Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease with an adult onset characterized by loss of both upper and lower motor neurons. In ~10% of cases, patients developed ALS with an apparent genetic linkage (familial ALS or fALS). Approximately 20% of fALS displays mutations in the SOD1 gene encoding superoxide dismutase 1. There are many proposed cellular and molecular mechanisms among which, mitochondrial dysfunctions occur early, prior to symptoms occurrence. In this review, we modeled the effect of mutant SOD1 protein via the formation of a toxic complex with Bcl2 on mitochondrial bioenergetics. Furthermore, we discuss that the shutdown of ATP permeation through mitochondrial outer membrane could lead to both respiration inhibition and temporary mitochondrial hyperpolarization. Moreover, we reviewed mitochondrial calcium signaling, oxidative stress, fission and fusion, autophagy and apoptosis in mutant SOD1-linked ALS.
Functional defects in mitochondria appear early before symptoms are manifested in ALS. Therefore, mitochondrial dysfunction is a promising therapeutic target in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common adult-onset motor neuron disease [1, 2], with an incidence of about 2 cases per 100,000 and a prevalence of 5 per 100,000 people per year worldwide [2]. ALS causes degeneration of upper motor neurons in the cerebral cortex and lower motor neurons in the brain stem and spinal cord, leading to muscle weakness, eventually progressing in muscle paralysis and atrophy. The most common reason of death for ALS patients is respiratory failure, usually within three to five years after the diagnosis [3, 4].
In approximately 90% of cases, patients developed ALS without apparent genetic linkage (sporadic ALS or sALS), while the remaining 10% of cases are familial (fALS). The first gene discovered with ALS-causative mutations was superoxide dismutase 1 (SOD1). More than 150 ALS-linked mutations have been reported in SOD1 over the course of 20 years, which are cumulatively responsible for approximately 20% of all fALS cases [5, 6]. In 2011, a genetic anomaly linked to a form of ALS associated with frontotemporal dementia (FTD) was identified as an aberrant number of expansions of a hexanucleotide repeat sequence (GGGGCC) in the non-coding region of the c9orf72 gene on chromosome 9 [7, 8]. In addition to being involved in ~40% of fALS cases, these intronic repeat expansions have been linked to ~10% of cases previously classified as sporadic [9], making this the most abundant ALS-causative gene so far. Several other mutated genes have been identified, mainly involved in non-traditional forms of fALS or have been found in just few families; including VAPB (Vesicle-associated membrane protein-associated protein B) [10], ALS2 (alsin) [11], VCP (valosin-containing protein) [12], OPTN (optineurin) [13], UBQLN2 (ubiquilin 2) [14], DAO (D-amino acid oxidase) [15], SPG11 [16], and hnRNPA2B1 and hnRNPA1 [17]. Cell and animal models incorporating different mutated genes have been developed, aiming at identifying molecular mechanisms of the disease. Among them, mice harboring mutations in the human SOD1 transgene are still the most common genetic animal models for this disease. In fact, most of our current understanding of the molecular mechanisms of ALS comes from studies done on the mutant SOD1 mouse models and will be the focus of the present review.
There is currently no cure for ALS. The only FDA approved drug, Riluzole, increases the survival in patients by few months [18, 19]. Preclinical ALS research is currently focused on the human mutant SOD1 transgenic mouse lines, which recapitulate many aspects of human ALS pathology and for which extended survival is one of the main predictors of preclinical success. Several compounds have been identified that provide some degree of improvement in survival, but none thus far has proved to be a substantial treatment option when translated in patients. There are multiple issues that could account for this discrepancy, including the study design of preclinical trials, the lack of additional animal models available for research, and insufficient insight into pathological causes. Furthermore, studying the mutant SOD1 transgenic mouse model has identified multiple cell types and molecular mechanisms that are affected, hence single treatments that target one pathway at a time may not be enough. Recently, a number of investigators have begun to test combination therapies, which can potentially enhance the effect of single pharmacological agents [20].
Many cellular and molecular mechanisms have been proposed to explain the loss of motor neurons seen in ALS, including glutamate-induced excitotoxicity, endoplasmic reticulum stress, proteasome inhibition, mitochondria-mediated damage, secretion of toxic factors by non-neuronal cells, oxidative stress, axonal disorganization, neuromuscular junction abnormalities, aberrant RNA processing [21]. In this article, we will review the role of mitochondria and mitochondria-mediated mechanisms of cell damage in ALS, focusing primarily on the function played by mitochondria in the pathogenesis of mutant SOD1-ALS, since most of the mechanistic studies on mitochondria dysfunction have been done using models of mutant SOD1-mediated ALS.
Mitochondria and ALS pathogenesis
That mitochondria are compromised in ALS is apparent from multiple studies performed using cellular or animal models of disease and in patients. Early studies on post-mortem tissues of ALS patients identified at the electron microscopic level structural and morphological abnormalities in mitochondria of skeletal muscle, liver, spinal cord neurons and motor cortex [22–24]. Defects in mitochondria Ca2+ buffering capacity [25–30] and in the activity of the electron transport protein complex occurring during the pre-symptomatic phase of disease were also reported in the spinal cord of mutant SOD1 mice [25–30]. Aberrant mitochondria have also been identified in more recent models of disease, as mutant FUS and TDP-43 expressing cells as well as flies, which exhibit aberrant, and perhaps, non-functional mitochondria [31]. In the SOD1-G93A mouse model it has been reported the appearance of mitochondria with dilated and disorganized cristae, both in the axons and dendrites of motor neurons at onset of disease [28]. In these mice, the onset of the disease is immediately preceded by a rapid increase in degenerating mitochondria with almost absent motor neuron death, which progressed during the symptomatic stage to a vacuolar pattern likely originating from expansion of the mitochondrial intermembrane space and extension of the outer mitochondrial membrane [32]. Mitochondria with displaying swollen morphology and increased cristae was reported in the soma and proximal axons of motor neurons in the anterior horns of patients with sporadic ALS (sALS) [33]. Since these morphological abnormalities appeared in mice before the onset of symptoms and motor neuron degeneration, it has been concluded that mitochondria impairment plays a key role in initiating motor neuron degeneration in ALS.
