Abstract
Purpose
This study explored the relationships between variations in cytokines genes and depressive symptoms in a sample of patients who were assessed prior to and for six months following breast cancer surgery. Phenotypic differences between Resilient (n=155) and Subsyndromal (n=180) depressive symptom classes, as well as variations in cytokine genes were evaluated.
Method
Patients were recruited prior to surgery and followed for six months. Growth mixture modeling was used to identify distinct latent classes based on Center for Epidemiological Studies Depression (CES-D) Scale scores. Eighty-two single nucleotide polymorphisms and 35 haplotypes among 15 candidate cytokine genes were evaluated.
Results
Patients in the Subsyndromal class were significantly younger, more likely to be married or partnered, and reported a significantly lower functional status. Variation in three cytokine genes (i.e., interferon gamma receptor 1 (IFNGR1 rs9376268), interleukin 6 (IL6 rs2069840), tumor necrosis factor alpha (TNFA rs1799964)), as well as age and functional status predicted membership in the Subsyndromal versus the Resilient class.
Conclusions
A variation in TNFA that was associated with Subsyndromal depressive symptoms in a sample of patients and their family caregivers was confirmed in this sample. Variations in cytokine genes may place these patients at higher risk for the development of Subsyndromal levels of depressive symptoms.
Keywords: depression, breast cancer, cytokines, genetics, depressive symptoms, subsyndromal depression, interleukin 6, tumor necrosis factor alpha, interferon gamma
Introduction
Depression is a common problem in patients with breast cancer. (Massie, 2004) Rates of depression are highest in the first six to twelve months following the diagnosis of breast cancer.(Helgeson et al., 2004; Lam et al., 2010) Most of the studies of depressive symptoms in women following breast cancer surgery have evaluated phenotypic predictors. Some of the strongest predictors of depressive symptoms in these patients included younger age,(Avis et al., 2012; Avis et al., 2013; Bardwell et al., 2006) lower level of education, (Torres et al., 2013) being single, caring for children at home, (Schlegel et al., 2012) feelings of pessimism,(Schou et al., 2004) feelings of neuroticism, higher levels of fatigue pre-operatively,(Den Oudsten et al., 2009) and financial difficulties.(Golden-Kreutz and Andersen, 2004)
Recent evidence suggests that inflammatory mechanisms may mediate some of the heterogeneity in depressive symptoms.(Belzeaux et al., 2010; Danese, 2008; Lau and Eley, 2010; Maes et al., 2009; Uddin et al., 2011) A number of meta-analyses or reviews have noted that serum levels of pro-inflammatory cytokines (e.g., tumor necrosis factor alpha (TNF-α), interleukin 1 beta (ILβ), IL-6) are elevated in patients with depression.(Dowlati et al., 2010; Hiles et al., 2012; Howren et al., 2009; Mossner et al., 2007; Wolkowitz et al., 2011; Zorrilla et al., 2001) In addition, the administration of inflammatory mediators (e.g., IL2, interferon-alpha (IFN-α)) results in the development of depressive symptoms.(Capuron et al., 2004; Eisenberger et al., 2010; Majer et al., 2008) Finally, the administration of antidepressants to humans reduces serum cytokine levels.(Raison et al., 2013; Tyring et al., 2006)
In patients with cancer, studies of the associations between depression and cytokine expression have yielded inconsistent results. In two studies, levels of IL6 or soluble IL2 receptors were increased in depressed cancer patients.(Jacobson et al., 2008; Musselman et al., 2001) However, in other studies, levels of pro-inflammatory cytokines were increased in patients who reported positive moods (Blomberg et al., 2009; Sepah and Bower, 2009) or no associations were found between serum cytokines and depression.(Steel et al., 2007) Reasons for these inconsistent findings may be related to diurnal variations in serum cytokines; the pleiotropic activity of cytokines; or the confounding effects that different cancer treatments may have on inflammatory processes.
Many of the studies cited above, that identified phenotypic predictors of depressive symptoms in breast cancer patients, were cross sectional (Avis et al., 2012; Bardwell et al., 2006; Golden-Kreutz and Andersen, 2004) or assessed the symptom only at the time of diagnosis and again at 12 months after surgery.(Den Oudsten et al., 2009; Schou et al., 2004) In addition, most studies reported mean depression scores for the entire sample or used variable cutoff scores to define cases. These limitations may partially explain the wide range of prevalence rates for depressive symptoms in breast cancer patients.
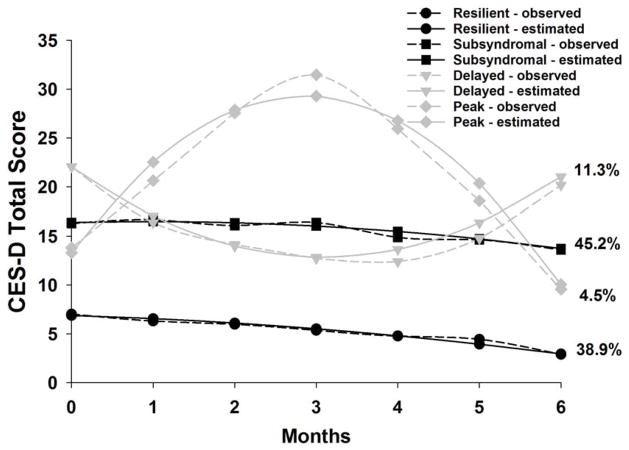
To overcome some of these limitations, in a recently completed a study, we used growth mixture modeling (GMM) to identify four subgroups of women with distinct depressive symptoms trajectories from before to six months after breast cancer surgery.(Dunn et al., 2011) In brief, patients (n=398) completed the Center for Epidemiological Studies Depression (CES-D) scale prior to surgery and monthly for a total of six months. Based on the GMM analysis of CES-D scores, the latent classes identified were named Resilient (38.9%), Subsyndromal (45.2%), Delayed (11.3%), and Peak (4.5%). Compared to the Resilient class, patients in the Subsyndromal class were significantly younger and more likely to have had an axillary lymph node dissection (ALND).
