Abstract
Tetralogy of Fallot and a complete atrioventricular septal defect are thought to arise by distinct mechanisms, yet their co-occurrence is a recognized association. Analysis of the prevalence of co-occurrence in Down syndrome suggests a common developmental basis. Trisomy 21 may perturb cardiac progenitor cells before they enter the heart tube.
Keywords: Trisomy 21, Down syndrome, second heart field, tetralogy of Fallot, atrioventricular septal defect
During embryonic development, cells from the cardiac crescent form the heart tube. Subsequently, cells from the second heart field are added to the arterial and venous poles (alternatively referred to as anterior or cranial and posterior or caudal, respectively). Defects of second heart field development are classified accordingly, and their investigation largely focuses upon events at either pole. For example, DiGeorge syndrome is associated with malformations such as tetralogy of Fallot (TOF) and truncus arteriosus. TBX1, the gene that underlies the DiGeorge cardiac phenotypes, regulates development at the anterior second heart field. Mutations of genes that perturb posterior development, such as Wnt2, cause an atrioventricular septal defect (AVSD) (1).
The co-occurrence of TOF and AVSD is associated with Down syndrome (2). The mechanism of co-occurrence is unknown but must involve either one or two developmental missteps. If each defect arises from two, independent events at the arterial and venous poles, then the prevalence of co-occurrence should equal the product of the prevalences of each defect in Down syndrome. If just one misstep causes both defects, then the prevalence should exceed that predicted by a two-misstep model. Statistical analyses of patients at our institution and in two population-based studies suggest a common developmental basis for the co-occurrence.
Methods
We calculated the prevalences among patients seen at St. Louis Children’s Hospital by querying the Clinical Investigation Data Exploration Repository (CIDER) at Washington University School of Medicine. CIDER contains the electronic medical records of >5 million adult and pediatric patients. The search was approved by the Institutional Review Board. Patients born on or after January 1, 1997 and seen by August 30, 2013 were searched by ICD9 codes and key words. We confirmed each instance of Down syndrome, AVSD, TOF and every combination of diagnoses by reading every cardiology and genetics clinic note (N > 5000), echocardiogram report (N = 4917) and operative summary (N = 1710). Fisher exact tests were performed to compare prevalences in our institution with published data. Chi-square tests were performed to test the two-misstep model. The expected number of co-occurrences in Down cases is given by: Expected number = (Down TOF cases/Down cases) × (Down AVSD cases/Down cases) × Down cases.
To obtain an independent estimate of the proportion of TOF-AVSD patients who have Down syndrome, we performed a meta-analysis of surgical case series that reported Down syndrome status. The reports were identified through PubMed and cited references.
Results
Among >630,000 patients evaluated at St. Louis Children’s Hospital, 1146 had Down syndrome. The prevalence of TOF was 4.4% (50/1146), which is greater than the 2.6% reported by the National Down Syndrome Project, a population-based study from the United States (P = 0.02) (3). The prevalence of AVSD was 17.4% (199/1146), which is greater than the reported 12.8% (P = 0.001). Consequently, the expected number of co-occurrences for a two-misstep model may be overestimated and biased against a one-misstep model.
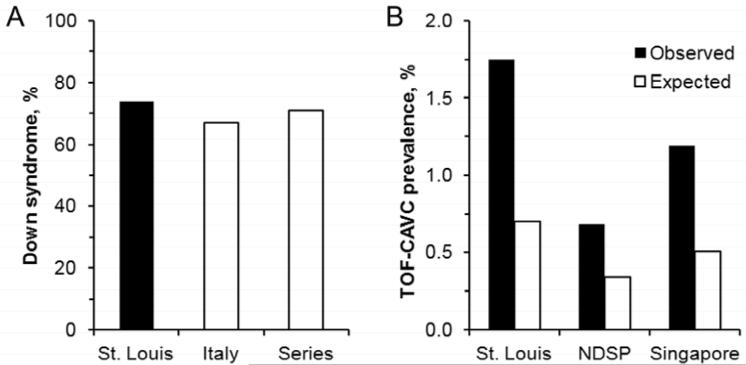
TOF and AVSD co-occurred in 1.8% of Down syndrome patients (20/1146). The prevalence exceeds the 0.68 and 1.2% reported by population-based studies in the National Down Syndrome Project and Singapore probably because children who have heart disease are more likely to be seen at a major center (3,4). Conversely, 74% of TOF-AVSD patients had Down syndrome (20/27). The percentage is similar to the 67% (43/64) reported in a multi-centered, Italian series (P = 0.6) (2). The percentage is also similar to the 71% (183/258) calculated from a meta-analysis of 16 surgical case series published from 1974 to 2009 (P = 0.8) (5-20). The similar proportions in our institution, Italy and surgical series suggest that the numerator, 20, is accurately estimated for testing the two-misstep model (Figure 1, A).
