Abstract
Membranous nephropathy (MN) is an immune complex-mediated cause of the nephrotic syndrome that can occur in all age groups, from infants to the elderly. While systemic disorders such as hepatitis B infection or lupus may more frequently cause secondary MN in the younger population, primary or “idiopathic” MN has generally been considered a disease of adults. Recent progress in our understanding of primary disease was recently made when the target antigen in primary MN was identified as the M-type phospholipase A2 receptor (PLA2R). Circulating anti-PLA2R antibodies may serve both as a diagnostic tool for distinguishing primary from secondary disease, and as a biomarker for monitoring the immunological activity of this organ-specific autoimmune disease during treatment. Whereas definitive therapy for secondary forms of MN should be targeted at the underlying cause, immunosuppressive therapy is often necessary for primary disease. Alkylating agents in combination with corticosteroids, as well as calcineurin inhibitors (+/− steroids), are first line agents due to randomized controlled trials in an adult population with relatively long durations of follow-up. However, rituximab, mycophenolate and ACTH have shown promise in smaller and/or observational studies. The optimal therapy for children and adolescents with MN is less well defined.
Keywords: membranous nephropathy, nephrotic syndrome, PLA2R, bovine serum albumin, neutral endopeptidase, autoimmunity
Introduction
Membranous nephropathy (MN) is one of the most common causes of adult nephrotic syndrome, but occurs less frequently in children. Despite its under-representation in the pediatric population, MN in its various forms can be found at any age from early infancy to the very elderly. Its underlying etiology may be primarily autoimmune (“idiopathic”) or instead secondary to infections, other systemic autoimmune processes, drugs, malignancy, or even dietary antigens. Although mild and self-resolving in certain cases, in others, MN can lead to major complications of the nephrotic syndrome and progression to end-stage kidney disease (ESKD).
Due to the relative infrequency of MN compared to other causes of pediatric nephrotic syndrome such as minimal change disease (MCD) or focal and segmental glomerulosclerosis (FSGS) [1], the current literature on pediatric MN is sporadic and limited. Much of what we know about treatment has been extrapolated from studies in the adult population. Since the most recent review of the topic [2], a renaissance in our understanding of MN, with implications for both adult and pediatric disease, has occurred with the identification of the M-type phospholipase A2 receptor 1 (PLA2R) as the major autoantigen in primary MN. This review will highlight these latest findings in MN, with emphasis on the pediatric population where possible, and the reader is additionally directed to several excellent reviews on adult and pediatric MN [2,3].
Histology and types of MN
It is important to recognize from the outset that the term “membranous nephropathy” merely describes a histopathologic pattern identified on kidney biopsy and not a unique disease entity. The thickened appearance of the glomerular basement membrane (GBM) resulting in the name “membranous” is a consequence of the antibody/antigen deposits that form beneath the podocyte as well as the extracellular matrix that forms around them. This newly-formed matrix gives rise to the spikes characteristically seen on Jones’ silver stain, whereas the deposits themselves result in the fine granular capillary loop pattern seen on immunofluorescence (IF) and the subepithelial electron dense deposits seen by electron microscopy (EM; see more below). MN has traditionally been broken into primary or “idiopathic” disease, and secondary disease due to other systemic illness or exposures. Whereas the majority of adult MN is primary in nature, and is often associated with autoantibodies against PLA2R, secondary disease appears to be more common in the pediatric population, comprising up to 75% of pediatric MN in selected cohorts, especially below the age of 10 [2]. Commonly reported causes of secondary disease in the pediatric population are hepatitis B virus (HBV) infection or systemic lupus erythematosus (SLE). Clues to a secondary cause may come from the biopsy: the presence of subendothelial deposits, a “full-house” pattern on immunofluorescence, or the absence of IgG4 (when stained for) as the predominant IgG subclass can all be suggestive of secondary MN [4].
The hallmark feature of MN is the presence of immune deposits in a subepithelial (early) or intramembranous (late) position and is best appreciated by EM. The injured podocyte increases synthesis of basement membrane components that accumulate around the deposits, which can be characterized by the Ehrenreich-Churg staging system [5]. Stage 1 MN is defined by small and sparse subepithelial deposits immediately adjacent to the podocyte foot processes. Stage 2 MN is characterized by global subepithelial deposits separated by projections of newly-formed matrix material. In stage 3, the intervening projections of GBM envelop the subepithelial deposits such that they become intramembranous deposits. Stage 4 reflects a remodeling phase, in which the deposits become more electron-lucent as they undergo absorption.
This staging system does not have precise clinical implications in either adult or pediatric populations, as no significant correlation with clinical presentation (eg degree of proteinuria or renal function) or prognosis has been found. Furthermore, “progression” from one stage to another (eg stage 2 to stage 3) does not necessarily indicate worsening disease but can also be seen with resolution [6,7]. It should be noted that a ‘stage 0’ has recently been defined as the presence of IgG ± C3 staining in a capillary-loop pattern in the absence of electron-dense deposits by EM [8]. This occurs in recurrent disease that is detected early in the course of transplantation by protocol biopsy and is due to the fact that IF staining is more sensitive than EM in the detection of miniscule immune deposits.
The light microscopic appearance of early MN may be unremarkable or show only subtle abnormalities. In more advanced stages there is diffuse thickening of the glomerular capillary walls, and as noted above, spikes and “craters” (non-staining spaces in tangential sections, corresponding to the deposits) may be noted on silver stain with advancing disease stage. By IF, IgG and C3 are seen in a granular, capillary loop pattern. The IgG4 subclass is dominant or co-dominant in primary or idiopathic disease, although IgG1 may predominate early in disease [9]. The presence of C1q, especially in the presence of IgA and IgM, should suggest a possible diagnosis of lupus or other secondary cause.