Mitochondria and Protein misfolding in ALS
One of the pathological hallmarks of ALS is aggregation of ubiquitinated proteins in motor neurons [34]. Most of the proteins encoded by the known ALS causative gene mutations have been identified as part of these ALS-linked cellular aggregates, which include SOD1, FUS, TDP-43, OPTN, UBQLN2 as well as the translational product of intronic repeats in C9ORF72. Mutations in proteins alter their normal conformation making them unstable [35]; segregation of this phenomenon with disease in familial and even sporadic cases (i.e. TDP43) [36] suggests a causal relationship between protein instability, aggregates and disease. However, whether aggregates represent a pathogenic or simply a pathological feature of the mutated protein is still debated. Whether there is a causal relationship between misfolded proteins and mitochondria dysfunction is still largely unknown for the newly discovered ALS-linked mutations such as FUS, TDP43 and C9ORF72, but it has been quite extensively studied in the case of mutant SOD1. Mutant SOD1 forms insoluble aggregates in mitochondria at the surface of outer membrane [37–39], raising the prospect of a direct cause-effect mechanism by which mutated SOD1 directly impact mitochondrial function, ultimately leading to cell death. Indeed, isolated mitochondria exposed in vitro to purified mutant SOD1 showed increased susceptibility to oxidative stress and structural damage, which ultimately caused cytochrome c release [39, 40].
While misfolding and oligomerization is pronounced in the perturbed mutant SOD1 protein, it was reported that also wild type SOD1 has the tendency to self-oligomerize, particularly in its apo state [41, 42]. Interestingly, the aggregation propensity of wild type SOD1 seemed to be balanced by the rate of self-dissociation [43]. This equilibrium may be disturbed by structural alterations introduced, for instance, by disease-causative mutations, or by age- and/or stress-induced posttranslational modifications, such as oxidation. Intriguingly, it has been reported that posttranslationally modified wild type SOD1 is a potential risk factor in ALS [44, 45]. Oxidized SOD1 (OxSOD1) is conformationally altered and is more prone to aggregation than its wild type counterpart [46], similarly to what has been described for mutant SOD1-G93A [47]. Oxidized forms of wild type SOD1 have also been identified in sALS and conformational alterations in wild type SOD1 have been proposed as a possible pathogenic link between sALS, or at least a subset of sALS cases, and mutant SOD1-linked fALS [47, 48]. OxSOD1 and mutant SOD1 may also hetero-oligomerize with wild type SOD1 to form aggregates that could potentially be harmful to cells, similarly to what has been reported in prion disease [49]. We previously reported that mutant SOD1 aberrantly binds to Bcl-2 in the mitochondrial outer membrane where it can trigger Bcl-2 mediated toxicity [40, 50]. Perturbations from the wild type state unrelated to the presence of mutations, can also increase SOD1 affinity for Bcl-2. For instance, an iperoxidized form of SOD1 found in a cohort of sporadic patients with bulbar ALS onset shares toxic features with mutant SOD1-G93A as it leads to an aberrant interaction with Bcl-2 in mitochondria and a conformational change in Bcl-2 similar to the one induced by mutant SOD1 [48].
Possible mechanisms of mitochondrial damage induced by mutant SOD1
One of the interacting proteins of mutant SOD1 is Bcl-2, which mediates, to some extent, the mutant SOD1 toxicity to mitochondria [37, 40, 50]. Upon docking to mitochondria, mutant SOD1 aberrantly interacts with Bcl-2 [37], which is converted into a toxic protein featuring exposure of the BH3 death domain [40]. Bcl-2 family proteins have been extensively characterized for their regulatory mechanisms on programmed cell death through the conformational reorganization of their respective BH domains [51]. They also have a dual role in regulating mitochondrial bioenergetics. For example, anti-apoptotic Bcl-2 family proteins, such as Bcl-xL and N-terminal truncated Mcl-1, promote mitochondrial respiration and oxidative metabolism; while pro-apoptotic Bcl-2 family proteins such as Bad and Noxa, when phosphorylated, enhance glycolysis (non-oxidative metabolism) and pentose phosphate pathway [52]. By mediating BH3 domain exposure in Bcl-2, mutant SOD1 could initially switch the bioenergetics states in affected motor neurons by favoring glycolysis and limiting mitochondrial respiration. In the short term, this change in metabolism may not necessarily have negative consequences. However, on a more extended time scale, neurons have been shown to be particularly sensitive to dysregulated energy metabolism because oxidative metabolism supplies the additional ATP necessary to sustain neuronal activity [53].
Systematic analysis of mitochondrial respiration kinetics revealed a drop of outer membrane permeability in spinal cord mitochondria of mutant SOD1 mice starting at pre-symptomatic stage [50], suggesting a direct inhibition by the mutant SOD1/Bcl2 complex on VDAC1 channel, a mitochondrial porin located on the mitochondrial outer membrane (MOM), which regulates mitochondria respiration and flow of ATP through the mitochondrial outer membrane (MOM) out into the cytosol and ADP into mitochondria for oxidative phosphorylation. VDAC1, a key player in mitochondria-mediated apoptosis and cell survival, is an anion-selective channel at full open state, but becomes cation selective when closed to sub-conducting states, with the pore diameter narrowing from 3 to 1.8 nm [54]. These changes of both size and selectivity of the channel have profound functional implications for the permeability to ions. For small and mono-valent ions such as K+, Cl−, H2PO4−, VDAC1 is a weak selective channel. In the closed (or sub-conducting) states, VDAC1 becomes a cation-selective channel with increased ion selectivity. For example, the permeability to Ca2+ ions increases dramatically at closed states [55]. In addition, the size change of the pore of the channel could introduce a physical barrier to large metabolites. As a result, the closed channel becomes virtually impermeable to large multi-valent anions such as ATP due to the electric barrier [56]. Thus, the change in gating of VDAC1 results in a mild reduction of conductance and a rather dramatic change in its selectivity [57] for multi-valent metabolites. Interestingly, reduced ATP production has been observed in motor neurons of mutant SOD1-G93A mice [25, 58], as well as in neuroblastoma cells expressing mutant SOD1-G37R [59].
