Abstract
Pharmacologic augmentation of endogenous cannabinoid (eCB) signaling is an emerging therapeutic approach for the treatment of a broad range of pathophysiological conditions. Thus far, pharmacological approaches have focused on inhibition of canonical eCB inactivation pathways, fatty acid amide hydrolase for anandamide and monoacylglycerol lipase for 2-arachidonoylglycerol. Here we review experimental evidence that cyclooxygenase-2-mediated eCB oxygenation represents a third mechanism for terminating eCB action at cannabinoid receptors. We describe the development, molecular mechanisms, and in vivo validation of “substrate-selective” COX-2 inhibitors that prevent eCB inactivation by COX-2 without affecting the prostaglandin generation from arachidonic acid. Lastly, we review recent data on the potential therapeutic applications of substrate-selective COX-2 inhibitors with a focus on neuropsychiatric disorders.
The endocannabinoid system
Two decades of intense scientific inquiry have defined a prominent role for central endogenous cannabinoid (eCB) signaling in a variety of physiological and pathophysiological processes [1, 2]. eCBs are arachidonate-containing lipid signaling molecules that exert biological actions via activation of cannabinoid type 1 and 2 receptors (CB1 and CB2), in addition to other targets including vanilloid receptor 1 (TRPV1), peroxisome proliferator-activated receptor (PPAR), and some ion channels [1]. The two most well studied eCBs, N-arachidonoylethanolamide (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), are synthesized and degraded by discrete sets of enzymes [3-5]. Elucidation of the molecular regulation of eCB metabolism has led to the development of pharmacological tools to enhance eCB signaling and probe the therapeutic utility of eCB augmentation for a variety of pathological conditions [6-8].
AEA is primarily degraded by fatty acid amide hydrolase (FAAH), and pharmacological inhibition of FAAH causes robust increases in brain AEA concentrations [9, 10]. However, FAAH also degrades a number of non-cannabinoid N-acylethanolamides (NAEs), which are elevated upon FAAH inhibition and active at molecular targets, such as PPARs [11-13]. In parallel to AEA, 2-AG is primarily degraded by monoacylglycerol lipase (MAGL) [14], which also metabolizes a series of monoacylglycerols (MAGs). Inhibition of FAAH or MAGL have demonstrated preclinical efficacy in models of neuropathic pain, neurodegeneration, anxiety and depression, hyperemesis, and drug withdrawal syndromes, many of which are mediated by CB receptor-dependent mechanisms [6, 15-18]. These studies demonstrate the pleiotropic therapeutic potential of eCB augmentation via FAAH and MAGL inhibition and the resulting augmentation of cannabinoid receptor signaling.
Here we will review the pharmacology and potential therapeutic implications of a third eCB metabolic pathway, the oxidative metabolism of AEA and 2-AG by cyclooxygenase-2 (COX-2). We review the molecular biology of COX-2, data defining its role as an eCB-metabolizing enzyme, the roles of eCB-derived COX-2 oxidative metabolites, and compare and contrast COX-2-mediated eCB metabolism with the canonical FAAH- and MAGL-mediated metabolic pathways. We then discuss recent advances in the development of “substrate-selective” COX-2 inhibitors (SSCIs), which prevent eCB oxygenation by COX-2 without inhibiting the oxygenation of arachidonic acid (AA) to prostaglandins (PGs). We review the evidence that this novel pharmacological strategy increases eCB tone without affecting AA-derived PG formation by COX-2 and could have fewer adverse side effects compared to either direct CB receptor activation or PG synthesis inhibition. Lastly, we will describe the development, validation, and proof-of-concept validation of the therapeutic potential of SSCIs in preclinical models of anxiety using the first-generation SSCI, LM-4131, as an example.
Molecular biology of COX-2
COX-2 is a homodimer encoded by Ptgs-2, an immediate-early gene that produces a 4 kb mRNA in response to a wide range of stimuli [19]. Upon synthesis, COX-2 localizes to the nuclear envelope and lumen of the endoplasmic reticulum [20, 21]. COX-2 contains two separate active sites: a cyclooxygenase active site, which catalyzes the oxygenation of polyunsaturated fatty acids to hydroperoxy endoperoxides, and a peroxidase active site, which reduces the hydroperoxide to an alcohol [22]. The cyclooxygenase reaction is initiated by the oxidation of the heme prosthetic group by hydroperoxide; the oxidized heme then oxidizes the active site Tyr-385 to the catalytic tyrosyl radical [23-25]. The tyrosyl radical then abstracts the 13-pro-(S)-hydrogen from the substrate to form a carbon-centered radical, which can be trapped at carbon 11 by molecular oxygen to produce an 11-(R)-peroxyl radical. The 11-(R)-peroxyl radical then undergoes two cyclizations to form a bicyclic endoperoxide and an allylic radical between carbons 13 and 15. The allylic radical then reacts with a second molecule of molecular oxygen to form a peroxyl radical at carbon 15. Finally, the peroxyl radical abstracts the phenolic hydrogen of Tyr-385 to generate hydroperoxy endoperoxide prostaglandin G2 (PGG2) and regenerate the tyrosyl radical, which can further catalyze fatty acid oxygenation. PGG2 then diffuses out of the cyclooxygenase active site and into the peroxidase active site, where the 15-hydroperoxide group is reduced to an alcohol to form prostaglandin endoperoxide H2 (PGH2). COX-2 action is regulated post-translationally by several mechanisms, including degradation by the endoplasmic reticulum-associated degradation system [26] and suicide-inactivation during catalysis [27]. Although COX-2 is generally considered an inducible enzyme, it is constitutively expressed in the brain, kidney, and spinal cord [28-30].
The catalytic activities of both COX-1 and COX-2 lead to the production of PGH2 from AA. PGH2 is metabolized by downstream synthases to form prostaglandins E2 (PGE2), D2 (PGD2), F2α (PGF2α), prostacyclin (PGI2), and thromboxane A2 (TxA2). Thus, COX enzymes catalyze the committed step in the formation of a series of lipid mediators that play crucial roles in immunity, inflammation, and a plethora of other biological responses [31, 32]. Non-steroidal anti-inflammatory drugs (NSAIDs) block the production of PGs from AA by inhibiting the cyclooxygenase reactions of COX-1 and COX-2, and this accounts for their myriad of pharmacological effects [33]. Because of the presence of COX-2 in inflammatory cells and neural tissue, it is the principal target for the anti-inflammatory and analgesic effects of NSAIDs [34].
