Abstract
Background
Versican is an extracellular matrix (ECM) proteoglycan that is present in the pericellular environment of most tissues and increases in many different diseases. Versican interacts with cells to influence the ability of cells to proliferate, migrate, adhere and assemble an ECM.
Scope of Review
The structure of the versican molecule is briefly reviewed and studies highlighting those factors that promote versican synthesis and degradation and their impact on cell phenotype in disease are discussed. Particular attention is given to vascular disease, but other diseases where versican is important are covered as well, most notably different forms of cancers. Attention is given to mechanisms(s) by which versican influences cell behaviors through either direct or indirect processes. Versican produced by either stromal cells or myeloid cells can have a major impact influencing immunity and inflammation. Finally, studies controlling versican accumulation that either delay or inhibit the progression of disease will be highlighted.
Major Conclusions
Versican is one component of the ECM that can influence the ability of cells to proliferate, migrate, adhere, and remodel the ECM. Targeting versican as a way to control cell phenotype offers a novel approach in the treatment of disease.
Significance
ECM molecules such as versican contribute to the structural integrity of tissues and interact with cells through direct and indirect means to regulate, in part, cellular events that form the basis of disease.
Keywords: Extracellular Matrix, Immunity, Inflammation, Migration, Proteoglycans, Proliferation, Versican
1. Introduction
The extracellular matrix (ECM) is a reinforced composite of collagens and elastic fibers embedded in a viscoelastic gel of proteoglycans, hyaluronan (HA), and water, together with a wide variety and arrangement of assorted glycoproteins [1–4]. These molecules interact by entanglement and crosslinking to form a bioactive polymer which, in part, regulates the biomechanical properties of tissues and the phenotype of the cells that live in those tissues. This regulation involves molecular interactions that govern the attachment of cells to their ECM scaffolds through integrin and non-integrin receptors, detachment of cells from those scaffolds and molecular rearrangements in the ECM that allow cells to change shape during morphogenetic and remodeling events that occur in development and disease. The amount and composition of the ECM is controlled by the co-ordinated and differential regulation of synthesis and turnover of each of the ECM components. It is becoming increasingly evident that individual components of the ECM can have dramatic effects on cell behavior.
Versican is a proteoglycan that is present in the ECM around cells and in the interstitial space between cells in most tissues [5–9]. In normal tissues, levels of versican are low but in disease versican increases dramatically, as is seen in the early intimal vascular lesions typical of developing human atherosclerosis (Figure 1) (see reviews, [10, 11]). In addition, versican accumulates in several types of cancers (see reviews [12–14]) and in many other diseases as well [10]. Versican is negatively charged due to its glycosaminoglycan (GAG) chains and attracts water, contributing to the viscoelasticity of the pericellular micro-environment [5, 8]. In addition, versican interacts with a number of ECM components near the cell surface including HA, tenascin-R and -C, thrombospondin 1, fibronectin, and fibrillin [8, 15–19] to create a mechanically active biopolymer around cells that influences the ability of cells to change shape, adhere, proliferate, migrate, and assemble other ECM components and survive. Versican, and ECM molecules that bind to versican, may modify the mechanical stiffness around cells contributing to alterations in mechanotransduction influencing cell behavior and phenotype [20–22]. Versican can also act as a reservoir for cytokines and growth factors to be released at various times, establishing fine control over cell activity and behavior [10, 15, 23, 24]. All of these activities attributed to versican are diagrammatically depicted in Figure 2 as a working hypothesis and will be used in this review to discuss the importance of versican in influencing cell behavior in the pathogenesis of disease [5, 11, 25, 26]. The focus will be primarily on vascular disease, but will highlight other diseases as well to show similarities for versican’s action on the disease phenotype of cells. The fact that versican plays a central role in a number of diseases makes it a prime candidate for therapeutic intervention in the treatment of diseases [27–29].