Additional disturbances in VDAC gating mediated by the toxic mutant SOD1/Bcl-2 can be predicted according to the known parameters of mitochondrial respiration. The single channel permeability to ATP drops from 0.7×105 ions/sec to essentially zero at closure of VDAC, while the permeability to creatine3−, HPO42−, succinate2− drops from 4–8×106 ions/sec to 3×105 ions/sec [57]. Instead, for monovalent ions such as pyruvate and H2PO4−, there is less permeability drop in the close states of VDAC [57]. Thus, in both open and closed states, ATP permeability is a limiting step for a functional mitochondrial respiration. As VDAC closes, perhaps through the toxic function of the mutant SOD1/Bcl-2 complex, ATP accumulates in the mitochondrial intermembrane space where it slows down ADP/ATP translocation through the adenine nucleotide translocator complex (ANT). However, since metabolites such as pyruvate can still permeate the MOM and proceed to the Kreb’s cycle, the proton gradient through the inner membrane cannot be dissipated. We can summarize these events in the following simplified biophysical model for proton motive force (illustrated in Fig.1).
Figure 1.
Kinetic model of generation of proton gradient (Not drawn to scale). ADP permeates through VDAC channels in the outer membrane, translocates through ANT in the inner membrane, where it is phosphorylated into ATP inside the matrix by ATP synthase through dissipation of proton gradient, with apparent kinetic constant of k2. The proton gradient is generated through respiratory complexes with apparent kinetic constant of k1 and subjected to the leakage through the inner membrane.
Where [H+]o and [H+]i are the proton concentration respectively outside and inside the matrix, k1 is the apparent kinetic constant of respiratory chain complex (assuming constant substrates and enzymes levels), k2 is the kinetic constant for ATP synthase, P is the permeability of the inner membrane to proton.
At steady state, k1[H+]i − k2[ADP][H+]o − P([H+]o − [H+]i) = 0.
Therefore,
Where Δψm is the mitochondrial membrane potential, Δp is the proton motive force. Closure of VDAC mediated by the mutant SOD1/Bcl-2 complex can, therefore, slow down the ADP/ATP permeation through the MOM, decreasing ADP levels available in the intermembrane space and matrix for ATP synthase to produce ATP and therefore dissipate the proton gradient, ultimately inducing mitochondrial hyperpolarization. In reality, mitochondrial substrate concentrations such as NAD/NADH ratio could affect the parameters above. This simplified model provides a quantitative approach to understand the effects of intermembrane space ADP concentration on the mitochondrial membrane potential and thus it is more related to the transient effects of VDAC closure on mitochondria.
We found that a specific SOD1-mimetic peptide can restore normal mitochondrial membrane potential and VDAC1 permeability by lessening the interaction between mutant SOD1 and Bcl-2. Studies in other pathological conditions would also seem consistent and supportive of this model. For example, the anti-cancer drug G3139, which closes VDAC channel and cause apoptosis in cancer cells, also induces mitochondrial hyperpolarization (Colombini M., personal communication). Hyperpolarization state can lead to reactive oxygen species (ROS) generation by Complex I [60]. These two important aspects of mitochondrial dysfunction have also been reported in ALS [50] and will be discussed in the next session.
Other lines of evidence pointing at defective mitochondrial bioenergetics as leading mechanism in mitochondria-mediated cell damage have also been identified in ALS, including mutation in cytochrome c oxidase subunit I [61], mutations in mitochondrial tRNA gene [62], complex I deficiency and decreased ATP/ADP ratio [63].
Calcium Signaling and Mitochondria in ALS
Increased oxidative phosphorylation and ATP production is required during action potential firing in neurons. During each cycle, Ca2+ is released from the endoplasmic reticulum (ER) stores, enters mitochondria, buffers in the matrix, and finally is extruded from the mitochondria and taken up by the ER. Therefore, there is a resilient; tightly regulated ER-mitochondrial coupled Ca2+ signaling in the neurons. Because neurons fire action potentials repeatedly in a millisecond time scale, they are also particularly susceptible to mitochondria and ER stress or impairment leading to calcium dysregulation.
The voltage-dependent anion channel VDAC actively participates in the mitochondria-ER Ca2+ coupling. VDAC channels (mainly VDAC1) are expressed close to the mitochondria-ER tether sites, where Ca2+ permeable channels such as inositol triphosphate receptors (IP3Rs) and ryanodine receptors (RyRs) are expressed at the ER side [64, 65]. There are direct local Ca2+ fluxes from ER to mitochondria, to expedite the whole process [64, 65]. Calcium ions enter mitochondrial matrix via the mitochondrial calcium uniporter (MCU) with the support of mitochondrial calcium uptake 1 (MICU1) by following the electrochemical gradient [66, 67]. Several lines of evidence showed that calcium uptake activity is impaired in ALS. For example, mutant SOD1 affects Ca2+ uptake in affected tissues such as spinal cord but not unaffected tissues such as liver [27]. It has recently been shown that the activity of MCU and MICU1 is depressed in the SOD1-G93A mouse model, even though the expression levels of these proteins were found to be higher than in control mice [68]. This may lead to a decreased Ca2+ buffering capacity in mitochondria of these mice. Indeed, evidence of defective Ca2+ buffering capacity has been reported prior to onset in neuronal mitochondria of SOD1-G93A mice [27]. In addition, SOD1-G93A motor neurons have higher intracellular calcium levels [69], leading to possible Ca2+ overload to mitochondria. It is well-established that Ca2+ overload results in mitochondrial permeability transition [70]. In line with this, multiple groups have examined how knockdown of CyPD, the only confirmed component of mitochondrial permeability transition pore, affects the survival of mutant SOD1 mice. Although the results are conflicting, it is the general consensus that Ca2+ overloads affect motor neuron survival [71–74].
Oxidative stress and mitochondria in ALS
Unrestrained production of ROS by mitochondria has been considered as one possible cause of neurodegeneration in ALS [75]. This belief is based on a number of circumstantial evidence: 1) Increased oxidative stress and ROS damage characterized by elevated proteins carbonylation and tyrosines nitration have been observed in CNS tissues from ALS patients [76–80]; 2) A conformation specific antibody (B8H10) recognizes misfolded mutant SOD1 proteins in ALS spinal cord mitochondria, which displayed ROS accumulation [39], confirming a role for misfolded mutant SOD1 in mitochondrial dysfunction and elevated ROS production; 3) ROS actively participates to the disease progression in mutant SOD1 ALS mouse models. SOD1-G93A transgenic mice crossed with mice deficient in the mitochondrial matrix antioxidant enzyme MnSOD (Sod2+/- mice) caused a decrease in lifespan that was associated with a reduced disease duration [81]. However, in the same report, MnSOD1 deficiency does not affect the disease states of metal deficient H46R/H48Q mutant SOD1 ALS mice [97]. Therefore, different ALS mutations may affect mitochondria through different mechanisms. ROS production appeared to participate in motor neuron death pathways in some ALS models, although perhaps not a determining factor.