In a subsequent study, with a different sample of oncology patients and their family caregivers (FCs),(Dunn et al., 2013) we confirmed the same four latent classes of distinct depressive symptoms trajectories identified in the patients with breast cancer (Dunn et al., 2011). In addition, because emerging evidence suggested that depressive symptoms are associated with molecular mechanisms involved in inflammatory responses,(Miller et al., 2013; Miller et al., 2009) we evaluated for variations in a number of pro- and anti-inflammatory cytokine genes between the Resilient and Subsyndromal classes of patients and FCs. Variations in three cytokine genes (i.e., IL1 receptor 2 (IL1R2), IL10, TNFA), as well as younger age and poorer functional status, were associated with membership in the Subsyndromal class.
Given the confirmation of the depressive symptom phenotypes, in our two independent samples (i.e., breast cancer patients (Dunn et al., 2011) and oncology patients and their FCs (Dunn et al., 2013)), as well as the identification of cytokines genes that appear to play a role in depressive symptoms in oncology patients and FCs,(Dunn et al., 2013) this study sought to explore the relationships between cytokine gene variations and depressive symptoms in an independent sample of patients who were assessed prior to and for six months following breast cancer surgery. Specifically, we evaluated for variations in cytokine genes between the Resilient and Subsyndromal depressive symptom classes. The Delayed and Peak classes were not evaluated in these analyses because of the small number of breast cancer patients in each class.
Materials and Methods
Patients and Settings
A detailed description of the sample is published elsewhere (Dunn et al., 2011). In brief, patients were recruited from Breast Care Centers located in a Comprehensive Cancer Center, two public hospitals, and four community practices. Patients were eligible to participate if they were women ≥18 years of age who underwent breast cancer surgery on one breast; were able to read, write, and understand English; agreed to participate; and gave written informed consent. Patients were excluded if they were having breast cancer surgery on both breasts and/or had distant metastasis at the time of diagnosis.
A total of 516 patients were approached to participate, 410 were enrolled in the study (response rate 79.5%), and 398 completed the baseline assessment. The most common reasons for refusal were: too busy, overwhelmed with the cancer diagnosis, or insufficient time available to do the baseline assessment prior to surgery.
Instruments
Demographic questionnaire obtained information on age, education, ethnicity, marital status, employment status, living situation, and menopausal status. In addition, functional status was evaluated using the Karnofsky Performance Status (KPS) scale (Karnofsky, 1977) and comorbid conditions were evaluated using the Self-Administered Comorbidity Questionnaire (SCQ) (Sangha et al., 2003). Scores on the SCQ can range from 0 to 39. The SCQ evaluates thirteen common medical conditions including depression. Patients were asked to indicate if they had the condition, did they receive treatment for it, and did it limit their activities.
Depression was evaluated using the CES-D scale that consists of 20 items that represents the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores ≥16 indicating the need for clinical evaluation for major depression. The CES-D has well established concurrent and construct validity.(Radloff, 1977) For this study, the Cronbach’s alpha for the CES-D ranged from .85 to .90.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Boards at each of the study sites. During the patient’s preoperative visit, a clinician explained the study to the patient and determined her willingness to participate. For those women who were willing to participate, the clinician introduced the patient to the research nurse. The research nurse met with the women, determined eligibility, and obtained written informed consent prior to surgery. After obtaining consent, patients completed the enrollment questionnaires on average four days prior to surgery and again at one, two, three, four, five, and six months after surgery. Medical records were reviewed for disease and treatment information.
Analyses of the Phenotypic Data
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 19 (SPSS, 2010) and STATA Version 9.(StataCorp, 2005) Descriptive statistics and frequency distributions were generated for sample characteristics. Independent sample t-tests, Mann-Whitney U tests, and Chi square analyses were used to evaluate for differences in demographic and clinical characteristics between the two latent classes. All calculations used actual values. Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest set of available data. A p-value of <.05 was considered statistically significant.
Unconditional GMM with robust maximum likelihood estimation was carried out to identify latent classes with distinct depressive symptom trajectories. These methods are described in detail elsewhere (Dunn et al., 2011). In brief, a single growth curve that represented the “average” change trajectory was estimated for the whole sample. Then, the number of latent growth classes that best fit the data was identified using guidelines recommended by a number of experts (Jung and Wickrama, 2008; Nylund et al., 2007; Tofighi and Enders, 2008).
Analyses of the Genomic Data
Gene selection
Cytokines and their receptors are classes of polypeptides that mediate inflammatory processes (Verri et al., 2006). Cytokine dysregulation is associated with an increase in depressive symptoms (Miller et al., 2009; Schiepers et al., 2005). These polypeptides are divided into pro- and anti-inflammatory cytokines. Pro-inflammatory cytokines promote systemic inflammation and include: interferon gamma 1 (IFNG1), IFNG receptor 1 (IFNGR1), IL1R1, IL2, IL8, IL17A, nuclear factor kappa beta (NFKB1), NFKB2, and TNFA. Anti-inflammatory cytokines suppress the activity of pro-inflammatory cytokines and include: IL1R2, IL4, IL10, and IL13 (24, 22). Of note, IFNG1, IL1B, and IL6 possess pro- and anti-inflammatory functions (Seruga et al., 2008).
