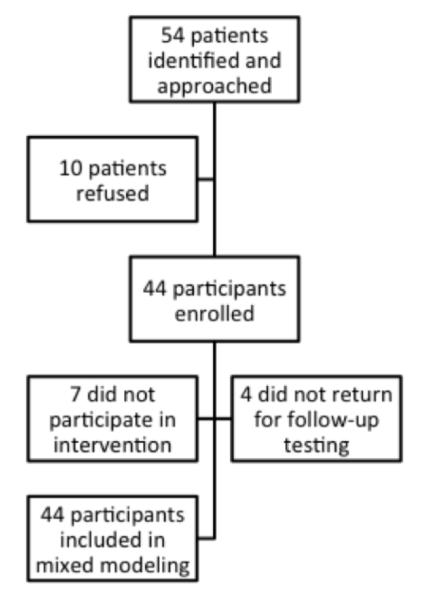
Study flow diagram.
Table 1.
Participant characteristics at baseline*
| Characteristic | N = 44 |
|---|---|
| Age | 15.1 ± 3.4 |
| Female, n (%) | 22 (50) |
| Race/ethnicity, n (%) | |
| White | 12 (27) |
| Hispanic | 20 (45) |
| Asian American | 8 (18) |
| African American | 4 (9) |
| CKD category, n (%) | |
| Transplant | 25 (57) |
| Dialysis | 7 (16) |
| Non-dialysis dependent CKD | 12 (27) |
| eGFR/CrCl, ml/min/1.73m2† | 60 (12, 146) |
| Body mass index, kg/m2 | 23.9 ± 4.8 |
| BMI-SD Score | 0.92 (0, 1.57) |
| Hemoglobin, g/dL | 12 ± 1.8 |
| Daily steps (mean over 7 days) | 5,976 (3309, 8,596) |
| Six-minute walk distance, m | 508 ± 100 |
| PF scale of PedsQL | 72.3 ± 17.3 |
Continuous variables are mean ± sd or median (25th, 75th). PF, Physical Function; PedsQL, Pediatric Quality of Life Inventory; BMI-SD, body mass index standard deviation, indicating the number of standard deviations from the age-and sex-standardized norm the patient’s BMI falls.
GFR estimated by the modified Schwartz formula for CKD and transplant recipients. In patients with ESRD, creatinine clearance was calculated using the 24-hour urine collection and normalized to 1.73m2.
Change in physical activity
Thirty-three participants wore the pedometers and provided step count data for all 12 weeks of the study. Seven participants did not wear the pedometers beyond the first week of baseline data collection; 4 participants provided data for part of the 12-week period (one each for 2, 4, 8 and 11 weeks). All available data were used in analyses, and overall, the participants did not increase their activity to a statistically significant extent. Specifically, the daily step count increased by 48 (95% CI −48 to 145) steps each week from a baseline of 6218 (95% CI 3637 to 9828) steps per day.
In multivariable analysis, there was a significant interaction between CKD category and change in steps per day, with transplant recipients and patients with CKD increasing their activity by 100 steps/day (95% CI −14 to 208) and 73 steps/day (95% CI −115 to 262) each week, respectively, and patients on dialysis decreasing by 133 steps/day (95% CI −325 to 58) after adjustment for age, sex and baseline steps per day (p-value for interaction 0.03; Table 2). Older participants tended to increase their activity to a lesser extent or decrease their activity compared with younger patients (−132 steps/day each week per 5 years of age, 95% CI −278 to 13, p=0.07).
Table 2.
Changes in physical activity (steps/d) over 12 weeks*
| Patient characteristic | Baseline daily steps, median (IQR) |
Change in daily steps per week, mean (95% CI) |
P-value |
|---|---|---|---|
| CKD category | |||
| Transplant | 8175 (4174 to 11,798) |
100 (−14 to 208) | 0.07 |
| CKD | 4978 (3058 to 6651) | 73 (−115 to 262) | 0.89† |
| Dialysis | 4566 (3500 to 9867) | −133 (−325 to 58) |
0.03† |
| Age, per 5 years | −132 (−278 to 13) |
0.07 |
Based on mixed modeling and also adjusted for baseline differences in activity related to age, sex, and CKD category. 95% CI: 95% confidence interval; IQR: interquartile range).
