Abstract
The cellular response to DNA double-stranded breaks (DSBs) involves a conserved mechanism of recruitment and activation of numerous proteins involved in this pathway. The events that trigger this response in mammalian cells involve several post-translational modifications, but the role of non-proteasomal ubiquitin signaling is particularly central to this pathway. Recent work has demonstrated that ubiquitination does not act alone, but in concert with other post-translational modifications, including phosphorylation, methylation, acetylation, ADP-ribosylation, and other ubiquitin-like modifiers, particularly SUMOylation. We review novel and exciting crosstalk mechanisms between ubiquitination and other post-translational modifications, many of which work synergistically with each other to activate signaling events and help recruit important DNA damage effector proteins, particularly BRCA1 and 53BP1, to sites of DNA damage.
Keywords: DNA damage, ubiquitin, chromatin, signaling
The double-stranded break response in the chromatin context
A double-stranded DNA break represents a difficult problem for any cell, and its proper repair is important for cell survival and the prevention of oncogenic translocations. In eukaryotes, an elaborate signaling pathway takes place on chromatin near the break site that coordinates ordered recruitment of specific factors, promotes DNA repair and cell cycle arrest. This response to double-stranded breaks (hereon referred to as the DSB response) relies heavily on post-translational modifications of both chromatin components (i.e., histones) and non-chromatin proteins. Thus, the basic organization of eukaryotic chromatin needs to be considered prior to understanding the crosstalk between the modifications that occur primarily on the chromatin landscape.
The fundamental unit of chromatin is the nucleosome, which contains eight histone subunits, consisting of two dimers of histones H2A and H2B, and a tetramer of histones H3 and H4. This octameric core of histones is combined with 146 base pairs of DNA to form the nucleosome core particle [1]. Each of the core histones has a similar overall structure, consisting of a globular, hydrophobic internal region that forms the histone fold, and N- and C-terminal extensions that are relatively unstructured [1]. These extensions, particularly those on the N-terminus, are sites for numerous post-translational modifications, which play key roles in the regulation of DNA template dependent processes, including DNA repair [2]. These modifications can alter the structure of the nucleosome directly, but their major function is to serve as signaling platforms for the recruitment of protein complexes that carry specific recognition modules (so-called ‘reader’ domains; see Boxes 1 and 2) recognizing specific modifications, either individually or in combination [2].
Box 1. Ubiquitin and SUMO reader domains in the DSB response.
A number of small (~50-100 amino acid) recognition modules are present in key DNA damage response factors that bind to specific post-translational modifications, mostly on or near chromatin, and are critical for orchestrating DSB response signaling. Because ubiquitination is critical for proper DSB signaling, numerous factors involved in this pathway contain ubiquitin binding domains (UBDs) that recognize ubiquitin chains. A prototypical example can be found in Rap80, which contains two ubiquitin interacting motifs (UIMs) in tandem [65-68]. Since ubiquitin can form multiple chain types, each of which possess distinct structures, different UBDs utilize these distinguishing features in recognizing specific ubiquitin linkage types. For example, Rap80 preferentially interacts with K63-linked ubiquitin over K48-linked chains. This specificity is not intrinsic to the UIMs themselves, but comes from the presence of a long linker helix that connects the UIMs in Rap80, which preferentially recognizes the more extended K63-linked chains compared to the more compact K48-linked chains [66, 68]. Like RNF4, Rap80 also contains a SUMO-interacting motif (SIM) that permits binding to both modifications, possibly simultaneously, thereby recognizing ubiquitin-SUMO mixed chains [25]. Like Rap80, other proteins that associate with BRCA1, such as Abraxas and BRCC36, also appear to contain SIM domains, although their functions have not been explored [25]. Other key proteins involved in DNA damage signaling, including RNF168, its paralog RNF169, as well as RAD18, also contain UBDs [69]. Interestingly, many of these UBDs appear to function with unique adjacent sequences that provide ligand specificity [69]. This is reminiscent of 53BP1, which recognizes nucleosomes that contain H2A monoubiquitinated at K15 and H4 dimethylated at K20 using adjacent domains [49]. It is very likely that other examples like 53BP1 exist where UBD specificity is determined by adjacent domains that may recognize other types of modifications that occur in close proximity.
Box 2. Phosphorylation and methylation reader domains in the DNA damage response.
Similar to ubiquitination specific interaction motifs, reader domain classes exist that recognize other types of post-translational modifications important to the DSB response. These include the BRCA1 C-terminal homology (BRCT) domain, which can be found in numerous DSB response proteins beyond BRCA1, including MDC1, 53BP1, and TopBP1 [6]. Traditionally, these are thought to mediate interactions with phosphorylated peptides, particularly the products of the ATM/ATR/DNA-PK serine-threonine kinases. Two canonical BRCT-phosphopeptide interactions include the BRCA1 BRCT domain interacting with the phosphorylated form of the BACH1 helicase, and the tandem BRCT domains of MDC1 interacting with γ-H2A.X [6, 71-73]. Recent work suggests that certain BRCT domains can also interact with ADP-ribose [60, 61]. The forkhead associated (FHA) domain is also another phosphopeptide interacting domain present in certain DSB response factors, such as RNF8 [7, 8]. As with the BRCT domain, the FHA domain has also been shown to interact with other modified ligands, specifically iso-ADP-ribose [60]. These data suggest that the BRCT and FHA domains have structural adaptability for different ligands. While much work has been done on the structural determinants of BRCT-phosphopeptide and FHA-phosphopeptide interactions [6], the interaction between these domains and ADP-ribose remains to be determined.
The recognition of methylated peptides, particularly lysines present on histones, can be accomplished by numerous reader domains. The most pertinent of these to the DSB response are the chromodomain and the Tudor domain, which are distantly related to each other and belong to the ‘Royal’ superfamily of methyl-lysine recognition modules [70]. These domains appear to be relatively specific to the degree of methylation (i.e., mono-, di-, or tri-methylation) present on the recognized lysine. For example, the 53BP1 tandem Tudor domains specifically recognize dimethylated H4K20, and the Tip60 chromodomain prefers trimethylated H3K9 [70]. The presence of these domains on proteins relevant to transcriptional regulation as well as the DSB response (e.g., the JMJD2 family of histone demethylases) strongly suggests the coordination of these two processes by these factors.