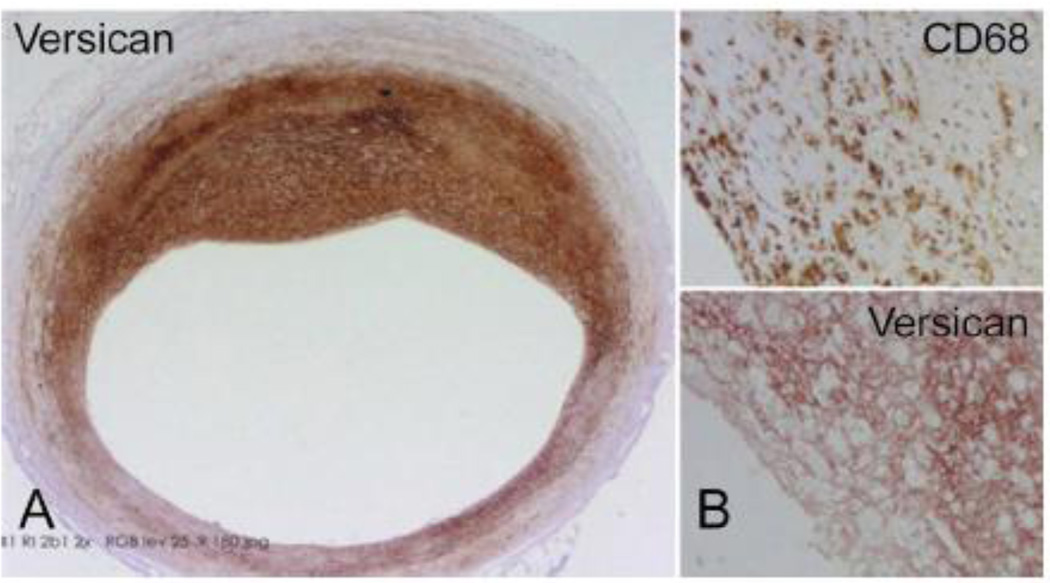
Figure 1.
A. A section through an early human coronary atherosclerotic lesion immunostained for versican illustrating marked accumulation of the ECM proteoglycan. B. Upper panel – These lesions frequently contain macrophages, as indicated by positive staining with antibody to CD68. Lower panel – Adjacent section immunostained for versican illustrating frequent colocalization of macrophage accumulation with versican. Sections kindly provided by Drs. Frank Kolodgie and Renu Virmani, CV Path Institute, Inc., Gaithersburg, MD.
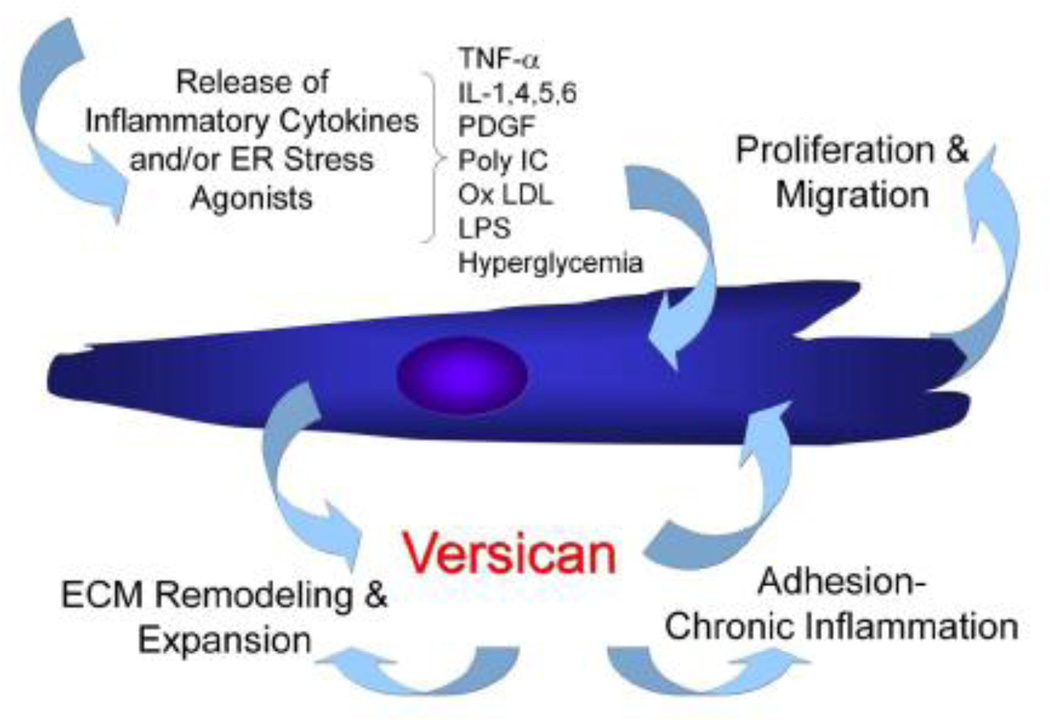
Figure 2.