Blood collection and genotyping
Of the 398 patients who completed the baseline assessment, 302 provided a blood sample for genomic analysis. Genomic DNA was extracted from peripheral blood mononuclear cells using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). DNA was quantitated with a Nanodrop Spectrophotometer (ND-1000) and normalized to a concentration of 50 ng/μL (diluted in 10 mM Tris/1 mM EDTA). Samples were genotyped using the Golden Gate genotyping platform (Illumina, San Diego, CA) and processed according to the standard protocol using GenomeStudio (Illumina, San Diego, CA).
SNP selection
A combination of tagging SNPs and literature driven SNPs were selected for analysis. Tagging SNPs were required to be common (defined as having a minor allele frequency ≥.05) in public databases (e.g., HapMap). In order to ensure robust genetic association analyses, quality control filtering of SNPs was performed. SNPs with call rates <95% or Hardy-Weinberg p<.001 were excluded.
As shown in Supplementary Table 1, a total of 82 SNPs among the 15 candidate genes passed all quality control filters and were included in the genetic association analyses. Potential functional roles of SNPs associated with depression were examined using PUPASuite 2.0 (Conde et al., 2006).
Statistical Analyses
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg equilibrium was assessed by the Chi-square or Fisher Exact tests. Measures of linkage disequilibrium (i.e., D′ and r2) were computed from the patients’ genotypes with Haploview 4.2. Linkage disequilibrium (LD)-based haplotype block definition was based on D′ confidence interval (Gabriel et al., 2002).
For SNPs that were members of the same haploblock, haplotype analyses were conducted in order to localize the association signal within each gene and to determine if haplotypes improved the strength of the association with the phenotype. Haplotypes were constructed using the program PHASE version 2.1 (Stephens et al., 2001). In order to improve the stability of haplotype inference, the haplotype construction procedure was repeated five times using different seed numbers with each cycle. Only haplotypes that were inferred with probability estimates of ≥.85, across the five iterations, were retained for downstream analyses. Haplotypes were evaluated assuming a dosage model (i.e., analogous to the additive model).
Ancestry informative markers (AIMs) were used to minimize confounding due to population stratification (Halder et al., 2008; Hoggart et al., 2003; Tian et al., 2008). Homogeneity in ancestry among patients was verified by principal component analysis (Price et al., 2006), using Helix Tree (Golden Helix, Bozeman, MT). Briefly, the number of principal components (PCs) was sought which distinguished the major racial/ethnic groups in the sample by visual inspection of scatter plots of orthogonal PCs (i.e., PC 1 versus PC2, PC2 versus PC3). This procedure was repeated until no discernible clustering of patients by their self-reported race/ethnicity was possible (data not shown). One hundred and six AIMs were included in the analysis. The first three PCs were selected to adjust for potential confounding due to population substructure (i.e., race/ethnicity) by including the three covariates in all logistic regression models.
For association tests, three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (i.e., delta <10%), the genetic model that best fit the data, by maximizing the significance of the p-value was selected for each SNP. Logistic regression analysis, that controlled for significant covariates, as well as genomic estimates of and self-reported race/ethnicity, was used to evaluate the association between genotype and depression class membership. A backwards stepwise approach was used to create the most parsimonious model. Except for genomic estimates of and self-reported race/ethnicity, only predictors with a p-value of <.05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted odds ratios were estimated using STATA version 9.
As was done in our previous studies (McCann et al., 2012; Miaskowski et al., 2012), based on recommendations in the literature (Hattersley and McCarthy, 2005; Rothman, 1990), the implementation of rigorous quality controls for genomic data, the non-independence of SNPs/haplotypes in LD, and the exploratory nature of the analyses, adjustments were not made for multiple testing. In addition, significant SNPs identified in the bivariate analyses were evaluated further using regression analyses that controlled for differences in phenotypic characteristics, potential confounding due to population stratification, and variation in other SNPs/haplotypes within the same gene. Only those SNPs that remained significant were included in the final presentation of the results. Therefore, the significant independent associations reported are unlikely to be due solely to chance. Unadjusted (bivariate) associations are reported for all SNPs passing quality control criteria in Supplementary Table 1 to allow for subsequent comparisons and meta-analyses.
Results
Differences in Demographic and Clinical Characteristics Between Latent Classes
Patients in the Resilient class had relatively low preoperative CES-D scores (mean= 6.8) that decreased slightly over the six months of the study. Patients in the Subsyndromal class had a mean preoperative CES-D score that was just above the clinically meaningful cutpoint (mean=17.1), that increased slightly and then decreased slightly over 6 months (Figure 1).
Figure 1.
Observed and estimated Center for Epidemiological Studies Depression Scale (CES-D) trajectories for the patients in each of the latent classes, as well as the mean CES-D scores for the total sample (Adapted from Dunn, et al. 2011).
No differences were found between the two classes for the majority of the demographic and clinical characteristics (Table 1). However, compared to the Resilient class, patients in the Subsyndromal class were younger (p=.001), more likely to be married/ partnered (p=.03), and reported a significantly lower KPS score (p<.0001). In terms of clinical characteristics, compared to the Resilient class, patients in the Subsyndromal class were more likely to have had an ALND (p=.03), had a higher number of lymph nodes removed (p=.01), and had received chemotherapy (CTX) during the first six months after surgery (p=.01).
Table 1.