The eukaryotic DSB response should be viewed within this chromatin context, because many key steps in the DSB response depend on specific chromatin modifications. The DSB response begins with the recognition of the double-stranded DNA break by the Mre11-Rad50-NBS1 (MRN) complex, which is thought to promote activation of signaling kinases including ATM, ATR, and DNA-PK [3]. These serine-threonine kinases target hundreds of proteins [4], but a key target is a conserved site (serine 139) at the C-terminus of the histone variant H2A.X, which replaces the canonical H2A in approximately 10% of the genome and is critical for orchestrating the DSB response [5]. Phosphorylated H2A.X (also known as γ-H2A.X) creates a ligand for MDC1, which recognizes γ-H2A.X via its tandem BRCT (BRCA1 C-terminal homology) domains [6]. MDC1 itself contains phosphorylation sites near its N-terminus that are targeted by the ATM/ATR kinases, and phosphorylation of these residues recruits the RNF8 E3 ubiquitin ligase via interaction with RNF8 FHA (Forkhead associated) domain [6]. RNF8 catalyzes K63-linked polyubiquitination, which promotes recruitment of another key E3 ubiquitin ligase, RNF168 [7-9]. Recent work has demonstrated that the E3 activity of RNF168 specifically targets lysines 13 and 15 in the N-termini of H2A and H2A.X [10, 11]. The RNF168-mediated ubiquitination of H2A/H2A.X is critical for the recruitment of 53BP1 and BRCA1 [12, 13], two key effectors of the DSB response which promote non-homologous end joining (NHEJ) and homologous recombination (HR), respectively [14, 15].
While it is well-established that RNF8/RNF168-mediated ubiquitin signaling is central to the recruitment of DSB effector complexes, recent work strongly supports a model where these ubiquitination events function in concert with other post-translational modifications in the DSB response cascade. In particular, evidence for ubiquitination crosstalk with the small ubiquitin-like modifier (SUMOylation), acetylation, methylation, and ADP-ribosylation has emerged, demonstrating complex mechanistic relationships between these modifications in the DSB response. In this review, we detail the molecular mechanisms of each of these crosstalk pairs and explore their effects on DSB repair.
Ubiquitination and SUMOylation
Like ubiquitination, SUMOylation is catalyzed by a cascade of enzymes initiated by E1 SUMO-activating enzymes (SAE1 and SAE2), a single E2 conjugating enzyme (Ubc9), and various E3 SUMO ligases, which are considered to be important for target selection [16]. Established examples of SUMOylation regulating ubiquitination pathways have been known for many years [17], and indirect evidence suggested a role for SUMOylation in the DSB response [18, 19]. Recently, two independent groups revealed the direct involvement of SUMOylation in the DNA damage response, demonstrating that all three SUMO isoforms (SUMO1, and the closely related SUMO2 and SUMO3) are highly enriched at sites of DNA damage in human cells [20, 21]. Upon DNA damage, the SUMO E2 ligase Ubc9 and the PIAS family of SUMO E3 ligases rapidly mobilize and localize to DNA damage foci [20, 21]. Knockdown of certain SUMO E3 ligases, particularly PIAS4, resulted in loss of SUMOylation at damage sites, as well as reduced accumulation of 53BP1, BRCA1, and K63-linked ubiquitin conjugated at damage sites without affecting MDC1 or RNF8 recruitment. Loss of MDC1 or RNF8 affected accumulation of all SUMO isoforms at damage sites, while loss of 53BP1 or BRCA1 selectively affected SUMO1 and SUMO2/3, respectively [20, 21]. These results strongly suggest that SUMOylation acts at multiple steps in the DSB response, but that its major role is upstream of the RNF168-mediated ubiquitination step.
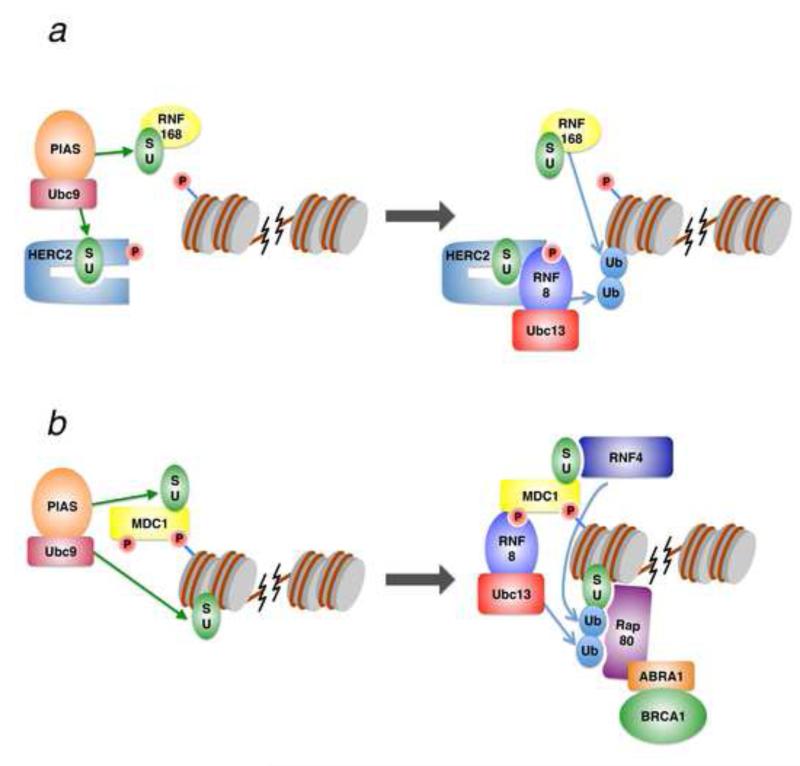
What could be the molecular mechanism behind the SUMO-dependent ubiquitination at sites of DNA damage? Although one study clearly demonstrated that the E3 ubiquitin ligase activity of BRCA1 is significantly increased upon SUMOylation [21], other targets upstream of BRCA1 must exist to explain the observed phenotypes becuase BRCA1 recruitment is itself dependent on SUMOylation. Recent work has provided evidence for a number of potential mechanisms that further explain the role of SUMOylation in promoting ubiquitination during the DSB response (Figure 1). It was demonstrated that RNF168 and another E3 ubiquitin ligase, HERC2 are modified by PIAS4-mediated SUMOylation during DNA damage [22]. PIAS4-mediated SUMOylation of HERC2 promotes its association with RNF8, likely by an intramolecular mechanism, and stabilizes the interaction between RNF8 and the E2 ubiquitin ligase Ubc13 (Figure 1a). Loss of PIAS4 also reduced the half-life of RNF168 protein [22], possibly due to a loss of HERC2 function, which was previously shown to affect RNF168 stability [23]. It should be noted that in the chicken DT40 cell system, HERC2 does not appear to function in the RNF8/RNF168 pathway, which may be due to a lack of functional conservation [24].