A working model for the involvement of the ECM molecule versican influencing the behavior of cells during injury, remodeling, and inflammation in disease. The postulate follows that initial stimuli associated with disease initiation stimulates stromal cells or leukocytes to synthesize an ECM enriched in versican, which in turn influences these cells or other cells to proliferate, migrate, adhere, and remodel the ECM, promoting phenotypic changes associated with disease progression.
The versican molecule exists in at least 4 different variants generated by alternative splicing of the two large internal exons that code for two chondroitin sulfate (CS) attachment domains in the core protein [5, 6] (Figure. 3). These variants differ in the size of the core protein and the number of attached CS chains. While the V0 and V1 variants of versican bear CS chains and are the major forms that accumulate in most diseases, the V3 variant lacks CS chains and is not elevated in disease to any significant extent. While the V0 and V1 isoforms promote events associated with inflammation and disease, we [30–34] and others [35–38] have found that V3 acts as a dominant negative by reducing the CS-carrying isoforms of versican that accumulate in tissue, thus inhibiting the effects of V0/V1 on cell phenotype and the inflammatory response (see below). Such findings have led to the proposed use of V3 as a means to reduce versican accumulation in tissues to inhibit the inflammatory response [27, 39].
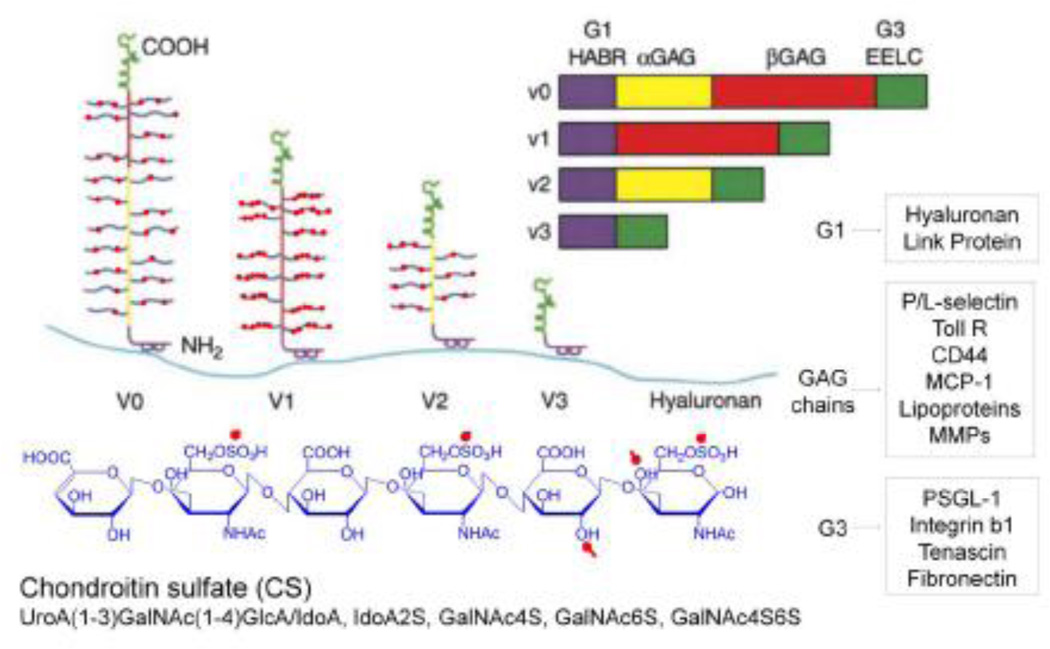
Figure 3.
