Abstract
Background
Numerous proteins and small leucine-rich proteoglycans (SLRPs) make up the composition of the extracellular matrix (ECM). Assembly of individual fibrillar components in the ECM, such as collagen, elastin, and fibronectin is understood at the molecular level. In contrast, the incorporation of non-fibrillar components and their functions in the ECM are not fully understood.
Scope of review
This review will focus on the role of the matricellular protein thrombospondin (TSP) 2 in ECM assembly. Based on findings in TSP2-null mice and in vitro studies, we describe the participation of TSP2 in ECM assembly, cell-ECM interactions, and modulation of the levels of matrix metalloproteinases (MMPs).
Major conclusions
Evidence summarized in this review suggests that TSP2 can influence collagen fibrillogenesis without being an integral component of fibrils. Altered ECM assembly and excessive breakdown of ECM can have both positive and negative consequences including increased angiogenesis during tissue repair and compromised cardiac tissue integrity, respectively.
General significance
Proper ECM assembly is critical for maintaining cell functions and providing structural support. Lack of TSP2 is associated with increased angiogenesis, in part, due to altered endothelial cell-ECM interactions. Therefore, minor changes in ECM composition can have profound effects on cell and tissue function. This article is part of a special issue, “Matrix-Mediated Cell Behavior and Properties.”
Highlights
TSP2 functions primarily as a modulator of cell-ECM interactions and can influence the assembly of ECM. More importantly, TSP2-null ECM enhances angiogenic responses. Therefore, strategies can be pursued to reduce TSP2 and generate novel ECM via decellularization techniques.
Keywords: Extracellular matrix, thrombospondin, collagen, decellularization
The extracellular matrix (ECM) consists of numerous proteins, including several collagen types, fibronectin, and laminin, which are arranged into a 3D network. Within the network structure of the ECM, other components such as proteoglycans (PG) contribute to its integrity and organization[1]. Alteration of major ECM proteins due to mutation, lack of translation, improper folding, or improper assembly, leads to significant changes in ECM properties. Clinically, Marfan’s syndrome, Ehlers-Danlos syndrome, and Stickler syndrome result from mutations in one or more of the collagen genes or collagen-processing enzymes[2]. Similarly, non-structural ECM components such as matricellular proteins play a major role in influencing ECM assembly. Matricellular proteins do not contribute directly to the stable structure of the ECM, however, they possess cellular binding sites that influence cell-matrix interactions and cellular signaling/behavior[3]. In addition, they are also able to sequester growth factors and modulate their bioavailability[4].
Even though the assembly of ECM proteins into a cohesive, functional matrix is integral for vertebral life, it is not fully understood how all components come together in an organized network. However, for specific matrix components, much has been determined regarding the transition from monomeric subunits to individual multimeric proteins. For example, collagen I (and other fibrillar collagens) has a multi-step selfassembly process that includes side by side alignment of triple helical tropocollagen monomers, the formation of microfibrillar units, the fusion of these microfibrillar units into larger fibrils, and continued fusion that leads to eventual formation of micrometer sized fibers that display collagen’s characteristic banding pattern and periodicity[5]. Proteoglycans, glycosaminoglycans, and minor collagen types also participate in fibrillogenesis. Fibronectin also displays a unique, step-wise, self-assembly process that is characterized by integrin-dependence and necessary actin cytoskeletal connections[6]. In addition to intramolecular interactions, ECM proteins utilize specific recognition sequences to bind to each other and contribute to matrix formation and stabilization. For example, fibronectin and SPARC bind collagen, and thrombospondin (TSP) 1 can bind laminin. Moreover, post-translational modifications such as cross-linking, glycosylation, and hydroxylation have been shown to influence ECM properties[1, 7, 8].
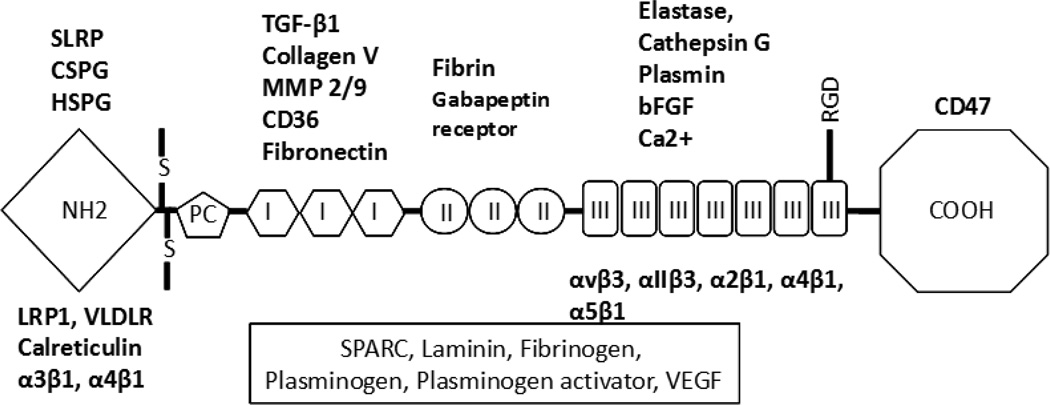
The TSPs are a family of five matricellular proteins, divided into groups based on their trimeric (TSP1 and TSP2) or pentameric (TSP3, TSP4, and TSP5) structure[9]. TSP1 and TSP2 are of particular interest in wound repair and tissue remodeling due to their anti-angiogenic activity. TSP1, is produced primarily by platelets and has been shown to be integral in the acute inflammatory phase of tissue repair[10]. Unlike TSP1, TSP2 is primarily produced by fibroblasts and smooth muscle cells, and is implicated in the later, remodeling phases of tissue repair[11]. TSP1 and TSP2 present 85% amino acid and structural conservation, thus, TSP2 has similar binding domains to TSP1[12]. Both proteins are synthesized and secreted as homotrimers consisting of N-terminal laminin G-like domain, a vWF pro-collagen-like domain, three type 1 properdin-like repeats, three EGF-like type II repeats, seven type III repeats, and a carboxy terminal lectin type domain (Fig. 1). These domains are involved in multiple interactions with cell surface receptors (LRP, CD36, CD 47, and numerous integrins), ECM components (decorin, fibronectin, HSPGs), growth factors (TGF-b, FGF2), enzymes (MMPs, elastase, cathepsin G), and calcium binding. The relative significance of specific TSP1/2 domain interactions in various cell functions and the molecular basis for the regulation of angiogenesis have been reviewed recently {Murphy-Ullrich, 2012 #663; Lawler, 2012 #668}. In this review, we will explore the role of TSP2 as a modulator of cell-matrix interactions and ECM assembly. In addition, we will focus on the novel properties of the TSP2-null ECM and its potential in therapeutic applications.