Figure 1.
(A) Approximately 70% of TOF-AVSD patients have Down syndrome in St. Louis, Italy (2), and a meta-analysis of 16 surgical case series (5-20). (B) The observed prevalences of TOF-AVSD in Down syndrome are twice that expected by a two-misstep model in St. Louis (20 vs. 8/1146, P = 2.1 × 10−5), the National Down Syndrome Project (10 vs. 5/1469, P = 0.02) and Singapore (7 vs. 3/588, P = 0.02). Percentages are shown for comparison; absolute numbers were used in Chi-squared tests.
The prevalence of co-occurrence of TOF and AVSD in Down syndrome consistently exceeds that expected by a two-misstep model (Figure 1, B). More than twice as many patients are observed than expected (P = 2 × 10−5). Similarly in the National Down Syndrome Project and Singapore the observed number of cases is twice the expected (P = 0.02 for both) (3,4).
Discussion
TOF and AVSD are thought to arise by distinct mechanisms, but the present results suggest that their co-occurrence in Down syndrome shares a common developmental basis. Recently, cellular subdomains of the second heart field, as delineated by their overlap with broad domains of Hoxb1, Hoxa1 and Hoxa3 expression, have been described in the early embryo (21). Hox transcription factors, which are encoded in four clusters on human chromosomes 2, 7, 12, and 17, pattern the embryonic anterior-posterior (cranial-caudal) axis. Hoxb1+/Hoxa1+/Hoxa3− and Hoxb1+/Hoxa1+/Hoxa3+ subdomains contribute primarily to the distal right ventricular outflow tract, whereas a Hoxb1-only subdomain gives rise to structures in both the right ventricular outflow tract and the atrioventricular junction (21). The abnormal development of these structures cause TOF and AVSD (22,23). We postulate that trisomy 21 perturbs the Hoxb1-only subdomain and so disrupts the future development of the two daughter cell populations that contribute to either pole of the heart (Figure 2). The model leaves unexplained the predilection for AVSD over TOF in Down syndrome, but it parsimoniously explains the association of each defect and their co-occurrence. Other genetic, epigenetic or environmental factors likely influence the specific cardiac presentation (24-28).
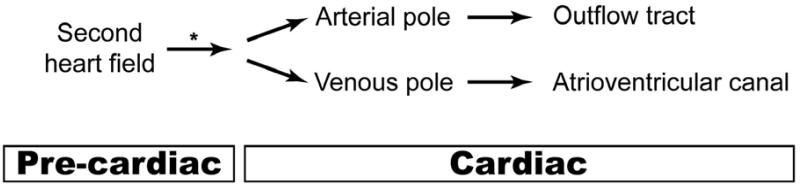
Figure 2.
One developmental misstep explains the co-occurrence of TOF and AVSD in Down syndrome. Trisomy 21 may perturb progenitor cells in the second heart field before they enter the heart tube (*). The defective cells then localize to the arterial or venous poles to make the structures malformed in TOF and AVSD. Alternatively, a modifier gene that increases the risk of both TOF and AVSD could act before or after cells from the second heart field have localized to either pole of the heart.
Alternatively, one unknown modifier gene could predispose to both TOF and AVSD in Down syndrome. Modifier genes do not cause but rather influence the presentation of a mutant phenotype. In a mouse model, alleles of modifier genes alter the risk of specific defects caused by mutations of the cardiac transcription factor NKX2-5. Some modifier genes may influence more than one type of defect (26,27). Rare human polymorphisms of CRELD1, HEY2 and genes in the VEGF pathway have been associated with AVSD in Down syndrome (24,25), but none is known for TOF. The one-gene and one-misstep hypotheses are not mutually exclusive. A hypothetical genetic polymorphism could exert its effect at the Hoxb1-only subdomain or simultaneously at the arterial and venous poles.
The analysis is limited mainly by the accuracy of the observed number of TOF-AVSD patients who have Down syndrome. Referral bias to a single, major pediatric center may cause an overestimate. Even if the observed number were overestimated by 50%, the two-misstep hypothesis would still be rejected. This high an overestimate is unlikely because the proportion of TOF-AVSD patients who have Down syndrome would fall to ~50%, which is much less than reported by other groups (2,5-20). Validation of the statistical result in two population-based studies suggests that any overestimate is not so large as to alter the main conclusion.
