Abstract
Objectives
To determine the impact of empiric ampicillin and gentamicin use in the first week of life on microbial colonization and diversity in preterm infants.
Study design
16s rDNA community profiling was used to compare the microbiota of 74 infants born ≤32 weeks gestational age by degree of antibiotic use in the first week of life. The degree of antibiotic use was classified as 0 days, 1-4 days and 5-7 days of antibiotic administration. . All of the antibiotic use was empiric, defined as treatment based solely on clinical suspicion of infection without a positive culture result.
Results
Infants who received 5-7 days of empiric antimicrobial agents in the first week had increased relative abundance of Enterobacter (p=0.016) and lower bacterial diversity in the second and third weeks of life. Infants receiving early antibiotics also experienced more cases of NEC, sepsis or death than those not exposed to antibiotics.
Conclusions
Early empiric antibiotics have sustained effects on the intestinal microbiota of preterm infants. Intestinal dysbiosis in this population has been found to be associated with elevated risk of necrotizing enterocolitis, sepsis, or death.
Keywords: diversity, empiric antibiotics, microbiome, necrotizing enterocolitis, preterm infant
Antibiotics are the most commonly prescribed medications administered to infants in neonatal intensive care units (NICUs) (1). Even though the incidence of early-onset sepsis is low, preterm infants often receive empiric antibiotic treatment in the first few days of life when infection is suspected (2, 3). Concerns about occult intrauterine infection precipitating spontaneous premature labor, premature rupture of membranes, and chorioamnionitis often prompt initiation of empiric antibiotic treatment (4). Although initiation of antibiotic treatment for premature infants may be prudent under these circumstances, the duration of treatment is often arbitrary, based not on positive culture results but on the clinician's perception of risk (5). When empiric antibiotic treatment is clinically warranted, many clinicians limit such treatment to two days as the standard. Nevertheless, empiric antibiotic treatment is sometimes continued for 5 days or more based on antepartum factors, such as prolonged rupture of membranes or suspected chorioamnionitis, or for non-specific postnatal signs of infection including need for resuscitation at delivery, respiratory distress, or feeding intolerance. Unfortunately, early antibiotic therapy has the potential to cause harm as well as benefit to the preterm infant by impeding initial microbial colonization (6, 7). The microbial community of preterm infants is known to consist of dramatically fewer beneficial species, lower bacterial diversity, and more pathogens than observed in healthy term infants (8-11), but it is not known to what extent this observation can be attributed to the high use of antimicrobial agents. Even though recent advances in culture-independent sequencing methods have revolutionized our understanding of the microbial communities that live on and within the human body, studies of preterm infants have not yet attempted to examine the impact of antimicrobial use on the gut microbiome.
Unrestricted antimicrobial use can have persistent, unintended consequences, including reduced diversity of the microbial community, and after use is discontinued, recovery of a healthy microbiome is not assured (12). Early empiric antibiotic use in preterm infants is associated with increased risk of necrotizing enterocolitis (NEC), sepsis and death (13-15). Although aberrant early intestinal microbial colonization is thought to contribute to the pathobiology of NEC (8, 11, 16 62), the impact of early empiric antibiotic use on the preterm microbial community is not well studied.
We sought to test the hypothesis that early intensive antibiotic use in preterm infants alters the ontogeny of the microbiota and decrease microbial diversity in a longitudinal study of intestinal colonization in 74 preterm infants. Serial stool samples were collected from days 4 to 23 of life to test any differences in the early establishment of the intestinal microbiome by early and intensive empiric antibiotic therapy.
METHODS
Study infants were enrolled from three level III NICUs in Cincinnati, Ohio as part of an ongoing cohort study of novel biomarkers for cases of NEC, sepsis, or death in infants ≤32 weeks gestational age. All infants remained free of NEC, sepsis, or death in the first postnatal week and had no identified congenital anomalies. The Institutional Review Boards at the three participating hospitals approved the study. Early empiric antibiotic exposure was defined as antibiotic treatment initiated within the first postnatal day (14). The duration of early empiric antibiotic therapy was defined as the total number of continuous days of administration of antibiotics with sterile culture results. Sepsis was defined as a positive blood, cerebrospinal fluid, urine, or sterile site culture. NEC was defined using modified Bell's stage II or III criteria (17)
In this cohort, early empiric antibiotic use consisted of ampicillin and gentamicin, using standard dosing. Antibiotic exposure groups were defined as no antibiotics (0 days), brief antibiotics (1-4 days of empiric antibiotic therapy), and intensive antibiotics (5-7 days of antibiotic therapy).