Figure 1. Structure of TSP1/2 monomer.
Schematic representation of the TSP1/2 monomer illustrating the various TSP domains and the putative interaction sites with various receptors, growth factors, extracellular matrix components, and Ca2+. Molecules in box are known to interact with TSPs but the interaction sites have not been identified. Not all interactions have been demonstrated for both TSP1 and TSP2. However, due to the overall similarity between the two proteins it is assumed that they could occur. Abbreviations: CSPG: Chondroitin sulfate proteoglycans, SLRP: small leucine-rich proteoglycans; HSPG: Heparan sulfate proteoglycans; TGF: Transforming growth factor; MMP: Matrix metalloproteinase; bFGF: Basic fibroblast growth factor, RGD: Arg-Gly-Asp; LRP1: Low density lipoprotein receptor-related protein 1, VLDLR: very low density lipoprotein receptor, SPARC: secreted protein acidic and rich in cysteine; VEGF: Vascular endothelial growth factor; S: disulfide bond.
Phenotype of TSP2-null mice
Connective tissue abnormalities
TSP2-null mice, generated over a decade ago, displayed normal physical appearance and reproduced at the expected Mendelian ratio. However, upon handling, it was noticed that the mice had lax skin and connective tissues including ligaments and tendons[14]. Tensile strength analysis of skin showed increased fragility and laxity. Further examination revealed a bleeding diathesis, increase in bone density, and increase in small to medium sized blood vessels. Interestingly, it was determined via light and electron microscopy that TSP2-null skin and tendons possessed disorganized collagen fibers and abnormally formed collagen fibrils. These findings highlighted a potential role for TSP2 in the maintenance of proper collagen fibrillogenesis, vascular density, bone growth, and hemostasis[14].
Other early findings also pointed towards TSP2’s role in inhibiting angiogenesis and modulating ECM remodeling. Specifically, in an excisional wound model it was found that the wounds of TSP2-null mice had irregular collagen organization, increased cellularity, and highly vascularized granulation tissue compared to WT wounds[15]. More importantly, wounds healed at an accelerated rate and exhibited minimal scarring. Similarly, in a foreign body response implant model, the response surrounding the implant in TSP2-null mice was characterized by a highly vascularized collagenous capsule composed of abnormally shaped collagen fibers[16]. Based on the expression patterns of TSP2, which peaked during vascular regression and ECM maturation, it is thought that it functions primarily as an inhibitor of angiogenesis and modulator of ECM remodeling. The former could be mediated by direct interaction of TSP2 with cell surface receptors on endothelial cells such as CD36 and CD47. The latter could involve both direct and indirect mechanisms including regulation of MMP levels leading to more stable ECM and sequestration of growth factors.
Cell adhesion, tissue transglutaminase and MMPs
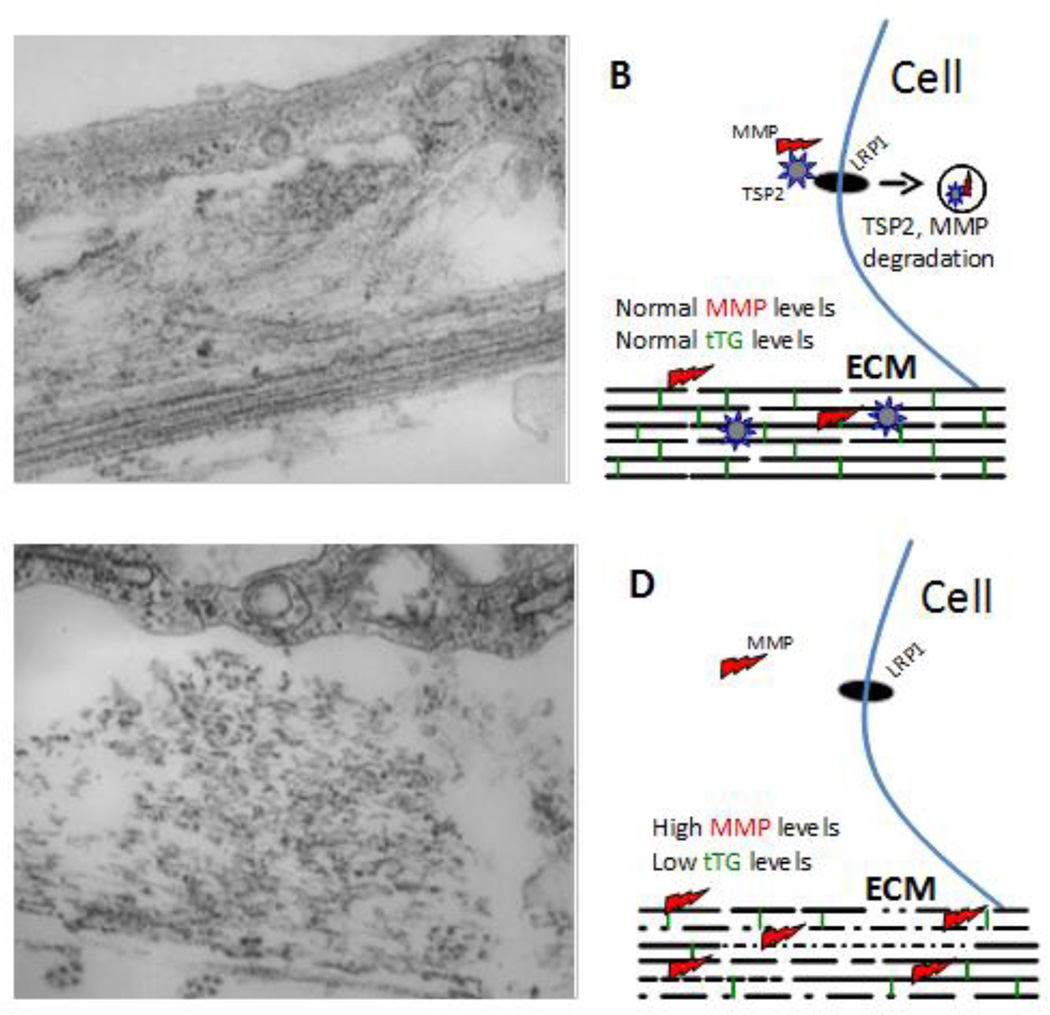