A histological variant of MN exists in which the deposits are present in a segmental fashion; such segmental MN was found to be more common in children (29%) than in adults (2.4%) [10]. The vast majority of these cases were positive for C1q and exhibited mesangial deposits, although there was no clinical or serological evidence of lupus. Although this pattern has been described in several cases as a “resolution phase” of global MN, the possibility of a separate entity is suggested by its stability on serial biopsies in some cases [10].
Prevalence and epidemiology
The prevalence of primary MN in the pediatric population is difficult to know with certainty, and most data come from single-center or national biopsy registries. Because many pediatric patients with steroid-sensitive nephrotic syndrome are never biopsied (since biopsies are typically performed only for steroid-resistant nephrotic syndrome and/or worsening renal function), the relative prevalence of MN versus MCD and FSGS is unclear. In one of the larger studies, a series from Pakistan that investigated 538 children who underwent biopsy for idiopathic nephrotic syndrome, the overall rate of MN was 8% [11]. Importantly, in this series there was a significant difference between the 3% rate of MN in children younger than 13 and the 18.5% rate found in adolescents (13–18 years old). An identical rate was found in adolescents with idiopathic nephrotic syndrome in a U.S. cohort [12]. Another study reported that the incidence of MN was 1% in children 1–12 years of age but increased to 22% in children between the ages of 13 and 19 years [13]. Summary prevalence rates in the 1–5% range [2] may therefore partly reflect the proportion of younger children within each cohort studied. For example, a recent retrospective chart review of children in New York found that MN accounts for approximately 3% of renal biopsies [14]. However, adolescents represented only a minority of MN cases; the mean age at presentation was 9.6±4.6 years (range: 4–17 years). This study also tabulated demographic data from other studies looking at the prevalence of pediatric MN, and found similar median ages of presentation, with onset of disease ranging from infancy to young adulthood. While not all studies showed a male predominance, the ratio of affected males to females in aggregate was 112:70 [14]. In a systematic review of the literature including 40 studies on the incidence of primary GN from Europe, North and South America, Canada, Australasia and the Middle East, the incidence rate of MN in children is estimated at around 0.1/100,000 per year compared to adults at 1.2/100,000 per year [15].
Clinical manifestations and prognosis
The majority of patients with MN have all the features of the nephrotic syndrome, including heavy proteinuria, tissue edema, hypoalbuminemia, hyperlipidemia and lipiduria. The onset of the edema in MN is typically more gradual than that seen in MCD or primary FSGS and thus the precise onset of disease may not be clear. The prevalence of edema in children is not well reported. One study reported a prevalence of 38%, which mirrored the percentage of cases who exhibited nephrotic-range proteinuria [14].
Due to the low prevalence of MN in the pediatric population and to disparate etiologies of disease, it is difficult to generalize about natural history or prognosis. Children do seem to have better outcome than adults [2], and patients of Asian ancestry seem to have a better long-term prognosis than other ancestries [16]. Renal function is almost always normal in children at presentation [14] and to a somewhat lesser extent in adults. However, while 30–40% of adult patients may eventually develop renal insufficiency [17] progression to renal failure in children is the exception [18]. Despite this, there remains a substantial concern that MN, if left untreated, will progress to worsening or loss of kidney function. Most studies have found that 21–29% of patients show decreased kidney function at final follow-up [14]. The U.S. Renal Data System 2012 Annual Data Report found that 0.6% of incident cases of pediatric ESKD are due to MN, with a median age for reaching ESKD of 17 [19]. Individually-based decisions about treatment (see below) are therefore paramount in order to minimize the risk of progression to renal failure.
Hematuria is not typically considered a feature of adult MN, although microscopic hematuria may be found in the setting of heavy proteinuria. In pediatric cases of MN, however, hematuria is more common and has been reported in up to 69% of patients [14]. Among those of Asian ancestry, the prevalence of hematuria has been reported to be as high as 92% and 100% in cohorts of MN cases from Japan and China, and gross hematuria was found in 30% and 28%, respectively [20,21].
Hypertension is seen at presentation in approximately one third of in adult patients with MN. Although reported rates in children are variable, hypertension appears to be less common in the pediatric MN population [7,14,21]. Thromboembolism is a significant life-threatening complication of nephrotic syndrome and thromboembolic events are more common in MN than in other causes of the nephrotic syndrome. Children (2.8%) are less likely than adults (26.7%) with nephrotic syndrome to develop thromboembolism [22]. Some of the major features that distinguish pediatric from adult disease are presented in Table 1.
Table 1.
Differences between pediatric and adult membranous nephropathy (MN)
| Pediatric MN | Adult MN | |
|---|---|---|
| Disease type/subtype: | ||
| Proportion of primary nephrotic syndrome cases that are MN | < 5% (children) 5–20% (adolescents) |
15–30% |
| MN that is primary (“idiopathic”) | Minority | Majority |
| Proportion of primary MN that is PLA2R-associated | 45% (more common in adolescents) | 70–80% |
| Demographic and clinical features: | ||
| Male predominance | Variable | Yes |
| Full nephrotic syndrome | 40–75% | 75% |
| Microscopic hematuria | 70–90% (can be macroscopic) | 50% |
| Hypertension | < 10% | 30% |
| Thromboembolic events | < 5% | 10–20% |
| Spontaneous remission | Common | 30% |
| Progressive renal impairment | < 25% | 30–40% |
| Pathological features: | ||
| Mesangial deposits | Up to 50% | 30% |
| Segmental distribution of deposits | Occasional | Very rare |
The Heymann nephritis animal model of MN
Our understanding of the pathogenetic mechanisms involved in MN has largely come from decades of work in the Heymann nephritis (HN) experimental rat model of MN, first described more than 50 years ago [23]. The longevity of this model is due to its faithful reproduction of the clinical and histological aspects of human disease. We will use HN to briefly introduce some of the concepts that will become important later in this review. For more information on this model, the reader is directed to a more comprehensive review of this topic [24].