P-value is for the difference over time compared to transplant group.
Associations between change in physical activity and changes in physical performance and function
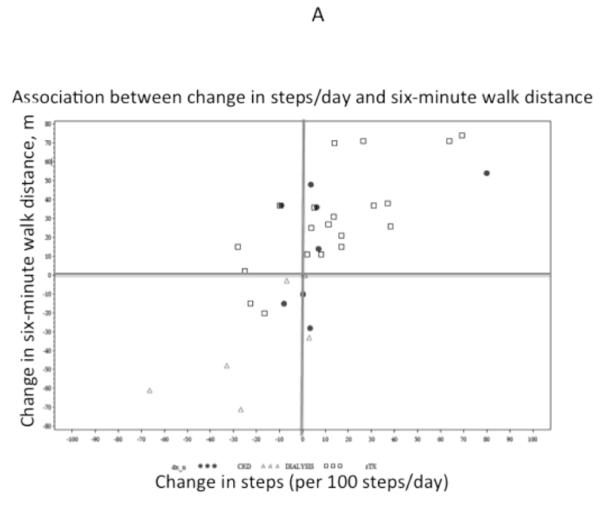
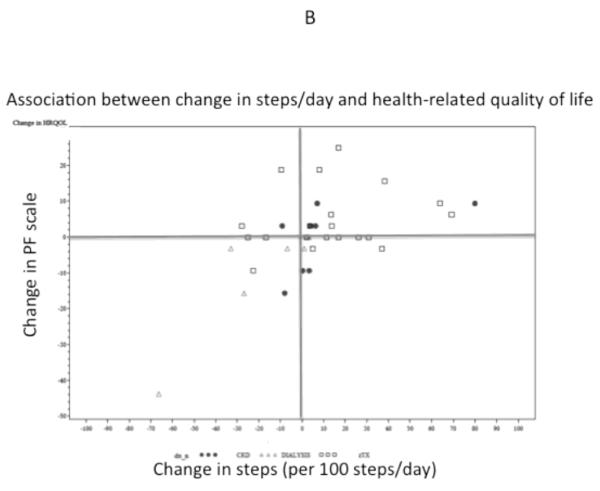
At the end of the 12-week intervention, 6-minute walk distance was significantly longer (mean increase of 16 m, 95% CI 4 to 28m), but self-reported physical function did not change significantly (−7, 95% CI −15 to +1 point). Change in physical activity was associated with change in 6-minute walk distance (r=0.74, p<0.001) and change in physical functioning (r=0.53, p=0.001) (Figure 2). CKD category was also associated with changes in 6-minute walk distance. Specifically, transplant recipients increased their 6-minute walk distance by 31m (95% CI 19, 42; p <0.0001), whereas patients on dialysis tended to decrease their walking distance overall (−28, 95% CI −60, 5; p=0.08) and differed from transplant recipients by −35m (95% CI −58, −12; p=0.003). CKD category was not significantly associated with change in physical functioning (P=0.18). Change in steps per day remained associated with change in 6-minute walk difference and physical functioning after adjustment for CKD category.
Figure 2.
Association of change in physical activity with change in six-minute walk distance (A) and health-related quality of life (B). PF, Physical Function Scale of the Pediatric Qualify of Life Index (PedsQL).
We planned to compare changes in 6-minute walk distance and physical function between patients who did and did not increase their steps/day by at least 1000 over the course of the study. Although the whole group did not substantially increase their physical activity, 13 participants (27%) did increase their activity by more than 1,000 steps per day over the course of the study, 12 of whom were transplant recipients and one of whom had CKD. eGFR among those who increased their activity was 38 (15, 72) compared with 68 (63,73) ml/min/1.73m2 among those who did not (p=0.03). Transplant recipients were more likely to increase steps by at least 1000 (p=0.002), and since only one non-transplant patient did so, we compared eGFR between transplant recipients who did (n=12) and did not (n=10) substantially increase steps and found no significant difference (68 [61, 82] vs 72 [55, 91] ml/min/1.73m2, p=0.97).