There are several types of ROS and reactive nitrogen species (RNS) that are formed in cells including superoxide anions, hydrogen peroxide, hydroxyl radicals, organic hydroperoxides, hypochlorous acid, peroxynitrite [82]. Endogenously, these compounds modulate different signaling pathways and their formation is tightly regulated [83, 84]. However, when produced at higher levels, they can target and react with lipid membranes, proteins and nucleotides, thus accelerating cell senescence and eventually cause cell death. Most of the oxidative species are formed from superoxide anions generated by mitochondrial respiration. Therefore, superoxide anion generation in mitochondria is the most critical point that directly initiates all the oxidative stress signals. Superoxide anions can be generated from both complex I and complex III, depending on the organs [85]. In the CNS, most superoxide is generated by complex I [85], whose activity is decreased in lymphocytes of ALS patients [63, 86–88]. The deficiency in complex I is associated with ROS production in the mitochondria [89–91]. Therefore, this defect may be the source of oxidative stress in ALS.
Fission and Fusion of mitochondria in ALS
Mitochondria are very dynamic and intensively interconnected organelles that form tube-like networks across the whole cell [92]. They constantly undergo fission and fusion in order to meet different cellular demands. This mitochondrial network remodels under stress conditions [93], changing in energy demand and Ca2+ levels [94]. Remodeling the mitochondria network serves also the purpose of repairing damaged mitochondria and coping with increases in Ca2+ waves [95]. Any alteration in this process of fission and fusion in the nervous system can cause cell damage and eventually lead to neurodegeneration [96]. For example, mutations in PINK1 and Parkin, two important proteins that promote mitochondrial fission and inhibit fusion [97], cause alterations in mitochondria dynamics and cause Parkinson’s disease (PD), thus suggesting an active role for dysfunctional mitochondrial fission and fusion dynamics in PD pathogenesis. Similar alterations have been reported in ALS, Pro-fusion OPA1 protein levels are decreased, while pro-fission phosphor-DRP1 protein levels are increased in mutant SOD1 mice [98]. Overexpression of Glutaredoxin 2, which regulates mitochondrial fragmentation, preserves mitochondrial function and dynamics by restoring DRP1 and OPA1 levels and strongly protects neuronal cells from apoptosis [98]. Increased mitochondrial fragmentation (fission) was recently reported in differentiated NSC34 cells transfected with different mutant SOD1 proteins [24, 99]. It is worth noting that the processes of mitochondrial fission/fusion and apoptosis/mitophagy share common mechanisms. For example, Bcl-XL increases both fission and fusion of mitochondria, which ultimately leads to a rise in mitochondrial biomass as net outcome [100]. Exposure of the BH3 domain in Bcl-2 by mutant SOD1 [40] could potentially affect the fission and fusion machinery in mitochondria as BH3 domain only proteins like Bax and Bak also increase mitochondrial fission. Changes in mitochondrial dynamics also involve both anterograde and retrograde transport of mitochondria in neurons that ultimately die in ALS [101] and are specific to mutant SOD1 motor neuron mitochondria, since they are absent in wild-type SOD1 motor neurons, they do not involve other organelles, and they are not found in neurons not affected by ALS, like cortical neurons [102].
Consequences of Mitochondrial Damage
Mutant SOD1 ALS-altered mitochondria may trigger apoptosis and ultimately cell death by inducing mitochondrial release of cytochrome c [103]. However, whether mitochondrial initiated apoptosis plays an essential role in neurodegeration is still a matter of dispute. Knocking out the pro-apoptotic protein Bax in mutant SOD1-G93A ALS mice prevents death of motor neurons and delay disease onset and progression, but mitochondrial degeneration still occurs [104], suggesting that mitochondrial initiated apoptosis in motor neurons could only be a secondary effect [105, 106]. Interestingly, knockout of both Bax and Bak (two pro-apoptotic Bcl-2 family proteins) significantly extends survival of SOD1-G93A ALS mice [107]. As Bax and Bak have similar function in forming pores in the mitochondrial outer membrane through which cytochrome c can be released [108], these results suggest a redundant mechanism of mitochondria-initiated toxicity between these two proteins, and point at the importance of mitochondrial altered permeabilization in the pathogenesis of ALS.
Mitochondrial are very abundant in nerve terminals where they serve the purpose of providing energy and regulate Ca2+ waves. Therefore, there must be robust coordination between synaptic activity, mitochondrial transport/distribution, and ATP generation. However, in mutant SOD1 mice, both anterograde and retrograde axonal transport are slowed down due to disruption of the neurofilament network [109, 110] and accumulation of insoluble proteins [111]. Therefore, deficiency in mitochondrial transport [112], hence inability to replace uncoupled mitochondria at the neuromuscular terminals, can trigger the disease through a “dying back” mechanism. For example, evidence of motor axon degeneration occurring prior to motor neuron death has been reported in mutant SOD1-linked ALS [111]. In the mutant SOD1 mice, quantitative analysis reveals the weakening of NMJ early during the presymptomatic stage of disease, followed by loss of motor axons at symptomatic stage [106]. This “dying back” mechanism has been observed in other neurodegenerative diseases as well [113] and is compatible with “mitochondrial-mediated” mechanism of neurodegeneration as enlarged mitochondria were first observed in the distal part of the nerve terminal at the pre-symptomatic stage [114]. These mitochondrial damages are also not limited to motor neurons, e.g., NMJ deterioration and axonal degeneration occurs when the efficiency of mitochondrial dependent oxidative phosphorylation is inhibited by overexpression of uncoupling protein 1 (UCP1) in the muscle cells [115]. Also, mitochondrial hyperpolarization, as predicted by the quantitative model, were observed in muscle cells prior to NMJ destruction [116], further proving mitochondria dysfunction is likely one of the early mechanism of the disease.