Figure 1.
Two potential mechanisms of crosstalk between ubiquitination and SUMOylation. (a) The PIAS family of SUMO E3 ligases, in conjunction with the Ubc9 SUMO E2, SUMOylate RNF168 as well as HERC2. This SUMOylation promotes an intramolecular interaction within HERC2, causing association with RNF8 and the formation of an active Ubc13-RNF8 complex. In conjunction with RNF168, this active form of RNF8 promotes ubiquitin chain formation at the site of DNA damage. (b) PIAS-Ubc9 proteins SUMOylate MDC1 and other proteins near the site of DNA damage. SUMOylated MDC1 helps to recruit RNF4, which, along with Ubc13-RNF8, form mixed SUMO-ubiquitin conjugates. These conjugates are recognized by Rap80 via its SIM and tandem UIM motifs, which then recruits BRCA1 through ABRA1/CCDC98.
Another mechanism involves the SUMO-targeted ubiquitin E3 ligase (STUbL), RNF4. STUbLs function by recognizing SUMOylated proteins, which are then targeted for ubiquitination. RNF4 contains SUMO-interacting motifs (SIMs) that, along with SUMO E3 ligases PIAS1 and PIAS4, are required for the recruitment of RNF4 to sites of DNA damage [25-27]. The RNF4 SIMs likely recognize multiple SUMOylated protein targets, but a key target is likely to be MDC1, which is SUMOylated at its C-terminus [26-28]. Loss of RNF4 reduces ubiquitin conjugation at damage sites, resulting in decreased 53BP1 and BRCA1 accrual there [25-27]. The mechanism behind these phenotypes likely involves reduced histone ubiquitination, although it is currently unclear whether RNF4 can catalyze histone ubiquitination directly. It is notable that RNF4 is thought to produce mixed ubiquitin-SUMO conjugates that are then preferentially recognized by Rap80, which associates with BRCA1 and is responsible for the recruitment of this effector protein (Figure 1b) [25]. Another potential mechanism involves the recruitment of proteasome components to DNA damage sites via RNF4 [27]. The recruitment of the proteasome could indirectly promote ubiquitin conjugation on chromatin by removing certain factors that may interfere with ubiquitin conjugation. At this time, it is difficult to determine which mechanism is primarily responsible for the crosstalk between SUMOylation and ubiquitination. However, many DSB response factors that regulate ubiquitination have functional SIMs, suggesting the paradigm established by RNF4 and Rap80 may be the favored model. Indeed, another STUbL, RNF111/Arkadia, has recently been identified to play a role in promoting ubiquitin conjugate formation at damage sites [29].
Ubiquitination and acetylation
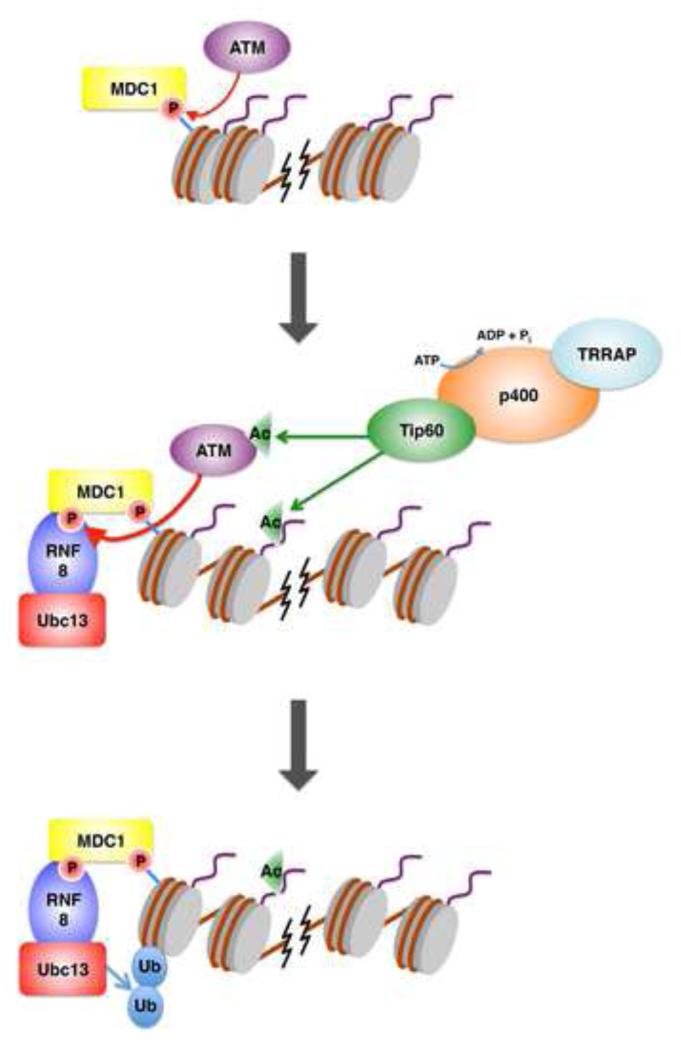
Histone acetylation plays an important role in the regulation of chromatin structure [30], and therefore it is not surprising that specific histone acetyltransferases (HATs) play key roles in the DSB response. One major HAT, Tip60, was originally found to be part of a multimeric complex, and this seminal study demonstrated a role for Tip60 in DNA repair [31]. Studies on the activity of Tip60 as a regulator of chromatin structure, and subsequently of histone ubiquitination events, are ideal examples of synergistic crosstalk between post-translational modifications in the DSB response (Figure 2). We explore this crosstalk by discussing three distinct targets of Tip60: Acetylation of histone H4, which in combination with the p400 ATPase promotes chromatin relaxation near DNA breaks and facilitates RNF8-mediated ubiquitination events [32]; acetylation of H2A.X, which promotes H2A.X polyubiquitination at K119 [33]; and acetylation of ATM, leading to ATM activation, which indirectly promotes ubiquitination by recruiting RNF8/RNF168 [34, 35].