A model of the different isoforms generated by alternative splicing of the mRNA transcript for versican. All isoforms interact with hyaluronan, forming different sized aggregates. Different colors denote specific domains in the gene and in the protein and carbohydrate product. Purple = hyaluronan binding region (HABR); yellow = α GAG exon and protein product; red = β GAG exon and protein product; green = two epidermal growth factor repeats (EE), a lectin binding domain (L) and a complement regulatory region (C). Bottom panel shows monosaccharides linked together to form the CS GAG with red dots denoting charged residues (blue). Versican interacts through specific domains in the molecule with a number of other molecules found on the surface of cells and within the ECM, some of these are depicted in the boxes to the right. These interactions form complexes which can also influence cell phenotypes. (Modified from Wight 2002) [Permission will be obtained.]
The CS GAG chains attached to versican may vary in size and composition, depending upon the tissue type, species of origin, cell type, and culture conditions. For example, CS chains isolated from versican synthesized by non-human primate arterial smooth muscle cells (ASMCs) have a chondroitin-6-sulfate to chondroitin-4-sulfate ratio (6S:4S) of 2 which increases to approximately 4 upon platelet-derived growth factor (PDGF) stimulation and proliferation of the ASMCs [40, 41]. The significance of such changes during the growth response are uncertain, although binding studies show that the 6S isoform binds with greater avidity to positively-charged proteins than the 4S isoform. Such stimulation also increases the length of the CS chains attached to versican, leading to an overall increase in the hydrodynamic size of the proteoglycan. Chain elongation increases the capacity of the versican molecule to bind lipoproteins, potentiating the capacity of versican to trap lipoproteins in the development of atherosclerosis [42–44]. Signaling pathways controlling transcription of the core protein in response to PDGF differ from the signaling pathways that regulate the post-translational processing of the CS chains attached to versican [40]. Other examples of post-translational modifications that can occur are in the degree of sulfation of the CS chains attached to versican. CS chains isolated from tumor tissue are frequently oversulfated [45] and such differences may be important in controlling key interactions of these molecules with ligands important in the progression of different types cancers. An excellent comprehensive review on factors that control CS synthesis is now available [46].
Attempts to approach functional aspects of versican’s control over cell behavior by gene knockout (KO) in chick embryos has been frustrating as a result of the early lethality of versican null mice [47]. However, some recent success has been achieved obtaining a partial KO of the versican gene, resulting in the expression of versican lacking the A subdomain of G1, which, in turn, significantly decreases versican accumulation and impacts cell growth and migration [48, 49].
2. Regulated Versican Synthesis and Impact on Cell Phenotype
The synthesis and degradation of versican in many tissues is highly regulated during events associated with disease [50]. There are several transcription factor binding sites in the versican promoter in addition to a classic TATA box sequence (see reviews [51–53]). In addition to positive regulatory elements, versican synthesis is under microRNA (miRNA) control as well. For example, myocardin is a transcription factor that regulates ASMC-specific gene expression and stimulates miRNA-143 expression. This miRNA binds to the 3′ untranslated region (UTR) of the versican gene and suppresses versican expression affecting ASMC migration [54]. These results indicate that part of the ASMC differentiation program involves decreased versican production. The 3′ UTR of the versican gene has other regulatory functions as well. For example, Burton Yang’s group has recently demonstrated that the versican 3′ UTR antagonizes other miRNAs [55] and most recently they have shown that this UTR can target miRNAs involved in regulating protein expression in growth control and facilitating tumor formation [56, 57].