We also compared outcomes between transplant recipients who did and did not increase steps. Six-minute walk distance improved among transplant recipients who increased their steps by more than 1000 steps/day compared to those who did not (37m [26, 71] vs 11m [−6.5, 30.5], p=0.006; Table 3). The change in physical functioning among transplant recipients who did and did not increase their steps by more than 1,000 steps/day was not significantly different (3.1 [0, 9.4] vs 0 [−1.6, 10.9], p=0.51).
Table 3.
Changes in physical activity, performance, and function among transplant recipients who did and did not increase physical activity by at least 1000 steps/day.
| Increase in daily step count by <1000 steps from baseline to week 12 |
|||
|---|---|---|---|
| Variable | Yes (n=12) Median change* |
No (n=10) Median change* |
P-value |
| Daily steps | 2,635 (1390, 3825) | −1312 (−2322, 415) | <0.0001 |
| 6-minute walk distance, m |
37 (26, 71) | 11 (−6.5, 30.5) | 0.02 |
| Physical Function, PedsQL |
3.1(0, 9.4) | 0 (−1.6, 10.9) | 0.40 |
| eGFR | 68 (61, 82) | 71 (46, 91) | 0.74 |
Abbreviations: IQR, interquartile range; PedsQL, Pediatric Quality of Life Inventory.
Numbers in parentheses are 25th and 75th percentiles.
Discussion
We found that a simple intervention aimed at children and adolescents with CKD – giving patients pedometers and counseling them to increase physical activity over 12 weeks – did not lead to an increase in the average number of steps walked per day. However, changes in physical activity – across the spectrum of increases and decreases among all patients – were correlated with changes in physical performance (6-minute walk distance) and self-reported physical function. In addition, transplant recipients who increased their average daily step count by more than 1000 over the 12 weeks increased their 6-minute walk distance significantly.
Most physical activity interventions aimed at children and adolescents have been undertaken in a school setting [18], and children and adolescents with CKD should be encouraged to participate in regular physical activity at school. However, because CKD is rare among children and may affect participation in physical activity, more individualized interventions integrated with clinical care may be warranted. A pilot study evaluated the effects of a highly individualized aerobic and strength exercise intervention during hemodialysis among children and young adults [19]. Half of the participants did not complete the program, but six-minute walk distance and lower extremity strength improved after three months of participation, suggesting that linking exercise to CKD treatment could be successful [19]. Another study included 20 children on HD or PD into a 12-week community-based vigorous exercise program and reported a very high dropout rate (75%) but improvements in VO2 peak and muscle strength among the five participants who completed the study [20].
Our intervention was designed to be accessible to patients with all stages of CKD, many living at a considerable distance from the nephrology clinic. In addition we sought to tailor the intervention to baseline levels of activity, allowing for a gradual increase similar to other pedometer-based interventions in children [21]. In our study, there was good adherence to wearing the pedometers and reporting the step counts, with three-quarters of the participants providing data for all twelve weeks of the study. The “disconnect” between adherence to wearing the pedometers and lack of adherence to the recommended increases in physical activity suggests that our intervention was not sufficient to motivate children and adolescents with CKD to increase their physical activity, a problem that was also encountered in prior studies of exercise in this population [19,20,22]. Indeed, lack of time and being too tired were the top two barriers cited by participants in our study, particularly among teenagers who worked. Other commonly cited reasons for not increasing physical activity included the perception that they were increasing their activity, inclement weather and lack of safe place to walk in the neighborhood.
We also observed that parental investment in exercise was an important factor in recruitment, retention and success of participants in the study. Higher maternal education was also associated with more steps/day in the children at baseline [5]. It is possible that future interventions that involve parents more directly in the educational and structural components could be more successful. Coupling a pedometer as a means of monitoring and encouraging activity with other interventions such as concrete incentives or with specific opportunities to increase activity (eg exercise classes or gym memberships) might also help overcome the motivational barriers [21]. Some possibilities might include a website- or smart phone-based intervention that would allow patients to track their activity relative to other patients and/or a system of points or rewards such as credits towards digital music or applications.
