Abstract
The mechanistic target of rapamycin complex I (mTORC1) is a central regulator of cellular and organismal growth and hyperactivation of this pathway is implicated in the pathogenesis of many human diseases, including cancer and diabetes. mTORC1 promotes growth in response to the availability of nutrients, such as amino acids, which drive mTORC1 to the lysosomal surface, its site of activation. How amino acid levels are communicated to mTORC1 is only recently coming to light by the discovery of a lysosome-based signaling system composed of the Rag GTPases and Ragulator, v-ATPase, GATOR and Folliculin complexes. An increased understanding of this pathway will not only provide insight into growth control, but also into the human pathologies triggered by its deregulation.
Keywords: Amino acid sensing, GATOR complex, Ragulator, Rag GTPases, Folliculin
Overview of mTORC1 signaling
Growth is a fundamental biological process that is highly influenced by an organism’s environment. For multicellular eukaryotes, including mammals, nutrient availability within the local environment is a major determinant of growth and is sensed through central signaling pathways that engage anabolic programs necessary to increase cell and body size. By coupling nutrient sensing to long-range growth factor and hormonal signaling networks, animals are able to readily adjust their growth and development programs to an ever-changing environment. One central nutrient sensing pathway is the mechanistic Target of Rapamycin (mTOR) pathway, which has emerged over the last twenty years as a master-regulator of cellular, organ and organismal growth (1).
mTOR is an atypical serine-threonine kinase (2, 3) that nucleates two distinct multi-protein complexes commonly known as mTORC1 and mTORC2. While mTORC2 promotes cell proliferation and survival (1), it is mTORC1 that that is generally associated with cell growth (1). mTORC1 is a 1 MDa (4) homodimer composed of the scaffolding subunit raptor (5, 6); two endogenous kinase inhibitors referred to as DEPTOR (7) and PRAS40 (8); and mLST8 (9) whose function remains cryptic. To stimulate cell growth, mTORC1 relies on its downstream effectors to coordinately promote anabolic programs such as mRNA translation (10) and repress catabolic programs such as autophagy (11), thereby avoiding a futile cycle of uncoordinated synthesis and degradation.
mTORC1 is regulated by the small GTPase Rheb (12-14), which resides at the lysosomal surface (15) where it functions as a potent stimulator of the mTORC1 kinase activity (8). Rheb in turn is negatively controlled by the trimeric Tuberous-Sclerosis complex (TSC), whose TSC2 component harbors GTPase activating protein (GAP) activity towards Rheb (14, 16) –converting it from the active GTP bound state to the inactive GDP bound state. The TSC complex, whose loss underlies a hamartomatous syndrome of the same name (17, 18), serves as a central hub for numerous extracellular and intracellular inputs including mitogen and growth factor signaling (19-23), energy levels (24), oxygen availability (25, 26) and genotoxic stress (27), which collectively exert their effects on the mTORC1 pathway by modulating the activity of the TSC complex.
In addition to these inputs, it has long been appreciated that amino acid levels are also critical for mTORC1 activation and represent one of the most conserved growth signals to this pathway. Despite progress in deciphering the TSC complex-Rheb axis, we have only begun to scratch the surface in uncovering how amino acids regulate mTORC1. Here, we focus on the rapidly evolving field of amino acid sensing and review how deregulation of this pathway contributes to human disease.
Amino acid signaling and mTORC1 localization
Early investigations revealed that amino acids were required to stimulate protein synthesis in rat skeletal muscles, (28) a process now known to be under the control of mTORC1. Subsequent studies in cultured mammalian cells confirmed that a mixture of all 20 amino acids activated mTORC1 and that the combination of amino acid and growth factor signaling was necessary for the phosphorylation of canonical mTORC1 substrates (29, 30). Whether all amino acids, one particular amino acid or an amino acid byproduct is being sensed remains unknown. Leucine and arginine are critical for mTORC1 activation but are insufficient for its activation in cells deprived of the remaining 18 amino acids (29). Dissecting the amino acid signal is further complicated by the fact that some plasma membrane amino acid transporters require additional amino acids to activate their cotransport mechanism (31); blurring the line between cellular transport and sensing.
Although it was clear for over a decade that amino acids were vital for mTORC1 activation, precisely how this signal functioned remained a mystery (32, 33). Careful cell biological analysis of this question revealed that amino acids regulate the intracellular localization of mTORC1 (34, 35). When cells are deprived of amino acids, mTORC1 is diffuse throughout the cytoplasm. However, upon addition of amino acids, mTORC1 rapidly translocates to the lysosomal surface where it is presumed to interact with the small GTPase Rheb (34). The localization of mTORC1 to the lysosome is mediated by the raptor component of mTORC1 (see below). Attachment of a lysosomal targeting sequence to raptor constitutively places mTORC1 on this surface (35), eliminating the need for the amino acid input to activate the pathway. Thus, it appears that the main purpose of the amino acid signal is to co-localize mTORC1 with its activator, Rheb (32, 36).
In budding yeast, TORC1 is localized to the vacuole, the equivalent of the mammalian lysosome (37). Although TORC1 kinase activity is responsive to amino acids in this system, it does not appear to shuttle in response to them (38). How amino acids actually activate TORC1 in yeast remains an open question that will certainly be addressed in the years to come.