At the cellular level, it was demonstrated that lack of TSP2 leads to abnormalities in fibroblast cell function. Specifically, primary dermal fibroblasts from TSP2-null mice were shown to have an adhesion defect[14]. This defect was further characterized, and it was found that these cells have an attachment and spreading defect on numerous ECM surfaces and produce significantly higher levels of MMP-2[17]. Increased MMP-2 levels were due to the lack of MMP-2 recycling via LRP, which was mediated by TSP2-MMP-2 interaction (Fig. 2) [18]. TSP2-null skin fibroblasts displayed increased proteolytic activity that was associated with compromised cellular adhesion. Moreover, this defect was rescued through treatment with an MMP inhibitor or addition of TSP2 [17].
Figure 2. Role of TSP2 in ECM assembly.
Representative transmission electron microscopy images of WT (A) and TSP2-null (C) dermal fibroblasts cultured for 10 days in the presence of ascorbic acid are shown. WT cells display robust and organized deposition of collagenous ECM characterized by layers of highly dense and parallel collagen fibrils (arrow in A) as well as formation of dense fibril bundles (arrowhead in A). In contrast, TSP2-null cells display suboptimal deposition of discontinuous collagen fibrils (arrow in C) and disorganized bundles (arrowhead in C). Asterisks (*) denote cell areas. B, D show schematic representations of the interplay between TSP2, MMPs, and tTG in the process of ECM assembly. In WT cells, TSP2-MMP complexes interact with LRP1 and are targeted for intracellular degradation allowing for balanced MMP and tTG activity. In TSP2-null cells, due to the lack of TSP2 there is an increased accumulation of MMP2 resulting in degradation of collagen and the crosslinking enzyme tTG. As a result, ECM assembly is compromised due to reduced presence of crosslinks (green vertical lines in B and D) and changes in collagen deposition (gaps in collagen fibrils). Original magnification 4,000× (A, C).
Tissue-transglutaminase (tTG) levels were also examined in TSP2-null mice. tTG, a collagen cross-linking enzyme, is a substrate for MMP-2[19]. It was determined that tTG levels and activity are decreased in TSP2-null dermal fibroblasts, and that tTG activity and isopeptide bond collagen cross-link levels are significantly lower in the skin of TSP2-null mice than in WT mice[19]. These findings indicated that tTG levels in TSP2-null mice were affected by increased levels and proteolytic activity of MMP-2 that could contribute to the irregular collagen fibrillogenesis in TSP2-null mice.
MMP-2 levels, along with MMP-9, TIMP1/2, and soluble (s)VEGF levels were found to be elevated in full-thickness excisional wound tissue isolated from TSP2-null mice[20]. Specifically, distribution of MMP-2 and MMP-9 was found to be much greater in the extracellular space of TSP2-null wounds, compared to the higher levels of cell-associated MMP-2/9 in WT wounds, which was consistent with the suboptimal clearance of MMPs[20]. TIMPs have been shown to complex with MMPs during secretion[21], and expectedly, levels of TIMP1/2 were found to be greater in TSP2-null wounds. Another difference in TSP2-null wounds included increased levels of soluble VEGF[20], which can be released from ECM by MMP activity[22]. Moreover, fibroblasts isolated from the TSP2-null wounds were compromised in their ability to contract collagen gels, suggesting that the reduced scarring seen in TSP2-null wounds could be due to lessened contraction[20].