Figure 2.
Crosstalk between the TRRAP-Tip60-p400 acetyltransferase complex and ubiquitination. Initially, ATM activation helps recruit MDC1 to sites of DNA damage (top). The Tip60 complex is subsequently recruited, which promotes chromatin remodeling via the p400 ATPase. Tip60 also acetylates multiple targets, including H4, H2A.X and ATM (middle). Acetylation of ATM further activates its kinase activity, and the hyperacetylated, more open chromatin configuration promotes RNF8-mediated ubiquitination (bottom).
A major target of Tip60 mediated acetylation is histone H4 [31]. Acetylation of H4 at lysine 16 was sufficient to inhibit the formation of the 30nm-like chromatin fibers, suggesting that this modification promotes chromatin relaxation [36]. Furthermore, Tip60 HAT acted in complex with its cofactor TRRAP to promote H4 acetylation near sites of DNA double-stranded breaks [37]. Cells lacking TRRAP did not recruit Tip60 and had reduced histone acetylation near these damage sites. As a result, these cells demonstrated an impaired efficiency to repair DNA, particularly via homologous recombination (HR) [37]. Consistently, loss of TRRAP reduced recruitment of 53BP1 and BRCA1 without affecting MDC1 recruitment. Importantly, the defect in repair protein recruitment and HR after TRRAP depletion was rescued by chemically inducing chromatin relaxation, suggesting a chromatin remodeling role for the Tip60-TRRAP HAT complex in promoting DNA repair [37]. The fact that 53BP1 and BRCA1 recruitment are affected downstream of MDC1 strongly suggests that this complex functions to promote histone ubiquitination downstream of γ-H2A.X. Indeed, Tip60 also acetylates H2A.X at K5 in response to ionizing radiation [33], and mutation of this residue was shown to reduce polyubiquitination of H2A.X without affecting γ-H2A.X formation. We should note, however, that another study did not find a reduction in H2A.X ubiquitination upon mutation of the K5 residue [38]. However, consistent with a role in H2A.X ubiquitination, Tip60 associated with the E2 ligase Ubc13 [33]. Interestingly, polyubiquitinated H2A.X is released from damaged chromatin in a manner dependent on the Tip60-Ubc13 pathway [33], suggesting histone eviction as a potential mechanism by which this HAT promotes chromatin relaxation.
The finding that the SWI/SNF-related ATPase p400 associates with the Tip60 HAT complex provides further understanding of the mechanism underlying the DSB-induced chromatin relaxation events mediated by the Tip60 complex [32]. This diverse family of ATPases may work to change chromatin structure by repositioning nucleosomes, altering DNA-histone interactions, or promoting histone exchange [39]. The p400 ATPase functions in conjunction with TRRAP and Tip60 acetylation to destabilize nucleosomes, and this destabilization is necessary to enable histone ubiquitination by RNF8/RNF168 [32]. Chemically induced hyperacetylation had no effect on chromatin relaxation in this study, suggesting that Tip60 needs to function in the context of the p400 complex to promote RNF8/RNF168 ubiquitination.
In addition to its roles in histone acetylation and ubiquitination-mediated chromatin remodeling, Tip60 has also been suggested to play a direct role in the activation of the protein kinase ATM. Tip60 acetylates K3016 of ATM, a residue adjacent to its kinase domain [40]. Mutation of this residue abolishes kinase activity induced by Tip60 upon DNA damage, resulting in decreased phosphorylation of downstream ATM targets and reduced survival. Since activated ATM phosphorylates MDC1 to recruit RNF8, Tip60 indirectly crosstalks with ubiquitination by promoting the recruitment of this E3 ligase. Tip60 is itself regulated by other post-translational modifications, particularly histone H3K9 methylation, which is recognized by Tip60 via its chromodomain [41]. The binding of Tip60 to this modification activates its HAT activity, thereby activating ATM. A recent report suggested that this binding to H3K9 methylation, and its resulting ability to activate ATM, is regulated by tyrosine phosphorylation of Tip60 itself [34]. Therefore, it appears that the function and regulation of Tip60 serves as a key nexus in promoting the DSB response pathway.
Ubiquitination and phosphorylation
As mentioned in the previous section, acetylation promotes RNF8 recruitment by activating the ATM kinase, which suggests crosstalk between ubiquitination and phosphorylation events during the DSB response. Not surprisingly, other crosstalk mechanisms between ubiquitination and phosphorylation also exist. Phosphorylation of MDC1 by these kinases promotes the recruitment of RNF8, which causes a feedback loop that promotes further ATM activation via RNF8 and another DSB response associated E3 ligase, CHFR [42]. Cells lacking both RNF8 and CHFR have a significantly reduced activation of ATM kinase, demonstrated by reduced phosphorylation of specific ATM targets in vivo and reduced ATM kinase activity in vitro. Similar to ATM deficient mice, RNF8-CHFR double-knockout (DKO) mice develop thymic lymphomas at a high rate, are impaired in T-cell development, and are significantly more sensitive to radiation [42]. The mechanism by which RNF8 and CHFR mediated ubiquitination promotes ATM activation may involve acetylation events mediated by Tip60 and another histone acetyltransferase, MOF. MRG15, a subunit common to the Tip60 and MOF acetyltranferase complexes, appears to recognize ubiquitinated histones, helping to target these histone modifying enzymes to chromatin, thereby promoting ATM activation through the mechanism described in the prior section on acetylation [42]. Another possible mechanism for how ubiquitination events promote ATM activation involves 53BP1. This effector protein contains two tandem BRCT motifs at its C-terminus, which can interact with phosphorylated Nbs1, promoting ATM phosphorylation events [43]. Since RNF8-mediated ubiquitination promotes 53BP1 recruitment, this recruitment could feed back to further amplify ATM activation. It is likely that a number of other feedback loops exist in the ATM pathway to help regulate DSB response signaling.