In HN, rats are either actively or passively (via heterologous antibodies raised in sheep) immunized against an antigenic fraction of rat proximal tubular brush border known as Fx1A. A prevailing theory at the time of this model’s inception suggested that the subepithelial deposits that ultimately formed beneath the podocytes were due to circulating immune complexes that possessed the appropriate physicochemical properties to pass through the endothelial layer and GBM and to deposit beneath the podocyte. Other potential mechanisms to explain the development of the subepithelial deposits were the presence of a putative intrinsic glomerular antigen in this location, or instead a circulating antigen that became “planted” in the subepithelial location. It was not until the late 1970s that two research groups independently demonstrated that the antibodies to the brush border antigenic fraction actually bound in situ to an intrinsic antigen that was likely a component of the podocyte foot process [25,26]. The pathogenic antigen in HN was later identified as the large transmembrane glycoprotein, megalin [27], which is involved in protein reuptake in the proximal tubular brush border of most mammalian species. In rats, megalin is additionally present on the podocyte foot process, a location that provided a rational explanation for the mechanism underlying the formation of the subepithelial deposits [28]. It was subsequently shown that immune deposits located at the base of the foot processes are rapidly shed from the cell membrane into the GBM [29]. Activation of the complement cascade by the immune complexes via the classical pathway leads to insertion of the terminal complement components C5b-9 into the podocyte cell membrane, causing sublethal injury, changes in cellular signaling and cell architecture, and proteinuria (reviewed in [30]). Such pathological events are considered to generally hold true in human disease as well, despite more limited evidence.
Fetomaternal alloimmune MN
Megalin, the HN antigen, is not expressed by human podocytes and with this realization began a long search for the antigenic target in human MN. The first demonstration of a relevant human antigen did not occur until 2002 when Debiec and colleagues reported a single case of MN that began before birth and was caused by the transplacental passage of maternal anti-neutral endopeptidase (NEP) antibodies [31]. This mother was genetically deficient in NEP, but had been alloimmunized to this protein during a prior pregnancy and miscarriage; the fetus had inherited a functional paternal gene and therefore expressed the protein. During her subsequent pregnancy, these pre-existing antibodies targeted the NEP antigen present at the cell membrane of the fetal podocytes, initiating in utero a pathophysiological process reminiscent of HN. The infant was born with massive proteinuria, oligoanuria and respiratory distress, and kidney biopsy later showed MN, the clinical features of which resolved after the infant cleared these maternal antibodies. The mother, lacking the antigen, remained unaffected by the circulating alloantibodies [32]. These authors found several other cases of fetomaternal alloimmune MN, all due to mothers deficient in NEP [33].
PLA2R-associated MN
A major advance in our understanding of MN came in 2009 with the identification of the M-type phospholipase A2 receptor (PLA2R) as the major antigenic target in adult disease [34]. Circulating anti-PLA2R autoantibodies were detected in 70% of adult primary MN patients, but in none of the patients with secondary MN, other glomerular or autoimmune disorders, or normal controls. PLA2R, a transmembrane glycoprotein and member of the mannose receptor family, is expressed by the glomerular podocyte, where its exact function remains unknown. In the same manner as megalin in the rat and NEP in humans, the expression of this target antigen in the podocyte supports a paradigm in which circulating autoantibodies target a glomerular antigen in situ. In biopsies of patients with MN, there was co-localization of the PLA2R antigen with IgG within the subepithelial immune deposits. Furthermore, anti-PLA2R antibodies could be eluted from biopsy samples of patients with primary MN, but not secondary MN or IgA nephropathy. IgG4 is the major subclass of anti-PLA2R, although smaller and varying proportions of IgG1, IgG2, and IgG3 are also present. The distinction is important, as IgG4 is felt unable to activate the classical complement pathway system. Although pathogenicity of anti-PLA2R antibodies has not yet been established due to the lack of an animal model, this report nonetheless heralded the start of a new era in the field of MN.
The sensitivity and specificity of anti-PLA2R for primary MN has been confirmed in a number of later studies, both in European and Asian cohorts [35–39]. The exact prevalence is dependent on the particular cohort studied: incident MN patients have a relatively high prevalence of anti-PLA2R, while cross-sectional cohorts that include active patients as well as those who have gone into remission, show lower rates of anti-PLA2R seropositivity. Immunoassays for anti-PLA2R have evolved from Western blotting techniques [34,40] to IF assays using PLA2R-transfected cells [36] to enzyme-linked immunosorbent assays (ELISA) using recombinant PLA2R [41,42]. The latter two types of assay are in clinical use in Europe, and will be available in the U.S. in the near future.