There are a variety of NSAID scaffolds (Figure 1a) that bind to COX-2 in distinct conformations. In the canonical binding mode, carboxylic acid-containing inhibitors form an ion-pair and hydrogen bond with Arg-120 and Tyr-355 at the base of the active site (e.g., flurbiprofen, naproxen, and indomethacin) (Figure 1b) [35, 36]. Alternatively, inhibitors can bind in an inverted fashion and form hydrogen bonds between their carboxylates and Tyr-385 and Ser-530 near the top of the active site (e.g., diclofenac and lumiracoxib) (Figure 1c) [37, 38]. Neutral NSAIDs, such as the COX-2 selective inhibitors celecoxib and rofecoxib, insert sulfonamide or sulfone groups into a side pocket of the COX-2 active site adjacent to Val-523 [36]. Thus, NSAIDs bind in several conformations within the COX-2 active site and form interactions with multiple residues.
Figure 1. Structures and binding mechanisms of COX-2 inhibitor scaffolds.
a) Structures of selected SSCI scaffolds: (R)-flurbiprofen, a member of the 2-arylpropionic class, lumiracoxib, a member of the arylacetic acid class, and LM-4131, a member of the arylacetic acid class, demonstrate the structural diversity of NSAIDs. b) Binding mode of the SSCI (R)-flurbiprofen to the cyclooxygenase active site of murine COX-2 in a canonical fashion with hydrogen bonding and ion pairing interactions with Arg-120 and Tyr-355 (PDB: 3RR3). c) Crystal structure of lumiracoxib bound to murine COX-2 in an inverted pose with hydrogen bonds to Tyr-385 and Ser-530 (PDB: 4LLZ).
Oxygenation of endocannabinoids by COX-2
In addition to the oxygenation of AA, COX-2 also catalyzes the oxygenation of AEA and 2-AG to form prostaglandin ethanolamides (PG-EAs) [39] and prostaglandin glycerol esters (PG-Gs), respectively (see Box 1) [40]. Although PGH2 is converted to PGE2, PGD2, PGF2α, PGI2, and TxA2 by downstream synthases, PGH2-EA and PGH2-G are not good substrates for thromboxane synthase; thus, they each only form four of the five downstream products [41]. The production of PG-EAs has been demonstrated in several settings, including in FAAH knockout mice treated with exogenous AEA [42], lipopolysaccharide-stimulated mouse dorsal root ganglia cultures [43], mouse renal medulla [44], rat spinal cord [45], and in mouse adipocytes [46]. PG-Gs have been detected in rat paws [47] and multiple stimulated macrophage cell lines including RAW 264.7 cells [48], resident peritoneal macrophages [49, 50], and J774 macrophages [51]. Several studies have demonstrated that PG-Gs are unstable due to enzymatic hydrolysis to PGs, which may account for the fewer reports of their formation in vivo compared to PG-EAs [52-54].
Emerging evidence reveals that PG-EAs and PG-Gs have discrete functions that appear to be mediated by receptors distinct from classical PG receptors (Box 2). Therefore, eCB-derived PGs form a bioactive signaling network discrete from AA-derived PGs. Efforts to categorize the effects of eCB-derived PG-EAs and PG-Gs are accelerating in part due to the availability of novel pharmacological tools including PGF2α-EA receptor agonists and antagonists (for review see [55]) as well as COX-2 inhibitors that differentially inhibit PG-EA and PG-G production by COX-2 without affecting AA-derived PGs.
Substrate-selective inhibition of COX-2
SSCIs represent a novel pharmacological approach to COX-2 inhibition by inhibiting the oxygenation of 2-AG and AEA but not AA by COX-2 (Box 3) [43, 76, 77]. The discovery of “substrate-selective” inhibition prompted several studies assessing the generalizability of this phenomenon among NSAIDs. The initial report identified ibuprofen, mefenamic acid, and 2’-des-methyl indomethacin (but not indomethacin) as SSCIs [76]. A more comprehensive investigation found that all rapidly reversible inhibitors are SSCIs, whereas all slow-tight binding inhibitors are non-SSCIs [43].
The structures of COX-2 complexes with some SSCIs have been determined by X-ray crystallography. The similarities between the structures of the bound SSCIs to analogs that are not SSCIs (e.g., (R)-flurbiprofen vs. (S)-flurbiprofen; diclofenac vs. lumiracoxib; desmethylflurbiprofen vs. (S)-flurbiprofen) indicate that the basis for substrate-selectivity cannot be elucidated by structural analysis alone. Interestingly, SSCIs can bind in either the canonical conformation, as in the case of (R)-flurbiprofen (shown in Figure 1b), or the inverted conformation as in the case of lumiracoxib (shown in Figure 1c). Comparison of the structures of AA and 1-AG bound in the active site of COX-2 reveal that the conformation of 1-AG is less favorable for catalysis [78]. Thus, even subtle differences in binding between substrate-selective and non-substrate-selective inhibitors could be sufficient to disrupt 1-AG or 2-AG oxidation but not AA oxidation by COX-2. Functional studies of site-directed mutants are underway to test the role of specific active site residues that may be key determinants of substrate-selective pharmacology [38, 43, 79].
Functional analyses of COX-2 inhibition have been used to design novel SSCIs. Site-directed mutagenesis to remove a hydrogen-bonding interaction between Tyr-355 or Arg-120 of COX-2 and the non-SSCI indomethacin causes it to become a SSCI against these mutant COX-2 proteins [77], suggesting inhibitor interactions with these residues are important for inhibition of AA conversion to PGs. As a corollary, reduction of hydrogen-bonding capacity between inhibitor molecules and Tyr-355 and Arg-120 by conversion of the carboxylate of indomethacin to a tertiary amide (e.g., LM-4131) results in a SSCI [77]. Thus, site-directed mutagenesis and functional COX-2 assays using both AA and eCBs as substrates could be used to develop novel SSCIs from a variety of scaffolds.
Different classes of SSCIs have been utilized in cellular settings to study the oxygenation of eCBs by COX-2. In particular, (R)-flurbiprofen has been utilized to demonstrate that COX-2 regulates eCB concentrations in stimulated primary dorsal root ganglia [43], that PGF2α-EA negatively regulates adipogenesis [46], and that PGD2-G has anti-inflammatory effects in macrophages [51]. In addition, LM-4131 increases 2-AG but does not affect AA or PG concentrations in stimulated RAW 264.7 macrophages [77]. These studies have validated the pharmacological profile of SSCIs in cellular settings, as (R)-flurbiprofen and LM-4131 selectively inhibit eCB oxygenation by COX-2 while displaying no inhibition of AA oxygenation by COX-2. The existence of multiple structurally diverse SSCIs provides complementary probes for the investigation of the role of COX-2 oxidation of eCBs in cellular systems.