Vascular Remodeling
Aside from an alteration in fibroblast phenotype, TSP2 has been shown to influence endothelial cell function, including angiogenesis. An in vitro 3D chord-formation assay employing HUVEC demonstrated that the addition of exogenous TSP2 greatly decreased chord-formation[11]. In vivo, using a hindlimb ischemia model, it was found that TSP2-null mice had enhanced recovery of blood flow and increased collateralization compared to WT mice. In WT mice, TSP2 protein expression in response to ischemic injury was highly induced at 1 week post-surgery and then again at 4 weeks post-procedure in a biphasic manner. Spatially, at 1 week, TSP2 was found in muscle fibers and the ECM, and at 4 weeks was elevated in muscle fibers but found more so in blood vessels and interstitial space cells. These findings highlight the role of TSP2 in maintenance of tissue homeostasis and vascular remodeling[11].
Bone Biology
TSP2 also plays multiple roles in bone development and fracture repair. Specifically, it influences bone neovascularization and promotes mesenchymal stem cell (MSC) differentiation, in part, through modulation of angiogenesis/oxygen tension[23, 24]. As is well known, oxygen tension and vascularity influence MSC lineage commitment; high oxygen tension stimulates osteoblast differentiation, while low oxygen tension stimulates chondrocyte commitment[25]. At the cellular level, mice lacking TSP2 have increased numbers of marrow-derived osteoprogenitors and stromal cells, leading to enhanced endosteal bone formation[23, 26]. In addition, TSP2-null mice show atypical bone remodeling patterns in response to mechanical load, depending on bone type. For example, in TSP2-null mice, endocortical bone formation rate was elevated compared to periosteal bone formation rate, despite greater periosteal strain. [27]. TSP2-null mice also exhibit decreased bone resorption and are protected against ovariectomy-induced bone loss[28]. In a murine bone fracture model, TSP2-null mice exhibited, at day 10 post-fracture, 30% more bone and 40% less cartilage than WT mice, along with a higher number of osteoblasts than chondrocytes[29]. In an ischemic fracture model, TSP2-null mice responded similarly, with TSP2-null mice having significantly higher bone volume, cell proliferation, vessel density, and a decrease in apoptosis at day 10 post-fracture, compared to WT mice[30]. Taken together, these findings suggest that the accelerated fracture repair in TSP2-null mice could be influenced by increased angiogenesis coupled to an alteration in MSC differentiation.
Interestingly, TSP2’s influence on MSC differentiation is exerted in a context-dependent and lineage-specific manner. In comparison to WT, MSCs isolated from TSP2-null mice demonstrated increased proliferation and less mineralization[23]. It was further shown that TSP2 induced osteoblastogenesis at the expense of adipogenesis, and that TSP2-null mice had increased adiposity due to a favoring of adipogenesis over osteoblastogenesis[31]. MC3T3-E1 pre-osteoblasts with TSP2 knocked down formed less mineralized ECM characterized by a disrupted collagen organization[32]. Similarly, TSP2-null MSCs undergoing osteoblastic differentiation in vitro produced less mature ECM that contained lower levels collagen, indicating that TSP2 promotes assembly of a “uniform” collagen-type I rich ECM[33].
Regulation of TSP2 expression
Surprisingly little is known about the transcriptional regulation of TSP2, but details regarding regulators have begun to emerge. For example, MacLauchlan et. al. demonstrated that nitric oxide (NO) is a negative regulator of TSP2 transcription[34]. Endothelial nitric oxide synthase (eNOS), is an enzyme that converts l-arginine to NO and is activated by Akt-1 in the PI3K/Akt pathway, with NO being integral for injury-induced angiogenesis and tissue repair. By analyzing hindlimb ischemia and dermal wound healing models in eNOS-null mice, it was found that eNOS activity inversely correlated with TSP2 protein expression. This suggested that the pro-angiogenic actions of NO occur, in part, though TSP2 inhibition. Moreover, eNOS/TSP2 double null mice displayed improved wound healing and normal recovery from ischemia, further solidifying the existence of a NO/TSP2 signaling axis[34]. Consistent with this suggestion, Akt-1-null mice also contained higher levels of TSP2 in dermal wounds, and these levels were normalized in Akt-1 mice harboring a constitutively active eNOS mutant[34].
CYP1B1, an enzyme from the cytochrome P450 family of proteins, has also been identified as a negative regulator of TSP2[35]. CYP1B1 is expressed during development and has been shown to modulate angiogenesis and blood flow[36–38]. Increased TSP2 expression has been linked to NADPH-dependent production of ROS, highlighting that its expression may be mediated through changes in cellular oxidative stress[39]. Tang et. al. found that CYP1B1 promotes a pro-angiogenic phenotype through the regulation of intracellular oxidative stress. Mice that lacked CYP1B1 demonstrated a reduced neovascular response, increased levels of cellular oxidative stress, and increased levels of TSP2[35]. Upon re-expression of CYP1B1 in CYP1B1−/− cells, TSP2 levels dropped. Taken together, these findings indicate that CYP1B1 is necessary for metabolizing intracellular oxidative stress products whose accumulation is associated with enhanced expression of TSP2; thus CYP1B1 serves as a negative regulator of TSP2[35].