Differences in Demographic and Clinical Characteristics Between the Resilient (n=155) and Subsyndromal (n=180) Classes
| Characteristic | Resilient Class n=155 (46.3%) Mean (SD) |
Subsyndromal Class n=180 (53.7%) Mean (SD) |
Statistic and p-value |
|---|---|---|---|
|
| |||
| Age (years) | 57.3 (11.0) | 53.0 (11.9) | t=3.50, p=.001 |
|
| |||
| Education (years) | 15.8 (2.5) | 15.9 (2.8) | t=−0.16, p=.87 |
|
| |||
| Karnofsky Performance Status score | 95.5 (8.7) | 91.1 (11.1) | t=3.93, p<.0001 |
|
| |||
| Self-administered Comorbidity Questionnaire score | 4.0 (2.5) | 4.6 (3.1) | t=−1.84, p=.07 |
|
| |||
| Center for Epidemiological Studies Depression score | 6.8 (4.7) | 17.1 (8.6) | t=−13.6, p<.0001 |
|
| |||
| Number of breast biopsies in past year | 1.5 (0.8) | 1.6 (0.9) | U, p=.29 |
|
| |||
| Number of positive lymph nodes | 0.9 (2.6) | 1.0 (2.0) | t=−0.34, p=.74 |
|
| |||
| Number of lymph nodes removed | 5.0 (5.9) | 7.0 (7.8) | t=−2.64, p=.01 |
|
| |||
| n (%) | n (%) | ||
|
| |||
| Ethnicity | |||
| White | 107 (69.5) | 112 (62.6) | |
| Black | 16 (10.4) | 16 (8.9) | x2=4.02, p=.26 |
| Asian/Pacific Islander | 18 (11.7) | 24 (13.4) | |
| Hispanic/Mixed ethnic background/Other | 13 (8.4) | 27 (15.1) | |
|
| |||
| Married/partnered (% yes) | 54 (35.1) | 84 (46.9) | FE, p=.03 |
|
| |||
| Work for pay (% yes) | 78 (50.3) | 83 (46.6) | FE, p=.51 |
|
| |||
| Lives alone (% yes) | 34 (22.1) | 41 (23.0) | FE, p=.90 |
|
| |||
| Gone through menopause (% yes) | 104 (68.0) | 104 (60.1) | FE, p=.17 |
|
| |||
| Current diagnosis of depression (% yes)* | 26 (16.8) | 43 (23.9) | FE, .14 |
|
| |||
| Receives treatment for depression (% yes)* | 20 (80.0) | 31 (75.6) | FE, .77 |
|
| |||
| Depression limits activities (% yes)* | 4 (15.4) | 12 (29.3) | FE, .25 |
|
| |||
| Stage of disease | |||
| 0 | 26 (16.8) | 34 (18.9) | |
| I | 68 (43.9) | 54 (30.0) | |
| IIA | 36 (23.2) | 49 (27.2) | |
| IIB | 14 (9.0) | 24 (13.3) | U, p=.12 |
| IIIA | 5 (3.2) | 14 (7.8) | |
| IIIB | 1 (0.6) | 1 (0.6) | |
| IIIC | 4 (2.6) | 4 (2.2) | |
| IV | 1 (0.6) | 0 (0.0) | |
|
| |||
| Surgical treatment | |||
| Breast conservation | 127 (81.9) | 142 (78.9) | FE, p=.50 |
| Mastectomy | 28 (18.1) | 38 (21.1) | |
|
| |||
| Sentinel node biopsy (% yes) | 133 (85.8) | 144 (80.0) | FE, p=.19 |
|
| |||
| Axillary lymph node dissection (% yes) | 52 (33.8) | 82 (45.6) | FE, p=.03 |
|
| |||
| Breast reconstruction at the time of surgery (% yes) | 28 (18.2) | 41 (22.8) | FE, p=.34 |
|
| |||
| Neoadjuvant chemotherapy (% yes) | 26 (16.9) | 44 (24.4) | FE, p=.11 |
|
| |||
| Radiation therapy during the first 6 months (% yes) | 93 (60.0) | 95 (52.8) | FE, p=.19 |
|
| |||
| Chemotherapy during the first 6 months (% yes) | 42 (27.1) | 73 (40.6) | FE, p=.01 |
Abbreviations: FE=Fisher Exact test, SD = standard deviation, U=Mann Whitney U test
Self-report data from the Self-Administered Comorbidity Questionnaire
Candidate Gene Analysis of the Two GMM Classes
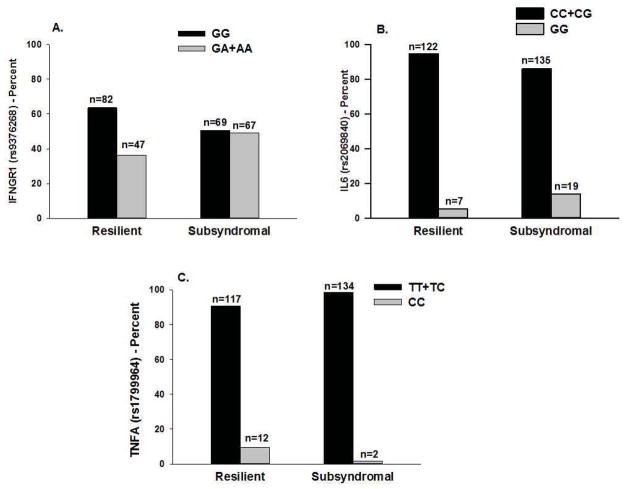
As summarized in Table 2, the minor allele frequency was significantly different between the two latent classes for three SNPs: IFNGR1 rs9376268, IL6 rs2069840, and TNFA rs1799964 and two haplotypes (IL6 HapA5 (p=.037), and TNFA HapA5 (p=.010)). For IFNGR1 rs9376268, a dominant model fit the data best (p=.047). For IL6 rs2069840 (p=.023) and TNFA rs1799964 (p=.005), a recessive model fit the data best (see Figure 2).
Table 2.