Serial stool samples were collected from infants during the first three weeks of life: at week 1 (4-7 days), week 2 (10-16 days), and week 3 (20-23 days of age). Samples were collected from soiled diapers, immediately refrigerated in the NICU, and transported to the laboratory where they remained in the refrigerator until processing with thioglycollate, and storage at -80° C.
As previously described (16) bacterial DNA was extracted from infant stool samples using one of two methods: phenol-chloroform or the QiaAmp DNA stool kit. Bacterial 16S rDNA sequences (Figure 1) were produced by the Broad Institute (Boston, MA) using production protocols established for the Human Microbiome Project (18). The V3 to V5 window of the 16S rRNA gene was amplified and the sequences were determined using the 454 FLX Titanium platform. A total of 1.3 M resulting sequences were processed using a data curation pipeline implemented in mothur for operational taxonomic unit clustering (19), complemented by abundant operational taxonomic unit (20), UCHIME for chimera detection (21) and NEWBLER for assembly-based error reduction (22, 23).
Figure 1.
Definitions for metagenomic sequencing and analyses.
Statistical Analyses
Sequence data was generated for 256 samples from 81 infants. Samples collected following NEC or sepsis were excluded from analysis. Five infants with only week 1 samples were also excluded. Thus, a total of 74 infants with 239 samples were available for study. Differences among groups were tested using Fisher's Exact test for categorical variables and analysis of variance (ANOVA) for continuous variables. The non-parametric Kruskal-Wallis (KW) test was used to compare differences in diversity between antibiotic groups.
An important metric to describe a microbiome is the diversity of bacterial species identified in that microbiome. We used the QIIME program (24) to calculate the Simpson diversity index, which measures both species richness (number of species present) and evenness of abundance. The entire data set was rarefied to 2200 reads per sample before alpha-diversity was calculated. This procedure was repeated 5 times. Alpha-diversity metrics were then averaged per sample across these 5 iterations.
In order to account for multiple samples per infant, generalized estimating equations (GEE) models of alpha diversity were used to analyze differences in Simpson diversity index between infants with differing levels of antibiotic use. GEE models were run with an exchangeable correlation structure using the geeglm function in the geepack package in R (25).
The association between early antibiotic use and the relative abundance of the two most common operational taxonomic units were examined using a linear regression model. These two most abundant operational taxonomic units were classified, respectively, as an Enterobacter and a Staphylococcus, and were selected based on initial evidence of change in relation to antibiotic use groups, and being sufficiently abundant to allow robust modeling. The much lower abundance of other operational taxonomic units disallowed modeling due to zero inflation and limited statistical power. Because data from the same individuals tend to be positively correlated, the regression model was modified using GEE. Because the distribution of the antibiotic use was different for different sampling time, we stratified the data related to sample collection time, either week 2 or week 3 of life. We also categorized the duration of antibiotic use into three categories: none (0 days); brief (1-4 days); or intensive (5-7 days). Prior to analysis, raw operational taxonomic unit data was transformed into log scale to conform with the normality assumption of the model. These models were controlled for birth weight, maternal hypertension of pregnancy, delivery mode, and extraction protocol.
As DNA extraction methods can affect the results of 16S rDNA studies, we undertook a series of analyses to identify potential effects in our data (16). Briefly, extraction protocol was associated with neither beta-diversity nor with Simpson index of alpha-diversity. Finally, we included extraction protocol in regression models and found that it did not confound the associations being modeled.