In vivo effects of substrate-selective COX-2 inhibition
Although in vitro and cellular studies clearly validate the pharmacology of SSCIs, whether this selectivity is retained in vivo is a critical question. Although (R)-flurbiprofen is an excellent probe in vitro, in mice (but to a lesser extent in rats, humans, or monkeys) it undergoes a unidirectional isomerization to the non-SSCI (S)-flurbiprofen, rendering it suboptimal for in vivo studies [84]. Therefore, we focused our initial in vivo SSCI validation studies on the morpholino amide of indomethacin, LM-4131 [77]. LM-4131 dose-dependently increases brain AEA concentrations to ~150% of control, while only marginally increasing 2-AG concentrations to ~110% of control. The non-selective COX-1/2 inhibitor indomethacin, the parent compound of LM-4131, and the COX-2 selective inhibitor NS398 also increase brain AEA and, to a lesser extent, 2-AG concentrations. Importantly, while all three inhibitors increased eCB concentrations, a clear distinction is evident between their effects on PG production: indomethacin and NS398 reduce brain PG and increase AA concentrations, while LM-4131 has no effect on either analyte [77].
The ability of LM-4131 to increase eCB concentrations is dependent on COX-2 activity because it does not increase eCB concentrations in COX-2–/– mice [77]. Importantly, COX-2–/– mice have basally elevated brain AEA, providing in vivo confirmation that COX-2 is a key mediator of basal brain AEA signaling. The effects of LM-4131 are mediated through COX-2 and not alternate mechanisms of action, such as FAAH and MAGL inhibition, because LM-4131 increases AEA concentrations in FAAH–/– mice and produces additive increases in brain AEA concentrations when co-administered with the irreversible FAAH inhibitor PF-3845. Similarly, LM-4131 produces additive increases in 2-AG concentrations when combined with the irreversible MAGL inhibitor JZL-184 [77]. These data provide compelling evidence that LM-4131 exhibits substrate-selective pharmacological properties in vivo and can increase eCB concentrations via a COX-2-dependent mechanism.
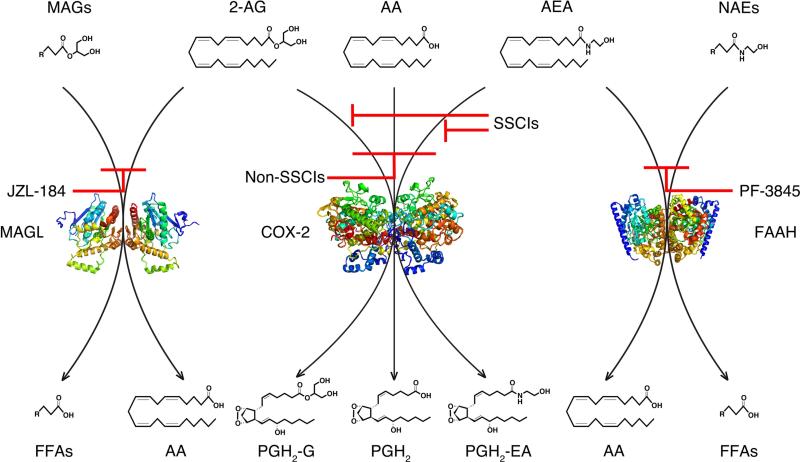
Comparative analyses of the effects of LM-4131 on NAE and MAG concentrations relative to PF-3845 and JZL-184 revealed divergent effects of COX-2 inhibition compared to FAAH or MAGL inhibition (Figure 2). FAAH inhibition increases concentrations of AEA and several non-cannabinoid NAEs in the brain and FAAH-rich tissues such as the liver. Similarly, MAGL inhibition increases the concentrations of 2-AG and several other non-cannabinoid MAGs. In contrast, LM-4131 only increases AEA and 2-AG concentrations, without affecting other NAEs or MAGs in any tissue. This remarkable selectivity for eCBs relative to non-eCB NAEs and MAGs is likely due to the substrate specificity of COX-2 for arachidonate-containing lipids. In contrast, FAAH catalyzes amidase and MAGL catalyzes esterase cleavages of a variety of fatty acid amides and glycerol esters, respectively. The selectivity of LM-4131 is mirrored by genetic COX-2 deletion, which results in a ~200% increase in brain AEA, but not other NAEs [77]. These data indicate that SSCIs can selectively augment eCB concentrations without affecting non-eCB NAEs or MAGs and highlights distinct differences between these three strategies for eCB augmentation.
Figure 2. Differential regulation of MAGs, eCBs, and NAEs by MAGL, COX-2, and FAAH.
MAGL catalyzes the hydrolysis of the glycerol moiety of 2-AG and other MAGs to AA and free fatty acids (left). COX-2 oxygenates AA, 2-AG, and AEA to PGs, PGGs, and PG-EAs, respectively (middle). FAAH catalyzes the hydrolysis of the ethanolamide moiety of AEA and other NAEs to AA and free fatty acids (right). Different classes of eCB degradation inhibitors and their effects on respective lipid metabolic pathways are depicted in red.
In addition to having central effects, LM-4131 increases AEA, but not other NAEs, in a variety of peripheral tissues including the lung, kidney, stomach, and small intestine, but not the liver or heart [77]. Thus, COX-2 plays a role in the regulation of eCBs in multiple peripheral organs. The differential tissue-specific effects likely relate to differences in COX-2 and FAAH expression as well as the differential rates of AEA biosynthesis between tissues. Importantly, LM-4131 does not affect PG concentrations in either central or peripheral tissues, whereas indomethacin profoundly reduces PGs in all tissues examined. Taken together, these data suggest an important role for COX-2 in the metabolism of eCBs and identify a third metabolic pathway for eCB inactivation in the brain and many peripheral tissues.
A growing literature indicates that COX-2 inhibition can enhance prototypical 2-AG-mediated forms of retrograde synaptic signaling in the form of depolarization-induced suppression of inhibition or excitation (DSI or DSE) [56, 75, 85]. Both pharmacological and genetic inhibition strategies indicate that COX-2 limits synaptic 2-AG signaling in the hippocampus; however, the role of COX-2 in the regulation of AEA-mediated synaptic plasticity remains to be investigated. One possible explanation for the lackluster effect of pharmacological or genetic inhibition of COX-2 on the concentration of 2-AG in the brain is that the bulk measurement of tissue 2-AG does not accurately reflect the effects of COX-2 inhibition at the cellular or synaptic level. Furthermore, alternative 2-AG metabolic pathways such as MAGL and α/β-hydrolase domain 6 or 12 may mitigate changes in bulk tissue concentrations after COX-2 inhibition [86], but allow for enhanced CB receptor activation prior to hydrolysis. In this scenario, COX-2 inhibition would cause higher concentrations of 2-AG postsynaptically, and increased presynaptic CB1 receptor activation followed by rapid presynaptic degradation by MAGL or other esterases. Thus, the effects of COX-2 inhibition may be transient and/or highly localized such that bulk tissue measurement does not capture effects that are measured in a more targeted fashion.