Ubiquitination and methylation
While histone methylation is canonically associated with transcriptional regulation, early studies revealed an evolutionarily conserved role for histone H4K20 methylation in recruiting 53BP1 to DNA damage sites [44]. 53BP1 binds to this methylation mark via its tandem Tudor domains [45]. While there are conflicting reports on which histone methyltransferase (HMTase) is primarily responsible for this modification [45-47], it is likely that many distinct HMTases contribute to H4K20 methylation and consequently 53BP1 recruitment. However, most reports have demonstrated that H4K20 methylation is not induced at damage sites, suggesting that another signal besides H4K20 methylation is responsible for promoting its recruitment [44, 47]. It had been known that RNF8/RNF168 mediated ubiquitination was critical for the recruitment of 53BP1 [48], but how this ubiquitination functioned with H4K20 methylation was unclear until recently.
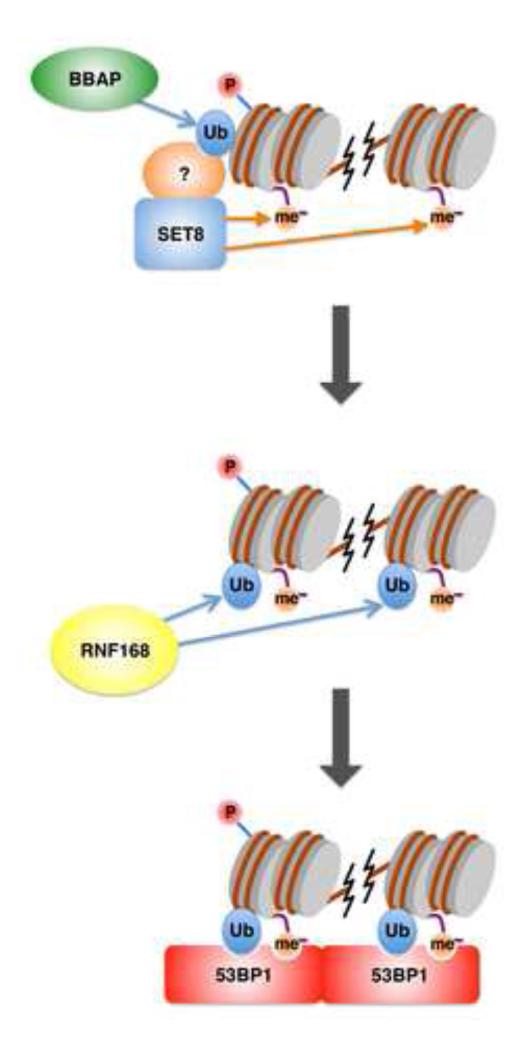
The finding that RNF168 directly ubiquitinates H2A/H2A.X at K13/K15 [10, 11] set the stage for the subsequent clarification of how methylation and ubiquitination work together to recruit 53BP1. It was discovered that 53BP1 contains an additional domain called the UDR (for ubiquitination-dependent recruitment domain) that is also critical for its recruitment to damage sites (Figure 3) [49]. This short motif resides downstream of the tandem Tudor domains and recognizes H2A monoubiquitinated at K15. Binding to monoubiquitinated H2A-K15 and H4K20me2 is not affected by mutations in the tandem Tudor domains and the UDR motif, respectively, suggesting that these two binding events are independent but equally important for 53BP1 recruitment [49]. Mutations that affect either domain also affect the ability of 53BP1 to regulate DNA end resection, a major function of this effector protein [50]. Since 53BP1 dimerizes with itself [49], a potential molecular mechanism for promoting non-homologous end joining and inhibition of resection could be that 53BP1 bridges the DNA break on either side by recognizing these two modifications, impeding nuclease access at specific DNA ends (Figure 3). While these studies clarify the molecular mechanism of 53BP1 recruitment to damaged chromatin, how RNF168-mediated H2A ubiquitination promotes BRCA1 recruitment is still unclear. It is possible that a BRCA1-associated factor functions like 53BP1 and preferentially recognizes ubiquitinated H2A. Genetic studies have suggested that 53BP1 and BRCA1 compete for the DNA break to inhibit or promote homologous recombination [50], respectively, suggesting that these two factors may recognize similar chromatin modification signatures. Further biochemical studies are necessary to test this possibility.
Figure 3.
Synergistic function of histone methylation and ubiquitination in the recruitment of 53BP1. BBAP mediates monoubiquitination of H4 at lysine 91, which promotes SET8 recruitment, possibly through an associated partner that recognizes monoubiquitinated H4 (top). SET8 then promotes H4K20 methylation. RNF168 mediates monoubiquitination of H2A at lysine 15 near sites of DNA damage (middle). This monoubiquitination works in concert with H4K20 methylation to recruit 53BP1 via its respective tandem Tudor domains and the adjacent UDR motif (bottom).