Summary of Single Nucleotide Polymorphisms Analyzed for Pro- and Anti-Inflammatory Cytokine Genes that Demonstrated Significant Bivariate Associations with the Growth Mixture Model Analysis for Depression
| Gene | SNP | Position | Chr | MAF | Alleles | Chi Square | p-value | Model |
|---|---|---|---|---|---|---|---|---|
| IFNGR1 | rs9376268 | 137574444 | 6 | .246 | G>A | FE | .047 | D |
| IL6 | rs2069840 | 22651652 | 7 | .333 | C>G | FE | .023 | R |
| IL6 | HapA5 | 6.608 | .037 | |||||
| TNFA | rs1799964 | 31542308 | 6 | .224 | T>C | FE | .005 | R |
| TNFA | HapA5 | 9.188 | .010 |
Abbreviations: A = additive model, Chr = chromosome, D = dominant model, FE = Fisher’s Exact, IFNG = interferon gamma, IL = interleukin, MAF = minor allele frequency, n/a = not assayed because SNP violated Hardy-Weinberg expectations (p<0.001), R = recessive model, SNP= single nucleotide polymorphism, TNFA = tumor necrosis factor alpha
Figure 2.
Figure 2A – Differences between the latent classes in the percentages of patients who were homozygous for the common allele (GG) or heterozygous of homozygous for the rare allele (GA+AA) for rs9376268 in interferon gamma receptor 1 (IFNGR1).
Figure 2B - Differences between the latent classes in the percentages of patients who were homozygous or heterozygous for the common allele (CC+CG) or homozygous for the rare allele (GG) for rs2069840 in interleukin 6 (IL6).
Figure 2C - Differences between the latent classes in the percentages of patients who were homozygous or heterozygous for the common allele (TT+TC) or homozygous for the rare allele (CC) for rs1799964 in tumor necrosis factor alpha (TNF-A).
Regression Analyses of IFNGR1, IL6, and TNFA Genotypes and Latent Class Membership
In order to better estimate the magnitude (i.e., odds ratio, OR) and precision (95% confidence interval, CI) of genotype on the odds of belonging to the Subsyndromal class as compared to the Resilient class, a multivariate logistic regression model was fit. In addition to genomic estimates of and self-reported race/ethnicity, the phenotypic variables evaluated in the initial model were; age (5 year increments), being married or partnered, functional status (estimated by the KPS score, in 10 point increments), having undergone an ALND, number of lymph nodes removed, and having received CTX at any time during the six month follow-up period. The only characteristics that remained significant in the final model were age and functional status.
The only genetic associations that remained significant in the multivariate logistic regression analyses were for IFNGR1 rs9376268, IL6 rs2069840, and TNFA rs1799964 (Table 3, Figures 2A, 2B, and 2C). In the regression analysis for IFNGR1 rs9376268, carrying one or two doses of the rare A allele (i.e., GG versus GA+AA) was associated with a 1.87-fold increase in the odds of belonging to the Subsyndromal class. In the regression analysis for IL6 rs2069840, being homozygous for the rare G allele (i.e., CC+CG versus GG) was associated with a 3.06-fold increase in the odds of belonging to the Subsyndromal class. In the regression analysis for TNFA rs1799964, being homozygous for the rare C allele (i.e., TT+TC versus CC) was associated with an 87% decrease in the odds of belonging to the Subsyndromal class.
Table 3.
Multiple Logistic Regression Analyses for Interferon Gamma Receptor 1(IFNGR1) rs9376268, Interleukin 6 (L6) rs2069840, and Tumor Necrosis Factor Alpha (TNFA) rs1800750 to Predict Subsyndromal Latent Class Membership
| Predictor | Odds Ratio | Standard Error | 95% CI | Z | p-value |
|---|---|---|---|---|---|
| IFNGR1 Genotype | 1.87 | 0.512 | 1.097, 3.201 | 2.30 | 0.022 |
| Age | 0.83 | 0.050 | 0.740, 0.938 | −3.02 | 0.003 |
| KPS score | 0.71 | 0.102 | 0.539, 0.943 | −2.37 | 0.018 |
| Overall model fit: χ2 = 27.60, p = 0.0011, R2 = 0.0772 | |||||
| IL6 Genotype | 3.06 | 1.511 | 1.165, 8.054 | 2.27 | 0.023 |
| Age | 0.83 | 0.050 | 0.734, 0.932 | −3.11 | 0.002 |
| KPS score | 0.73 | 0.103 | 0.553, 0.963 | −2.23 | 0.026 |
| Overall model fit: χ2 = 27.84, p = 0.0010, R2 = 0.0779 | |||||
| TNFA Genotype | 0.13 | 0.105 | 0.026, 0.635 | −2.52 | 0.012 |
| Age | 0.84 | 0.051 | 0.748, 0.948 | −2.84 | 0.005 |
| KPS score | 0.69 | 0.101 | 0.522, 0.923 | −2.51 | 0.012 |
| Overall model fit: χ2 = 31.11, p = 0.0003, R2 = 0.0870 | |||||
Multiple logistic regression analysis of candidate gene associations with resilient versus subsyndromal classes. For each model, the first three principal components identified from the analysis of ancestry informative markers as well as self-report race/ethnicity were retained in all models to adjust for potential confounding due to race or ethnicity (data not shown). Predictors evaluated in each model included genotype (IFNGR1 rs9376268: GG versus GA+AA; IL6 rs2069840: CC+CG versus GG; TNFA rs1799964: TT+TC versus CC), age (in 5 year increments), and functional status at baseline (estimated by the KPS score, in 10 point increments).
Abbreviations; CI = confidence interval; KPS = Karnofsky Performance Status
Discussion
This study is the first to attempt to evaluate for associations between pro- and anti-inflammatory cytokine genes and distinct depressive symptom trajectories in a relatively large sample of women who underwent breast cancer surgery. An evaluation of differences in phenotypic characteristics between the Resilient and Subsyndromal classes of patients with breast cancer is described in detail in our previous report (Dunn et al., 2011). In brief, in both groups of patients, the trajectory of depressive symptoms remained relatively stable across the six months of the study. This stability within each latent class may indicate a predisposition for a better or worse mental health status. While younger age, having poorer functional status, being married/partnered, and having more extensive treatment was associated with being in the Subsyndromal class, additional phenotypic predictors reported in previous studies, like personality (Den Oudsten et al., 2009; Schou et al., 2004) need to be evaluated in future studies.