Therapeutic implications of SSCIs
Augmenting eCB signaling has shown preclinical efficacy in reducing behavioral signs of anxiety in laboratory animals [10, 87-91]. Seminal studies by Piomelli and coworkers demonstrate that inhibiting FAAH reduces anxiety in multiple preclinical models via a CB1 receptor-dependent mechanism [10]. Importantly, some studies suggest that COX-2 inhibition may have anxiolytic effects in preclinical models [92, 93], and some clinical studies have identified therapeutic effects of adjunctive COX-2 inhibition in individuals with major depression [94-96].
Based on these data, we tested the hypothesis that the prototypic SSCI, LM-4131, has anxiolytic effects mediated by eCB augmentation and CB receptor activation. Acute treatment with LM-4131 reduces anxiety measures in the open-field assay, elevated plus-maze, and light-dark box exploration assay [77]. Pharmacological and genetic approaches identified that the anxiolytic effects of LM-4131 are mediated via CB1 receptor activation and are COX-2-dependent [77]. These studies provide the first proof-of-concept support that SSCIs exert behavioral effects mediated via eCB activation of CB1 receptors.
Although the in vivo therapeutic effects of SSCIs have not been examined in detail, given the wide array of pathophysiological processes modulated by eCB signaling, the therapeutic potential of SSCIs could be extensive. Augmentation of eCBs via FAAH or MAGL inhibition has shown therapeutic potential for mitigating drug withdrawal syndromes, neuropathic pain, hyperemesis, neurodegeneration, seizure, excitotoxicity, and tumor progression, among other promising effects [97]. Future studies aimed at determining the changes in COX-2 expression patterns and the therapeutic potential of SSCIs in the treatment of a broad range of disorders will help define the promise of augmenting eCBs by COX-2 inhibition.
Additionally, SSCIs may provide similar therapeutic effects as NSAIDs given their similar molecular target of COX-2. NSAIDs have long been used as analgesic and antipyretic agents [98, 99]. NSAIDs also have potent anti-inflammatory actions and have been utilized clinically in inflammatory disorders including osteoarthritis, rheumatoid arthritis, inflammatory arthropathies, and acute gout [100-103]. COX-2 inhibitors have also been utilized as anti-oncogenic agents in breast, colon, bladder, and prostate cancers, among others [104-107]. COX inhibitors have also shown promise in combating infection and sepsis [108, 109]. A critical fundamental question is whether these diverse therapeutic effects of NSAIDs are mediated by PG inhibition, PG-G and PG-EA inhibition, and/or eCB augmentation. SSCIs are ideal probes to determine the differential impact of exclusively inhibiting PG-EA and PG-G synthesis, and consequently increasing AEA and 2-AG concentrations, while not modulating PGs. The use of SSCIs in combination with tools such as PG receptor knockout animals should help to dissect the importance of COX-2-eCB oxidation in a range of physiological and pathophysiological effects.
Predicting the adverse effect profile of SSCIs in the CNS and beyond
The mechanism of action of SSCIs predicts two potential sets of adverse effects. First, adverse effects such as gastrointestinal and cardiovascular or cerebrovascular toxicity are associated with most NSAIDs and are mediated by the inhibition of PG synthesis by COX-1, COX-2, or both enzymes [110-113]. Second, adverse cognitive, metabolic, and motoric side effects are associated with direct CB1 receptor activation. The selective inhibition of eCB-derived PGs but not AA-derived PGs suggests that SSCIs may present a more favorable side effect profile relative to other NSAIDs.
A major clinical concern for the chronic use of COX inhibitors is cardiovascular and cerebrovascular toxicity manifested by an increased incidence of heart attack and stroke. Cardiovascular side effects are exhibited by most NSAIDs, regardless of their selectivity for COX-2, due to a reduction in vascular PGI2 biosynthesis [114, 115]. As SSCIs do not affect central or peripheral concentrations of PGs, including PGI2, it is possible that this pharmacological class of inhibitors could be devoid of or exhibit significantly reduced cardio/cerebrovascular toxicity compared to traditional NSAIDs. Indeed, clinical trials conducted with the SSCI (R)-flurbiprofen suggest that it does not increase cardiovascular events [116, 117].
Gastrointestinal bleeding is also a well-known adverse effect of COX inhibition and is mediated by inhibition of gastroprotective PG synthesis in the gut [118, 119]. The SSCI LM-4131 does not cause overt gastrointestinal bleeding after acute administration, whereas indomethacin causes significant overt bleeding at the same dose [77]. Additionally, the SSCI (R)-flurbiprofen does not display gastrointestinal toxicity in humans [116, 117]. These studies suggest that SSCI's lack of effect on PG synthesis may render them less prone to or even devoid of the complicating side effects of traditional COX inhibitors.
Because SSCIs lead to CB1 receptor activation in the CNS, and possibly in other tissues, cannabimimetic side effects could occur. Common adverse effects of direct acting cannabinoid agonists include motor suppression, cognitive impairment, hyperphagia, and dependence liability. Initial studies with the SSCI LM-4131 found a notable lack of motor suppression or object recognition memory deficits [77]. This may be in part be due to the relative preference of SSCIs to elevate AEA over 2-AG, since MAGL inhibition or combined MAGL and FAAH inhibition produces cannabimimetic effects such as pronounced motoric inhibition [120].
In addition to the potential lack of adverse cannabimimetic effects of SSCIs, distinct differences in the tissue-specificity of COX-2 relative to FAAH suggests that SSCIs may lack some of the side effects of FAAH inhibition. FAAH inhibition in the liver causes robust increases in AEA and non-eCB NAE concentrations [77]. In contrast, LM-4131 does not affect liver AEA or NAE concentrations [77]. Activation of hepatic CB1 receptors contributes to diet-induced metabolic pathology [121, 122], and genetic FAAH deletion promotes a pre-diabetic state and adversely affects energy metabolism [123]. The lack of effect of LM-4131 on hepatic AEA indicates that SSCIs may lack adverse metabolic side effects relative to other indirect or direct acting cannabinoid agonists [77]. Moreover, one might predict a general theme that in tissues where FAAH expression is high and can act as an alternate eCB metabolic pathway, adverse cannabimimetic effects of SSCI would likely be minimized. Lastly, AEA is also an agonist at TRPV1 receptors [124], and as such, SSCIs could potentially result in overstimulation of TRPV1 under some conditions. The biological significance of this potential mechanism needs to be evaluated in subsequent studies. Ongoing studies aimed at comparing and contrasting the relative cannabimimetic profiles of the three distinct eCB augmentation strategies will clarify the relative side-effect profiles of the three different enzymatic inhibition strategies.