RESULTS
Of the 74 infants included for study, 13 (18%) had no antibiotics, 48 (64%) had a brief course of antibiotics, and 13 (18%) had intensive antibiotics in the first week of life. Empiric antibiotics were initiated and duration of therapy determined based on clinician's perceived risk of infection. Throughout this study ampicillin and gentamicin were the universally prescribed combination for early empiric treatment. Infants who received intensive antibiotics in the first week of life were delivered at lower birth weights and less mature gestational age, and were more likely to have had premature rupture of membranes (>18 hours prior to delivery) (Table). Also, infants who were born to mothers with hypertension or pre-eclampsia were significantly (p<0.001) less likely to receive empiric antibiotics. Intensive antibiotic exposure was significantly higher in the first week of life among 14 case infants who subsequently developed NEC or sepsis or died (p=0.044; Table). Cases included NEC alone (n=4); NEC with sepsis (n=2); NEC with death (n=3); NEC with sepsis and death (n=1); and sepsis alone (n=2); and death alone (n=2). However, because all cases of NEC, sepsis, or death in our study occurred among infants < 29 weeks gestational age, the higher use of antibiotics in this group could be confounding the association. When we examined antibiotic receipt in only subjects < 29 weeks gestation, antibiotic receipt during the first week did not differ significantly between cases compared with controls (p=0.208).
Table.
Demographic and clinical characteristics of study subjects
| Initial empirical antibiotic therapy, n = infants (% of total study infants) | ||||
|---|---|---|---|---|
| Variable, description | 0 Days n = 13 (17.6) | 1-4 Days, n = 48 (64.8) | 5-7 Days n = 13 (17.6) | P value* |
| Infant baseline characteristics | ||||
| Birth weight, median (range) (g) | 1305 (890-1500) | 1065 (520-1480) | 750 (510-1440) | 0.001 |
| Gestational age, median (range) (wk) | 31 (24-32) | 28 (24-32) | 25 (23-31) | < 0.001 |
| Non-Hispanic black, no. (%) | 3 (23.1) | 10 (20.8) | 7 (53.8) | 0.071 |
| Male, no. (%) | 2 (15.4) | 26 (54.2) | 6 (46.2) | 0.046 |
| Cases, no. (%) | 0 (0) | 9 (18.8) | 5 (38.5) | 0.044 |
| Maternal and prenatal information | ||||
| Prenatal steroids given (any), no. (%) | 13 (100) | 42 (87.5) | 11 (84.6) | 0.553 |
| Hypertension / Pre-eclampsia, no. (%) | 12 (92.3) | 6 (12.5) | 4 (30.8) | < 0.001 |
| Prenatal care (≥ 1 prenatal visit), no. (%) | 13 (100) | 47 (97.9) | 13 (100) | 1 |
| Antepartum hemorrhage, no. (%) | 0 (0) | 12 (25) | 1 (7.7) | 0.069 |
| Multiple birth, no. (%) | 5 (38.5) | 17 (35.4) | 2 (15.4) | 0.417 |
| Antepartum antibiotic therapy, no. (%) | 4 (30.8) | 25 (52.1) | 8 (61.5) | 0.311 |
| Labor and delivery information | ||||
| Cesarean delivery, no. (%) | 12 (92.3) | 31 (64.6) | 8 (61.5) | 0.151 |
| Chorioamnionitis, no. (%) | 0 (0) | 5 (10.4) | 2 (15.4) | 0.443 |
| Delivery room resuscitation, no. (%) | 8 (61.5) | 29 (60.4) | 12 (92.3) | 0.100 |
| Rupture of membranes > 18 hrs, no. (%) | 0 (0) | 9 (18.8) | 7 (53.8) | 0.003 |
P-values calculated by Fisher exact test for categorical values (%) and ANOVA for continuous variables (birth weight, gestational age)
Stool samples were immediately refrigerated in the NICU and transported to the laboratory where they remained in the refrigerator until processing and freezing. The median time to freezing was 27 hours, with 91% of samples frozen by 72 hours. To test for the potential confounding effect of time to freezing we tested bacterial abundance and diversity of specimens processed before and after 24 hours. With the exception of Lactococcus, no differences were noted in relative abundance of bacterial genera between samples processed before versus after 24 hours following collection. Likewise, microbial diversity measures were not confounded by time to freezing.