Characterization and properties of TSP2-null ECM
As mentioned above, it was noted that TSP2-null mice have lax tendons and ligaments and increased skin fragility suggestive of abnormal collagen fibrillogenesis. Electron microscopy analysis of the skin and tail tendons from TSP2-null mice revealed disorganized collagen fiber weave and collagen fibrils with uneven contours and larger diameters compared to that of WT mice[14]. These findings highlighted that TSP2 is necessary for the proper formation and organization of both collagen fibrils and fibers, in the skin, tendons, and potentially the extracellular matrix. It should be noted that immunohistochemical analysis of both embryonic and adult tissues did not reveal association of TSP2 with collagen fibers, which is consistent with its matricellular nature[40, 41]. Subsequent transmission electron microcopy studies in early postnatal WT and TSP2-null mice revealed that collagen fiber-forming compartments and fibroblast-defined compartments within tendons of TSP2-null mice were less well-defined and organized[41]. Specifically, the cytoplasmic processes that are hallmarks of these compartments were shorter and less regular in orientation in TSP2-null mice. Moreover, transmission electron microscopy analysis of cell-derived TSP2-null ECM produced by dermal fibroblasts in vitro also revealed irregular collagen fibrillogenesis[11]. Specifically, collagen fibrils in the TSP2-null ECM lacked definition and displayed disrupted periodicity. In addition, immunohistochemical analysis of the TSP2-null ECM revealed the presence of increased cryptic collagen epitopes normally found on degraded collagens[11]. Despite these apparent structural differences, quantitatively, the deposition of ECM components in WT and TSP2-null dermal fibroblasts appeared to be similar. Specifically, analysis of fibronectin and collagen distribution in decellularized ECM revealed no significant differences between WT and TSP2-null samples. More importantly, decellularized WT samples retained TSP2 in a diffuse pattern that did not overlap with that of structural ECM proteins (unpublished observation). Based on these observations, we speculate that the presence of TSP2 in the ECM is critical for mediating cell-ECM interactions and ECM assembly.
Consistent with the abnormal assembly, TSP2-null ECM was found to induce unique cellular behaviors. In contrast to decellularized WT ECM, TSP2-null ECM provided an ideal substrate for endothelial cell attachment[11]. Specifically, HUVEC plated on decellularized TSP2-null ECM spread and assumed morphological patterns that resembled chord formation and were able to migrate more efficiently [11]. In addition, platelets fail to aggregate on TSP2-null ECM in vitro and in vivo (unpublished observations). Therefore, this ECM has a unique combination of properties including being pro-angiogenic and non-thrombogenic.
While our data points toward insignificant compositional differences between WT and TSP2-null ECM, except TSP2, the striking differences in morphology and the cell behavior they induce suggest alterations in ECM assembly. As was discussed above, the elevated levels of MMPs could be linked to this aspect of the TSP2-null phenotype. MMP-2/9 are known to cleave collagens I and III at specific sites, leaving a mix of one quarter and three quarter length chain fragments[42]. These chain fragments are highly vulnerable to further degradation and unfolding. Therefore, matrix stability, tensile strength, and cross-linked collagen content may be altered due to the higher levels of MMPs. As mentioned earlier, it was also determined that tTG, an enzyme that introduces isopeptide crosslinks into matrix, is a proteolytic substrate for MMP-2[19]. Without tTG to form extensive crosslinks in maturing matrix, the ECM could become more easily digested, less stiff, and possess a looser organization.
TSP2 levels and ECM abnormalities
TSP2 and cardiac ECM integrity
Is matrix-incorporated TSP2 always a villain, however? What about instances when matrix structural integrity and architecture needs to be maintained? In myocardial remodeling, such as during left ventricular hypertophy or the progression of cardiomyopathy, both the cardiac cells and ECM experience large amounts of stress. Surprisingly, TSP2 has been shown to be cardioprotective in many instances. Scroen et. al. demonstrated that while chronically elevated levels of TSP2 are detrimental and contribute significantly to the progression of heart failure, TSP2-null mice were ill-equipped to deal with increased cardiac load and most suffered either cardiac rupture or rapid cardiac decompensation. This highlighted the necessity of TSP2 presence in matrix to adapt to an increased left ventricular pressure load[43]. Similarly, TSP2-null mice treated with doxorubicin had increased mortality and cardiomyocyte apoptosis, decreased cardiac function, and increased matrix damage due to lack of structural integrity and increase of MMPs[44]. Left alone, without ventricular load manipulation or drug treatment, >55% of TSP2-null mice, aged 24–60 weeks, compared to <10% WT mice, died, exhibiting severe dilated cardiomyopathy and increased fibrosis. At this age, TSP2-null mice displayed increased MMP-2 activity leading to decreased collagen crosslinking and loss of matrix structural integrity. This caused cardiomyocyte dropout and failure, all of which were rescued by introduction of TSP2-producing adenovirus, indicating the protective role of TSP2 in the heart[45]. It should be noted however, that this observation is limited to a single TSP2-null colony. We did not observe this susceptibility in TSP2-null mice in three different genetic backgrounds: pure 129/SvJ, pure C57Bl/6, and mixed 129/SvJ-C57Bl/6, which in our facilities survive normally for over 24 months[14]. In fact, we were able to study wound healing responses in TSP2-null mice that were over 24 months old[46]. Nevertheless, TSPs are appreciated as significant modulators of cardiac adaptation and disease[47].
TSP2 and scleroderma
Altered levels of TSP2 are also observed in Systemic Sclerosis (SSc), also known as scleroderma, which is characterized by inflammation, vascular damage, and fibrosis of the skin and internal organs[48]. TSP2 levels in serum samples from SSc patients are significantly elevated, despite the fact that mRNA and protein levels in SSc fibroblasts are decreased compared to WT fibroblasts. The increase in accumulation of extracellular TSP2 may be significantly contributing to the fibrosis found in SSC, due to TSP2’s ability to induce collagen synthesis. Moreover, knockdown of TSP2 led to down-regulation of collagen I synthesis and rescued the phenotype in vitro [48].