Concluding remarks
Despite the recent elucidation of COX-2 as a key regulator of eCB signaling, several fundamental questions remain unanswered. For example, why is there significant redundancy in eCB metabolic pathways? It is now clear that both FAAH and COX-2 can metabolize AEA; blockade of either enzyme can elevate AEA levels; and the effects of FAAH and COX-2 inhibition are additive. In many cases this occurs in tissues and/or cells where both enzymes are co-expressed, as they are in neurons [125]. These data suggest the intriguing possibility that there are distinct metabolic pools of AEA that are segregated with regard to metabolic pathways; if this were not the case one would not expect to see increases in AEA after COX-2 blockade, since shunting through FAAH would be expected to metabolize the “excess” AEA. What then defines these distinct metabolic pools? Do distinct synthetic pathways determine them or are they physically segregated within cells, or both? An alternative to this differential pool hypothesis could be that COX-2-mediated AEA metabolism represents a salvage pathway operating to limit AEA signaling only under conditions where FAAH activity is already near maximal efficiency. Differentiating between these two possibilities could provide important insight into the basic biology of endocannabinoid signaling and potential differential therapeutic implications of pharmacological FAAH and COX-2 inhibition.
Furthermore, although COX-2 eCB oxidative metabolites have been identified and some of their biological effects elucidated, the vast majority of these findings are based on application of exogenous PG-EAs or PG-Gs to biological systems. We suggest elucidation of the physiological triggers of endogenous PG-EA and PG-G synthesis and degradation are critically important. In addition, elucidation of the receptors by which PG-EAs and PG-Gs exert biological actions represents a key open question that, if answered, could reveal a new network of endogenous bioactive lipid signaling systems amenable to pharmacological manipulation through both inhibition of COX-2 as well as antagonism of these receptors. Modulation of this system could potentially have therapeutic implications for neuropsychiatric, pain, and metabolic disorders.
The data summarized herein highlight the emerging role of COX-2 in the regulation of central and peripheral eCB metabolism and suggest that, in addition to the canonical FAAH and MAGL pathways, COX-2-mediated eCB oxygenation represents a third eCB metabolic mechanism. We propose that SSCIs provide a novel pharmacological strategy to augment eCB signaling without affecting PG formation, which will enhance therapeutic opportunities surrounding eCB augmentation and provide tools to elucidate differential fundamental biology of eCB- and AA-derived COX-2 products. Optimizing the in vivo pharmacological profile of SSCIs and elucidating the therapeutic potential of this class of compounds could ultimately have broad implications.
Highlights.
Endocannabinoid augmentation may have broad therapeutic implications
COX-2 is a third endocannabinoid metabolic mechanism
Substrate-selective COX-2 inhibition increases central endocannabinoid signaling
Substrate-selective COX-2 inhibition decreases anxiety
Box 1: COX-2 as an eCB-inactivating enzyme.
A connection between COX-2 action and eCB inactivation has been suggested by converging data. Inhibition of COX-2 potentiates retrograde eCB synaptic signaling in the hippocampus [56] and decreases excitatory responses in a CB1-dependent manner [57], revealing a functional tie between COX-2 activity and eCB tone at central synapses. NSAIDs inhibit the metabolism of AEA by rat cerebellar membrane preparations [58] and extend the stability of exogenous AEA in mouse brain [59], suggesting that COX-2 directly metabolizes AEA in vivo. COX-2 is constitutively expressed in the spinal cord and mediates tissue injury-induced hyperalgesia [60]. The NSAIDs indomethacin and nimesulide produce eCB-mediated spinal antinociception as evidenced by the blockade of their antinociceptive effects by the CB1 receptor antagonist AM251 [61, 62]. Moreover, the peripheral antinociceptive effects of AEA and NSAIDs are synergistic [63] and ibuprofen interacts with AEA in both acute and inflammatory pain models [64]. COX-2 selective inhibitors, but not COX-1 selective inhibitors, also reverse spinal neuron hyperexcitability in a CB1 receptor-dependent manner and reduce the breakdown of 2-AG [65]. These studies, combined with the data reviewed below, indicate that COX-2 plays a fundamental role as an eCB-metabolizing enzyme in multiple settings and tissues.
Box 2: Biological targets and actions of PG-EAs and PG-Gs.
PG-EAs and PG-Gs have little or no activation of the canonical EP1-4, DP, FP, TP, and IP prostanoid receptors [66, 67], with the exception of PGE2-EA which binds to the EP1-4 receptors [68], indicating that they form a discrete bioactive signaling network from AA- derived PGs. The receptors that mediate the biological actions of most PG-EAs and PG Gs have not been elucidated, however, the PGF2α-EA receptor has been identified as a heterodimer containing a FP receptor and a FP receptor splice variant [69]. Several studies have identified biological actions of some PG-Gs and PG-EAs. PGE2-G potently induces calcium mobilization in RAW 264.7 macrophages and H1819 cells [67, 70], induces hyperalgesia through modulation of NF-κB [47], enhances miniature excitatory post-synaptic currents in glutamatergic neurons [71], and exacerbates malonate-induced neurotoxicity [72]. In contrast, PGD2-G exhibits anti-inflammatory activity in isolated macrophages and in vivo [51]. PGF2α-EA and its analog, bimatoprost, are ocular hypotensive agents used for treating glaucoma [73]. PGF2α-EA has also been shown to negatively regulate adipogenesis [46] and increase the firing of nociceptive neurons and reduce hot plate paw withdrawal latency in mice after spinal application [45]. PGE2-G, PGD2-G, PGF2α-G, and PGD2-EA increase the frequency of miniature inhibitory postsynaptic currents in GABAergic hippocampal neurons [74]. PGE2-G, PGE2-EA, and PGF2α-EA elevate long-term potentiation in the hippocampus [75]. Thus, PG-Gs and PG-EAs produce a plethora of biological effects in multiple settings through their activation of as of yet unidentified receptors.
Box 3: Substrate-selective inhibition of COX-2.

Figure I: Mechanism of substrate-selective inhibition of COX-2. Binding of a SSCI to the allosteric subunit (Eallo) results in non-competitive inhibition of eCB oxygenation in the catalytic subunit (Ecat) but no inhibition of AA oxygenation. Binding of a second SSCI molecule to the catalytic subunit results in inhibition of AA.