There is considerably less known about whether the synthesis of the different isoforms of versican is differentially regulated in disease. The V1 isoform is the most abundant in most adult tissues and seems to be the isoform that is most highly regulated in events associated with repair and remodeling in injury and disease, with the exception of diseases of the nervous system (see below). The V0 isoform appears to be more prominent during embryonic development [58] and less abundant in adult tissues [59]. The V2 isoform is mostly found in the central nervous system (CNS) [60], but has been found in other tissues as well. A number of studies show mRNA expression of V3 in a variety of tissues and synthesized by a variety of cells [59], but few studies have identified native V3 protein in tissue and cells. However, V3 has been identified when overexpressed in different experimental conditions. For example, forced expression of V3 in a chondrogenic cell line disrupts the deposition and organization of V0/V1 isoforms and inhibits mesenchymal condensation and chondrogenesis [35]. Kern and colleagues [36] transduced mouse embryonic cardiomyocytes with V3 and noticed a marked reduction in proliferation of the cardiomyocytes and a significant increase in myocardial cell-cell association. Furthermore, injection of an adenovirus that contained the V3 gene into a developing mouse heart led to an increase in the outflow track myocardium and at least a two-fold increase in the compact layer of the ventricular myocardium. Notably, this study found that when only the G1 domain of versican was expressed, opposite effects were seen [36]. Given that V3 may resist degradation, these findings suggest that V3 may have dominant effects on phenotype when compared to degraded forms of versican. We have found that forced V3 expression in ASMCs and fibroblasts creates dramatic effects on cell phenotypes [30–34] (see below). Recently, a new versican isoform, V4, consisting of the G1 domain, the first 398 amino acids of the β-GAG region and the G3 domain has been found to be upregulated in human breast cancer lesions [61]. Other isoforms potentially may exist, such as a V5 isoform, consisting of essentially only the G1 domain, found by new gene discovery techniques and listed as a reference sequence for mouse versican in Entrez Gene.
Proteoglycans are also synthesized by leukocytes and may play a role in the inflammatory response [62], also see reviews [23, 39]. A number of studies have identified versican as a gene in monocytes which is upregulated in a number of pro-inflammatory states (see reviews, [10, 23, 39]. Versican synthesis is increased when monocytes differentiate into macrophages along with other proteins involved in the inflammatory response [63] (Figure 4). Versican has been identified as a gene that is differentially expressed in M1 macrophages as opposed to M2, but it is not clear if versican is critical for M1 macrophage differentiation [64, 65]. Versican expressed by macrophages binds to matrix metalloproteinases (MMPs) such as MMP9 [66] and chemokines such as CCL2 [67, 68], suggesting a role for versican in determining macrophage phenotype in inflammation. In addition, macrophages in tumors express versican and can regulate mesenchymal to epithelial transitions and metastasis through effects on tumor cell proliferation, as recently shown [69, 70].
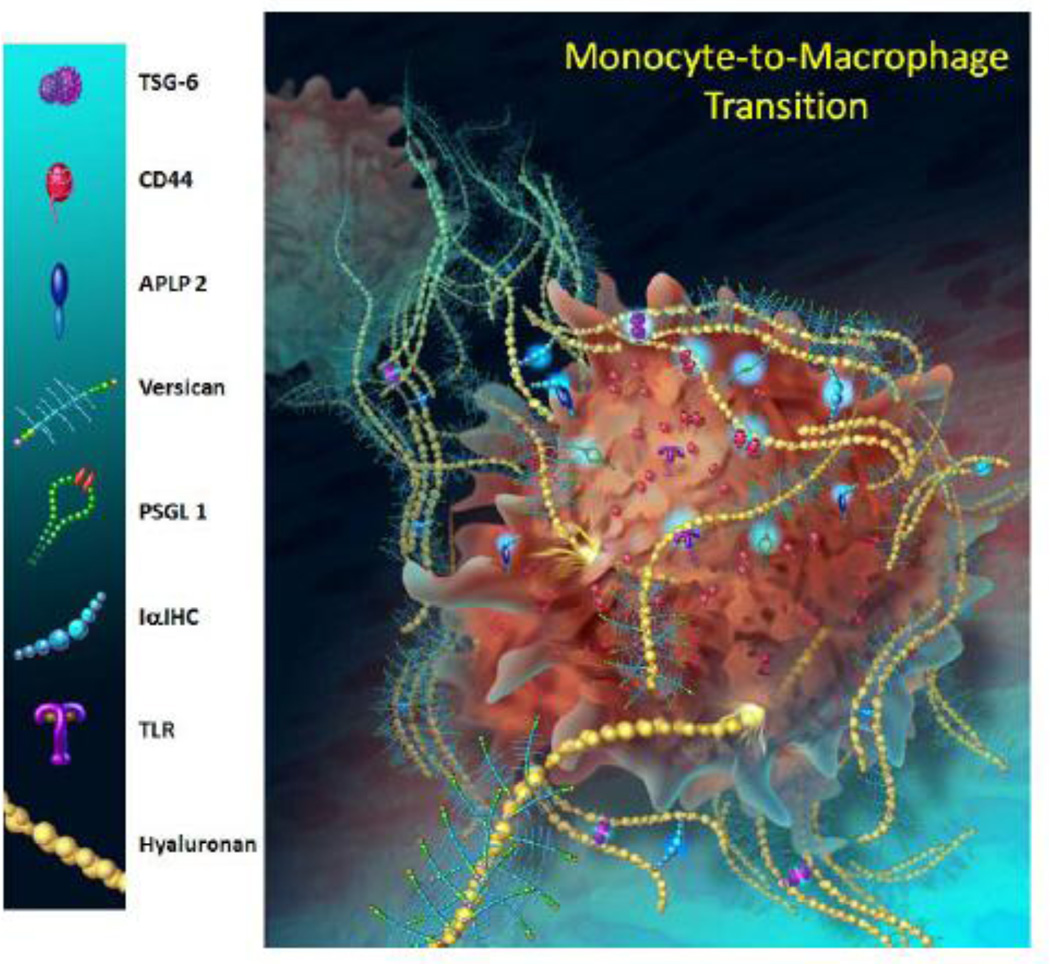
Figure 4.