Mouse models with abnormal collagen/ECM phenotypes
Other prominent ECM components such as decorin, lumican, and fibromodulin have been shown to produce similar phenotypes when knocked-out in murine models. Decorin, a member of the small leucine-rich proteoglycan (SLRP) family and aptly named for its “decoration” of collagen fibrils, has been demonstrated to influence fibrillogenesis, matrix assembly, and growth factor availability[49]. It has also been shown that decorin, in soluble form, acts as a signaling proteoglycan through tyrosine kinase inhibition[50]. Danielson et. al. characterized decorin-null mice and discovered that while mice homozygous for the decorin-null mutation yielded viable offspring with no skeletal, anatomical, hematological, or behavioral issues, there did exist some striking skin abnormalities. Specifically, decorin-null skin was found to be fragile, lax, and prone to rupture. Further investigation highlighted dermal thinning and detachment, loose connective tissue, and reduced tensile strength. Ultrastructural analysis revealed chaos at the collagen fibril level. Compared to WT skin and tail tendon, the collagen fibrils found in decorin-null skin and tail tendon were unorganized, lacked a uniform cross-sectional shape, and varied greatly in thickness and size. These findings led the authors to conclude that decorin is developmentally significant for collagen fibrillogenesis, potentially due to its role in fibril fusion or maintenance of fibril uniformity[51].
Similar to decorin-null mice, mice lacking lumican or fibromodulin displayed phenotypes highlighted by collagen abnormalities[52–54]. Specifically, lumican-null mice exhibited collagen fibril abnormalities and skin laxity, along with bilateral corneal opacification[52]. Fibromodulin-null mice exhibited abnormal collagen fiber morphology within the tail and Achilles tendons, along with increased lumican deposition in tendons[53]. While not an SLRP, collagen V, a minor fibrillar collagen, was shown to produce a similar phenotype when the col5-2α gene was mutated in a murine model[55]. Collagen V-mutant mice presented with perinatal mortality and exhibited skin fragility, reduced collagenous dermal thickness, and spinal deformities. Ultrastructural analysis revealed abnormally sized collagen fibrils that were disorganized and not tightly packed. Altered fibrillogenesis was the result of collagen V trimers that contained the mutated α2 subunit which lacked the N-teleopeptide of collagen V, indicating its critical role in the assembly and growth of collagen I fibrils[55].
It is important to consider that while TSP2, collagen V, and SLRPs are not major structural components of the matrix, they are integral in modulating and ensuring proper matrix assembly, especially with respect to the major structural collagens. Consistent with this suggestion, clinically, mutations in collagen V and/or decorin can manifest as Ehlers-Danlos Syndrome (EDS), characterized by connective tissue abnormalities, bruising/bleeding, and collagen synthesis issues[56, 57]. It should be noted that the phenotype of the TSP2-null mice is far more complex than the mice described above and includes additional abnormalities in diverse processes including synapse formation, adiposity, platelet formation, fibrosis and inflammation[31, 58–62].
Cell-Derived ECM as a potential substrate for in vitro studies and in vivo applications
Decellularization of tissues and cell cultures has allowed for tissue- and cell-derived ECM to be employed as a bioactive substrate to investigate cell function[63, 64]. Methods of decellularization can be broken down into three main categories: chemical, enzymatic, or physical, with combinations of methods often used in various protocols. Chemical decellularization methods tend to employ a mixture of basic and hypo/hypertonic solutions, plus an ionic, non-ionic, or zwitterionic detergent that help disrupt cellular membranes. Enzymatic decellularization can occur with trypsin or nuclease treatment. Physical decellularization employs techniques such as freeze/thaw, mechanical agitation, or sonication to help dislodge cells. Decellularization can be performed on a single layer of ECM or the solution can be perfused throughout entire isolated organs[65]. After decellularization, cell-derived ECM can be further manipulated for proper use or storage. For example, cell-derived ECM or ECM scaffolds can be lyophilized and stored for extended periods of time with little change in their structural properties[66]. Lyophilized ECM can also be ground and mixed into a hydrogel, making an injectable or insertable biomaterial. It is now recognized that hydrogels consisting exclusively of collagen are inferior to those composed of complete cell-derived ECM because of the diverse protein composition and ability to retain growth factors[67–73]. It has also been shown that tissue-derived hydrogels have a high degree of success due to subtle matrix differences between tissues. For example, a recent study has demonstrated that myocardium-derived hydrogels injected into a porcine myocardial infarction model provide tissue-specific cues for infiltrating cells, are biocompatible and biodegradable, and greatly increase rate of recovery[74].
Based on the unique properties of the TSP2-null ECM, it is intriguing to consider its potential uses as a pro-angiogenic treatment and in vascular applications. As discussed above, the lack of TSP2 could limit the presentation of direct anti-angiogenic signals. In addition, the abnormally assembled ECM could allow enhanced neovascularization. Moreover, the inability to promote platelet aggregation suggests that this ECM could be useful as coating for vascular grafts or other blood-contacting constructs where thrombosis might be a complication. Therefore, TSP2-null ECM, formed either by isolated TSP2-null fibroblasts or WT fibroblasts treated with TSP2 siRNA/shRNA, could be used to generate therapeutic coatings for vascular grafts and hydrogels for treating wounds and ischemic tissues. Significant advantages of such ECM include the elimination of immunogenic cellular components and the need to supply exogenous pro-angiogenic growth factors, both of which are common in most tissue engineered constructs. Depending on the preparation method, TSP2-null ECM could be grafted or seeded onto chronic wounds to promote repair. In such a scenario, the orientation of the ECM could promote endothelial cell migration leading to enhanced neovascularization. Ongoing studies in our laboratory are focused on determining the efficacy of such interventions in dermal repair. Because of the complex effects of TSP2 on MSC differentiation in the bone microenvironment, we speculate that TSP2-null ECM might not be suitable as a substrate for bone repair. In parallel, studies aimed at deciphering the role of TSP2 in collagen fibrillogenesis and ECM assembly should expand our understanding of cell-ECM interactions in tissue repair.