The two subunits of COX-2 are sequence homodimers, but the heme prosthetic group binds to only a single monomer, creating functional heterodimers (Figure I). The heme- containing subunit is the catalytic subunit, whereas the non-heme-containing subunit is the allosteric subunit [80, 81]. Binding of substrates, activators, and inhibitors to the allosteric subunit alters binding in the catalytic subunit through subunit communication via the dimer interface [82]. COX-2 inhibitors bind in one of two kinetic modes, rapid- reversible or slow-tight binding. Compounds that are rapid-reversible inhibitors of COX- 2 inhibit eCB oxygenation at concentrations that are orders of magnitude lower than the concentrations required for inhibition of AA oxygenation, a phenomenon termed “substrate-selective” COX-2 inhibition [76]. Substrate-selective inhibitors bind in the allosteric subunit and induce a conformational change that blocks eCB oxidation in the catalytic subunit. Binding of a second inhibitor molecule in the catalytic subunit blocks AA oxygenation, but this typically occurs at inhibitor concentrations orders of magnitude higher than the concentrations that block eCB oxygenation [76]. Slow, tight-binding inhibitors bind in the catalytic subunit and block the oxygenation of all substrates at similar concentrations [35, 83].
Acknowledgments
These studies were supported by National Institutes of Health Grants K08MH090412, R01MH100096 (S.P.), P30GM15431, R01CA89450 (L.J.M.), and F31DA031572 (D.J.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kano M, et al. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annual review of psychology. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 3.Ahn K, et al. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chemical reviews. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 5.Di Marzo V. Endocannabinoids: synthesis and degradation. Reviews of physiology, biochemistry and pharmacology. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 6.Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- 7.Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- 8.Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacological reviews. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravatt BF, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nature medicine. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 11.Khasabova IA, et al. Peroxisome proliferator-activated receptor alpha mediates acute effects of palmitoylethanolamide on sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12735–12743. doi: 10.1523/JNEUROSCI.0130-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu J, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill MN, et al. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci. 2009;30:484–493. doi: 10.1016/j.tips.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Roques BP, et al. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat Rev Drug Discov. 2012;11:292–310. doi: 10.1038/nrd3673. [DOI] [PubMed] [Google Scholar]
- 17.Mulvihill MM, Nomura DK. Therapeutic potential of monoacylglycerol lipase inhibitors. Life sciences. 2013;92:492–497. doi: 10.1016/j.lfs.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bari M, et al. New insights into endocannabinoid degradation and its therapeutic potential. Mini reviews in medicinal chemistry. 2006;6:257–268. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- 19.Kosaka T, et al. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. European journal of biochemistry / FEBS. 1994;221:889–897. doi: 10.1111/j.1432-1033.1994.tb18804.x. [DOI] [PubMed] [Google Scholar]
- 20.Morita I, et al. Different intracellular locations for prostaglandin endoperoxide H synthase-1 and -2. The Journal of biological chemistry. 1995;270:10902–10908. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- 21.Regier MK, et al. Localization of prostaglandin endoperoxide synthase-1 to the endoplasmic reticulum and nuclear envelope is independent of its C-terminal tetrapeptide-PTEL. Archives of biochemistry and biophysics. 1995;317:457–463. doi: 10.1006/abbi.1995.1188. [DOI] [PubMed] [Google Scholar]
- 22.Picot D, et al. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 23.Dietz R, et al. Higher oxidation states of prostaglandin H synthase. Rapid electronic spectroscopy detected two spectral intermediates during the peroxidase reaction with prostaglandin G2. European journal of biochemistry / FEBS. 1988;171:321–328. doi: 10.1111/j.1432-1033.1988.tb13793.x. [DOI] [PubMed] [Google Scholar]
- 24.Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chemical reviews. 2003;103:2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 25.van der Donk WA, et al. The cyclooxygenase reaction mechanism. Biochemistry. 2002;41:15451–15458. doi: 10.1021/bi026938h. [DOI] [PubMed] [Google Scholar]
- 26.Mbonye UR, et al. The 19-amino acid cassette of cyclooxygenase-2 mediates entry of the protein into the endoplasmic reticulum-associated degradation system. The Journal of biological chemistry. 2006;281:35770–35778. doi: 10.1074/jbc.M608281200. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, et al. A mechanistic study of self-inactivation of the peroxidase activity in prostaglandin H synthase-1. The Journal of biological chemistry. 1999;274:9231–9237. doi: 10.1074/jbc.274.14.9231. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann WE, et al. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris RC, et al. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. The Journal of clinical investigation. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshikawa K, et al. Preferential localization of prostamide/prostaglandin F synthase in myelin sheaths of the central nervous system. Brain research. 2011;1367:22–32. doi: 10.1016/j.brainres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Smith WL, et al. Cyclooxygenases: structural, cellular, and molecular biology. Annual review of biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 32.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacology & therapeutics. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New biology. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 34.Vane JR, et al. Cyclooxygenases 1 and 2. Annu Rev Pharmacol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Duggan KC, et al. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. The Journal of biological chemistry. 2010;285:34950–34959. doi: 10.1074/jbc.M110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurumbail RG, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 37.Rowlinson SW, et al. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. The Journal of biological chemistry. 2003;278:45763–45769. doi: 10.1074/jbc.M305481200. [DOI] [PubMed] [Google Scholar]
- 38.Windsor MA, et al. Exploring the molecular determinants of substrate-selective inhibition of cyclooxygenase-2 by lumiracoxib. Bioorganic & medicinal chemistry letters. 2013;23:5860–5864. doi: 10.1016/j.bmcl.2013.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu M, et al. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. The Journal of biological chemistry. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 40.Kozak KR, et al. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. The Journal of biological chemistry. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 41.Kozak KR, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. The Journal of biological chemistry. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 42.Weber A, et al. Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. Journal of lipid research. 2004;45:757–763. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Duggan KC, et al. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nature chemical biology. 2011;7:803–809. doi: 10.1038/nchembio.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritter JK, et al. Production and actions of the anandamide metabolite prostamide E2 in the renal medulla. The Journal of pharmacology and experimental therapeutics. 