Acknowledgments
We thank David Wilson and members of the Jay laboratory for helpful comments.
P.J. is supported by an Established Investigator Award from the American Heart Association, the Lawrence J. & Florence A. DeGeorge Charitable Trust, the Children’s Discovery Institute of Washington University, St Louis Children’s Hospital, the Children’s Heart Foundation, the National Institutes of Health (NIH; R01 HL105857), and the Washington University Institute of Clinical and Translational Sciences (NIH National Center for Advancing Translational Sciences UL1 TR000448).
Abbreviations
- TOF
Tetralogy of Fallot
- CIDER
Clinical Investigation Data Exploration Repository
- AVSD
Atrioventricular septal defect
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- 2.Vergara P, Digilio MC, Zorzi AD, Carlo DD, Capolino R, Rimini A, et al. Genetic heterogeneity and phenotypic anomalies in children with atrioventricular canal defect and tetralogy of Fallot. Clin Dysmorphol. 2006;15:65–70. doi: 10.1097/01.mcd.0000198925.94082.ea. [DOI] [PubMed] [Google Scholar]
- 3.Freeman SB, Bean LH, Allen EG, Tinker SW, Locke AE, Druschel C, et al. Ethnicity, sex, and the incidence of congenital heart defects: a report from the National Down Syndrome Project. Genet Med. 2008;10:173–80. doi: 10.1097/GIM.0b013e3181634867. [DOI] [PubMed] [Google Scholar]
- 4.Tan M, Xu C, Sim SK, Seow AL, Tan TH, Quek SC. Types and distribution of congenital heart defects associated with trisomy 21 in Singapore. J Paediatr Child Health. 2013;49:223–7. doi: 10.1111/jpc.12129. [DOI] [PubMed] [Google Scholar]
- 5.Tandon R, Moller JH, Edwards JE. Tetralogy of Fallot associated with persistent common atrioventricular canal (endocardial cushion defect) Br Heart J. 1974;36:197–206. doi: 10.1136/hrt.36.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uretzky G, Puga FJ, Danielson GK, Feldt RH, Julsrud PR, Seward JB, et al. Complete atrioventricular canal associated with tetralogy of Fallot. Morphologic and surgical considerations. J Thorac Cardiovasc Surg. 1984;87:756–66. [PubMed] [Google Scholar]
- 7.Alonso J, Nunez P, Perez de LJ, Sanchez PA, Villagra F, Gomez R, et al. Complete atrioventricular canal and tetralogy of Fallot: surgical management. Eur J Cardiothorac Surg. 1990;4:297–9. doi: 10.1016/1010-7940(90)90205-e. [DOI] [PubMed] [Google Scholar]
- 8.Gatzoulis MA, Shore D, Yacoub M, Shinebourne EA. Complete atrioventricular septal defect with tetralogy of Fallot: diagnosis and management. Br Heart J. 1994;71:579–83. doi: 10.1136/hrt.71.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu IS, Hung CR, Wang JK, Wu MH, Chu SH. Surgical treatment of complete atrioventricular septal defect associated with tetralogy of Fallot. Int J Cardiol. 1995;48:225–30. doi: 10.1016/0167-5273(94)02243-c. [DOI] [PubMed] [Google Scholar]
- 10.Bertolini A, Dalmonte P, Bava GL, Calza G, Lerzo F, Zannini L, et al. Surgical management of complete atrioventricular canal associated with tetralogy of Fallot. Cardiovasc Surg. 1996;4:299–302. doi: 10.1016/0967-2109(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 11.Delius RE, Kumar RV, Elliott MJ, Stark J, de Leval MR. Atrioventricular septal defect and tetralogy of Fallot: a 15-year experience. Eur J Cardiothorac Surg. 1997;12:171–6. doi: 10.1016/s1010-7940(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 12.Najm HK, Van Arsdell GS, Watzka S, Hornberger L, Coles JG, Williams WG. Primary repair is superior to initial palliation in children with atrioventricular septal defect and tetralogy of Fallot. J Thorac Cardiovasc Surg. 1998;116:905–13. doi: 10.1016/S0022-5223(98)70040-6. [DOI] [PubMed] [Google Scholar]
- 13.O’Blenes SB, Ross DB, Nanton MA, Murphy DA. Atrioventricular septal defect with tetralogy of Fallot: results of surgical correction. Ann Thorac Surg. 1998;66:2078–82. doi: 10.1016/s0003-4975(98)00975-8. [DOI] [PubMed] [Google Scholar]