Acknowledgements
This work was supported by NIH (HL107205) and the Gruber Science Fellowship (to N.E.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat Rev Genet. 2009;10:173–183. doi: 10.1038/nrg2520. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Current opinion in cell biology. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 4.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 5.Fratzl P. Collagen: Structure and Mechanics. New York: Springer; 2008. [Google Scholar]
- 6.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Leeming DJ, Bay-Jensen AC, Vassiliadis E, Larsen MR, Henriksen K, Karsdal MA. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2011;16:193–205. doi: 10.3109/1354750X.2011.557440. [DOI] [PubMed] [Google Scholar]
- 8.Miner JH. Extracellular Matrix in Development and Disease. London: Elsevier; 2005. [Google Scholar]
- 9.Adams JC, Lawler J. The thrombospondins. Cold Spring Harbor perspectives in biology. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krady MM, Zeng J, Yu J, Maclauchlan S, Skokos EA, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 Modulates Extracellular Matrix Remodeling during Physiological Angiogenesis. Am J Pathol. 2008;173:879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3:215–225. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy-Ullrich JE, Iozzo RV. Thrombospondins in physiology and disease: new tricks for old dogs. Matrix Biol. 2012;31:152–154. doi: 10.1016/j.matbio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 16.Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acad Sci U S A. 1999;96:4449–4454. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 19.Agah A, Kyriakides TR, Bornstein P. Proteolysis of cell-surface tissue transglutaminase by matrix metalloproteinase-2 contributes to the adhesive defect and matrix abnormalities in thrombospondin-2-null fibroblasts and mice. Am J Pathol. 2005;167:81–88. doi: 10.1016/S0002-9440(10)62955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maclauchlan S, Skokos EA, Agah A, Zeng J, Tian W, Davidson JM, Bornstein P, Kyriakides TR. Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF. J Histochem Cytochem. 2009;57:301–313. doi: 10.1369/jhc.2008.952689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nature reviews. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hankenson KD, Bornstein P. The secreted protein thrombospondin 2 is an autocrine inhibitor of marrow stromal cell proliferation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17:415–425. doi: 10.1359/jbmr.2002.17.3.415. [DOI] [PubMed] [Google Scholar]
- 24.Burke D, Dishowitz M, Sweetwyne M, Miedel E, Hankenson KD, Kelly DJ. The role of oxygen as a regulator of stem cell fate during fracture repair in TSP2-null mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31:1585–1596. doi: 10.1002/jor.22396. [DOI] [PubMed] [Google Scholar]
- 25.Hirao M, Tamai N, Tsumaki N, Yoshikawa H, Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J Biol Chem. 2006;281:31079–31092. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- 26.Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, Bornstein P. Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:851–862. doi: 10.1359/jbmr.2000.15.5.851. [DOI] [PubMed] [Google Scholar]
- 27.Hankenson KD, Ausk BJ, Bain SD, Bornstein P, Gross TS, Srinivasan S. Mice lacking thrombospondin 2 show an atypical pattern of endocortical and periosteal bone formation in response to mechanical loading. Bone. 2006;38:310–316. doi: 10.1016/j.bone.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Hankenson KD, James IE, Apone S, Stroup GB, Blake SM, Liang X, Lark MW, Bornstein P. Increased osteoblastogenesis and decreased bone resorption protect against ovariectomy-induced bone loss in thrombospondin-2-null mice. Matrix Biol. 2005;24:362–370. doi: 10.1016/j.matbio.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DK, Meganck JA, Terkhorn S, Rajani R, Naik A, O'Keefe RJ, Goldstein SA, Hankenson KD. Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:1043–1054. doi: 10.1359/jbmr.090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miedel E, Dishowitz MI, Myers MH, Dopkin D, Yu YY, Miclau TS, Marcucio R, Ahn J, Hankenson KD. Disruption of thrombospondin-2 accelerates ischemic fracture healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31:935–943. doi: 10.1002/jor.22302. [DOI] [PubMed] [Google Scholar]
- 31.Shitaye HS, Terkhorn SP, Combs JA, Hankenson KD. Thrombospondin-2 is an endogenous adipocyte inhibitor. Matrix Biol. 2010;29:549–556. doi: 10.1016/j.matbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alford AI, Terkhorn SP, Reddy AB, Hankenson KD. Thrombospondin-2 regulates matrix mineralization in MC3T3-E1 pre-osteoblasts. Bone. 2010;46:464–471. doi: 10.1016/j.bone.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alford AI, Golicz AZ, Cathey AL, Reddy AB. Thrombospondin-2 facilitates assembly of a type-I collagen-rich matrix in marrow stromal cells undergoing osteoblastic differentiation. Connective tissue research. 2013;54:275–282. doi: 10.3109/03008207.2013.811236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLauchlan S, Yu J, Parrish M, Asoulin TA, Schleicher M, Krady MM, Zeng J, Huang PL, Sessa WC, Kyriakides TR. Endothelial nitric oxide synthase controls the expression of the angiogenesis inhibitor thrombospondin 2. Proc Natl Acad Sci U S A. 2011;108:E1137–E1145. doi: 10.1073/pnas.1104357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poulos TL. Cytochrome P450: molecular architecture, mechanism, and prospects for rational inhibitor design. Pharmaceutical research. 1988;5:67–75. doi: 10.1023/a:1015920931701. [DOI] [PubMed] [Google Scholar]
- 37.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation. 2004;109:178–183. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]