Recent work suggests two alternative mechanisms by which ubiquitination and methylation may crosstalk with each other. The ubiquitin E3 ligase BBAP and its binding partner BAL have been shown to play a role in the DSB response [51, 52]. Interestingly, BBAP was shown to be an E3 ligase that targets histone H4 monoubiquitination at lysine 91 [51]. Loss of BBAP selectively affects 53BP1 foci formation without affecting MDC1 or BRCA1 recruitment, suggesting that it functions distinctly relative to the RNF8/RNF168 pathway. Mechanistically, BBAP functions to recruit the H4K20 methyltransferase SET8 to chromatin (also known as PR-Set7 or KMT5A; Figure 3). How H4 ubiquitination affects SET8 recruitment is unclear, as SET8 does not have an obvious ubiquitin binding domain. In another example of such crosstalk, the histone demethylase LSD1 plays a role in the DNA damage response and is recruited to sites of DNA damage by RNF168 [53]. Consistent with its role in RNF168-mediated ubiquitination, loss of LSD1 affects H2A ubiquitination, reducing 53BP1 and BRCA1 foci formation. It is unclear how LSD1 promotes H2A ubiquitination, although we surmise that its histone demethylase activity may create a chromatin environment conducive to RNF168-mediated ubiquitination.
Ubiquitination and ADP-ribosylation
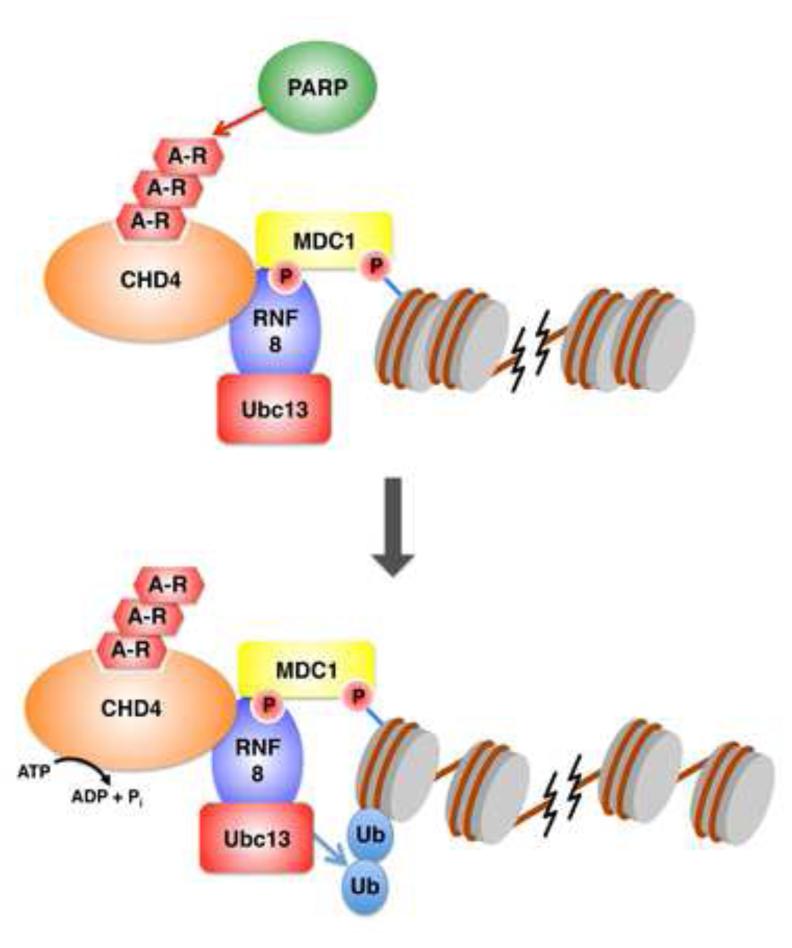
It is accepted that ADP-ribosylation plays a role in the DSB response, amongst many other signaling pathways [54]. Activation of poly(ADP-ribose) polymerase (PARP) enzymes is an early event in the DSB response [54], and recent work has demonstrated that ADP-ribosylation promotes the recruitment of multiple chromatin modifying enzymes to damage sites [55-57]. The most prominent of these is the CHD4/NuRD complex, which is another member of the SWI/SNF-like ATPases that remodels chromatin [58]. Originally identified as a key component of a histone deacetylase complex that promotes transcriptional repression [58], CHD4/NuRD binds directly to poly-ADP-ribose and requires PARP activity for its recruitment to damage sites [55, 56]. Loss of this complex reduces ubiquitination signaling at sites of damage subsequent to the recruitment of RNF8 [57] (Figure 4). Interestingly, CHD4/NuRD also associates with RNF8 itself, and RNF8 functions with ADP-ribosylation to promote CHD4/NuRD recruitment independent of its E3 ligase activity [59]. Similar to p400, CHD4/NuRD induces chromatin decondensation, resulting in efficient ubiquitin conjugation, and thereby promoting BRCA1 and 53BP1 recruitment [59]. How CHD4/NuRD chromatin remodeling promotes ubiquitination remains unknown. Nevertheless, the CHD4/NuRD complex appears to act as an intermediary factor that links ADP-ribosylation to RNF8-mediated ubiquitination events.
Figure 4.
Mechanism of crosstalk between ADP-ribosylation and ubiquitination. Poly-ADP-ribose polymerase (PARP) enzymes act early during DNA damage signaling and help recruit the CHD4 chromatin remodeling complex (top). CHD4 recruitment is also promoted by its interaction with RNF8. Subsequently, CHD4 promotes chromatin remodeling, which promotes ubiquitination via Ubc13-RNF8 (bottom).
Recently, studies suggest that ADP-ribosylation also serves as a ligand for certain FHA and BRCT domains and may promote ubiquitination by alternative mechanisms [60]. Specifically, the BRCT domains of BARD1, a major partner of BRCA1, binds directly to poly(ADP-ribose) [61]. PARP inhibition suppresses this early recruitment of the BRCA1/BARD1 complex to damage sites, consistent with the notion that PARP activity promotes early events in the DSB response [61]. The direct recruitment of this complex by poly(ADP-ribose) may promote ubiquitination by the BRCA1/BARD1 E3 ligase. While the relevant ubiquitination targets of BRCA1 are not known, expression of an H2A-ubiquitin chimeric protein is able to largely rescue the genomic instability seen in the absence of BRCA1 [62]. Therefore, ADP-ribosylation may help initiate ubiquitination events during the DSB response by directly recruiting E3 ligases.