2012;342:770–779. doi: 10.1124/jpet.112.196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatta L, et al. Discovery of prostamide F2alpha and its role in inflammatory pain and dorsal horn nociceptive neuron hyperexcitability. PloS one. 2012;7:e31111. doi: 10.1371/journal.pone.0031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silvestri C, et al. Anandamide-derived prostamide F2alpha negatively regulates adipogenesis. The Journal of biological chemistry. 2013;288:23307–23321. doi: 10.1074/jbc.M113.489906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu SS, et al. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. British journal of pharmacology. 2008;153:1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kingsley PJ, et al. Simultaneous analysis of prostaglandin glyceryl esters and prostaglandins by electrospray tandem mass spectrometry. Analytical biochemistry. 2005;343:203–211. doi: 10.1016/j.ab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Rouzer CA, et al. Zymosan-induced glycerylprostaglandin and prostaglandin synthesis in resident peritoneal macrophages: roles of cyclo-oxygenase-1 and -2. The Biochemical journal. 2006;399:91–99. doi: 10.1042/BJ20060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouzer CA, Marnett LJ. Glycerylprostaglandin synthesis by resident peritoneal macrophages in response to a zymosan stimulus. The Journal of biological chemistry. 2005;280:26690–26700. doi: 10.1074/jbc.M501021200. [DOI] [PubMed] [Google Scholar]
- 51.Alhouayek M, et al. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17558–17563. doi: 10.1073/pnas.1314017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozak KR, et al. Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamides in vitro and in vivo. The Journal of biological chemistry. 2001;276:36993–36998. doi: 10.1074/jbc.M105854200. [DOI] [PubMed] [Google Scholar]
- 53.Xie S, et al. Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2. Chemical research in toxicology. 2010;23:1890–1904. doi: 10.1021/tx1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang R, et al. Identification of Palmitoyl Protein Thioesterase 1 in Human THP1 Monocytes and Macrophages and Characterization of Unique Biochemical Activities for This Enzyme. Biochemistry. 2013;52:7559–7574. doi: 10.1021/bi401138s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woodward DF, et al. Recent progress in prostaglandin F2alpha ethanolamide (prostamide F2alpha) research and therapeutics. Pharmacological reviews. 2013;65:1135–1147. doi: 10.1124/pr.112.007088. [DOI] [PubMed] [Google Scholar]
- 56.Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nature neuroscience. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- 57.Slanina KA, Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology. 2005;49:653–659. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Fowler CJ, et al. Ibuprofen inhibits the metabolism of the endogenous cannabimimetic agent anandamide. Pharmacology & toxicology. 1997;80:103–107. doi: 10.1111/j.1600-0773.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 59.Glaser ST, Kaczocha M. Cyclooxygenase-2 mediates anandamide metabolism in the mouse brain. The Journal of pharmacology and experimental therapeutics. 2010;335:380–388. doi: 10.1124/jpet.110.168831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghilardi JR, et al. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:2727–2732. doi: 10.1523/JNEUROSCI.5054-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guhring H, et al. A role for endocannabinoids in indomethacin-induced spinal antinociception. European journal of pharmacology. 2002;454:153–163. doi: 10.1016/s0014-2999(02)02485-8. [DOI] [PubMed] [Google Scholar]
- 62.Staniaszek LE, et al. Effects of COX-2 inhibition on spinal nociception: the role of endocannabinoids. British journal of pharmacology. 2010;160:669–676. doi: 10.1111/j.1476-5381.2010.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guindon J, et al. Synergistic antinociceptive effects of anandamide, an endocannabinoid, and nonsteroidal anti-inflammatory drugs in peripheral tissue: a role for endogenous fatty-acid ethanolamides? European journal of pharmacology. 2006;550:68–77. doi: 10.1016/j.ejphar.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 64.Guindon J, et al. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85–93. doi: 10.1016/j.pain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 65.Telleria-Diaz A, et al. Spinal antinociceptive effects of cyclooxygenase inhibition during inflammation: Involvement of prostaglandins and endocannabinoids. Pain. 2010;148:26–35. doi: 10.1016/j.pain.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 66.Matias I, et al. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. The Journal of pharmacology and experimental therapeutics. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- 67.Nirodi CS, et al. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW264.7 cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1840–1845. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ross RA, et al. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. The Journal of pharmacology and experimental therapeutics. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- 69.Liang Y, et al. Identification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexes. British journal of pharmacology. 2008;154:1079–1093. doi: 10.1038/bjp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richie-Jannetta R, et al. Structural determinants for calcium mobilization by prostaglandin E2 and prostaglandin F2alpha glyceryl esters in RAW 264.7 cells and H1819 cells. Prostaglandins Other Lipid Mediat. 2010;92:19–24. doi: 10.1016/j.prostaglandins.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sang N, et al. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. Journal of neurochemistry. 2007;102:1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- 72.Valdeolivas S, et al. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: a potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell death & disease. 2013;4:e862. doi: 10.1038/cddis.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodward DF, et al. Bimatoprost: a novel antiglaucoma agent. Cardiovascular drug reviews. 2004;22:103–120. doi: 10.1111/j.1527-3466.2004.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 74.Sang N, et al. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. The Journal of physiology. 2006;572:735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang H, et al. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci. 2008;37:682–695. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prusakiewicz JJ, et al. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry. 2009;48:7353–7355. doi: 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hermanson DJ, et al. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nature neuroscience. 2013;16:1291–1298. doi: 10.1038/nn.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vecchio AJ, Malkowski MG. The Structural Basis of Endocannabinoid Oxygenation by Cyclooxygenase-2. Journal of Biological Chemistry. 2011;286:20736–20745. doi: 10.1074/jbc.M111.230367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Windsor MA, et al. Substrate-Selective Inhibition of Cyclooxygenase-2: Development and Evaluation of Achiral Profen Probes. ACS medicinal chemistry letters. 2012;3:759–763. doi: 10.1021/ml3001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong L, et al. Human Cyclooxygenase-2 Is a Sequence Homodimer That Functions as a Conformational Heterodimer. Journal of Biological Chemistry. 2011;286:19035–19046. doi: 10.1074/jbc.M111.231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kulmacz RJ, Lands WEM. Prostaglandin-H Synthase - Stoichiometry of Heme Cofactor. Journal of Biological Chemistry. 1984;259:6358–6363. [PubMed] [Google Scholar]