Due to the high (but not 100%) specificity of anti-PLA2R for primary MN, the question arises whether its presence is sufficient to rule out secondary disease. Several authors have described cases of what was considered secondary MN that surprisingly tested positive for either circulating anti-PLA2R or the presence of the PLA2R antigen within immune deposits on kidney biopsy (see below) [4,38]. Secondary disease is often suspected when MN occurs in the presence of diseases or exposures historically known to be associated with MN, and lengthy tables exist in many reviews and textbooks. However, it is very difficult to truly rule out the possibility that two disparate disease processes are occurring coincidentally. There is some evidence to suggest that the reported cases of anti-PLA2R-positive “secondary” disease actually represent primary autoimmune MN in the presence of another disease such as lupus or hepatitis B infection. Qin and colleagues looked at the presence of the IgG4 subclass within immune deposits on biopsy as indicative of primary disease [38]. These authors found that in most cases of MN secondary to lupus, hepatitis B, and malignancy, there was no circulating anti-PLA2R and no IgG4 present on biopsy, both of which would be typical of secondary MN. In those few cases that had been initially classified as secondary MN but were positive for circulating anti-PLA2R, there were IgG4 deposits similar to and suggestive of primary disease. In a histopathology study, Larsen and colleagues found an absence of the PLA2R antigen in cases of lupus-associated MN, but interestingly found that the majority of MN cases that had been classified as secondary due to association with hepatitis C or sarcoidosis had PLA2R within the immune deposits [4]. Future studies that use association with PLA2R to establish primary vs. secondary MN may help us narrow down the list of diseases and exposures that are truly causative of secondary MN.
In addition to its role in diagnosis and classification, it has become clear that the presence of circulating anti-PLA2R indicates ongoing immunological disease activity. Levels are high during the initial episode of nephrotic syndrome, fall to undetectable in remission, and re-appear when clinical relapse occurs [35]. It is likely that there is a lag time after the decline and disappearance of anti-PLA2R before improvement in clinical parameters occurs [40,43], although this may not be a universal phenomenon [44]. Nonetheless, monitoring of immunological activity (ie anti-PLA2R) during therapy, such as has been shown with the anti-B cell agent rituximab in primary MN, may provide a more rapid assessment of the response to immunosuppressive therapy [34,35,38,40].
As described above, in patients with anti-PLA2R-associated primary MN, the PLA2R antigen can be detected within the immune deposits in kidney biopsy (see Figure 1) [34,37]. This has turned out to be a useful diagnostic test for the renal pathologist to help distinguish primary, anti-PLA2R-associated MN from secondary disease [4,37,45]. There are cases in which a proteinuric MN patient who is seronegative for circulating anti-PLA2R autoantibodies may be found to have detectable PLA2R antigen within immune deposits on biopsy [37]. The most likely explanation for this scenario is that the patient has already had a remission of their immunologic disease, but has yet to fully clear the PLA2R- and IgG-containing subepithelial immune deposits, leading to continued clinical manifestations of disease. From a therapeutic point of view, it would be reasonable to withhold immunosuppressive treatment and give only conservative, anti-proteinuric and diuretic therapy to such a patient. However, there also exists the possibility that, very early in the course of disease, the kidney acts as a sink to bind and remove all anti-PLA2R from the circulation. Therefore, in the case of seronegativity for anti-PLA2R in the face of positive PLA2R staining of the immune deposits, close monitoring of proteinuria is necessary to distinguish between these two possibilities.
Figure 1.
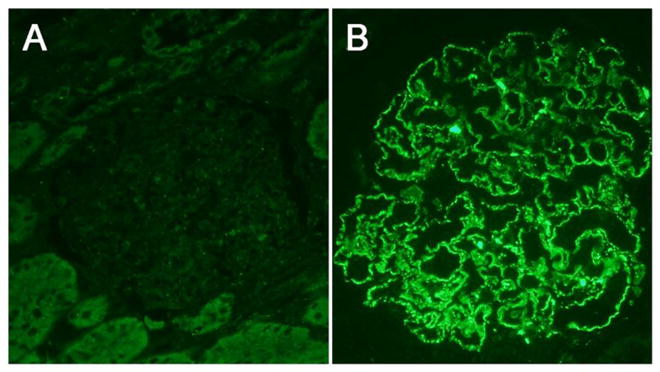
Immunofluorescence staining for PLA2R in kidney biopsy specimens from an 11 year-old male (A) and a 74 year-old male (B) with membranous nephropathy. Note the absence of granular staining in panel A which is indicative of non-PLA2R-associated MN. The finding of granular PLA2R staining in a capillary loop pattern (B) is indicative of primary PLA2R-associated membranous nephropathy. Images by R. Ayalon, 400x.
An important role of PLA2R in the pathogenesis of IMN in adults was supported by the association of idiopathic MN with a single nucleotide polymorphism (SNP) within the PLA2R1 gene in a genome-wide association study in three European cohorts [46]. A SNP within HLA-DQA1 encoding the alpha chain of the class II major histocompatibility protein HLA-DQ yielded an even more highly-significant signal, and the odds ratio for an individual being homozygous for both PLA2R1 and HLA-DQA1 risk alleles and having MN was nearly 80. The genetic risk associated with the PLA2R1 gene has been shown in Caucasian, Chinese, and Korean populations, although the pathophysiological significance of this finding is not yet understood. Despite the sequencing of all exons within PLA2R1, no specific coding mutation has been found, even in PLA2R-associated disease [47]. These authors speculate that a combination of genetic risk alleles in PLA2R1 and one or more HLA loci, and perhaps an acquired environmental factor as well, may all be necessary for the development of disease. Genotype-phenotype correlations have also recently been observed in terms of the presence of circulating autoantibody. In a European population, anti-PLA2R titer correlates with the HLA-DQA1*05:01 molecular genotype, as well as with HLA-DQB1*02:01 [44]. Additionally, among Chinese patients with PLA2R-associated disease, those homozygous for a high-risk haplotype consisting of two PLA2R1 alleles and one HLA-DQA1 allele had a much higher percentage of anti-PLA2R antibodies at the time of biopsy than did the group homozygous for the low-risk haplotype [48].