Concluding remarks
The molecular mechanisms involved in protein recruitment and activation during the DSB response are noticeably complex. The crosstalk mechanisms described here reflect only a small portion of this complexity, and crosstalk mechanisms that involve other modifications most definitely exist. For example, recent work has suggested that protein neddylation is also important for promoting ubiquitination events at sites of damage [63]. This complexity and various crosstalk mechanisms likely arose due to the numerous different requirements for a proper DSB response, which beyond the well-described cell cycle arrest and recruitment of effector proteins, includes transcriptional repression of the local chromatin and coordination with DNA replication. It is therefore not surprising that the post-translational modifications that function during the DSB response are carefully coordinated and interdependent, leading to important implications in DSB response biology. Specifically, the association of 53BP1 and BRCA1 at sites of damage appears to behave as a serial circuit, where numerous sequential events function to promote recruitment, each of which appear to be essential. The numerous points of feedback and crosstalk between these modifications may explain this behavior because loss of a particular modifying enzyme affects downstream modifications indirectly, many of which feed into ubiquitination.
Quantitative biochemical approaches, such as the identification of the coordinate function of the 53BP1 Tudor and UDR domains, have helped to clarify some of the specific combinations of DSB-associated chromatin modifications. It will be interesting to determine whether distinct combinations of specific modifications regulate the choice between different repair mechanisms, such as homologous recombination versus non-homologous end joining. For example, while H4 methylation and H2A monoubiquitination directly recruit 53BP1 to repair via non-homologous end joining, K63-linked polyubiquitination is thought promote recruitment of BRCA1 to repair via homologous recombination. Recent work has suggested that H3K4 methylation may play a role in promoting homologous recombination [64], and it will be interesting to determine whether any factors associated with BRCA1 directly bind to methylated H3K4 to help recruit this effector protein. The combination of K63 polyubiquitination and H3K4 methylation may potentially serve as the ‘histone code’ for BRCA1 recruitment, and hence, promote homologous recombination. Future work is necessary to shed light on the molecular mechanisms responsible for recruiting repair factors and deploying distinct pathways of DSB repair.
Highlights.
Ubiquitination is central to DNA double-stranded break (DSB) signaling
Other modifications, such as methylation, crosstalk with ubiquitination on chromatin
Effector protein recruitment depends on coordination between specific modifications
Acknowledgments
We wish to thank Barry Sleckman and Andrea Bredemeyer for critical reading and feedback on this manuscript. We also wish to thank the numerous scientists who have contributed significantly to our understanding of ubiquitination and its role in the DNA damage response, and we apologize that we could not cite all pertinent papers due to space limitations. This work was supported by the NIH/National Cancer Institute (K08 CA158133), the American Cancer Society (IRG-58-010-56), as well as the Department of Pathology and Immunology at Washington University in St. Louis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- 1.Luger K, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Gardner KE, et al. Operating on chromatin, a colorful language where context matters. Journal of molecular biology. 2011;409:36–46. doi: 10.1016/j.jmb.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sirbu BM, Cortez D. DNA damage response: three levels of DNA repair regulation. Cold Spring Harbor perspectives in biology. 2013;5:a012724. doi: 10.1101/cshperspect.a012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 5.Celeste A, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhardt HC, Yaffe MB. Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response. Nat Rev Mol Cell Biol. 2013;14:563–580. doi: 10.1038/nrm3640. [DOI] [PubMed] [Google Scholar]
- 7.Huen MS, et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mailand N, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Kolas NK, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattiroli F, et al. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell. 2012;150:1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Gatti M, et al. A novel ubiquitin mark at the N-terminal tail of histone H2As targeted by RNF168 ubiquitin ligase. Cell cycle. 2012;11:2538–2544. doi: 10.4161/cc.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doil C, et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell. 2009;136:435–446. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Stewart GS, et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Huen MS, et al. BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol. 2010;11:138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann M, de Lange T. 53BP1: pro choice in DNA repair. Trends in cell biology. 2013 doi: 10.1016/j.tcb.2013.09.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jentsch S, Psakhye I. Control of Nuclear Activities by Substrate-Selective and Protein-Group SUMOylation. Annual review of genetics. 2013;47:167–186. doi: 10.1146/annurev-genet-111212-133453. [DOI] [PubMed] [Google Scholar]
- 17.Sriramachandran AM, Dohmen RJ. SUMO-targeted ubiquitin ligases. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbamcr.2013.08.022. in press. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Blobel G. A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc Natl Acad Sci U S A. 2005;102:4777–4782. doi: 10.1073/pnas.0500537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mabb AM, et al. PIASy mediates NEMO sumoylation and NF-kappaB activation in response to genotoxic stress. Nat Cell Biol. 2006;8:986–993. doi: 10.1038/ncb1458. [DOI] [PubMed] [Google Scholar]
- 20.Galanty Y, et al. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature. 2009;462:935–939. doi: 10.1038/nature08657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JR, et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature. 2009;462:886–890. doi: 10.1038/nature08593. [DOI] [PubMed] [Google Scholar]
- 22.Danielsen JR, et al. DNA damage-inducible SUMOylation of HERC2 promotes RNF8 binding via a novel SUMO-binding Zinc finger. The Journal of cell biology. 2012;197:179–187. doi: 10.1083/jcb.201106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekker-Jensen S, et al. HERC2 coordinates ubiquitin-dependent assembly of DNA repair factors on damaged chromosomes. Nat Cell Biol. 2010;12:80–86. 81–12. doi: 10.1038/ncb2008. sup. [DOI] [PubMed] [Google Scholar]