- 38.Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes N, Gregg D, Vasudevan S, Hassanain H, Goldschmidt-Clermont P, Kovacic H. Thrombospondin 2 regulates cell proliferation induced by Rac1 redoxdependent signaling. Mol Cell Biol. 2003;23:5401–5408. doi: 10.1128/MCB.23.15.5401-5408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyriakides TR, Zhu YH, Yang Z, Bornstein P. The distribution of the matricellular protein thrombospondin 2 in tissues of embryonic and adult mice. J Histochem Cytochem. 1998;46:1007–1015. doi: 10.1177/002215549804600904. [DOI] [PubMed] [Google Scholar]
- 41.Bornstein P, Kyriakides TR, Yang Z, Armstrong LC, Birk DE. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J Investig Dermatol Symp Proc. 2000;5:61–66. doi: 10.1046/j.1087-0024.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 42.Bigg HF, Rowan AD, Barker MD, Cawston TE. Activity of matrix metalloproteinase-9 against native collagen types I and III. The FEBS journal. 2007;274:1246–1255. doi: 10.1111/j.1742-4658.2007.05669.x. [DOI] [PubMed] [Google Scholar]
- 43.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 44.van Almen GC, Swinnen M, Carai P, Verhesen W, Cleutjens JP, D'Hooge J, Verheyen FK, Pinto YM, Schroen B, Carmeliet P, Heymans S. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. Journal of molecular and cellular cardiology. 2011;51:318–328. doi: 10.1016/j.yjmcc.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D'Hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, Verheyen FK, VandenDriessche T, Chuah MK, Westermann D, Paulus WJ, Van de Werf F, Schroen B, Carmeliet P, Pinto YM, Heymans S. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- 46.Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22:539–547. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiological reviews. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kajihara I, Jinnin M, Yamane K, Makino T, Honda N, Igata T, Masuguchi S, Fukushima S, Okamoto Y, Hasegawa M, Fujimoto M, Ihn H. Increased accumulation of extracellular thrombospondin-2 due to low degradation activity stimulates type I collagen expression in scleroderma fibroblasts. Am J Pathol. 2012;180:703–714. doi: 10.1016/j.ajpath.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 49.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 54.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrikopoulos K, Liu X, Keene DR, Jaenisch R, Ramirez F. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet. 1995;9:31–36. doi: 10.1038/ng0195-31. [DOI] [PubMed] [Google Scholar]
- 56.Parapia LA, Jackson C. Ehlers-Danlos syndrome--a historical review. British journal of haematology. 2008;141:32–35. doi: 10.1111/j.1365-2141.2008.06994.x. [DOI] [PubMed] [Google Scholar]
- 57.Wu J, Utani A, Endo H, Shinkai H. Deficiency of the decorin core protein in the variant form of Ehlers-Danlos syndrome with chronic skin ulcer. Journal of dermatological science. 2001;27:95–103. doi: 10.1016/s0923-1811(01)00102-5. [DOI] [PubMed] [Google Scholar]
- 58.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Kyriakides TR, Rojnuckarin P, Reidy MA, Hankenson KD, Papayannopoulou T, Kaushansky K, Bornstein P. Megakaryocytes require thrombospondin-2 for normal platelet formation and function. Blood. 2003;101:3915–3923. doi: 10.1182/blood.V101.10.3915. [DOI] [PubMed] [Google Scholar]
- 60.Reinecke H, Robey TE, Mignone JL, Muskheli V, Bornstein P, Murry CE. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2013;22:91–95. doi: 10.1016/j.carpath.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lange-Asschenfeldt B, Weninger W, Velasco P, Kyriakides TR, von Andrian UH, Bornstein P, Detmar M. Increased and prolonged inflammation and angiogenesis in delayed-type hypersensitivity reactions elicited in the skin of thrombospondin-2--deficient mice. Blood. 2002;99:538–545. doi: 10.1182/blood.v99.2.538. [DOI] [PubMed] [Google Scholar]
- 62.Park YW, Kang YM, Butterfield J, Detmar M, Goronzy JJ, Weyand CM. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am J Pathol. 2004;165:2087–2098. doi: 10.1016/S0002-9440(10)63259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gospodarowicz D, Delgado D, Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A. 1980;77:4094–4098. doi: 10.1073/pnas.77.7.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pham QP, Kasper FK, Scott Baggett L, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29:2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 65.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freytes DO, Tullius RS, Valentin JE, Stewart-Akers AM, Badylak SF. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J Biomed Mater Res A. 2008;87:862–872. doi: 10.1002/jbm.a.31821. [DOI] [PubMed] [Google Scholar]
- 67.Iozzo RV. Proteoglycans: structure, biology, and molecular interactions. New York: Marcel Dekker; 2000. [Google Scholar]
- 68.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–1637. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 69.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. 2010;16:2017–2027. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8:3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rompre P, Auger FA, Germain L, Bouvard V, Lopez Valle CA, Thibault J, Le Duy A. Influence of initial collagen and cellular concentrations on the final surface area of dermal and skin equivalents: a Box-Behnken analysis. In vitro cellular & developmental biology : journal of the Tissue Culture Association. 1990;26:983–990. doi: 10.1007/BF02624473. [DOI] [PubMed] [Google Scholar]
- 72.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 73.Helary C, Bataille I, Abed A, Illoul C, Anglo A, Louedec L, Letourneur D, Meddahi-Pelle A, Giraud-Guille MM. Concentrated collagen hydrogels as dermal substitutes. Biomaterials. 2010;31:481–490. doi: 10.1016/j.biomaterials.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 74.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science translational medicine. 2013;5:173ra125. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]