A recent study among schoolchildren in the United Kingdom using pedometers and step goals similar to ours showed that physical activity could be increased when pedometer step goals were combined with peer-modeling materials or rewards [23]. The group that got rewards increased their steps more during the six-month intervention. These results provide some encouragement that a more intensive intervention using pedometers might be effective, although it should not be assumed that interventions that are useful among healthy children will be equally effective in children with chronic diseases such as CKD. Even within our study we found some differences in physical activity in response to our intervention based on severity of disease. Specifically, kidney function and CKD category were also associated with change in physical activity and physical performance. Transplant recipients were more active at baseline than patients with CKD or patients on dialysis, and patients on dialysis did not respond as well as the other groups to our intervention. Because transplant recipients differ substantially from patients on dialysis, including having higher eGFR, we could not distinguish whether the reason for the differences between these two groups was related to differences in kidney function or other factors. It is possible that the more limited cardiorespiratory fitness of patients on dialysis compared to those who receive a transplant, which is related to worse kidney function [24], presents a barrier to increasing physical activity. On the other hand, the time commitment required for dialysis could impinge on time needed to achieve physical activity goals. In addition to treatment or stage of CKD, we assessed age as a patient characteristic potentially related to change in activity, since physical activity in the general population peaks at around age six and then appears to gradually decline throughout childhood and into youth [25]. We found that the change in steps per week was lower among older participants (−132 steps/week for every 5 years of age, 95% CI −278 to 13, p=0.07).
We also found that changes in physical performance and self-reported physical function over the 12-week study period mirrored changes in physical activity. It is certainly possible, even likely, that differences in clinical status might drive both changes in physical activity and changes in physical functioning and performance. Nevertheless, these associations serve to highlight the tight link between physical activity and physical function, even among children who have a much more limited spectrum of comorbid conditions than do adults with CKD. Our findings raise the intriguing possibility that interventions that increase physical activity could lead to better functioning in the short term, in addition to the anticipated long-term benefits associated with increasing physical activity. Our study was limited by enrollment of a relatively small group of participants from a single center who were heterogeneous in their CKD stage and kidney function (Table 1). In addition, our study did not have a comparison group that was not given pedometers and encouraged to be more active. We chose this design because of the modest number of children with CKD available, because a major goal of our study was to determine the feasibility and tolerability of the intervention, and because a cross-over design is not practical for an exercise intervention. However, without a control group we cannot be certain that variations in activity observed during the study period were related to our intervention. In addition, without a control group we cannot rule out a practice effect as the cause of the improvement in six-minute walk distance we observed from baseline to week 12. However, practice-related change would be expected to be fairly consistent across participants and would not be likely to be associated with changes in physical activity or to be different among participants who did and did not increase their steps by more than 1,000 per day. We also cannot rule out weather-related changes in activity during the study, but recruitment of participants throughout the year makes it less likely that consistent differences in weather are responsible for the lack of increase in physical activity we observed. An additional limitation was that we relied on participants’ report of step counts without verification that pedometers were reset and worn at all times.
In conclusion, children and adolescents with CKD had low levels of physical activity and did not significantly increase their activity over 12 weeks of a pedometer-based intervention. However, changes in physical activity were associated with changes in physical functioning and performance, and performance increased among those who did increase their activity. Further studies are needed to determine whether more intensive interventions can increase physical activity and whether such an increase is associated with improved functioning and survival.
Acknowledgements
Dr Akber was supported by the University of California, San Francisco, Nephrology Division T32 fellowship training grant. Dr Johansen was supported by grant K24DK05153 from the National Institute of Diabetes and Digestive and Kidney Diseases. This project was also supported by National Institutes of Health/National Center for Research Resources University of California, San Francisco-Clinical & Translational Science Institute grant UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosure Dr Johansen serves on the National Nephrology Advisory Board of Amgen and as Deputy Editor for the Clinical Journal of the American Society of Nephrology. Dr Portale receives research support from Genzyme Corp and Abbott Laboratories.