The main focus of this paper was to determine if findings from our previous study that evaluated associations between similar depressive symptom trajectories and cytokine candidate genes (Dunn et al., 2013), could be identified in a different sample. In both of our studies, the trajectories of depressive symptoms in the Resilient and Subsyndromal classes were identical. However, in the previous study (Dunn et al., 2013), the mean CES-D scores for the Resilient and Subsyndromal classes on enrollment were 4.6 and 14.7, respectively. Compared to the breast cancer patients, the lower CES-D scores for both classes may be related to the heterogeneity of the sample that included patients with a variety of cancer diagnoses (i.e., breast, prostate, lung, brain) and both males and females.
In the current study, patients who were homozygous for the rare allele in TNFA rs1799964 had an 87% decrease in the odds of belonging to the Subsyndromal class. While no studies were identified that evaluated for an association between this SNP and depressive symptoms, our finding is consistent with a recent report that showed an association with rs1799964, located in the promoter region of the TNFA gene and another common symptom, namely decreased pain in lung cancer patients (Rausch et al., 2012).
It is not entirely clear why the associations between Subsyndromal class membership and the two SNPs in TNFA (i.e., rs2229074, rs1800629) identified in our previous study (Dunn et al., 2013) were not found in the patients with breast cancer. Of note, in the recent study that evaluated patients with breast cancer (Kim et al., 2012), the association between depressive symptoms and SNPs in TNFA that were evaluated in our study were either not significant (i.e., rs1800629, rs1799724) or not evaluated (rs1799964, rs2229074). One potential explanation for these inconsistent findings is that when a tagSNP approach is employed to test for genetic associations, subtle variations in LD among measured and unmeasured SNPs can occur in different samples. Therefore, different tagSNPs in different samples can be surrogate markers for an unmeasured causal SNP. Given the fact that serum levels of TNF-α are associated with depressive symptoms (Dowlati et al., 2010) and responses to antidepressants (Powell et al., 2012; Raison et al., 2013), additional research is warranted with larger samples to determine the role of TNFA polymorphisms in the development and maintenance of depressive symptoms in oncology patients
Two candidate genes (i.e., IFNGR1, IL6) not found in our sample of patients and FCs (Dunn et al., 2013) were identified in the current sample of patients with breast cancer. For IFNGR1 rs9376268, being homozygous or heterozygous for the rare allele was associated with a 1.9-fold increase in the odds of belonging to the Subsyndromal class. This SNP is located in the intronic region of the gene and has no known function. While no studies were found that evaluated the role of the IFNGR1 in depression, it is known that this receptor modulates the effects of other pro- and anti-inflammatory genes (Schroder et al., 2004).
Individuals homozygous for the rare G allele in IL6 rs2069840 had a 3.6-fold increase in the odds of belonging to the Subsyndromal class. This SNP is located in the intronic region of the gene and has no known function. However, several lines of evidence suggest that IL6 is involved in neuropsychiatric disorders including depression. For example, compared to healthy controls, depressed patients had higher levels of IL6 (Schlatter et al., 2001). In addition, higher levels of IL6 were found in the cerebrospinal fluid of patients who attempted suicide (Lindqvist et al., 2009). Equally important and warranting investigation in future studies is evidence that suggests that IL6 has both neurodegenerative (Morales et al., 2010) and neuroprotective (Godbout and Johnson, 2004; Peng et al., 2005) effects.
Consistent with a previous report by Kim and colleagues (Kim et al., 2012), no associations were found between depressive symptoms and SNPs in IL4, IL8, and IL10. In contrast, we did not confirm an association between IL1B and higher levels of depressive symptoms that was found in the study by Kim and colleagues. However, we did validate associations between SNPs in both IL6 and TNFA and higher levels of depressive symptoms. Reasons for these inconsistent findings may be related to differences in the methods used to assess depression; the timing of the depressive symptom measures; the primary ethnicity of the study participants; and/or the approach to classifying subgroups of patients with different levels of the depressive symptoms. Additional research is warranted to clarify the role of variations in pro- and anti-inflammatory cytokine genes and the development and maintenance of depressive symptoms in oncology patients across the disease trajectory.
Lastly, in our previous study (Dunn et al., 2013), SNPs in IL10, IL1R2 were associated with the Subsyndromal phenotype. The reasons why these associations were not found in the current study may be related to differences in sample characteristics particularly gender; the timing of the measures of depressive symptoms in relationship to the diagnosis of cancer; variations in cancer diagnoses; and differences in cancer treatments. Additional research is warranted to confirm or refute these genetic associations.
Individuals who are categorized as having subsyndromal depression have depressive symptoms but do not meet the criteria for a depressive disorder (Pietrzak et al., 2012). Patients in this class are at increased risk for poorer outcomes because they are under-diagnosed and may not be receiving proper treatment for their symptoms. In fact, patients with subsyndromal depression are at greater risk for transitioning into a major depressive disorder (Yi et al., 2012). While prevalence rates for subsyndromal depression range from 2.9% to 9.9% in primary care and from 1.4% to 17.2% in community settings (Rodriguez et al., 2012), findings from our two studies of oncology patients and their FCs suggest prevalence rates of 32.5% (Dunn et al., 2013) to 45.2% (Dunn et al., 2011). Consistent with findings from a recent systematic review (Rodriguez et al., 2012), breast cancer patients who were married, had poorer functional status, and higher treatment-related comorbidity were more likely to be in the Subsyndromal class. Given the recent findings that different gene expression profiles are evident in patients with subsyndromal depression compared to patients with major depressive disorder (Yi et al., 2012), additional research is warranted on the identification of oncology patients with different subtypes of depressive symptoms, as well as on the molecular characterization of these subtypes and their differential responsiveness to pharmacologic interventions (Cattaneo et al., 2013).