- 82.Yuan C, et al. Cyclooxygenase Allosterism, Fatty Acid-mediated Cross-talk between Monomers of Cyclooxygenase Homodimers. The Journal of biological chemistry. 2009;284:10046–10055. doi: 10.1074/jbc.M808634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kulmacz RJ, Lands WE. Stoichiometry and kinetics of the interaction of prostaglandin H synthase with anti-inflammatory agents. The Journal of biological chemistry. 1985;260:12572–12578. [PubMed] [Google Scholar]
- 84.Leipold DD, et al. Bioinversion of R-flurbiprofen to S-flurbiprofen at various dose levels in rat, mouse, and monkey. Chirality. 2004;16:379–387. doi: 10.1002/chir.20053. [DOI] [PubMed] [Google Scholar]
- 85.Straiker A, et al. COX-2 and fatty acid amide hydrolase can regulate the time course of depolarization-induced suppression of excitation. British journal of pharmacology. 2011;164:1672–1683. doi: 10.1111/j.1476-5381.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marrs WR, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nature neuroscience. 13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinsey SG, et al. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 2010;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Busquets-Garcia A, et al. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–486. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 89.Sciolino NR, et al. Pharmacol Res. 2011;Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moreira FA, et al. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2007 doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. The Journal of pharmacology and experimental therapeutics. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 92.Kumari B, et al. Protective effect of non-selective and selective COX-2-inhibitors in acute immobilization stress-induced behavioral and biochemical alterations. Pharmacological reports : PR. 2007;59:699–707. [PubMed] [Google Scholar]
- 93.Dhir A, et al. Protective effect of naproxen (non-selective COX-inhibitor) or rofecoxib (selective COX-2 inhibitor) on immobilization stress-induced behavioral and biochemical alterations in mice. European journal of pharmacology. 2006;535:192–198. doi: 10.1016/j.ejphar.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 94.Akhondzadeh S, Jafari S. A double-blind, placebo-controlled trial of celecoxib adjunctive treatment to fluoxetine in major depression. Brit J Clin Pharmaco. 2010;70:291–292. [Google Scholar]
- 95.Akhondzadeh S, et al. Clinical Trial of Adjunctive Celecoxib Treatment in Patients with Major Depression: A Double Blind and Placebo Controlled Trial. Depress Anxiety. 2009;26:607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 96.Muller N, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatr. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 97.Hermanson DJ, Marnett LJ. Cannabinoids, endocannabinoids, and cancer. Cancer Metast Rev. 2011;30:599–612. doi: 10.1007/s10555-011-9318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Futaki N, et al. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. General pharmacology. 1993;24:105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- 99.Engelhardt G, et al. Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflammation research : official journal of the European Histamine Research Society ... [et al.] 1995;44:423–433. doi: 10.1007/BF01757699. [DOI] [PubMed] [Google Scholar]
- 100.Crilly MA, Mangoni AA. Non-steroidal anti-inflammatory drug (NSAID) related inhibition of aldosterone glucuronidation and arterial dysfunction in patients with rheumatoid arthritis: a cross-sectional clinical study. BMJ open. 2011;1:e000076. doi: 10.1136/bmjopen-2011-000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Macdonald TM, et al. Methodology of a large prospective, randomised, open, blinded endpoint streamlined safety study of celecoxib versus traditional non-steroidal anti-inflammatory drugs in patients with osteoarthritis or rheumatoid arthritis: protocol of the standard care versus celecoxib outcome trial (SCOT). BMJ open. 2013;3 doi: 10.1136/bmjopen-2012-002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dougados M, et al. Celecoxib, a COX-2 specific inhibitor in ankylosing spondylitis (AS). A 6-week efficacy study. Arthritis Rheum. 2000;43:S103–S103. doi: 10.1002/1529-0131(200101)44:1<180::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 103.Scopelitis E, Mcgrath H. Nsaid-Masked Gout. Southern Med J. 1987;80:1464–1465. doi: 10.1097/00007611-198711000-00035. [DOI] [PubMed] [Google Scholar]
- 104.Marnett LJ, DuBois RN. COX-2: A target for colon cancer prevention. Annu Rev Pharmacol. 2002;42:55–+. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 105.Retsky M, et al. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Current medicinal chemistry. 2013;20:4163–4176. doi: 10.2174/09298673113209990250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wagner M, et al. COX-2 expression and effects of selective inhibition in prostate cancer cell lines. J Urology. 2002;167:135–136. [Google Scholar]
- 107.Kelly JD, Hall E. Boxing Bladder Cancer with COX-2-specific Inhibition. Cancer Prev Res. 2011;4:1534–1535. doi: 10.1158/1940-6207.CAPR-11-0409. [DOI] [PubMed] [Google Scholar]
- 108.Eisen DP. Manifold beneficial effects of acetyl salicylic acid and nonsteroidal anti-inflammatory drugs on sepsis. Intens Care Med. 2012;38:1249–1257. doi: 10.1007/s00134-012-2570-8. [DOI] [PubMed] [Google Scholar]
- 109.Stables MJ, et al. Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria. Blood. 2010;116:2950–2959. doi: 10.1182/blood-2010-05-284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patrono C. Molecular and clinical determinants of the cardiovascular effects of selective COX-isozyme inhibition. Ann Rheum Dis. 2005;64:39–40. [Google Scholar]
- 111.Fitzgerald GA, et al. COX-2 and mPGES-1 derived eicosanoids and cardiovascular function. Prostag Oth Lipid M. 2006;79:157–157. [Google Scholar]
- 112.Mitchell JA, Warner TD. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–85. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- 113.Cannon CP, Cannon PJ. COX-2 Inhibitors and Cardiovascular Risk. Science. 2012;336:1386–1387. doi: 10.1126/science.1224398. [DOI] [PubMed] [Google Scholar]
- 114.Yu Y, et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Science translational medicine. 2012;4:132ra154. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet. 2008;371:270–273. doi: 10.1016/S0140-6736(08)60137-3. [DOI] [PubMed] [Google Scholar]
- 116.Wilcock GK. Disease-modifying treatments for Alzheimer's disease: a perspective based on experience with R-Flurbiprofen. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2006;2:150–152. doi: 10.1016/j.jalz.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 117.Wilcock GK, et al. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer's disease: a randomised phase II trial. Lancet neurology. 2008;7:483–493. doi: 10.1016/S1474-4422(08)70090-5. [DOI] [PubMed] [Google Scholar]
- 118.Goldstein JL, et al. Significantly improved upper gastrointestinal (UGI) tolerability with celecoxib, a COX-2 specific inhibitor, compared with conventional NSAIDs. The SUCCESS trial. Gastroenterology. 2001;120:A105–A105. [Google Scholar]
- 119.Burkhardt F, et al. COX-2 selectivity: Its clinical relevance for NSAID-gastrointestinal toxicity. Clin Pharmacol Ther. 1997;61:Pii13–Pii13. [Google Scholar]
- 120.Long JZ, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology. 2012;142:1218–1228. e1211. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cinar R, et al. Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology. 2014;59:143–153. doi: 10.1002/hep.26606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J, et al. Monounsaturated fatty acids generated via stearoyl CoA desaturase-1 are endogenous inhibitors of fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18832–18837. doi: 10.1073/pnas.1309469110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cristino L, et al. Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience. 2008;151:955–968. doi: 10.1016/j.neuroscience.2007.11.047. [DOI] [PubMed] [Google Scholar]