A model to depict some of the ECM molecules expressed by monocytes, including versican, as they differentiate into macrophages. Illustration by Kate Sweeney, University of Washington; based on data presented in Chang et al, 2012.
3. Regulated Versican Degradation and Impact on Cell Phenotype
The degradation of versican is associated with several tissue remodeling and inflammatory events in disease. Once bound to the versican-containing ECM, leukocytes may degrade the ECM to generate pro-inflammatory fragments that further drive the inflammatory response [71–75]. For example, the G3 domain of versican interacts with P-selectin glycoprotein-1 (PSGL-1) on the surface of macrophages to promote macrophage adhesion and aggregation [76].
Versican is degraded by a number of different proteases, including several MMPs, plasmin, and at least 5 members of the ADAMTS (A Disintegrin And Metalloproteinase with Thrombospondin Motifs) family of proteases (see reviews [77, 78]). The versican cleavage site for these ADAMTS enzymes is in the G1 domain of versican at the Glu441-Ala422 bond which generates a 70 kDa fragment that can be recognized by an antibody against the neoepitope sequence DPEAAE [79]. Altering the capacity of ADAMTS proteases to degrade versican leads to increases in versican in the pericellular matrix of fibroblasts and an increase in the myofibroblastic phenotype [80]. Such studies highlight a potentially significant role of versican in fibrosis. In addition, areas of increased ADAMTS-1 and -4 in early developing lesions of the vascular wall correlate with greater versican degradation and production of the amino terminal DPEAAE-containing versican fragment [79]. Alteration of blood flow in a baboon model of vascular graft repair promotes regression of vascular lesions by increasing versican degradation through ADAMTS activity [81]. The increase in cleaved versican correlates with regression of neointimal thickenings and loss of versican [81]. Interestingly, these changes in versican integrity also correlate with cell death in the regressed lesions [82]. It remains to be shown however, whether versican fragments promotes ASMC death in this model. These findings are of interest because of studies demonstrating that cleaved versican regulates apoptosis during mammalian inter-digital web regression [83]. Of further interest is the finding that proteolytic cleavage products of versican are present in human plaques from endarterectomy segments, consistent with their generation in a pro-inflammatory microenvironment [84]. Notably, an MMP-12-derived versican fragment has been identified in plasma samples from patients with atherosclerotic heart disease, confirming versican degradation as part of the atherosclerotic response [85]. ADAMTS-1 mRNA transcript is also abundant in human aorta and increases as ASMCs migrate and proliferate in vitro [86]. A polymorphism in the ADAMTS-1 gene has been associated with an increase in cardiovascular disease in two separate studies [87, 88]. Furthermore, high levels of ADAMTS-1 in brain tissues are associated with neurodegenerative diseases such as Down syndrome, Alzheimer’s, and Pick’s disease [89]. However, a direct causal link for versican in these diseases awaits further experimentation. On the other hand, single nucleotide polymorphisms (SNPs) and haplotype analyses of the versican gene in intracranial aneurysms revealed a strong association [90, 91].