- 14.Tlaskal T, Hucin B, Kostelka M, Chaloupecky V, Marek J, Tax P, et al. Repair of tetralogy of Fallot associated with atrioventricular septal defect. Cardiol Young. 1998;8:105–12. doi: 10.1017/s1047951100004728. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y, Tatsuno K, Kikuchi T, Takahashi Y, Shimokawa T. Complete atrioventricular septal defect associated with tetralogy of fallot: surgical indications and results. Jpn Circ J. 1999;63:889–92. doi: 10.1253/jcj.63.889. [DOI] [PubMed] [Google Scholar]
- 16.Prifti E, Crucean A, Bonacchi M, Bernabei M, Luisi VS, Murzi B, et al. Total correction of complete atrioventricular septal defect with tetralogy of Fallot. J Heart Valve Dis. 2003;12:640–8. [PubMed] [Google Scholar]
- 17.Hoohenkerk GJ, Schoof PH, Bruggemans EF, Rijlaarsdam M, Hazekamp MG. 28 years’ experience with transatrial-transpulmonary repair of atrioventricular septal defect with tetralogy of Fallot. Ann Thorac Surg. 2008;85:1686–9. doi: 10.1016/j.athoracsur.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Canale LS, Monteiro AJ, Rangel I, Pinto DF, Soares P, Barbosa RC, et al. Mid-to-long term follow-up after surgical repair of atrioventricular septal defect with common atrioventricular junction and ventricular shunting associated with tetralogy of Fallot. Cardiol Young. 2008;18:100–4. doi: 10.1017/S1047951107001874. [DOI] [PubMed] [Google Scholar]
- 19.Sarioglu T, Erek E, Yalcinbas YK, Turkekul Y, Saygili A, Sarioglu A, et al. Combination of complete atrioventricular septal defect and tetralogy of Fallot: surgical management and its results. Turk Kardiyol Dern Ars. 2008;36:461–6. [PubMed] [Google Scholar]
- 20.Brancaccio G, Michielon G, Filippelli S, Perri G, Di CD, Iorio FS, et al. Transannular patching is a valid alternative for tetralogy of Fallot and complete atrioventricular septal defect repair. J Thorac Cardiovasc Surg. 2009;137:919–23. doi: 10.1016/j.jtcvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand N, Roux M, Ryckebusch L, Niederreither K, Dolle P, Moon A, et al. Hox genes define distinct progenitor sub-domains within the second heart field. Dev Biol. 2011;353:266–74. doi: 10.1016/j.ydbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson RH, Spicer DE, Giroud JM, Mohun TJ. Tetralogy of Fallot: nosological, morphological, and morphogenetic considerations. Cardiol Young. 2013;23:857–65. doi: 10.1017/S1047951113001686. [DOI] [PubMed] [Google Scholar]
- 23.Briggs LE, Phelps AL, Brown E, Kakarla J, Anderson RH, Van Den Hoff MJ, et al. Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circ Res. 2013;112:1420–32. doi: 10.1161/CIRCRESAHA.112.300821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackerman C, Locke AE, Feingold E, Reshey B, Espana K, Thusberg J, et al. An excess of deleterious variants in VEGF-A pathway genes in Down-syndrome-associated atrioventricular septal defects. Am J Hum Genet. 2012;91:646–59. doi: 10.1016/j.ajhg.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Cherry S, Klinedinst D, Deleon V, Redig J, Reshey B, et al. Genetic Modifiers Predisposing to Congenital Heart Disease in the Sensitized Down Syndrome Population. Circ Cardiovasc Genet. 2012;5:301–8. doi: 10.1161/CIRCGENETICS.111.960872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winston JB, Erlich JM, Green CA, Aluko A, Kaiser KA, Takematsu M, et al. Heterogeneity of genetic modifiers ensures normal cardiac development. Circulation. 2010;121:1313–21. doi: 10.1161/CIRCULATIONAHA.109.887687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winston JB, Schulkey CE, Chen IB, Regmi SD, Efimova M, Erlich JM, et al. Complex Trait Analysis of Ventricular Septal Defects Caused by Nkx2-5 Mutation. Circ Cardiovasc Genet. 2012;5:293–300. doi: 10.1161/CIRCGENETICS.111.961136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malinge S, Chlon T, Dore LC, Ketterling RP, Tallman MS, Paietta E, et al. Development of acute megakaryoblastic leukemia in Down syndrome is associated with sequential epigenetic changes. Blood. 2013;122:e33–e43. doi: 10.1182/blood-2013-05-503011. [DOI] [PMC free article] [PubMed] [Google Scholar]