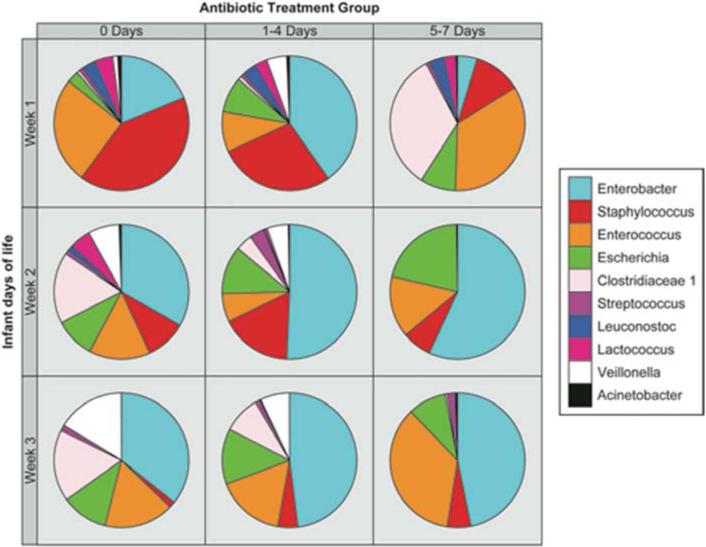
Pie charts of the most common genera are shown in Figure 2 in relation to antibiotic use and day of stool specimen collection. During the first week of life, among infants who received no antibiotics, Staphylococcus (41%), Enterococcus (26%), and Enterobacter (19%) were the most abundant genera. Infants who received a brief course of antibiotics had a greater relative abundance of Enterobacter (40%), and had less Enterococcus and Staphylococcus compared with infants who received no antibiotics. Infants who received intensive antibiotics had a different profile of the most abundant genera in samples from the first week of life, which were Enterococcus (34%) and Clostridium (33%), followed by Staphylococcus (11%) and Escherichia (8%). Concurrent with antimicrobial exposure, there was a striking variability in the genera comprising the intestinal microbiota.
Figure 2.
Pie graphs depicting relative abundance of bacterial genera detected in stool specimens from study infants as a function of antibiotic exposure over the first three weeks of life.
During week two of life, the stool of infants who received any antibiotics in the first week of life contained Enterobacter as the most common genus. The next three common genera, Staphylococcus, Enterococcus and Escherichia, were similar in relative abundance. For infants who received a brief course of antibiotics in the first week, the most common genera seen in week 2 samples were Enterobacter (51%), Staphylococcus (17%), and Escherichia (12%). Among infants receiving intensive antibiotics in the first week, only four genera - Enterobacter (57%), Escherichia (21%), Enterococcus (15%), and Staphylococcus (7%) - comprised >99% of the microbiome. Overall, in the second week of life, there was a trend toward increased abundance of Enterobacter among infants exposed to antibiotics compared with infants who did not receive antibiotics.
For samples from week three of life, Enterobacter (36%), Enterococcus (17%), Clostridia (17%), Veillonella (16%), and Escherichia (11%) were the most common genera present from infants who did not receive antibiotics. A similar pattern was observed for infants who received brief antibiotics, in which Enterobacter (48%) and Enterococcus (16%) were the most common genera followed by Escherichia (13%), Clostridia (9%) and Veillonella (7%). Among infants who received intensive antibiotics, the most common genera were Enterobacter (47%) followed closely by Enterococcus (35%).
To examine the statistical significance of these changes in microbiota, GEE models quantified the association between early antibiotic exposure and relative abundance of the two most abundant operational taxonomic units representing Enterobacter and Staphylococcus. Enterobacter significantly increased with empiric antibiotic use in week 2 with exposure to intensive antibiotics [p=0.016] and brief antibiotics [p=0.006], after controlling for birth weight, maternal hypertension of pregnancy, delivery mode, and extraction protocol. However, Enterobacter did not significantly increase in week 3 of postnatal life with more intensive antibiotic use during the first week. Intensive antibiotic use in week 1 was also associated with lower relative abundance of Staphylococcus in week 2 [p<0.001], but not through week 3. Brief antibiotics compared with no antibiotics were associated with a higher relative abundance of Staphylococcus in week 3 [p=0.004].