It is important to note that anti-PLA2R autoantibodies have been found in children as young as twelve years old (manuscript in preparation) although their exact prevalence in pediatric patients has yet to be established. Based on differences in the prevalence of MN in adolescence vs. childhood, the associations with primary PLA2R-associated MN are also likely to differ in these two age groups. The ability to stain archival biopsy specimens for PLA2R has allowed an analysis of the frequency of PLA2R-associated disease in a pediatric population. A recent analysis in biopsies from cases ranging in age from 4 to 17 has shown that 10/22 (45%) of pediatric biopsies exhibited PLA2R staining in a granular capillary loop pattern; the youngest case was from a ten year-old [49]. Those cases negative for PLA2R had more evidence of secondary features, such as mesangial deposits, the presence of C1q, and predominance of an IgG subclass other than IgG4. However, with average follow-up of 38 months in 17 patients, none was found to develop a secondary cause of MN such as lupus. These seminal findings indicate that primary MN may be more common in the pediatric population than had once been thought.
Other autoimmune targets in MN
Other targets for autoantibodies in adult IMN have been described, such as aldose reductase, manganese superoxide dismutase and alpha enolase [50], although the prevalence of such antibodies is lower than that for anti-PLA2R. The fact that these are intracellular antigens and are not found in high amounts in the normal glomerulus (so called “neoantigens’) raises the question of whether these antigens become targets of the humoral immune system only after podocyte damage has already begun, through the process of inter-molecular epitope spreading. A common target antigen to explain the approximately 20–30% of cases of idiopathic MN that are not PLA2R-associated has yet to be identified.
Pediatric MN and anti-cationic BSA antibodies
A remarkable case series of pediatric MN has recently been described, which happens to recapitulate to another animal model of MN. Debiec and colleagues were able to demonstrate antibodies to dietary cationic bovine serum albumin (cBSA) in young infants with MN, and showed co-localization of cBSA with IgG within the immune deposits. It was hypothesized that the cBSA represented a modified food-derived antigen that was absorbed from the relatively-underdeveloped pediatric intestinal tract in a partially-digested or undigested form. Cationic BSA had been used in several previous animal models of MN, since its positive charge allows it to deposit on the subepithelial side of the anionic GBM and thereby serve as a planted antigen. Anti-BSA antibodies subsequently bind to this antigen to form subepithelial immune complexes [51], which is likely what happened in these infantile cases.
Subepithelial granular deposits of BSA in biopsy specimens were detected only in children who had high levels of circulating cationic BSA. Anti-BSA antibodies eluted from a kidney biopsy specimen in one case were reactive with bovine, but not human serum albumin. The investigators have shown that during remission, the levels of circulating bovine serum albumin and BSA antibodies were substantially decreased. Although rare, the detection of antibodies to cBSA in an infant with MN would likely be treated by the avoidance of cow-derived milk products rather than immunosuppression.
Secondary causes of MN in children
The most important secondary causes of MN, both in children and adults, include SLE and chronic hepatitis B infection (especially in East Asia)[52]. There are a number of other secondary associations that have been associated with MN, although it is often difficult to establish a cause-and-effect relationship vs a coincidental occurrence of two disease processes, as noted above [53].
The relationship of MN with HBV is highlighted by the epidemiology of the viral infection itself. The reported prevalence of HBV-associated MN closely parallels the geographic patterns of prevalence of HBV, and the rarity of HBV-associated nephropathy in developed countries probably reflects the rarity of HBV infection, particularly in children [54]. National vaccination programs for HBV have clearly influenced the rates of associated MN. For example, a universal HBV vaccination program started in 1984 in Taiwan, a highly HBV endemic area, reduced the hepatitis B surface antigen (HBsAg)-carrier rate in children from 9.8% to 1.3% in 10 years and to less than 1% in 20 years. Studies have shown that the incidence of HBV-associated MN significantly declined accordingly, as was the case with HBV-associated hepatocellular carcinoma, which has decreased in frequency as well [55]. Studies from China [56] and South Africa [55] show similar results.
Due to the decreasing prevalence of HBV-associated MN, more of the pediatric secondary MN cases can currently be attributed to SLE; however, pediatric class V lupus nephritis still remains an uncommon disease and very little published data are available on its prevalence. Among nine series of children and adolescents with any type of lupus nephritis (LN), the overall prevalence of World Health Organization (WHO) class V (“membranous”) LN was 9%, which is similar to the rate reported in adult series (6–27%) [57]. A retrospective study from Memphis, Tennessee on 44 children with LN, 82% of whom were African Americans, reported a higher (30%) prevalence of class V LN; all of these cases were female [58]. This clear female predominance in lupus-associated MN is highlighted by a striking 12:1 female to male ratio among Chinese children with class V LN [59].