References
- 1.Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, Kent-Braun JA. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 2.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164–1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varni JW, Burwinkle TM, Sherman SA, Hanna K, Berrin SJ, Malcarne VL, Chambers HG. Health-related quality of life of children and adolescents with cerebral palsy: hearing the voices of the children. Dev Med Child Neurol. 2005;47:592–597. [PubMed] [Google Scholar]
- 4.Johansen K, Kaysen GA, Dalrymple LS, Grimes BA, Glidden D, Anand S, Chertow GM. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8:248–253. doi: 10.2215/CJN.08560812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akber A, Portale A, Johansen K. Pedometer-assessed physical activity among children and young adults with chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:720–726. doi: 10.2215/CJN.06330611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz J, Pinero J, Artero E, Ortega F, Sjostrom M, Suni J, Castillo M. Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med. 2009;43:909–923. doi: 10.1136/bjsm.2008.056499. [DOI] [PubMed] [Google Scholar]
- 7.Hasslestrom H, Hansen S, Froberg K, Andersen L. Physical fitness and physical activity during adolescence as predictors of cardiovascular disease risk in young adulthood. Danish Youth and Sports Study. An eight-year follow-up study. Int J Sports Med. 2002;23:S27–S31. doi: 10.1055/s-2002-28458. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan S, Bao W, Wattitgney W, Berenson G. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996;45:235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 9.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple L, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B. Physical activity and rapid decline in kidney function among older adults. Arch Intern Med. 2009;169:2116–2123. doi: 10.1001/archinternmed.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosas S, Reese P, Huan Y, Doria C, Cochetti P, Doyle A. Pretransplant physical activity predicts all-cause mortality in kidney transplant recipients. Am J Nephrol. 2012;35:17–23. doi: 10.1159/000334732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varni JW, Burwinkle TM, Limbers CA, Szer IS. The PedsQL as a patient-reported outcome in children and adolescents with fibromyalgia: an analysis of OMERACT domains. Health Qual Life Outcomes. 2007;5:9. doi: 10.1186/1477-7525-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein SL, Graham N, Burwinkle T, Warady B, Farrah R, Varni JW. Health-related quality of life in pediatric patients with ESRD. Pediatr Nephrol. 2006;21:846–850. doi: 10.1007/s00467-006-0081-y. [DOI] [PubMed] [Google Scholar]
- 16.Tudor-Locke C, Pangrazi RP, Corbin CB, Rutherford WJ, Vincent SD, Raustorp A, Tomson LM, Cuddihy TF. BMI-referenced standards for recommended pedometer-determined steps/day in children. Prev Med. 2004;38:857–864. doi: 10.1016/j.ypmed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Tudor-Locke C, Bassett DR., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 18.van Sluijs E, McMinn A, Griffin S. Effectiveness of interventions to promote physical activity in children and adolescents: systematic review of controlled trials. Br J Sports Med. 2008;42:653–657. [PubMed] [Google Scholar]
- 19.Goldstein SL, Montgomery LR. A pilot study of twice-weekly exercise during hemodialysis in children. Pediatr Nephrol. 2009;24:833–839. doi: 10.1007/s00467-008-1079-4. [DOI] [PubMed] [Google Scholar]
- 20.van Bergen M, Takken T, Engelbert R, Groothoff J, Nauta J, van Hoeck K, Helders P, Lilien M. Exercise training in pediatric patients with end-stage renal disease. Pediatr Nephrol. 2009;24:619–622. doi: 10.1007/s00467-008-1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubans D, Morgan P, Tudor-Locke C. A systematic review of studies using pedometers to promote physical activity among youth. Prev Med. 2009;48:307–315. doi: 10.1016/j.ypmed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Clapp E, Bevington A, Smith A. Exercise for children with chronic kidney disease and end-stage renal disease. Pediatr Nephrol. 2012;27:165–172. doi: 10.1007/s00467-010-1753-1. [DOI] [PubMed] [Google Scholar]
- 23.Hardman C, Horne P, Lowe F. Effects of rewards, peer-modelling and pedometer targets on children’s physical activity: A school-based intervention study. Psychol Health. 2011;26:3–21. doi: 10.1080/08870440903318119. [DOI] [PubMed] [Google Scholar]
- 24.Painter P, Krasnoff J, Kuskowski M, Frassetto L, Johansen K. Effects of modality change and transplant on peak oxygen uptake in patients with kidney failure. Am J Kidney Dis. 2011;57:113–122. doi: 10.1053/j.ajkd.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps per day in US children and youth. Med Sci Sports Exerc. 2010;42:2244–2250. doi: 10.1249/MSS.0b013e3181e32d7f. [DOI] [PubMed] [Google Scholar]