Perspectives and conclusions
Since the first reports on mitochondria abnormalities in tissues from ALS patients, several lines of evidence gathered from studies subsequently done on the mutant SOD1 mouse and cell models expressing mutant SOD1 proteins have pointed at mitochondrial dysfunction as cause of motor neuron death in ALS. One example is the recent observation that VDAC1 channel can enter less conductive (closed) states by directly interacting with mutant SOD1 [117]. Bcl-2 is a mitochondria outer membrane protein that directly regulates VDAC activity and these two proteins are very tightly associated. In this respect, we showed that at the mitochondria, mutSOD1 forms a toxic complex with Bcl-2, which is then converted into a toxic protein via a structural rearrangement that exposes its toxic BH3 domain (Pedrini et al., 2010). The formation of this toxic complex with Bcl-2 is the primary event in mutSOD1-induced mitochondrial dysfunction, inhibiting mitochondrial permeability to ADP and inducing mitochondrial hyperpolarization. In mutSOD1-G93A cells and mice, the newly exposed BH3 domain in Bcl-2 alters the normal interaction between Bcl-2 and VDAC1 thus reducing permeability of the outer mitochondrial membrane. In motor neuronal cells, the mutSOD1/Bcl-2 complex causes mitochondrial hyperpolarization leading to cell loss [50]. In addition, alterations in mitochondrial metabolism could be one of the disease mechanism, as knocking down VDAC1 in mutant SOD1 ALS mice, accelerates the onset of the disease [117], suggesting that mitochondrial bioenergetics is critical for the good health of motor neurons. Increased mitochondrial Ca2+ storage capacity reduces aggregation of misfolded SOD1 and motor neuron cell death. Surprisingly, this does not seem to extend survival in mouse models of inherited ALS [72]. At minimum, motor neuron death is, however, an essential measurement of disease progression, but it is possible that the final muscle paralysis event is independent from motor neuron loss. Nevertheless, this result is puzzling and needs further cross-examination, since at least in multiple sclerosis, a similar approach do protect motor axons and enhances recovery [118].
Mitochondrial dysfunction is not only observed in SOD1 ALS but has also seen in familial cases involved with FUS, TDP43 and sporadic cases [119]. In summary, the wealth of evidence published thus far leads to the conclusion that mitochondrial dysfunction occurs early in disease and it is one key mechanism responsible for the degeneration of motor neurons in ALS.
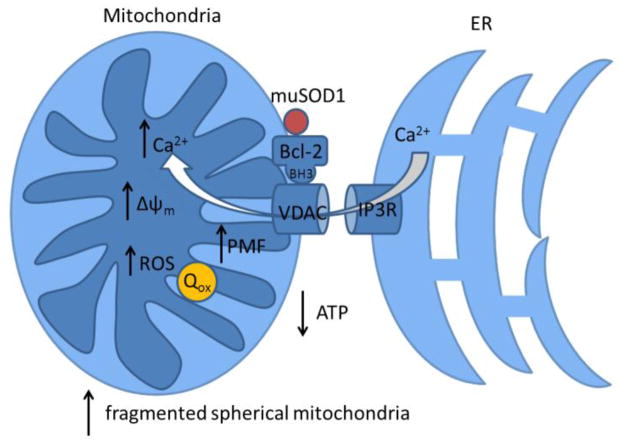
Figure 2.
Summarized impact of mutant SOD1/Bcl2 on mitochondria. Mutant SOD1 binds to Bcl2, expose its BH3 domain, alters VDAC conducting states, resulting in reduced ATP production, enhanced calcium signaling, increased mitochondrial potential and ROS production. We also propose that Quinone from Complex I is trapped in the oxidized form.
Highlights.
We reviewed role of mitochondrial dysfunctions in mutant SOD1-linked ALS.
We discussed and modeled the effect of mutant SOD1 on mitochondrial bioenergetics.
We discussed the impact of ATP permeation disruption in mutant SOD1-linked ALS.
Acknowledgments
This work was supported by the National Institute of Health, grants RO1-NS044292 and RO1-NS064488 to DT, RO1-NS051488 to PP. The Weinberg Unit for ALS research is also supported by the Farber Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nature reviews Neuroscience. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 2.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the ‘common’ neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 3.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. The New England journal of medicine. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 4.Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, Ince PG, Lin C, Miller RG, Mitsumoto H, Nicholson G, Ravits J, Shaw PJ, Swash M, Talbot K, Traynor BJ, Van den Berg LH, Veldink JH, Vucic S, Kiernan MC. Controversies and priorities in amyotrophic lateral sclerosis. Lancet neurology. 2013;12:310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddique T, Deng HX. Genetics of amyotrophic lateral sclerosis. Human molecular genetics. 1996;5:1465–1470. doi: 10.1093/hmg/5.supplement_1.1465. [DOI] [PubMed] [Google Scholar]
- 6.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) The Cochrane database of systematic reviews. 2012;3:CD001447. [Google Scholar]
- 7.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Consortium I, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majounie E, Traynor BJ, Chio A, Restagno G, Mandrioli J, Benatar M, Taylor JP, Singleton AB. Mutational analysis of the VCP gene in Parkinson’s disease. Neurobiology of aging. 2012;33:209 e201–202. doi: 10.1016/j.neurobiolaging.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, Skehel P, Zatz M. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. American journal of human genetics. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, Hung WY, Ouahchi K, Yan J, Azim AC, Cole N, Gascon G, Yagmour A, Ben-Hamida M, Pericak-Vance M, Hentati F, Siddique T. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JO, Mandrioli J, Benatar M, Abramzon Y, Van Deerlin VM, Trojanowski JQ, Gibbs JR, Brunetti M, Gronka S, Wuu J, Ding J, McCluskey L, Martinez-Lage M, Falcone D, Hernandez DG, Arepalli S, Chong S, Schymick JC, Rothstein J, Landi F, Wang YD, Calvo A, Mora G, Sabatelli M, Monsurro MR, Battistini S, Salvi F, Spataro R, Sola P, Borghero G, Galassi G, Scholz SW, Taylor JP, Restagno G, Chio A, Traynor BJ. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, Ogasawara K, Hirano A, Takumi T, Kusaka H, Hagiwara K, Kaji R, Kawakami H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 14.Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Donkervoort S, Bigio EH, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell J, Paul P, Chen HJ, Morris A, Payling M, Falchi M, Habgood J, Panoutsou S, Winkler S, Tisato V, Hajitou A, Smith B, Vance C, Shaw C, Mazarakis ND, de Belleroche J. Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7556–7561. doi: 10.1073/pnas.0914128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daoud H, Zhou S, Noreau A, Sabbagh M, Belzil V, Dionne-Laporte A, Tranchant C, Dion P, Rouleau GA. Exome sequencing reveals SPG11 mutations causing juvenile ALS. Neurobiology of aging. 2012;33:839, e835–839. doi: 10.1016/j.neurobiolaging.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, Kanagaraj AP, Carter R, Boylan KB, Wojtas AM, Rademakers R, Pinkus JL, Greenberg SA, Trojanowski JQ, Traynor BJ, Smith BN, Topp S, Gkazi AS, Miller J, Shaw CE, Kottlors M, Kirschner J, Pestronk A, Li YR, Ford AF, Gitler AD, Benatar M, King OD, Kimonis VE, Ross ED, Weihl CC, Shorter J, Taylor JP. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495:467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. The New England journal of medicine. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 19.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 20.Kong Q, Carothers S, Chang Y, Glenn Lin CL. The importance of preclinical trial timing - a potential reason for the disconnect between mouse studies and human clinical trials in ALS. CNS neuroscience & therapeutics. 2012;18:791–793. doi: 10.1111/j.1755-5949.2012.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, Hersmus N, Kusters B, Van Den Bosch L, Van Damme P, Richardson WD, Robberecht W. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain : a journal of neurology. 2013;136:471–482. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano A, Nakano I, Kurland LT, Mulder DW, Holley PW, Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. Journal of neuropathology and experimental neurology. 1984;43:471–480. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki S, Iwata M. Ultrastructural change of synapses of Betz cells in patients with amyotrophic lateral sclerosis. Neuroscience letters. 1999;268:29–32. doi: 10.1016/s0304-3940(99)00374-2. [DOI] [PubMed] [Google Scholar]
- 24.Menzies FM, Cookson MR, Taylor RW, Turnbull DM, Chrzanowska-Lightowlers ZM, Dong L, Figlewicz DA, Shaw PJ. Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain : a journal of neurology. 2002;125:1522–1533. doi: 10.1093/brain/awf167. [DOI] [PubMed] [Google Scholar]
- 25.Mattiazzi M, D’Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. The Journal of biological chemistry. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 26.Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Flint Beal M, Manfredi G. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. Journal of neurochemistry. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- 28.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamata H, Manfredi G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mechanisms of ageing and development. 2010;131:517–526. doi: 10.1016/j.mad.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung C, Higgins CM, Xu Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. Journal of neurochemistry. 2002;83:535–545. doi: 10.1046/j.1471-4159.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 31.Traub R, Mitsumoto H, Rowland LP. Research advances in amyotrophic lateral sclerosis, 2009 to 2010. Current neurology and neuroscience reports. 2011;11:67–77. doi: 10.1007/s11910-010-0160-0. [DOI] [PubMed] [Google Scholar]
- 32.Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC neuroscience. 2003;4:16. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki S, Iwata M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. Journal of neuropathology and experimental neurology. 2007;66:10–16. doi: 10.1097/nen.0b013e31802c396b. [DOI] [PubMed] [Google Scholar]
- 34.Stieber A, Gonatas JO, Gonatas NK. Aggregation of ubiquitin and a mutant ALS-linked SOD1 protein correlate with disease progression and fragmentation of the Golgi apparatus. Journal of the neurological sciences. 2000;173:53–62. doi: 10.1016/s0022-510x(99)00300-7. [DOI] [PubMed] [Google Scholar]
- 35.Richards RI. Dynamic mutations: a decade of unstable expanded repeats in human genetic disease. Human molecular genetics. 2001;10:2187–2194. doi: 10.1093/hmg/10.20.2187. [DOI] [PubMed] [Google Scholar]
- 36.Janssens J, Van Broeckhoven C. Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD-ALS spectrum disorders. Human molecular genetics. 2013;22:R77–87. doi: 10.1093/hmg/ddt349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, Brannstrom T, Gredal O, Wong PC, Williams DS, Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Pickles S, Destroismaisons L, Peyrard SL, Cadot S, Rouleau GA, Brown RH, Jr, Julien JP, Arbour N, Vande Velde C. Mitochondrial damage revealed by immunoselection for ALS-linked misfolded SOD1. Human molecular genetics. 2013;22:3947–3959. doi: 10.1093/hmg/ddt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedrini S, Sau D, Guareschi S, Bogush M, Brown RH, Jr, Naniche N, Kia A, Trotti D, Pasinelli P. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Human molecular genetics. 2010;19:2974–2986. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banci L, Bertini I, D’Amelio N, Libralesso E, Turano P, Valentine JS. Metalation of the amyotrophic lateral sclerosis mutant glycine 37 to arginine superoxide dismutase (SOD1) apoprotein restores its structural and dynamical properties in solution to those of metalated wild-type SOD1. Biochemistry. 2007;46:9953–9962. doi: 10.1021/bi700620r. [DOI] [PubMed] [Google Scholar]
- 42.Banci L, Bertini I, Boca M, Calderone V, Cantini F, Girotto S, Vieru M. Structural and dynamic aspects related to oligomerization of apo SOD1 and its mutants. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6980–6985. doi: 10.1073/pnas.0809845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bille A, Jonsson SAE, Akke M, Irback A. Local unfolding and aggregation mechanisms of SOD1: a Monte Carlo exploration. The journal of physical chemistry B. 2013;117:9194–9202. doi: 10.1021/jp404500b. [DOI] [PubMed] [Google Scholar]
- 44.Ezzi SA, Urushitani M, Julien JP. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. Journal of neurochemistry. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 45.Gruzman A, Wood WL, Alpert E, Prasad MD, Miller RG, Rothstein JD, Bowser R, Hamilton R, Wood TD, Cleveland DW, Lingappa VR, Liu J. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakhit R, Cunningham P, Furtos-Matei A, Dahan S, Qi XF, Crow JP, Cashman NR, Kondejewski LH, Chakrabartty A. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. The Journal of biological chemistry. 2002;277:47551–47556. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 47.Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, Frosch MP, Agar JN, Julien JP, Brady ST, Brown RH., Jr Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nature neuroscience. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, Trotti D, Pasinelli P. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polymenidou M, Cleveland DW. The seeds of neurodegeneration: prion-like spreading in ALS. Cell. 2011;147:498–508. doi: 10.1016/j.cell.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan W, Naniche N, Bogush A, Pedrini S, Trotti D, Pasinelli P. Small peptides against the mutant SOD1/Bcl-2 toxic mitochondrial complex restore mitochondrial function and cell viability in mutant SOD1-mediated ALS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11588–11598. doi: 10.1523/JNEUROSCI.5385-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends in cell biology. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 52.Andersen JL, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Molecular cell. 2013;49:399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gjedde A, Marrett S. Glycolysis in neurons, not astrocytes, delays oxidative metabolism of human visual cortex during sustained checkerboard stimulation in vivo. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:1384–1392. doi: 10.1097/00004647-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Molecular and cellular biochemistry. 2004;256–257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 55.Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochimica et biophysica acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rostovtseva T, Colombini M. ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. The Journal of biological chemistry. 1996;271:28006–28008. doi: 10.1074/jbc.271.45.28006. [DOI] [PubMed] [Google Scholar]