It is important to recognize that class V LN can be the initial presentation of SLE, only later to be followed by the appearance of extra-renal manifestations. Pure class V LN presents with proteinuria, with or without the nephrotic syndrome. In a recent cohort of pediatric LN, nephrotic syndrome was present in 30.8% of the cases of class V LN [58]. Class V LN typically presents with preserved kidney function and other clinical/serological manifestations of SLE (such as hypocomplementemia or anti-double stranded DNA antibodies) are often lacking. When accompanied by proliferative GN (class III [focal] or class IV [diffuse] LN), there is an increased likelihood of kidney dysfunction, the proteinuria is accompanied by hematuria, and typical serological manifestations are usually present. Several pathological features suggest the presence of lupus as the underlying etiology of membranous nephropathy: mesangial hypercellularity on light microscopy; positive “full house” IF staining for IgG, IgA and IgM, C3 and C1q; and the presence of subendothelial and mesangial immune deposits, as well as endothelial tubuloreticular structures by EM.
Treatment of MN
Due to the low prevalence of MN in the pediatric population, there are very limited studies that can define the optimal treatment of children with MN and consequently, a more personalized therapeutic approach needs to be adopted. In children with non-nephrotic amounts of proteinuria who are at low risk for complications such as progression of renal disease, supportive therapy (renin-angiotensin-aldosterone inhibition, diuretics and salt restriction) and avoidance of immunosuppression is a reasonable strategy. Similar to adult disease, pediatric MN may undergo spontaneous remission and it is worth following urinary protein and serum albumin to see if there is longitudinal improvement. If a child is found to be anti-PLA2R-positive, serial titers can also be helpful in assessing whether or not the disease might be entering a spontaneous remission.
The management of children with frank nephrotic syndrome is often empiric and begins with 4–8 weeks of oral corticosteroids to assess responsiveness. Those children who undergo remission with such treatment are not biopsied and are largely considered to have had minimal change disease. It should be noted that included in this group could also be undiagnosed mild or steroid-responsive MN. Biopsy is generally reserved for steroid-resistant nephrotic syndrome, and it is then that cases of FSGS or MN are identified. Thus, those nephrotic children in whom a diagnosis of MN has been made have often already been treated with several weeks of corticosteroids.
In adults, the first-line therapeutic agents for those who require immunosuppressive therapy are alkylating agents such as cyclophosphamide or chlorambucil, in conjunction with corticosteroids, or calcineurin inhibitors such as cyclosporine or tacrolimus, with or without steroids. The latest treatment recommendations and strength of evidence behind them has recently been detailed in the KDIGO Glomerulonephritis Guidelines [60], and this document contains a brief section on the treatment of pediatric disease. The consensus of this committee and of many pediatric nephrologists is that similar treatment choices can be made in severe cases of pediatric MN. The majority of published case series have described treatment regimens that include cyclophosphamide. In a pediatric population, there is always the added concern of inducing infertility, however the use of a limited course of low-dose cyclophosphamide can avoid cumulative doses that would be concerning for gonadal toxicity. One option that provides a relatively low exposure to cyclophosphamide (total dose of < 200 mg/kg) involves a 12 week regimen using daily cyclophosphamide (2 mg/kg/day) with alternate-day steroids and was used successfully in a small uncontrolled study [61]. Calcineurin inhibitors may also be effective but, based on experience in adults, they require 6–12 months of therapy with a slow taper to avoid relapse. Other agents such as the B-cell depleting agent rituximab, mycophenolate and adrenocorticotrophic hormone (ACTH) have been used in small and/or non-randomized studies in adults, but no evidence exists as to their use or appropriateness in the pediatric population.
Summary
Membranous nephropathy may be an uncommon cause of pediatric nephrotic syndrome, but it should always be considered in the differential diagnosis when a child or adolescent presents with proteinuria and/or edema. The recent identification of the anti-PLA2R antibodies has quickly led to new serologic and histologic tests to distinguish primary from secondary disease and to monitor disease activity in those with PLA2R-associated MN, which is known to occur in adolescents and pre-teens. Although antigens such as BSA or NEP can very rarely be the cause of MN in neonates, the etiology of much of pediatric MN remains to be elucidated. Secondary causes such as lupus or hepatitis B infection are common, and treatment should be targeted at an underlying systemic cause if identified. For primary disease, supportive treatment alone is sometimes enough to allow a spontaneous remission, although for more severe or progressive disease, courses of oral corticosteroids, calcineurin inhibitors, or alkylating agents may be necessary as in adult disease. Awareness of the disparate causes of pediatric MN should allow improved diagnosis and more individualized therapies for children, as findings from recent studies in adult MN are translated to the pediatric population.