The lysosome: key site of amino acid sensing
Extracellular amino acids must cross the plasma membrane to reactivate mTORC1 after their depletion from cell culture media (31). Nevertheless, treating cells with cycloheximide, a protein synthesis inhibitor, preserves sufficient intracellular pools of amino acids to rescue mTORC1 signaling even in the absence of extracellular amino acids. This finding argues that the sensing mechanism must occur within the cell and not at its periphery (34). The use of a cell-free reconstitution assay suggested that the amino acid signal initiates from within the lysosomal lumen (39). Depleting lysosomal amino acid stores by disrupting the lysosomal membrane with detergents or ionophores inhibits amino acid-dependent recruitment of mTORC1 to purified lysosomes. Amino acids accumulate in the lysosome after their extracellular addition (39) further supporting luminal sensing in cells. Furthermore, over-expression of PAT1, a lysosomal amino acid exporter, drains the lysosomal lumen of amino acids (40), turning off mTORC1 signaling even in the presence of amino acids. Intuitively, it makes sense for mTORC1 signaling to occur at the lysosome because this organelle is the end point of many catabolic pathways including autophagy, thus offering mTORC1 a window into the metabolic state of the cell.
The Rag GTPases mediate the amino acid signal to mTORC1
For a long time it was believed that the amino acid signal impinged on the TSC complex-Rheb axis, however, the development of TSC2−/− mice suggested otherwise. mTORC1 signaling remained sensitive to a change in amino acid levels in MEFs obtained from these animals (41, 42), implicating an alternative route for sensing. This alternative pathway, identified by biochemical and genetic screens (34, 43), centers around the Rag GTPases which lay the molecular foundation for amino acid signaling to mTORC1.
Loss of function studies in mammalian, fly and yeast cells indicates the requirement of Rag GTPases in communicating amino acid availability to mTORC1 (34, 38, 43). Rag GTPases lie downstream of amino acids and in their absence, mTORC1 cannot translocate to the lysosome. The Rag subfamily is unique among all small GTPase subfamilies because they function as obligate heterodimers (34, 43-46). Mammalian systems contain four members of the Rag subfamily: RagA and RagB (RagA/B) are functionally redundant and bind to the highly similar RagC and RagD (RagC/D) (44-46), suggesting the existence of four possible independent heterodimeric pairs. In yeast, only two Rag orthologs exist: GTR1 is the equivalent of RagA/B (44) and binds to GTR2 the ortholog of RagC/D (45, 47). Interestingly, the Rags also localize to the lysosomal surface where they recruit raptor in an amino acid dependent manner (35), substantiating their role as a docking site for mTORC1 at this compartment (34). Linking amino acids to mTORC1 recruitment is dependent on the nucleotide bound state of the Rags; RagA/B binds GDP during amino acid starvation and is quickly exchanged for GTP after re-stimulation (34). The importance of GTP-bound RagA/B was made clear in cells or animals expressing a GTP-locked RagA/B mutant, where mTORC1 was found constitutively localized to the lysosome regardless of amino acid levels (34, 36, 43).
Unlike other small GTPases, Rags do not contain lipid modifications commonly used for intracellular protein targeting. Rather, they rely on a pentameric complex referred to as Ragulator to function as its lysosomal tether (35, 48). Ragulator was identified as a Rag interacting complex and its basic architecture consists of the central Lamtor1 component that functions as a scaffold for two obligate heterodimers composed of Lamtor2-Lamtor3 and Lamtor4-Lamtor5. Myristoylation and palmitoylation on the N-terminus of Lamtor1 (49) promotes the localization of Ragulator and Rag GTPases to lipid rafts on lysosomal surfaces. In cells lacking or depleted of Ragulator components, Rag GTPases no longer attach to lysosomes, preventing mTORC1 shuttling to this surface, resulting in pathway inactivation (35, 48). The functional ortholog of Ragulator in yeast is likely the heterodimeric EGO1-3 complex that sits at the vacuolar surface, analogously localizing GTRs and TORC1 to this membrane (38, 50, 51). Although EGO1-3 and Ragulator members share no primary sequence identity, EGO3 adopts a nearly identical fold with Lamtor2/3 and Lamtor4/5 (52, 53) (see Box 1).
Box 1. Structural studies of amino acid sensing machinery.
Detailed structural studies of amino acid sensing components have provided a wealth of mechanistic insights. Perhaps the most surprising result has been the prevalence of the roadblock domain in this pathway, found in four out of five Ragulator proteins and all four Rag GTPases (48, 75-77). At its most basic form, the roadblock domain adopts a profilin-like fold after homo- or heterodimerization of two Roadblock containing proteins. While the function of this domain is still poorly understood, it is often associated with regulation of GTPases (78) as made evident by its presence on Ragulator and the bacterial GAP MglB (79).
The crystal structure of the yeast GTRs has also offered clues into a potentially new area of study, intra-Rag regulation. The GTRs are stitched together by their C-terminal domains containing the aforementioned roadblock domain, with the N-terminus occupied by rather dynamic nucleotide binding domains (75, 80). When both GTRs are bound to GTP, the G domains face away from each other, however, when GTR2 becomes GDP loaded, a dramatic rearrangement occurs, with the G domain of GTR2 swinging 28° to face the G domain of GTR1 (80). The significance of this structural re-arrangement remains to be determined, but given that heterodimeric GTPases such as the SRP-SRP receptor are known to control the nucleotide state of each other (81), this large movement raises the possibility that the Rag GTPases also partake in this form of self-regulation.