Limitations of this study need to be acknowledged. While ratings of depressive symptoms were obtained using a valid and reliable self-report measure, future studies need to incorporate a structured clinical evaluation of pre-existing and concurrent psychiatric conditions. Additional phenotypic characteristics including personality traits and previous and concurrent life stressors that could influence latent class membership warrant evaluation in future studies. Finally, the single diagnosis of breast cancer limits the generalizability of the study’s findings.
In conclusion, findings from this study suggest that subsyndromal depressive symptoms are a significant clinical problem for patients with breast cancer. More detailed characterization of this phenotype in conjunction with a comprehensive evaluation of the molecular markers of inflammation may identify oncology patients at higher risk for poorer outcomes, as well as potential therapeutic targets.
Supplementary Material
Acknowledgments
This study was funded by grants from the National Cancer Institute (CA107091 and CA118658). Dr. Dunn received funding from the Mount Zion Health Fund. Dr. Christine Miaskowski is an American Cancer Society (ACS) Clinical Research Professor. Dr. Dhruva is funded through NIH Mentored Patient-Oriented Research Career Development Award (K23 AT005340). Dr. Langford is supported by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship. Dr. Merriman was supported by an NINR fellowship (F31 NR012604), an ACS Doctoral Degree Scholarship (DSCN-10-087), an Oncology Nursing Society Doctoral Scholarship, and a UCSF Nursing Alumni Association Scholarship. Dr. Baggott is funded by an American Cancer Society Mentored Research Scholar Award (MRSG 12-01-PCSM). This project was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Footnotes
Conflicts of interest: None
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avis NE, Levine B, Naughton MJ, Case DL, Naftalis E, Van Zee KJ. Explaining age-related differences in depression following breast cancer diagnosis and treatment. Breast Cancer Research and Treatment. 2012;136:581–591. doi: 10.1007/s10549-012-2277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avis NE, Levine B, Naughton MJ, Case LD, Naftalis E, Van Zee KJ. Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Research and Treatment. 2013 doi: 10.1007/s10549-013-2513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell WA, Natarajan L, Dimsdale JE, Rock CL, Mortimer JE, Hollenbach K, Pierce JP. Objective cancer-related variables are not associated with depressive symptoms in women treated for early-stage breast cancer. Journal of Clinical Oncology. 2006;24:2420–2427. doi: 10.1200/JCO.2005.02.0081. [DOI] [PubMed] [Google Scholar]
- Belzeaux R, Formisano-Treziny C, Loundou A, Boyer L, Gabert J, Samuelian JC, Feron F, Naudin J, Ibrahim EC. Clinical variations modulate patterns of gene expression and define blood biomarkers in major depression. Journal of Psychiatry Research. 2010;44:1205–1213. doi: 10.1016/j.jpsychires.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Blomberg BB, Alvarez JP, Diaz A, Romero MG, Lechner SC, Carver CS, Holley H, Antoni MH. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: An exploratory study. Journal of Psychosomatic Research. 2009;67:369–376. doi: 10.1016/j.jpsychores.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner F, Bachmann LM, Weber U, Kessels AG, Perez RS, Marinus J, Kissling R. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskeletal Disorders. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behavior and Immunity. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM. Candidate Genes Expression Profile Associated with Antidepressants Response in the GENDEP Study: Differentiating between Baseline ‘Predictors’ and Longitudinal ‘Targets’. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieza A, Geyh S, Chatterji S, Kostanjsek N, Ustun BT, Stucki G. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Medical Research Methodology. 2006;6:36. doi: 10.1186/1471-2288-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, Schymkowitz J, Dopazo J. PupaSuite: finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Research. 2006;34:W621–625. doi: 10.1093/nar/gkl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A. Genetic opportunities for psychiatric epidemiology: on life stress and depression. Epidemiologia e Psichiatria Sociale. 2008;17:201–210. doi: 10.1017/s1121189x00001299. [DOI] [PubMed] [Google Scholar]
- Den Oudsten BL, Van Heck GL, Van der Steeg AF, Roukema JA, De Vries J. Predictors of depressive symptoms 12 months after surgical treatment of early-stage breast cancer. Psychooncology. 2009;18:1230–1237. doi: 10.1002/pon.1518. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dunn LB, Aouizerat BE, Langford DJ, Cooper BA, Dhruva A, Cataldo JK, Baggott CR, Merriman JD, Dodd M, West C, Paul SM, Miaskowski C. Cytokine gene variation is associated with depressive symptom trajectories in oncology patients and family caregivers. European Journal of Oncology Nursing. 2013;17:346–353. doi: 10.1016/j.ejon.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Cooper BA, Neuhaus J, West C, Paul S, Aouizerat B, Abrams G, Edrington J, Hamolsky D, Miaskowski C. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychology. 2011;30:683–692. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Interleukin-6 in the aging brain. Journal of Neuroimmunology. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psychooncology. 2004;13:211–220. doi: 10.1002/pon.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Human Mutation. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366:1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychology. 2004;23:3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behavior and Immunity. 2012;26:1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, McKeigue PM. Control of confounding of genetic associations in stratified populations. American Journal of Human Genetics. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Jacobson CM, Rosenfeld B, Pessin H, Breitbart W. Depression and IL-6 blood plasma concentrations in advanced cancer patients. Psychosomatics. 2008;49:64–66. doi: 10.1176/appi.psy.49.1.64. [DOI] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- Karnofsky D. Performance scale. Plenum Press; New York: 1977. [Google Scholar]