References
- 1.Churg J, Habib R, White RH. Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet. 1970;760:1299–1302. doi: 10.1016/s0140-6736(70)91905-7. [DOI] [PubMed] [Google Scholar]
- 2.Menon S, Valentini RP. Membranous nephropathy in children: clinical presentation and therapeutic approach. Pediatr Nephrol. 2010;25:1419–1428. doi: 10.1007/s00467-009-1324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldman M, Austin HA. Treatment of idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1617–1630. doi: 10.1681/ASN.2012010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 5.Ponticelli C. Membranous nephropathy. J Nephrol. 2007;20:268–287. [PubMed] [Google Scholar]
- 6.Forland M, Spargo BH. Clinicopathological correlations in idiopathic nephrotic syndrome with membranous nephropathy. Nephron. 1969;6:498–525. doi: 10.1159/000179748. [DOI] [PubMed] [Google Scholar]
- 7.Latham P, Poucell S, Koresaar A, Arbus G, Baumal R. Idiopathic membranous glomerulopathy in Canadian children: a clinicopathologic study. J Pediatr. 1982;101:682–685. doi: 10.1016/s0022-3476(82)80290-4. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez EF, Cosio FG, Nasr SH, Sethi S, Fidler ME, Stegall MD, Grande JP, Fervenza FC, Cornell LD. The pathology and clinical features of early recurrent membranous glomerulonephritis. Am J Transplant. 2012;12:1029–1038. doi: 10.1111/j.1600-6143.2011.03903.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang CC, Lehman A, Albawardi A, Satoskar A, Brodsky S, Nadasdy G, Hebert L, Rovin B, Nadasdy T. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol. 2013;26:799–805. doi: 10.1038/modpathol.2012.237. [DOI] [PubMed] [Google Scholar]
- 10.Obana M, Nakanishi K, Sako M, Yata N, Nozu K, Tanaka R, Iijima K, Yoshikawa N. Segmental membranous glomerulonephritis in children: comparison with global membranous glomerulonephritis. Clin J Am Soc Nephrol. 2006;1:723–729. doi: 10.2215/CJN.01211005. [DOI] [PubMed] [Google Scholar]
- 11.Mubarak M, Kazi JI, Lanewala A, Hashmi S, Akhter F. Pathology of idiopathic nephrotic syndrome in children: are the adolescents different from young children? Nephrol Dial Transplant. 2012;27:722–726. doi: 10.1093/ndt/gfr221. [DOI] [PubMed] [Google Scholar]
- 12.Hogg RJ, Silva FG, Berry PL, Wenz JE. Glomerular lesions in adolescents with gross hematuria or the nephrotic syndrome. Report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol. 1993;7:27–31. doi: 10.1007/BF00861557. [DOI] [PubMed] [Google Scholar]
- 13.Moxey-Mims MM, Stapleton FB, Feld LG. Applying decision analysis to management of adolescent idiopathic nephrotic syndrome. Pediatr Nephrol. 1994;8:660–664. doi: 10.1007/BF00869080. [DOI] [PubMed] [Google Scholar]
- 14.Chen A, Frank R, Vento S, Crosby V, Chandra M, Gauthier B, Valderrama E, Trachtman H. Idiopathic membranous nephropathy in pediatric patients: presentation, response to therapy, and long-term outcome. BMC Nephrol. 2007;8:11. doi: 10.1186/1471-2369-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 16.Shiiki H, Saito T, Nishitani Y, Mitarai T, Yorioka N, Yoshimura A, Yokoyama H, Nishi S, Tomino Y, Kurokawa K, Sakai H Research Group on Progressive Renal Diseases in J. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int. 2004;65:1400–1407. doi: 10.1111/j.1523-1755.2004.00518.x. [DOI] [PubMed] [Google Scholar]
- 17.du Buf-Vereijken PW, Branten AJ, Wetzels JF. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis. 2005;46:1012–1029. doi: 10.1053/j.ajkd.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Cameron JS. Membranous nephropathy in childhood and its treatment. Pediatr Nephrol. 1990;4:193–198. doi: 10.1007/BF00858840. [DOI] [PubMed] [Google Scholar]
- 19.US Renal Data System. US Renal Data System, USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute for Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. Available from http://www.usrds.org/archive.aspx. [Google Scholar]
- 20.Tsukahara H, Takahashi Y, Yoshimoto M, Hayashi S, Fujisawa S, Suehiro F, Akaishi K, Nomura Y, Morikawa K, Sudo M. Clinical course and outcome of idiopathic membranous nephropathy in Japanese children. Pediatr Nephrol. 1993;7:387–391. doi: 10.1007/BF00857546. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Huang JP, Zhu BZ, Yao Y, Xiao HJ. Clinicopathological feature and treatment of idiopathic membranous nephropathy in children. Zhonghua Er Ke Za Zhi. 2011;49:311–315. [PubMed] [Google Scholar]
- 22.Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and Pathophysiology of Nephrotic Syndrome-Associated Thromboembolic Disease. Clin J Am Soc Nephrol. 2012;7:513–520. doi: 10.2215/CJN.10131011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heymann W, Hackel DB, Harwood S, Wilson SG, Hunter JL. Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959;100:660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- 24.Cybulsky AV, Quigg RJ, Salant DJ. Experimental membranous nephropathy redux. Am J Physiol Renal Physiol. 2005;289:F660–671. doi: 10.1152/ajprenal.00437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Damme BJ, Fleuren GJ, Bakker WW, Vernier RL, Hoedemaeker PJ. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978;38:502–510. [PubMed] [Google Scholar]
- 26.Couser WG, Steinmuller DR, Stilmant MM, Salant DJ, Lowenstein LM. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978;62:1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerjaschki D, Farquhar MG. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982;79:5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farquhar MG, Saito A, Kerjaschki D, Orlando RA. The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol. 1995;6:35–47. doi: 10.1681/ASN.V6135. [DOI] [PubMed] [Google Scholar]