Regulation of the Rag GTPases
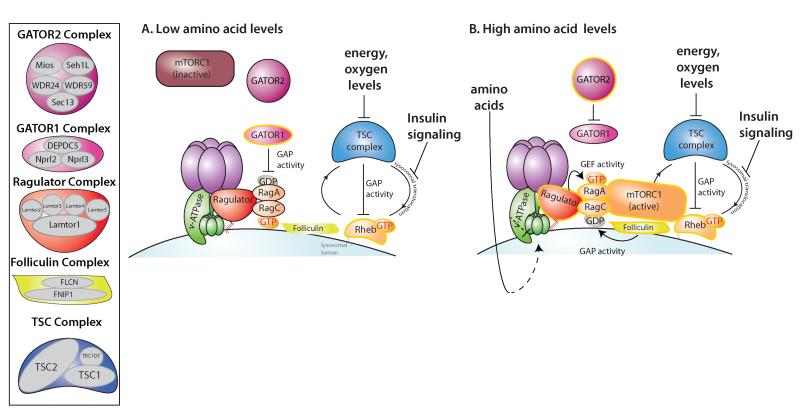
The Rags are critical for proper amino acid sensing as their tight coordination with amino acid levels prevents deregulation of mTORC1 signaling. This coordination depends on Rag GTPase activators and inhibitors, which modulate their nucleotide bound state. The recent identification of some of these regulators highlights a complex signaling network upstream of the Rag GTPases (Figure 1).
Figure 1. The mTORC1 amino acid sensing pathway.
A. Under low amino acid conditions Ragulator is found in an inhibitory state with the v-ATPase and GATOR1 exerts its GAP activity towards RagA, keeping this GTPase in the inactive GDP-bound state that is not sufficient to recruit mTORC1. Insulin signaling inhibits TSC complex translocation to the lysosomal surface where it functions as a GAP for Rheb, inactivating this G protein. B. Upon amino acid stimulation, GATOR1 may be inhibited by GATOR2 and Ragulator and v-ATPase undergo a conformational change unleashing the GEF activity of Ragulator towards RagA, while the folliculin complex promotes RagC GTP hydrolysis. The now active heterodimer, consisting of GTP-bound RagA and GDP-loaded RagC, recruits mTORC1 to the lysosomal surface, where it interacts with and is activated by Rheb.
Ragulator is a GEF for RagA and RagB
Dominant active mutations in the RagA/B proteins have led to the conclusion that a critical step in the amino acid sensing pathway is their conversion from the inactive GDP-bound state to the active GTP-bound state. In cells, GDP dissociation and GTP binding is mediated by guanine nucleotide exchange factors (GEFs) (54). In vitro experiments with the Rag GTPases suggested that their rates of GDP dissociation were not physiologically relevant, indicating the need for a GEF. Early experiments with Ragulator hinted at the auxiliary roles of this complex: Ragulator preferentially bound to Rags in their inactive state, an interaction that was driven by the nucleotide state of RagA/B (48). Clarifying the molecular nature of this observation, in vitro and in vivo data demonstrated a strong preference for Ragulator binding to Rags devoid of nucleotide, a characteristic of GEF-GTPase interactions. Using a system that allowed for the preferential loading of one Rag GTPase with guanine nucleotide in the context of the Rag heterodimer, it was revealed that Ragulator indeed functions as a GEF for RagA/B; however, it did not display any activity towards RagC or an unrelated GTPase (48). Moreover, the GEF activity of Ragulator appears to be shared across multiple surfaces of the pentameric complex, evoking comparisons to the TRAPP1 complex, which also requires multiple subunits for its GEF activity towards YPT1 (55, 56).
The v-ATPase controls Ragulator
Since the prominence of the lysosome for mTORC1 signaling is established, a limited RNAi screen in Drosophila cells was undertaken (39) to determine if additional lysosomal proteins partake in amino acid sensing. This screen led to the discovery that reducing the levels of lysosomal v-ATPase components severely inhibits dTORC1 signaling. Complementing the RNAi results, the use of v-ATPase specific chemical inhibitors in mammalian cells verified the importance of this complex in mediating the amino acid signal to mTORC1. The v-ATPase is composed of two multi-protein complexes termed V1 and V0 and is best appreciated for its role in lysosomal lumen acidification (57). While this acidification appears to be dispensable for mTORC1 signaling, the v-ATPase engages in extensive amino acid dependent interactions with Ragulator (39). Interestingly, the interactions between the two complexes during times of starvation are mimicked by pharmacological inhibition of the v-ATPase, offering a model in which the GEF activity of Ragulator is blocked during amino acid starvation but is fully reactivated after amino acids induce a conformational change between the v-ATPase and Ragulator (39, 48). This model raises the question of whether the v-ATPase is a direct amino acid sensor; an answer that will be forthcoming through the application of advanced biophysical and in vitro reconstitution assays.
Folliculin: A tumor suppressor complex that regulates RagC and RagD
While the key importance of RagA/B in controlling mTORC1 is established, the functional significance of RagC/D to this pathway has remained largely unanswered. Recently, studies employing new Rag-raptor in vitro and in vivo binding assays, indicated that the nucleotide state of RagC but not RagA governs the raptor-Rag GTPase interaction. Specifically, when RagC is bound to GDP, the Rag heterodimer strongly interacts with raptor, whereas GTP loading of RagC abolishes this interaction (58). These results raise the question of how GTP binding to RagA/B activates the heterodimer and in turn mTORC1 (see Box 1).