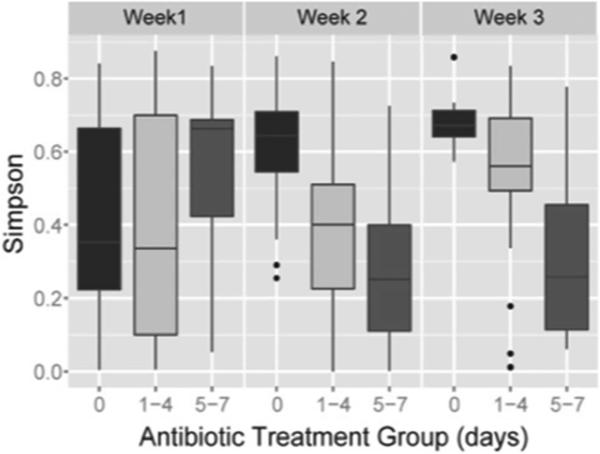
Simpson diversity index (Figure 3) was related to antibiotic receipt. For stool samples from week 1, Simpson's diversity index did not differ by antibiotic exposure. By week 2, decreasing diversity was observed after receiving either brief or intensive early antibiotics (p<0.001, Kruskal-Wallis test). By week 3, Simpson's diversity index remained significantly different across treatment groups (p<0.004). To control for potential confounding, the Simpson's index was modeled by GEE to account for multiple samples per individual, and to control for potential confounding factors (birth weight, maternal hypertension of pregnancy, delivery mode, and extraction protocol). Week two samples displayed significantly less diversity in infants who received brief (p=0.035) and intensive antibiotics (p=0.001). Similarly, in week 3, there was less diversity in infants who received brief (p=0.023) and intensive antibiotics (< 0.001).
Figure 3.
Simpson diversity index depicted in relation to antibiotic receipt (No, 0 days; brief, 1-4 days; or intensive antibiotics, 5-7 days) during the first three weeks of life.
DISCUSSION
The data from this study support the hypothesis that intensive early antibiotic administration to premature infants is associated with profound alterations in the intestinal microbiota and potentially, increased risk of NEC, sepsis, or death. A striking reduction in microbial diversity was observed within days of receiving intensive antibiotics and persisted over the 3-week study. More intensive antibiotic use increased representation by Enterobacter. Understanding the succession of the microbiota as well as the factors that influence the formation of the microbiota in preterm infants might provide insight into ontogeny of the microbiota and the pathogenesis of life threatening neonatal conditions such as NEC and sepsis. This is a small study and it is possible that results could be confounded by differences between cases and controls in factors such as gestational age. However, our findings are consistent with larger studies of antibiotic use and increased risk of these outcomes (13-15).
Others have reported low bacterial diversity in preterm infants. In a case control study of 20 preterm infants with and without NEC, Wang et al demonstrated low bacterial diversity that was most pronounced among infants who developed NEC (8) with an increase in Proteobacteria around the time of NEC. Our group and others also found that dysbiosis in early colonizing organisms precedes NEC, and domination by Proteobacteria, specifically Enterobacter, was found in some infants who subsequently developed NEC (16, 26). Here we report a high relative abundance of Enterobacter that is most striking in infants receiving intensive antibiotics. Antibiotic use may select forEnterobacter, which include pathogenic species commonly encountered in nosocomial infections in hospitalized patients. Enterobacter species are also frequently resistant to beta-lactams though a constitutive AmpC beta-lactamase (27). Although antibiotic sensitivity patterns and genetic profiles were beyond the scope of this study, it is key to study these residual colonizing species with regard to their degree of antimicrobial resistance. A new plasmid that confers resistance to both beta-lactams and aminoglycosides, the antibiotics used in this study, has been described (27). Animal studies report aggregation of resistance gene clusters, a core antimicrobial resistome, in the gut microbiome of antibiotic-fed swine (28). Drug resistance has been rapidly increasing in sepsis cases, and broad-spectrum antibiotic use strongly increases risk of invasive fungal infection (29, 30).