- 29.Kerjaschki D, Miettinen A, Farquhar MG. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987;166:109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salant DJ, Quigg RJ, Cybulsky AV. Heymann nephritis: mechanisms of renal injury. Kidney Int. 1989;35:976–984. doi: 10.1038/ki.1989.81. [DOI] [PubMed] [Google Scholar]
- 31.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschenes G, Ronco PM. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053–2060. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 32.Ronco P, Debiec H. Target antigens and nephritogenic antibodies in membranous nephropathy: of rats and men. Semin Immunopathol. 2007;29:445–458. doi: 10.1007/s00281-007-0091-2. [DOI] [PubMed] [Google Scholar]
- 33.Debiec H, Nauta J, Coulet F, van der Burg M, Guigonis V, Schurmans T, de Heer E, Soubrier F, Janssen F, Ronco P. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet. 2004;364:1252–1259. doi: 10.1016/S0140-6736(04)17142-0. [DOI] [PubMed] [Google Scholar]
- 34.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 37.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 38.Qin W, Beck LH, Jr, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh YJ, Yang SH, Kim DK, Kang SW, Kim YS. Autoantibodies against Phospholipase A2 Receptor in Korean Patients with Membranous Nephropathy. PLoS One. 2013;8:e62151. doi: 10.1371/journal.pone.0062151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dähnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, Wetzels JF, Hofstra JM, Hoxha E, Stahl RA, Lambeau G, Stöcker W, Schlumberger W. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013;421C:213–218. doi: 10.1016/j.cca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Beck LH, Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int. 2010;77:765–770. doi: 10.1038/ki.2010.34. [DOI] [PubMed] [Google Scholar]
- 44.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83:940–948. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 45.Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 46.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 47.Coenen MJ, Hofstra JM, Debiec H, Stanescu HC, Medlar AJ, Stengel B, Boland-Augé A, Groothuismink JM, Bockenhauer D, Powis SH, Mathieson PW, Brenchley PE, Kleta R, Wetzels JF, Ronco P. Phospholipase A2 Receptor (PLA2R1) Sequence Variants in Idiopathic Membranous Nephropathy. J Am Soc Nephrol. 2013;24:677–683. doi: 10.1681/ASN.2012070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv J, Hou W, Zhou X, Liu G, Zhou F, Zhao N, Hou P, Zhao M, Zhang H. Interaction between PLA2R1 and HLA-DQA1 Variants Associates with Anti-PLA2R Antibodies and Membranous Nephropathy. J Am Soc Nephrol. 2013;24:1323–1329. doi: 10.1681/ASN.2012080771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cossey LN, Walker PD, Larsen CP. Phospholipase A2 receptor staining in pediatric idiopathic membranous glomerulopathy. Pediatr Nephrol. 2013;28:2307–2311. doi: 10.1007/s00467-013-2574-9. [DOI] [PubMed] [Google Scholar]
- 50.Murtas C, Bruschi M, Candiano G, Moroni G, Magistroni R, Magnano A, Bruno F, Radice A, Furci L, Argentiero L, Carnevali ML, Messa P, Scolari F, Sinico RA, Gesualdo L, Fervenza FC, Allegri L, Ravani P, Ghiggeri GM. Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:1394–1400. doi: 10.2215/CJN.02170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschenes G, Remuzzi G, Ulinski T, Ronco P. Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med. 2011;364:2101–2110. doi: 10.1056/NEJMoa1013792. [DOI] [PubMed] [Google Scholar]
- 52.Zeng CH, Chen HM, Wang RS, Chen Y, Zhang SH, Liu L, Li LS, Liu ZH. Etiology and clinical characteristics of membranous nephropathy in Chinese patients. Am J Kidney Dis. 2008;52:691–698. doi: 10.1053/j.ajkd.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Kleinknecht C, Levy M, Gagnadoux MF, Habib R. Membranous glomerulonephritis with extra-renal disorders in children. Medicine (Baltimore) 1979;58:219–228. doi: 10.1097/00005792-197905000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Fang ZL, Harrison TJ, Yang JY, Chen QY, Wang XY, Mo JJ. Prevalence of hepatitis B virus infection in a highly endemic area of southern China after catch-up immunization. J Med Virol. 2012;84:878–884. doi: 10.1002/jmv.23278. [DOI] [PubMed] [Google Scholar]
- 55.Liao MT, Chang MH, Lin FG, Tsai IJ, Chang YW, Tsau YK. Universal hepatitis B vaccination reduces childhood hepatitis B virus-associated membranous nephropathy. Pediatrics. 2011;128:e600–604. doi: 10.1542/peds.2010-3137. [DOI] [PubMed] [Google Scholar]
- 56.Xu H, Sun L, Zhou LJ, Fang LJ, Sheng FY, Guo YQ. The effect of hepatitis B vaccination on the incidence of childhood HBV-associated nephritis. Pediatr Nephrol. 2003;18:1216–1219. doi: 10.1007/s00467-003-1277-z. [DOI] [PubMed] [Google Scholar]
- 57.Cameron JS. Lupus and lupus nephritis in children. Adv Nephrol Necker Hosp. 1993;22:59–119. [PubMed] [Google Scholar]
- 58.Lau KK, Jones DP, Hastings MC, Gaber LW, Ault BH. Short-term outcomes of severe lupus nephritis in a cohort of predominantly African-American children. Pediatr Nephrol. 2006;21:655–662. doi: 10.1007/s00467-006-0060-3. [DOI] [PubMed] [Google Scholar]
- 59.Wong SN, Chan WK, Hui J, Chim S, Lee TL, Lee KP, Leung LC, Tse NK, Yuen SF. Membranous lupus nephritis in Chinese children--a case series and review of the literature. Pediatr Nephrol. 2009;24:1989–1996. doi: 10.1007/s00467-009-1257-z. [DOI] [PubMed] [Google Scholar]
- 60.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 61.Valentini RP, Mattoo TK, Kapur G, Imam A. Membranous glomerulonephritis: treatment response and outcome in children. Pediatr Nephrol. 2009;24:301–308. doi: 10.1007/s00467-008-1005-9. [DOI] [PubMed] [Google Scholar]