While the function of RagC has greatly expanded, the positive and negative regulators of RagC are only beginning to emerge. One such regulator is the tumor suppressor folliculin (FLCN) that functions as a RagC/D GAP. FLCN is not a new member of the mTORC1 pathway; truncating mutations in the protein are known to underlie a hamartoma- like syndrome referred to as Birt-Hogg-Dubé (BHD), which is characterized by aberrant mTORC1 activity (59, 60). Paradoxically, acute loss of FLCN, in human and fly cells, inactivates this pathway (58, 61) suggesting that FLCN could function as either a GEF for RagA/B or a GAP for RagC/D. In vitro studies established that FLCN along with its binding partner FNIP1 function as a GAP for RagC and RagD but not as a GEF for RagA/B, thus providing another avenue of control over mTORC1 translocation (58). In light of these new studies, the mechanism by which the loss of FLCN in BHD triggers mTORC1 pathway activation along with its control by amino acids must be revisited.
Other positive regulators: VAM6 and LRS
In addition to the role of Ragulator and FLCN in regulating mTORC1 activity via RagC/D, other novel regulators of TORC1 activity in yeast have been identified including Vam6 (38). While VAM6 has traditionally been recognized as part of the HOPS endosome/lysosome maturation pathway and previously thought to be a GEF for the GTPase Ypt7 (62, 63), new evidence suggests it also has GEF activity towards GTR1 (37). The GEF activity of VAM6 might only be conserved to lower eukaryotes, as its mammalian ortholog hVPS39 is neither a RagA GEF or an interacting protein (48). However, it is important to consider that deletion of VAM6 severely disrupts endosomal trafficking (38), a process known to be critical for proper mTORC1 signaling (64), implying that TORC1 may be indirectly regulated by VAM6. Resolving the differences in how Rag GTPases become activated in these two systems will be critical for our understanding of this pathway.
Recently, two independent studies revealed another positive regulator of mTORC1 activity, the tRNA charging enzyme, leucyl tRNA-synthetase (LRS), which was found to mediate the leucine signal to mTORC1 (65, 66). In yeast, LRS was identified as a GTR1 interacting protein that positively regulates TORC1 by blocking its inactivation by an unknown negative regulator upon LRS binding to leucine (66). Meanwhile, in mammalian cells, LRS may bind to RagD and function as a GAP for this GTPase in a leucine dependent manner (66). While this study identifies a critical region in LRS analogous to GAP domains found in Arf GAPs and eschew the widely held belief that GAP domains are highly divergent among different GTPase families, the GAP activity of LRS towards RagD has not been reproduced in a subsequent study (58). The alternative preferences of LRS for RagA/B or RagD in yeast and mammalian cells, respectively, is a point of contention that must also be reconciled with the function of other positive regulators of Rags
The GATOR complex is a GAP for RagA and RagB
While our understanding of how amino acid stimulation activates the Rags has evolved, the identity of negative regulators of these GTPases has eluded the field. Recently, an octomeric complex that interacts with the Rag GTPases and GTRs, called GATOR (GAP activity towards Rags) (67) in humans and SEA (Seh1-associated) in yeast (68) has been identified. GATOR is comprised of two distinct interacting subcomplexes known as GATOR1 and GATOR2. While the GATOR orthologs in yeast are identifiable, they differ in their hierarchical organization as they exist in stoichiometric ratios in SEA, forming one complex as opposed to two (69). Consistent with the localization of Rags to the lysosome, components from both GATOR subcomplexes have been found there via immunofluorescence and organellar mass-spectrometry studies (67, 68, 70). However, only GATOR1 was found to directly interact with the Rags. Loss of function studies in both species revealed a surprising bi-functional role for this complex: GATOR1 negatively regulates mTORC1, conferring complete insensitivity to amino acid starvation when deleted in cancer cell lines, whereas GATOR2 functions as a positive regulator. This bipartite regulation was explained through epistasis analysis placing GATOR1 downstream of GATOR2, emphasizing that the positive function of GATOR2 stems from its inhibition of GATOR1. Confirming the strong genetic evidence for its negative role in this pathway, GATOR1 was discovered to have GAP activity towards RagA/B similar to its yeast counterpart towards GTR1, converting these G proteins to an inactive state incapable of supporting mTORC1 signaling (67, 68). The exact GATOR1 subunit that confers GAP activity remains to be determined. Mutations in GATOR1 components occur in human tumors (67, 71) (see Box 2), suggesting that the ability to maintain mTORC1 activity in tumor microenvironments, where reduced nutrient concentrations would otherwise not support this type of signaling, may confer a selective advantage to cancer cells that have lost these negative regulators (72).
Box 2. Deregulation of amino acid signaling in human pathologies.
Since mTORC1 controls a variety of cellular processes, it is not surprising that deregulation of this pathway underlies many human pathologies including immunodeficiencies and various cancer types. Although diseases stemming from mutation of the TSC complex-Rheb axis are well appreciated, emerging evidence suggests mutations in components of the amino acid branch may also underlie several human diseases as well.
A previously unknown primary immune disorder has been linked to a reduction in the protein levels of the Ragulator component, Lamtor2 (p14). Although complete absence of Lamtor2 results in embryonic lethality (82), its reduction in humans leads to a decrease in the function of neutrophils, B cells, cytotoxic T cells and melanocytes (83). Consistent with a positive regulation of organismal size by mTORC1, affected individuals also display significant growth defects with growth profiles below the first percentile when compared to healthy age-matched peers (83). Moreover, in cells isolated from patients, mTORC1 activity was drastically reduced (35), making this disorder the first human disease associated with a reduction in a positive component of mTORC1.