- 24.Oestergaard VH, et al. RNF8 and RNF168 but not HERC2 are required for DNA damage-induced ubiquitylation in chicken DT40 cells. DNA Repair (Amst) 2012;11:892–905. doi: 10.1016/j.dnarep.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Guzzo CM, et al. RNF4-dependent hybrid SUMO-ubiquitin chains are signals for RAP80 and thereby mediate the recruitment of BRCA1 to sites of DNA damage. Science signaling. 2012;5:ra88. doi: 10.1126/scisignal.2003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin Y, et al. SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes & development. 2012;26:1196–1208. doi: 10.1101/gad.189274.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galanty Y, et al. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes & development. 2012;26:1179–1195. doi: 10.1101/gad.188284.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo K, et al. Sumoylation of MDC1 is important for proper DNA damage response. EMBO J. 2012;31:3008–3019. doi: 10.1038/emboj.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poulsen SL, et al. RNF111/Arkadia is a SUMO-targeted ubiquitin ligase that facilitates the DNA damage response. The Journal of cell biology. 2013;201:797–807. doi: 10.1083/jcb.201212075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annual review of biochemistry. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 31.Ikura T, et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, et al. The p400 ATPase regulates nucleosome stability and chromatin ubiquitination during DNA repair. The Journal of cell biology. 2010;191:31–43. doi: 10.1083/jcb.201001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikura T, et al. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol Cell Biol. 2007;27:7028–7040. doi: 10.1128/MCB.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaidi A, Jackson SP. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature. 2013;498:70–74. doi: 10.1038/nature12201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Sun Y, et al. Tip60: connecting chromatin to DNA damage signaling. Cell cycle. 2010;9:930–936. doi: 10.4161/cc.9.5.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shogren-Knaak M, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 37.Murr R, et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 38.Chen WT, et al. Systematic identification of functional residues in mammalian histone H2AX. Mol Cell Biol. 2013;33:111–126. doi: 10.1128/MCB.01024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Euskirchen G, et al. SWI/SNF chromatin-remodeling factors: multiscale analyses and diverse functions. J Biol Chem. 2012;287:30897–30905. doi: 10.1074/jbc.R111.309302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, et al. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol Cell Biol. 2007;27:8502–8509. doi: 10.1128/MCB.01382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, et al. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat Cell Biol. 2009;11:1376–1382. doi: 10.1038/ncb1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, et al. Chfr and RNF8 synergistically regulate ATM activation. Nat Struct Mol Biol. 2011;18:761–768. doi: 10.1038/nsmb.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JH, et al. 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 2010;29:574–585. doi: 10.1038/emboj.2009.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders SL, et al. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell. 2004;119:603–614. doi: 10.1016/j.cell.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Botuyan MV, et al. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127:1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pei H, et al. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature. 2011;470:124–128. doi: 10.1038/nature09658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartlerode AJ, et al. Impact of histone H4 lysine 20 methylation on 53BP1 responses to chromosomal double strand breaks. PLoS One. 2012;7:e49211. doi: 10.1371/journal.pone.0049211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Attikum H, Gasser SM. Crosstalk between histone modifications during the DNA damage response. Trends in cell biology. 2009;19:207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Fradet-Turcotte A, et al. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. Nature. 2013;499:50–54. doi: 10.1038/nature12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunting SF, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan Q, et al. BBAP monoubiquitylates histone H4 at lysine 91 and selectively modulates the DNA damage response. Mol Cell. 2009;36:110–120. doi: 10.1016/j.molcel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan Q, et al. BAL1 and its partner E3 ligase, BBAP, link Poly(ADP-ribose) activation, ubiquitylation, and double-strand DNA repair independent of ATM, MDC1, and RNF8. Mol Cell Biol. 2013;33:845–857. doi: 10.1128/MCB.00990-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosammaparast N, et al. The histone demethylase LSD1/KDM1A promotes the DNA damage response. The Journal of cell biology. 2013;203:457–470. doi: 10.1083/jcb.201302092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalisch T, et al. New readers and interpretations of poly(ADP-ribosyl)ation. Trends in biochemical sciences. 2012;37:381–390. doi: 10.1016/j.tibs.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou DM, et al. A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci U S A. 2010;107:18475–18480. doi: 10.1073/pnas.1012946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polo SE, et al. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J. 2010;29:3130–3139. doi: 10.1038/emboj.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smeenk G, et al. The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. The Journal of cell biology. 2010;190:741–749. doi: 10.1083/jcb.201001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen HF, et al. The NuRD architecture. Cellular and molecular life sciences: CMLS. 2013;70:3513–3524. doi: 10.1007/s00018-012-1256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luijsterburg MS, et al. A new non-catalytic role for ubiquitin ligase RNF8 in unfolding higher-order chromatin structure. EMBO J. 2012;31:2511–2527. doi: 10.1038/emboj.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li M, et al. The FHA and BRCT domains recognize ADP-ribosylation during DNA damage response. Genes & development. 2013;27:1752–1768. doi: 10.1101/gad.226357.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Yu X. Function of BRCA1 in the DNA damage response is mediated by ADP-ribosylation. Cancer cell. 2013;23:693–704. doi: 10.1016/j.ccr.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Q, et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477:179–84. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma T, et al. RNF111-dependent neddylation activates DNA damage-induced ubiquitination. Mol Cell. 2013;49:897–907. doi: 10.1016/j.molcel.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung VG, et al. Genetics. Genetic control of hotspots. Science. 2010;327:791–2. doi: 10.1126/science.1187155. [DOI] [PubMed] [Google Scholar]
- 65.Sobhian B, et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science. 2007;316:1198–1202. doi: 10.1126/science.1139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato Y, et al. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by tandem UIMs of RAP80. EMBO J. 2009;28:2461–2468. doi: 10.1038/emboj.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang B, et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science. 2007;316:1194–1198. doi: 10.1126/science.1139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sims JJ, Cohen RE. Linkage-specific avidity defines the lysine 63-linked polyubiquitin-binding preference of rap80. Mol Cell. 2009;33:775–783. doi: 10.1016/j.molcel.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panier S, et al. Tandem protein interaction modules organize the ubiquitin-dependent response to DNA double-strand breaks. Mol Cell. 2012;47:383–395. doi: 10.1016/j.molcel.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 70.Blus BJ, et al. Epigenetic virtues of chromodomains. Critical reviews in biochemistry and molecular biology. 2011;46:507–526. doi: 10.3109/10409238.2011.619164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manke IA, et al. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–9. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- 72.Stucki M, et al. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-stranded breaks. Cell. 2005;123:1213–26. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 73.Yu X, et al. The BRCT domain is a phospho-protein binding domain. Science. 2003;302:639–42. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]