- 57.Hodge T, Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. The Journal of membrane biology. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 58.Browne SE, Yang L, DiMauro JP, Fuller SW, Licata SC, Beal MF. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiology of disease. 2006;22:599–610. doi: 10.1016/j.nbd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Coussee E, De Smet P, Bogaert E, Elens I, Van Damme P, Willems P, Koopman W, Van Den Bosch L, Callewaert G. G37R SOD1 mutant alters mitochondrial complex I activity, Ca(2+) uptake and ATP production. Cell calcium. 2011;49:217–225. doi: 10.1016/j.ceca.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Comi GP, Bordoni A, Salani S, Franceschina L, Sciacco M, Prelle A, Fortunato F, Zeviani M, Napoli L, Bresolin N, Moggio M, Ausenda CD, Taanman JW, Scarlato G. Cytochrome c oxidase subunit I microdeletion in a patient with motor neuron disease. Annals of neurology. 1998;43:110–116. doi: 10.1002/ana.410430119. [DOI] [PubMed] [Google Scholar]
- 62.Borthwick GM, Taylor RW, Walls TJ, Tonska K, Taylor GA, Shaw PJ, Ince PG, Turnbull DM. Motor neuron disease in a patient with a mitochondrial tRNAIle mutation. Annals of neurology. 2006;59:570–574. doi: 10.1002/ana.20758. [DOI] [PubMed] [Google Scholar]
- 63.Ghiasi P, Hosseinkhani S, Noori A, Nafissi S, Khajeh K. Mitochondrial complex I deficiency and ATP/ADP ratio in lymphocytes of amyotrophic lateral sclerosis patients. Neurological research. 2012;34:297–303. doi: 10.1179/1743132812Y.0000000012. [DOI] [PubMed] [Google Scholar]
- 64.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca(2)(+) and reactive oxygen species signaling. Journal of cell science. 2013;126:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallilankaraman K, Gandhirajan RK, Hawkins BJ, Madesh M. Visualization of vascular Ca2+ signaling triggered by paracrine derived ROS. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuchs A, Kutterer S, Muhling T, Duda J, Schutz B, Liss B, Keller BU, Roeper J. Selective mitochondrial Ca2+ uptake deficit in disease endstage vulnerable motoneurons of the SOD1G93A mouse model of amyotrophic lateral sclerosis. The Journal of physiology. 2013;591:2723–2745. doi: 10.1113/jphysiol.2012.247981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guatteo E, Carunchio I, Pieri M, Albo F, Canu N, Mercuri NB, Zona C. Altered calcium homeostasis in motor neurons following AMPA receptor but not voltage-dependent calcium channels’ activation in a genetic model of amyotrophic lateral sclerosis. Neurobiology of disease. 2007;28:90–100. doi: 10.1016/j.nbd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiological reviews. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 71.Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Experimental neurology. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parone PA, Da Cruz S, Han JS, McAlonis-Downes M, Vetto AP, Lee SK, Tseng E, Cleveland DW. Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4657–4671. doi: 10.1523/JNEUROSCI.1119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim HJ, Magrane J, Starkov AA, Manfredi G. The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis. Brain : a journal of neurology. 2012;135:2865–2874. doi: 10.1093/brain/aws208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peixoto PM, Kim HJ, Sider B, Starkov A, Horvath TL, Manfredi G. UCP2 overexpression worsens mitochondrial dysfunction and accelerates disease progression in a mouse model of amyotrophic lateral sclerosis. Molecular and cellular neurosciences. 2013;57:104–110. doi: 10.1016/j.mcn.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 76.Abe K, Pan LH, Watanabe M, Kato T, Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neuroscience letters. 1995;199:152–154. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]
- 77.Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Annals of neurology. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 78.Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Jr, Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. Journal of neurochemistry. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 79.Beal MF. Oxidatively modified proteins in aging and disease. Free radical biology & medicine. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki S, Shibata N, Komori T, Iwata M. iNOS and nitrotyrosine immunoreactivity in amyotrophic lateral sclerosis. Neuroscience letters. 2000;291:44–48. doi: 10.1016/s0304-3940(00)01370-7. [DOI] [PubMed] [Google Scholar]
- 81.Muller FL, Liu Y, Jernigan A, Borchelt D, Richardson A, Van Remmen H. MnSOD deficiency has a differential effect on disease progression in two different ALS mutant mouse models. Muscle & nerve. 2008;38:1173–1183. doi: 10.1002/mus.21049. [DOI] [PubMed] [Google Scholar]
- 82.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free radical biology & medicine. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 83.Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 84.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxidants & redox signaling. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 85.Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of physiology. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Q, Vande Velde C, Israelson A, Xie J, Bailey AO, Dong MQ, Chun SJ, Roy T, Winer L, Yates JR, Capaldi RA, Cleveland DW, Miller TM. ALS-linked mutant superoxide dismutase 1 (SOD1) alters mitochondrial protein composition and decreases protein import. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21146–21151. doi: 10.1073/pnas.1014862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murray J, Zhang B, Taylor SW, Oglesbee D, Fahy E, Marusich MF, Ghosh SS, Capaldi RA. The subunit composition of the human NADH dehydrogenase obtained by rapid onestep immunopurification. The Journal of biological chemistry. 2003;278:13619–13622. doi: 10.1074/jbc.C300064200. [DOI] [PubMed] [Google Scholar]