Growing evidence suggests metabolic pathways play a large role in regulating tumor growth. The identification of GATOR1 as a novel negative regulator of mTORC1, suggested tumor suppressors might exist in the amino acid sensing pathway. Indeed, approximately 3% of glioblastoma and 2% of ovarian cancers analyzed contain inactivating mutations in two GATOR1 components (DEPDC5 and NPRL2) and analysis of NPRL3 still remains to be completed (67). Future large scale sequencing endeavors are likely to uncover even more cancers with mutations in GATOR1 genes and those cancers that over-express GATOR2 components, the negative regulator of GATOR1. Intriguingly, GATOR1-null cells with hyperactive mTORC1 signaling are highly sensitive to treatment with the mTORC1 inhibitor rapamycin (67), suggesting the use of GATOR1 mutations as biomarkers to identify tumors in patients that might be sensitive to mTORC1 inhibitors.
In the past several years, deregulation of the mTORC1 pathway has been appreciated to be an important contributor to epilepsy (84), a notion underscored by the fact that a majority of TSC patients suffer from at least one epileptic seizure during their lifetimes (85). Connecting amino acid sensing to epilepsy, two independent studies reported that mutations in the GATOR1 component DEPDC5 are responsible for many cases of familial focal epilepsy with variable foci, an autosomal dominant form of epilepsy (86, 87). These new studies coupled with previous research on TSC patients suggest that mTORC1 inhibitors may be beneficial for treating this disease.
SH3BP4 is a negative modulator of RagB
In addition to GATOR1, SH3BP4 (SH3 binding protein 4) was found to interact with the Rags and reduce mTORC1 signaling by increasing both RagB GTP hydrolysis and preventing RagB GDP dissociation; in short, this protein ensures that RagB is kept inactive (73). In contrast to all previously identified regulators, SH3BP4 is not conserved to lower eukaryotes, and its effect on mTORC1 signaling is more similar to that of a modulator. Therefore, it will be interesting to understand how SH3BP4 fits into the existing amino acid signaling pathway.
Spatial regulation of the TSC complex
A new rigorous study shows that that like mTORC1 the TSC complex translocates to and from the lysosomal surface in response to insulin signaling, but not to amino acid levels (74). Akt-dependent phosphorylation of TSC2, presumed by many to inhibit TSC complex GAP activity is responsible for driving the TSC complex off the lysosomal surface, allowing for mTORC1 activation by removing TSC from Rheb, the target of its GAP activity (74). Given the number of signals upstream of mTORC1 that converge on TSC complex phosphorylation, it is likely that other pathways, like the AMPK pathway, also affect TSC shuttling to and from the lysosome (74).
Concluding Remarks
This is an exciting time to study how amino acids are sensed by mTORC1. With the discovery of so many new pathway components, there remain many more questions than answers. Clearly, understanding the interplay between positive and negative regulators and the existence of additional human pathologies associated with these factors are of high interest (see Box 3). With the use of a combination of bioinformatic and systems biology approaches along with more traditional discovery platforms, the identity of the long sought amino acid sensor finally seems within reach.
Box 3. Outstanding questions.
The complexity of how amino acids are sensed by mTORC1 raises more questions than answers. Below we list a few outstanding questions that will be increasingly important to address in the years to come.
What is the identity of the amino acid sensor? While there is evidence suggesting that the v-ATPase may be an amino acid sensor, it remains to be determined whether this is the sole sensing mechanism or if additional sensors exist that modulate the activity of GATOR1 and GATOR2.
How does GATOR2 regulate GATOR1? Studies in both yeast and mammalian cells established a clear genetic and biochemical interaction between the two complexes, yet at the molecular level it remains unclear how GATOR2 inactivates GATOR1, presumably doing so under conditions of amino acid sufficiency.
Is there cross-talk between different Rag regulators? In GATOR1-null cell lines, mTORC1 is hyperactive and non-responsive to amino acid regulation. Interestingly, pharmacological inhibition of the v-ATPase does not reduce mTORC1 activity in these cell lines, formally suggesting that the v-ATPase/Ragulator arm functions either upstream or in parallel to GATOR1 (67). How these multi-component signaling complexes actually communicate with each other represents a ripe area for future study.