Increased abundance of Enterobacter observed with antibiotic therapy might occur though alternative mechanisms, notably modification of innate immunity. In studies comparing germ-free mice to conventionalized littermates, El Aidy et al characterized the dynamic temporal and region-specific mucosal responses to colonizing microbiota (31). These developmental changes included induction of innate immune function followed by stimulation and expansion of adaptive immune responses and ultimately a more tolerant state of immune homeostasis. Despite reaching a state of homeostasis, RegIII peptide expression remains high in the small intestines but declines to germ free levels in the colon after development of tolerant immune homeostasis. These finding suggest that RegIIIα and RegIIIγ play an important function, keeping microbes at bay in the small intestine. Disrupted expression of RegIII and other innate molecules by antibiotic exposure could potentially adversely impact immune-associated inflammatory pathways. Moreover, Reikvam and coworkers reported that broad-spectrum antibiotic treatment reduced the expression of antimicrobial factors to a level similar to that of germ-free mice and altered the expression of 517 genes in the colonic epithelium (32).
Infants who received a brief course of antibiotics, displayed a decrease in microbial diversity. However, by week 3 the diversity approached that of samples from the first week. The initial short antibiotic exposure could have suppressed the microbiota diversity only temporarily, in contrast to infants who received intensive antibiotics having a sustained reduction in diversity, a change not previously reported.
The infants with no antibiotic exposure displayed the increase in overall diversity reported by others (33). Intensive antibiotic use was significantly greater in infants who were affected by NEC or sepsis, or who died (13-15). It is interesting to speculate that the mechanism underlying this association may be related to antimicrobial killing of protective commensal bacteria with resultant growth of pathogenic bacteria. This mechanism is supported by the reduction of NEC associated with probiotic administration (34, 35).
Other potential determinants of the intestinal microbiota composition include delivery mode and feeding type. Staphylococcus is an early colonizer of the infant gastrointestinal tract. The early large abundance of Staphylococcus in the no antibiotic group may be a reflection of their predominant (but not statistically significant) delivery mode being cesarean delivery. Common indications for preterm birth by cesarean delivery include pre-eclampsia or eclampsia and intrauterine growth restriction (36), whose influence on infant intestinal microbiota is not known. Enteral feeding practices certainly contribute to variation in colonization of the gastrointestinal tract. However, in this study variation attributable to enteral feeding practices was probably limited because enteral feeding type and advancement were driven by a standardized feeding protocol for infants weighing 1500 g or less at birth in participating NICUs. Over the time course of this study more than 90% of infants received human milk either mothers-own-milk or donor human milk starting in the first two days of life and advancing to full feeds over about two weeks (37).
Bifidobacterium was the most common genera in a cohort of term infants in the Netherlands (38) measured by 16s PCR, and Bifidobacterium and Bacteroides frequency decreased with oral antibiotic use (mainly amoxicillin). Clostridium species increased with oral antibiotic use in hospitalized premature infants, which included late preterm infants 34 to 37 weeks gestational age. Lack of bifidobacteria in our cohort of preterm infants may be a function of greater prematurity, antibiotic use, illness, or possibly that Bifidobacterium is not as abundant in premature infants as previously thought, even when they receive human milk. Most infants in our study received either pasteurized donor milk or their own mother's human milk during the study.
Our study includes a larger number of infants relative to previously published studies in premature infants. Limitations of our study include the infrequent early defecation by premature infants in the early days of life, and the few samples obtained may not fully capture the effect of antibiotics on the microbiota. In addition, adverse outcomes including NEC, sepsis and death were too few to determine associations with antibiotic exposure and limited the number of samples included in the study. Antibiotic resistance patterns or genetic activity for resistance were not studied, but remain a promising undertaking for the future.
The duration of early empiric administration of ampicillin and gentamicin to preterm infants correlates with a decrease in intestinal microbial diversity. Among premature infants receiving intensive empiric antibiotics, the reduction in microbial diversity of the gastrointestinal tract continued through the 3 weeks of the study. It is plausible that early intensive antibiotic use may perturb the infant microbiota during a critical period, thus altering the course of ontogeny of the microbiota. This supports the hypothesis that consequent dysbiosis could precede, and perhaps precipitate sepsis and NEC, suggesting a need to assess the long-term impact of antibiotics on the microbiota, as well as their impact on disease outcomes such as NEC.
ACKNOWLEDGMENTS
We gratefully acknowledge Estelle Fischer for study management, Cathy Grisby, Barbara Alexander, Lenora Jackson, Kristin Kirker and Greg Muthig for clinical data collection and data entry, Barbara Davidson for sample collection planning and management oversight, Myra Johnson, Amy LeFevers, Maria Hughes for sample collection and management, and Donna Wuest for assistance with manuscript preparation.