- 88.Willis JH, Capaldi RA, Huigsloot M, Rodenburg RJ, Smeitink J, Marusich MF. Isolated deficiencies of OXPHOS complexes I and IV are identified accurately and quickly by simple enzyme activity immunocapture assays. Biochimica et biophysica acta. 2009;1787:533–538. doi: 10.1016/j.bbabio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Pitkanen S, Robinson BH. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. The Journal of clinical investigation. 1996;98:345–351. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seaton TA, Cooper JM, Schapira AH. Free radical scavengers protect dopaminergic cell lines from apoptosis induced by complex I inhibitors. Brain research. 1997;777:110–118. doi: 10.1016/s0006-8993(97)01034-2. [DOI] [PubMed] [Google Scholar]
- 91.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. The Biochemical journal. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferree A, Shirihai O. Mitochondrial dynamics: the intersection of form and function. Advances in experimental medicine and biology. 2012;748:13–40. doi: 10.1007/978-1-4614-3573-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. The EMBO journal. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Molecular cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 95.Chan WH. Citrinin induces apoptosis via a mitochondria-dependent pathway and inhibition of survival signals in embryonic stem cells, and causes developmental injury in blastocysts. The Biochemical journal. 2007;404:317–326. doi: 10.1042/BJ20061875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho B, Choi SY, Cho HM, Kim HJ, Sun W. Physiological and Pathological Significance of Dynamin-Related Protein 1 (Drp1)-Dependent Mitochondrial Fission in the Nervous System. Experimental neurobiology. 2013;22:149–157. doi: 10.5607/en.2013.22.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferri A, Fiorenzo P, Nencini M, Cozzolino M, Pesaresi MG, Valle C, Sepe S, Moreno S, Carri MT. Glutaredoxin 2 prevents aggregation of mutant SOD1 in mitochondria and abolishes its toxicity. Human molecular genetics. 2010;19:4529–4542. doi: 10.1093/hmg/ddq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raimondi A, Mangolini A, Rizzardini M, Tartari S, Massari S, Bendotti C, Francolini M, Borgese N, Cantoni L, Pietrini G. Cell culture models to investigate the selective vulnerability of motoneuronal mitochondria to familial ALS-linked G93ASOD1. The European journal of neuroscience. 2006;24:387–399. doi: 10.1111/j.1460-9568.2006.04922.x. [DOI] [PubMed] [Google Scholar]
- 100.Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB, 3rd, Goebbels S, Nave KA, Arnold BA, Jonas EA, Pineda FJ, Hardwick JM. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. The Journal of cell biology. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, Brownlees J, Ackerley S, Shaw PJ, McLoughlin DM, Shaw CE, Leigh PN, Miller CC, Grierson AJ. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Human molecular genetics. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Magrane J, Sahawneh MA, Przedborski S, Estevez AG, Manfredi G. Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:229–242. doi: 10.1523/JNEUROSCI.1233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naniche N, Sau D, Pasinelli P. In vivo and in vitro determination of cell death markers in neurons. Methods in molecular biology. 2011;793:9–21. doi: 10.1007/978-1-61779-328-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fischer LR, Glass JD. Axonal degeneration in motor neuron disease. Neuro-degenerative diseases. 2007;4:431–442. doi: 10.1159/000107704. [DOI] [PubMed] [Google Scholar]
- 106.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Experimental neurology. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 107.Reyes NA, Fisher JK, Austgen K, VandenBerg S, Huang EJ, Oakes SA. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. The Journal of clinical investigation. 2010;120:3673–3679. doi: 10.1172/JCI42986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell death and differentiation. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 109.Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. The Journal of cell biology. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ligon LA, LaMonte BH, Wallace KE, Weber N, Kalb RG, Holzbaur EL. Mutant superoxide dismutase disrupts cytoplasmic dynein in motor neurons. Neuroreport. 2005;16:533–536. doi: 10.1097/00001756-200504250-00002. [DOI] [PubMed] [Google Scholar]
- 111.Tateno M, Kato S, Sakurai T, Nukina N, Takahashi R, Araki T. Mutant SOD1 impairs axonal transport of choline acetyltransferase and acetylcholine release by sequestering KAP3. Human molecular genetics. 2009;18:942–955. doi: 10.1093/hmg/ddn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magrane J, Manfredi G. Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxidants & redox signaling. 2009;11:1615–1626. doi: 10.1089/ars.2009.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fischer-Hayes LR, Brotherton T, Glass JD. Axonal degeneration in the peripheral nervous system: implications for the pathogenesis of amyotrophic lateral sclerosis. Experimental neurology. 2013;246:6–13. doi: 10.1016/j.expneurol.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 114.Siklos L, Engelhardt J, Harati Y, Smith RG, Joo F, Appel SH. Ultrastructural evidence for altered calcium in motor nerve terminals in amyotropic lateral sclerosis. Annals of neurology. 1996;39:203–216. doi: 10.1002/ana.410390210. [DOI] [PubMed] [Google Scholar]
- 115.Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, Halter B, Huze C, Schaeffer L, Bouillaud F, Loeffler JP. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PloS one. 2009;4:e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dupuis L, Loeffler JP. Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models. Current opinion in pharmacology. 2009;9:341–346. doi: 10.1016/j.coph.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 117.Israelson A, Arbel N, Da Cruz S, Ilieva H, Yamanaka K, Shoshan-Barmatz V, Cleveland DW. Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron. 2010;67:575–587. doi: 10.1016/j.neuron.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, Yu X, Fowlkes J, Rahder M, Stem K, Bernardi P, Bourdette D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cozzolino M, Ferri A, Valle C, Carri MT. Mitochondria and ALS: implications from novel genes and pathways. Molecular and cellular neurosciences. 2013;55:44–49. doi: 10.1016/j.mcn.2012.06.001. [DOI] [PubMed] [Google Scholar]