Acknowledgements
We thank Lynne Chantranupong and Shuyu Wang for helpful suggestions and Tom DeCesare for figure design. This work was supported by grants from the NIH (CA103866 and AI47389) and Department of Defense (W81XWH-07-0448) to D.M.S. L.B.P is the Lallage Feazel Wall Fellow of the Damon Runyon Cancer Research Foundation (DRG-2178-14). D.M.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. published online EpubApr 13 (10.1016/j.cell.2012.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. published online EpubJun 10 (10.1126/science.1199498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. published online EpubJun 10 (10.1126/science.1199484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–774. doi: 10.1016/j.molcel.2010.05.017. published online EpubJun 11 (10.1016/j.molcel.2010.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. published online EpubJul 26 ( [DOI] [PubMed] [Google Scholar]
- 6.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a Binding Partner of Target of Rapamycin (TOR), Mediates TOR Action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 7.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. published online EpubMay 29 (10.1016/j.cell.2009.03.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. published online EpubMar 23 (10.1016/j.molcel.2007.03.003) [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. published online EpubApr ( [DOI] [PubMed] [Google Scholar]
- 10.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. published online EpubMay (10.1038/nrm2672) [DOI] [PubMed] [Google Scholar]
- 11.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. published online EpubDec 3 (10.1126/science.1193497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. published online EpubJun (10.1038/ncb996) [DOI] [PubMed] [Google Scholar]
- 13.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. published online EpubJun (10.1038/ncb995) [DOI] [PubMed] [Google Scholar]
- 14.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. published online EpubAug 1 (10.1101/gad.1110003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, Finan PM, Kwiatkowski DJ, Murphy LO, Manning BD. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47:535–546. doi: 10.1016/j.molcel.2012.06.009. published online EpubAug 24 (10.1016/j.molcel.2012.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. published online EpubJun (10.1038/ncb999) [DOI] [PubMed] [Google Scholar]
- 17.Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. published online EpubDec 31 ( [DOI] [PubMed] [Google Scholar]
- 18.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. published online EpubAug 8 ( [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. published online EpubApr 22 (10.1016/j.cell.2005.02.031) [DOI] [PubMed] [Google Scholar]
- 20.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. published online EpubSep (10.1038/ncb840) [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. published online EpubJul ( [DOI] [PubMed] [Google Scholar]
- 22.Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–35370. doi: 10.1074/jbc.M205838200. published online EpubSep 20 (10.1074/jbc.M205838200) [DOI] [PubMed] [Google Scholar]
- 23.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. published online EpubSep (10.1038/ncb839) [DOI] [PubMed] [Google Scholar]
- 24.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. published online EpubNov 26 ( [DOI] [PubMed] [Google Scholar]
- 25.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. published online EpubDec 1 (10.1101/gad.1256804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18:2879–2892. doi: 10.1101/gad.322704. published online EpubDec 1 (10.1101/gad.322704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. published online EpubAug 8 (10.1016/j.cell.2008.06.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preedy VR, Garlick PJ. The response of muscle protein synthesis to nutrient intake in postabsorptive rats: the role of insulin and amino acids. Biosci Rep. 1986;6:177–183. doi: 10.1007/BF01115004. published online EpubFeb ( [DOI] [PubMed] [Google Scholar]
- 29.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. published online EpubJun 5 ( [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334(Pt 1):261–267. doi: 10.1042/bj3340261. published online EpubAug 15 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. published online EpubFeb 6 (10.1016/j.cell.2008.11.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. published online EpubMar (10.1038/nrm3522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524–533. doi: 10.1016/j.molmed.2012.05.007. published online EpubSep (10.1016/j.molmed.2012.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. published online EpubJun 13 (10.1126/science.1157535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. published online EpubApr 16 (10.1016/j.cell.2010.02.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. published online EpubJan 31 (10.1038/nature11745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binda M, PÈli-Gulli M-P, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF Controls TORC1 by Activating the EGO Complex. Mol Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Binda M, Peli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. published online EpubSep 11 (10.1016/j.molcel.2009.06.033) [DOI] [PubMed] [Google Scholar]
- 39.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. published online EpubNov 4 (10.1126/science.1207056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sagne C, Agulhon C, Ravassard P, Darmon M, Hamon M, El Mestikawy S, Gasnier B, Giros B. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci U S A. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. published online EpubJun 19 (10.1073/pnas.121183498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roccio M, Bos JL, Zwartkruis FJ. Regulation of the small GTPase Rheb by amino acids. Oncogene. 2006;25:657–664. doi: 10.1038/sj.onc.1209106. published online EpubFeb 2 (10.1038/sj.onc.1209106) [DOI] [PubMed] [Google Scholar]