- Kim JM, Stewart R, Kim SY, Kang HJ, Jang JE, Kim SW, Shin IS, Park MH, Yoon JH, Park SW, Kim YH, Yoon JS. A one year longitudinal study of cytokine genes and depression in breast cancer. Journal of Affective Disorders. 2012;148:57–65. doi: 10.1016/j.jad.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Lam WW, Bonanno GA, Mancini AD, Ho S, Chan M, Hung WK, Or A, Fielding R. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psychooncology. 2010;19:1044–1051. doi: 10.1002/pon.1658. [DOI] [PubMed] [Google Scholar]
- Lau JY, Eley TC. The genetics of mood disorders. Annu Rev Clin Psychol. 2010;6:313–337. doi: 10.1146/annurev.clinpsy.121208.131308. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biological Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic Brain Disorders. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behavior and Immunity. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. Journal of the National Cancer Institute. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- McCann B, Miaskowski C, Koetters T, Baggott C, West C, Levine JD, Elboim C, Abrams G, Hamolsky D, Dunn L, Rugo H, Dodd M, Paul SM, Neuhaus J, Cooper B, Schmidt B, Langford D, Cataldo J, Aouizerat BE. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. Journal of Pain. 2012;13:425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Dhruva A, Dunn LB, Langford DJ, Cataldo JK, Baggott CR, Merriman JD, Dodd M, Lee K, West C, Paul SM, Aouizerat BE. Evidence of associations between cytokine genes and subjective reports of sleep disturbance in oncology patients and their family caregivers. PLoS One. 2012;7:e40560. doi: 10.1371/journal.pone.0040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depression and Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales I, Farias G, Maccioni RB. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation. 2010;17:202–204. doi: 10.1159/000258724. [DOI] [PubMed] [Google Scholar]
- Mossner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Muller N, Fallgatter AJ, Riederer P. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World Journal of Biological Psychiatry. 2007;8:141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. American Journal of Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Peng YP, Qiu YH, Lu JH, Wang JJ. Interleukin-6 protects cultured cerebellar granule neurons against glutamate-induced neurotoxicity. Neuroscience Letters. 2005;374:192–196. doi: 10.1016/j.neulet.2004.10.069. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Kinley J, Afifi TO, Enns MW, Fawcett J, Sareen J. Subsyndromal depression in the United States: prevalence, course, and risk for incident psychiatric outcomes. Psychological Medicine. 2012:1–14. doi: 10.1017/S0033291712002309. [DOI] [PubMed] [Google Scholar]
- Powell TR, Schalkwyk LC, Heffernan AL, Breen G, Lawrence T, Price T, Farmer AE, Aitchison KJ, Craig IW, Danese A, Lewis C, McGuffin P, Uher R, Tansey KE, D’Souza UM. Tumor necrosis factor and its targets in the inflammatory cytokine pathway are identified as putative transcriptomic biomarkers for escitalopram response. European Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch SM, Gonzalez BD, Clark MM, Patten C, Felten S, Liu H, Li Y, Sloan J, Yang P. SNPs in PTGS2 and LTA predict pain and quality of life in long term lung cancer survivors. Lung Cancer. 2012;77:217–223. doi: 10.1016/j.lungcan.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12:181. doi: 10.1186/1471-244X-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuropsychopharmacology, Biology, and Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schlatter J, Ortuno F, Cervera-Enguix S. Differences in interleukins’ patterns between dysthymia and major depression. European Psychiatry. 2001;16:317–319. doi: 10.1016/s0924-9338(01)00585-5. [DOI] [PubMed] [Google Scholar]
- Schlegel RJ, Manning MA, Molix LA, Talley AE, Bettencourt BA. Predictors of depressive symptoms among breast cancer patients during the first year post diagnosis. Psycholofy & Health. 2012;27:277–293. doi: 10.1080/08870446.2011.559232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou I, Ekeberg O, Ruland CM, Sandvik L, Karesen R. Pessimism as a predictor of emotional morbidity one year following breast cancer surgery. Psychooncology. 2004;13:309–320. doi: 10.1002/pon.747. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Sepah SC, Bower JE. Positive affect and inflammation during radiation treatment for breast and prostate cancer. Brain Behavior and Immunity. 2009;23:1068–1072. doi: 10.1016/j.bbi.2009.06.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Natures Review Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- SPSS. IBM SPSS for Windows (Version 19) SPSS, Inc; Chicago, Illinois: 2010. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 9. Stata Corporation; College Station, Texas: 2005. [Google Scholar]
- Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. Journal of Clinical Oncology. 2007;25:2397–2405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Human Molecular Genetics. 2008;17:R143–150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. Information Age Publishing; Charlotte, NC: 2008. [Google Scholar]
- Torres MA, Pace TW, Liu T, Felger JC, Mister D, Doho GH, Kohn JN, Barsevick AM, Long Q, Miller AH. Predictors of depression in breast cancer patients treated with radiation: Role of prior chemotherapy and nuclear factor kappa B. Cancer. 2013 doi: 10.1002/cncr.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Uddin M, Koenen KC, Aiello AE, Wildman DE, de los Santos R, Galea S. Epigenetic and inflammatory marker profiles associated with depression in a community-based epidemiologic sample. Psychological Medicine. 2011;41:997–1007. doi: 10.1017/S0033291710001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacology and Therapeutics. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues in Clinical Neuroscience. 2011;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Li Z, Yu S, Yuan C, Hong W, Wang Z, Cui J, Shi T, Fang Y. Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PLoS One. 2012;7:e31283. doi: 10.1371/journal.pone.0031283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behavior and Immunity. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.