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (HD059140, HD13021, and HD27853), National Institute of Environmental Health Sciences (T32 ES010957), National Center for Research Resources, National Institutes of Health (U01 RR026314), National Human Genome Research Institute, National Institutes of Health (HG005969), Danone Research (PLF-5972-GD), and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (contract HHSN272200900018C).
Sequence data generated for this work is deposited under the NCBI bioproject ID 63661.
ABBREVIATIONS (ALPHABETICAL)
- ANOVA
analysis of variance
- GEE
generalized estimating equations
- KW
Kruskal-Wallis
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- OTU
operational taxonomic unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–87. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. coli Disease Continues. Pediatrics. 2011;127(5):817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 5.Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol. 2003;24(9):662–6. doi: 10.1086/502270. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72(3):317–21. [PubMed] [Google Scholar]
- 7.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91(441):48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. Isme J. 2009;3(8):944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morowitz MJ, Denef VJ, Costello EK, Thomas BC, Poroyko V, Relman DA, et al. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc Natl Acad Sci U S A. 2011;108(3):1128–33. doi: 10.1073/pnas.1010992108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallstrom M, Eerola E, Vuento R, Janas M, Tammela O. Effects of mode of delivery and necrotising enterocolitis on the intestinal microflora in preterm infants. Eur J Clin Microbiol Infect Dis. 2004;23(6):463–70. doi: 10.1007/s10096-004-1146-0. [DOI] [PubMed] [Google Scholar]
- 11.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. The Journal of clinical investigation. 2010;120(12):4332–41. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empiric antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empiric antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–5. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–7. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1:13. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evaluation of 16S rDNA-based community profiling for human microbiome research. PLoS One. 2012;7(6):e39315. doi: 10.1371/journal.pone.0039315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Y. Identification and Quantification of Abundant Species from Pyrosequences of 16S rRNA by Consensus Alignment. Proceedings (IEEE Int Conf Bioinformatics Biomed) 2011;2010:153–7. doi: 10.1109/BIBM.2010.5706555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Blaxter ML. Comparing de novo assemblers for 454 transcriptome data. BMC genomics. 2010;11:571. doi: 10.1186/1471-2164-11-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JR, Koren S, Sutton G. Assembly algorithms for next-generation sequencing data. Genomics. 2010;95(6):315–27. doi: 10.1016/j.ygeno.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halekoh U, Hojsgaard S, Yan J. The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software. 2006;15(2):1–11. [Google Scholar]
- 26.Claud EC, Keegan KP, Bruic JM, Lu L, Bartels D, Glass E, et al. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome. 2013;(20):1. doi: 10.1186/2049-2618-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alavi MR, Antonic V, Ravizee A, Weina PJ, Izadjoo M, Stojadinovic A. An Enterobacter plasmid as a new genetic background for the transposon Tn1331. Infect Drug Resist. 2011;4:209–13. doi: 10.2147/IDR.S25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Looft T, Johnson TA, Allen HK, Bayles DO, Alt DP, Stedtfeld RD, et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc Natl Acad Sci U S A. 2012;109(5):1691–6. doi: 10.1073/pnas.1120238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brecht M, Clerihew L, McGuire W. Prevention and treatment of invasive fungal infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2009;94(1):F65–9. doi: 10.1136/adc.2007.133769. [DOI] [PubMed] [Google Scholar]
- 30.Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK., Jr. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118(2):717–22. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 31.El Aidy S, van Baarlen P, Derrien M, Lindenbergh-Kortleve DJ, Hooiveld G, Levenez F, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5(5):567–79. doi: 10.1038/mi.2012.32. [DOI] [PubMed] [Google Scholar]
- 32.Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6(3):e17996. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122(4):693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 35.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125(5):921–30. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delfosse NM, Ward L, Lagomarcino AJ, Auer C, Smith C, Meinzen-Derr J, et al. Donor human milk largely replaces formula-feeding of preterm infants in two urban hospitals. J Perinatol. 2013;33(6):446–51. doi: 10.1038/jp.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]