- 42.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. published online EpubMay 13 (10.1074/jbc.M414499200) [DOI] [PubMed] [Google Scholar]
- 43.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. published online EpubAug (10.1038/ncb1753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirose E, Nakashima N, Sekiguchi T, Nishimoto T. RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. J Cell Sci. 1998;111(Pt 1):11–21. doi: 10.1242/jcs.111.1.11. published online EpubJan ( [DOI] [PubMed] [Google Scholar]
- 45.Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem. 2001;276:7246–7257. doi: 10.1074/jbc.M004389200. published online EpubMar 9 (10.1074/jbc.M004389200) [DOI] [PubMed] [Google Scholar]
- 46.Schurmann A, Brauers A, Massmann S, Becker W, Joost HG. Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. J Biol Chem. 1995;270:28982–28988. doi: 10.1074/jbc.270.48.28982. published online EpubDec 1 ( [DOI] [PubMed] [Google Scholar]
- 47.Nakashima N, Noguchi E, Nishimoto T. Saccharomyces cerevisiae putative G protein, Gtr1p, which forms complexes with itself and a novel protein designated as Gtr2p, negatively regulates the Ran/Gsp1p G protein cycle through Gtr2p. Genetics. 1999;152:853–867. doi: 10.1093/genetics/152.3.853. published online EpubJul ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator Is a GEF for the Rag GTPases that Signal Amino Acid Levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. published online EpubSep 14 (10.1016/j.cell.2012.07.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, Okada M. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. Embo J. 2009;28:477–489. doi: 10.1038/emboj.2008.308. published online EpubMar 4 (10.1038/emboj.2008.308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao M, Kaiser CA. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat Cell Biol. 2006;8:657–667. doi: 10.1038/ncb1419. published online EpubJul ( [DOI] [PubMed] [Google Scholar]
- 51.Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell. 2005;19:15–26. doi: 10.1016/j.molcel.2005.05.020. published online EpubJul 1 ( [DOI] [PubMed] [Google Scholar]
- 52.Zhang T, Peli-Gulli MP, Yang H, De Virgilio C, Ding J. Ego3 functions as a homodimer to mediate the interaction between Gtr1-Gtr2 and Ego1 in the ego complex to activate TORC1. Structure. 2012;20:2151–2160. doi: 10.1016/j.str.2012.09.019. published online EpubDec 5 (10.1016/j.str.2012.09.019) [DOI] [PubMed] [Google Scholar]
- 53.Kogan K, Spear ED, Kaiser CA, Fass D. Structural conservation of components in the amino acid sensing branch of the TOR pathway in yeast and mammals. J Mol Biol. 2010;402:388–398. doi: 10.1016/j.jmb.2010.07.034. published online EpubSep 17 (10.1016/j.jmb.2010.07.034) [DOI] [PubMed] [Google Scholar]
- 54.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. published online EpubJun 1 (10.1016/j.cell.2007.05.018) [DOI] [PubMed] [Google Scholar]
- 55.Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz EM, Ferro-Novick S, Reinisch KM. The Structural Basis for Activation of the Rab Ypt1p by the TRAPP Membrane-Tethering Complexes. Cell. 2008;133:1202–1213. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol. 2000;151:289–296. doi: 10.1083/jcb.151.2.289. published online EpubOct 16 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. published online EpubNov (10.1038/nrm2272) [DOI] [PubMed] [Google Scholar]
- 58.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The Folliculin Tumor Suppressor Is a GAP for the RagC/D GTPases That Signal Amino Acid Levels to mTORC1. Mol Cell. 2013 doi: 10.1016/j.molcel.2013.09.016. published online EpubOct 2 (10.1016/j.molcel.2013.09.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, Patel V, Igarashi P, Alvord WG, Leighty R, Yao M, Bernardo M, Ileva L, Choyke P, Warren MB, Zbar B, Linehan WM, Schmidt LS. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst. 2008;100:140–154. doi: 10.1093/jnci/djm288. published online EpubJan 16 (10.1093/jnci/djm288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, Turner M, Choyke P, Merino MJ, Pinto PA, Steinberg SM, Schmidt LS, Linehan WM. BHD mutations, clinical and molecular genetic investigations of Birt- Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. published online EpubJun (10.1136/jmg.2007.054304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J Cell Biol. 2013;202:1107–1122. doi: 10.1083/jcb.201307084. published online EpubSep 30 (10.1083/jcb.201307084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class CVps complex couples nucleotide exchange on the Ypt7 GTPase to SNAREdependent docking and fusion. J Cell Biol. 2000;151:551–562. doi: 10.1083/jcb.151.3.551. published online EpubOct 30 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20:1654–1659. doi: 10.1016/j.cub.2010.08.002. published online EpubSep 28 (10.1016/j.cub.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 64.Flinn RJ, Yan Y, Goswami S, Parker PJ, Backer JM. The late endosome is essential for mTORC1 signaling. Mol Biol Cell. 2010;21:833–841. doi: 10.1091/mbc.E09-09-0756. published online EpubMar 1 (10.1091/mbc.E09-09-0756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. published online EpubApr 13 (10.1016/j.cell.2012.02.044) [DOI] [PubMed] [Google Scholar]
- 66.Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46:105–110. doi: 10.1016/j.molcel.2012.02.009. published online EpubApr 13 (10.1016/j.molcel.2012.02.009) [DOI] [PubMed] [Google Scholar]
- 67.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. published online EpubMay 31 (10.1126/science.1232044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panchaud N, Peli-Gulli MP, De Virgilio C. Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the Rag family GTPase Gtr1. Sci Signal. 2013;6:ra42. doi: 10.1126/scisignal.2004112. published online EpubMay 28 (10.1126/scisignal.2004112) [DOI] [PubMed] [Google Scholar]
- 69.Dokudovskaya S, Waharte F, Schlessinger A, Pieper U, Devos DP, Cristea IM, Williams R, Salamero J, Chait BT, Sali A, Field MC, Rout MP, Dargemont C. A conserved coatomer-related complex containing Sec13 and Seh1 dynamically associates with the vacuole in Saccharomyces cerevisiae. Mol Cell Proteomics. 2011;10:M110–006478. doi: 10.1074/mcp.M110.006478. published online EpubJun (10.1074/mcp.M110.006478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schroder B, Wrocklage C, Pan C, Jager R, Kosters B, Schafer H, Elsasser HP, Mann M, Hasilik A. Integral and associated lysosomal membrane proteins. Traffic. 2007;8:1676–1686. doi: 10.1111/j.1600-0854.2007.00643.x. published online EpubDec (10.1111/j.1600-0854.2007.00643.x) [DOI] [PubMed] [Google Scholar]
- 71.Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000;60:6116–6133. published online EpubNov 1 ( [PubMed] [Google Scholar]
- 72.Menon S, Manning BD. Cell signalling: nutrient sensing lost in cancer. Nature. 2013;498:444–445. doi: 10.1038/498444a. published online EpubJun 27 (10.1038/498444a) [DOI] [PubMed] [Google Scholar]
- 73.Kim YM, Stone M, Hwang TH, Kim YG, Dunlevy JR, Griffin TJ, Kim DH. SH3BP4 is a negative regulator of amino acid-Rag GTPase-mTORC1 signaling. Mol Cell. 2012;46:833–846. doi: 10.1016/j.molcel.2012.04.007. published online EpubJun 29 (10.1016/j.molcel.2012.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial Control of the TSC Complex Integrates Insulin and Nutrient Regulation of mTORC1 at the Lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. published online EpubFeb 13 (10.1016/j.cell.2013.11.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong R, Li L, Liu Y, Wang P, Yang H, Wang L, Cheng J, Guan KL, Xu Y. Crystal structure of the Gtr1p-Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Genes Dev. 2011;25:1668–1673. doi: 10.1101/gad.16968011. published online EpubAug 15 (10.1101/gad.16968011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Saez I, Lacroix FB, Blot D, Gabel F, Skoufias DA. Structural characterization of HBXIP: the protein that interacts with the anti-apoptotic protein survivin and the oncogenic viral protein HBx. J Mol Biol. 2011;405:331–340. doi: 10.1016/j.jmb.2010.10.046. published online EpubJan 14 (10.1016/j.jmb.2010.10.046) [DOI] [PubMed] [Google Scholar]
- 77.Kurzbauer R, Teis D, de Araujo ME, Maurer-Stroh S, Eisenhaber F, Bourenkov GP, Bartunik HD, Hekman M, Rapp UR, Huber LA, Clausen T. Crystal structure of the p14/MP1 scaffolding complex: how a twin couple attaches mitogen-activated protein kinase signaling to late endosomes. Proc Natl Acad Sci U S A. 2004;101:10984–10989. doi: 10.1073/pnas.0403435101. published online EpubJul 27 (10.1073/pnas.0403435101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koonin EV, Aravind L. Dynein light chains of the Roadblock/LC7 group belong to an ancient protein superfamily implicated in NTPase regulation. Curr Biol. 2000;10:R774–776. doi: 10.1016/s0960-9822(00)00774-0. published online EpubNov 2 ( [DOI] [PubMed] [Google Scholar]
- 79.Miertzschke M, Koerner C, Vetter IR, Keilberg D, Hot E, Leonardy S, Sogaard-Andersen L, Wittinghofer A. Structural analysis of the Ras-like G protein MglA and its cognate GAP MglB and implications for bacterial polarity. Embo J. 2011;30:4185–4197. doi: 10.1038/emboj.2011.291. published online EpubOct 19 (10.1038/emboj.2011.291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeong JH, Lee KH, Kim YM, Kim DH, Oh BH, Kim YG. Crystal structure of the Gtr1p(GTP)-Gtr2p(GDP) protein complex reveals large structural rearrangements triggered by GTP-to-GDP conversion. J Biol Chem. 2012;287:29648–29653. doi: 10.1074/jbc.C112.384420. published online EpubAug 24 (10.1074/jbc.C112.384420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akopian D, Shen K, Zhang X, Shan SO. Signal Recognition Particle: An Essential Protein-Targeting Machine. Annu Rev Biochem. 2013 doi: 10.1146/annurev-biochem-072711-164732. published online EpubFeb 13 (10.1146/annurev-biochem-072711-164732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teis D, Taub N, Kurzbauer R, Hilber D, de Araujo ME, Erlacher M, Offterdinger M, Villunger A, Geley S, Bohn G, Klein C, Hess MW, Huber LA. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J Cell Biol. 2006;175:861–868. doi: 10.1083/jcb.200607025. published online EpubDec 18 (10.1083/jcb.200607025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bohn G, Allroth A, Brandes G, Thiel J, Glocker E, Schaffer AA, Rathinam C, Taub N, Teis D, Zeidler C, Dewey RA, Geffers R, Buer J, Huber LA, Welte K, Grimbacher B, Klein C. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med. 2007;13:38–45. doi: 10.1038/nm1528. published online EpubJan (10.1038/nm1528) [DOI] [PubMed] [Google Scholar]
- 84.Russo E, Citraro R, Constanti A, De Sarro G. The mTOR signaling pathway in the brain: focus on epilepsy and epileptogenesis. Mol Neurobiol. 2012;46:662–681. doi: 10.1007/s12035-012-8314-5. published online EpubDec (10.1007/s12035-012-8314-5) [DOI] [PubMed] [Google Scholar]
- 85.van Eeghen AM, Black ME, Pulsifer MB, Kwiatkowski DJ, Thiele EA. Genotype and cognitive phenotype of patients with tuberous sclerosis complex. Eur J Hum Genet. 2012;20:510–515. doi: 10.1038/ejhg.2011.241. published online EpubMay (10.1038/ejhg.2011.241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dibbens LM, de Vries B, Donatello S, Heron SE, Hodgson BL, Chintawar S, Crompton DE, Hughes JN, Bellows ST, Klein KM, Callenbach PM, Corbett MA, Gardner AE, Kivity S, Iona X, Regan BM, Weller CM, Crimmins D, O’Brien TJ, Guerrero-Lopez R, Mulley JC, Dubeau F, Licchetta L, Bisulli F, Cossette P, Thomas PQ, Gecz J, Serratosa J, Brouwer OF, Andermann F, Andermann E, van den Maagdenberg AM, Pandolfo M, Berkovic SF, Scheffer IE. Mutations in DEPDC5 cause familial focal epilepsy with variable foci. Nat Genet. 2013;45:546–551. doi: 10.1038/ng.2599. published online EpubMay (10.1038/ng.2599) [DOI] [PubMed] [Google Scholar]
- 87.Ishida S, Picard F, Rudolf G, Noe E, Achaz G, Thomas P, Genton P, Mundwiller E, Wolff M, Marescaux C, Miles R, Baulac M, Hirsch E, Leguern E, Baulac S. Mutations of DEPDC5 cause autosomal dominant focal epilepsies. Nat Genet. 2013;45:552–555. doi: 10.1038/ng.2601. published online EpubMay (10.1038/ng.2601) [DOI] [PMC free article] [PubMed] [Google Scholar]