4. Versican and Cell Proliferation
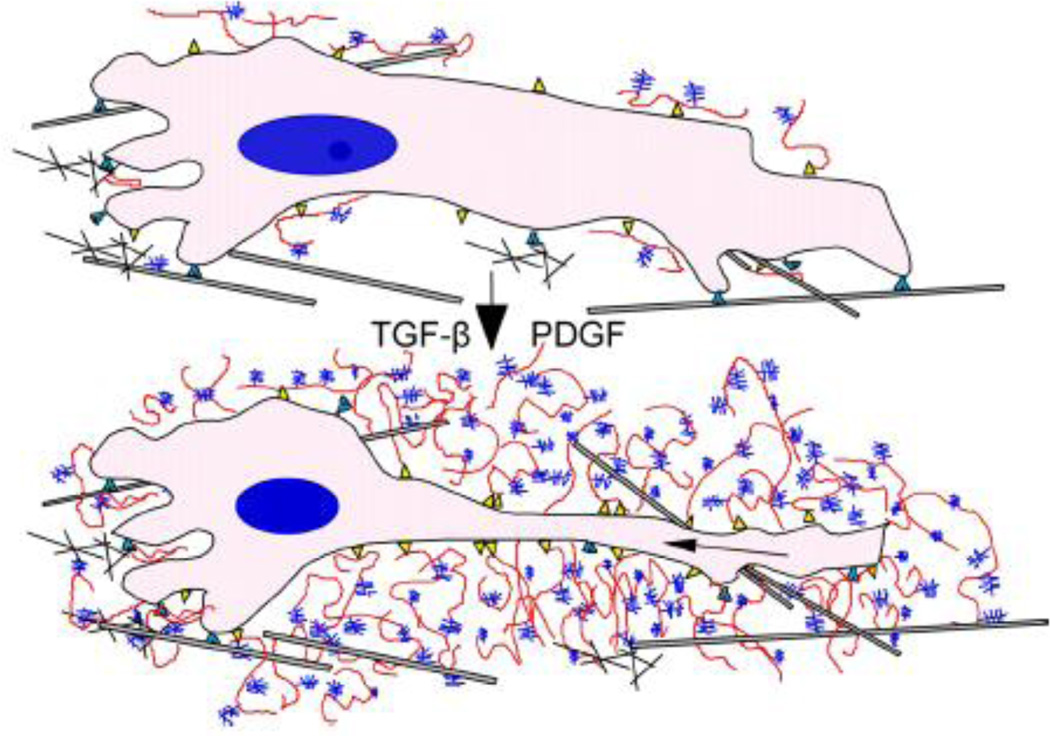
Accumulation of versican occurs in tissues undergoing cellular proliferation. For example, mitogens such as PDGF upregulate versican expression in ASMCs as they are stimulated to divide [7, 41, 92, 93]. Together with HA, versican contributes to the expansion of the pericellular ECM that is required for the proliferation of these cells to occur [7, 8, 92]. These complexes increase the viscoelastic nature of the pericellular matrix, creating a highly malleable extracellular environment influencing mechanotransduction and supporting a cell-shape change necessary for cell proliferation and migration to take place [8] (Figure 5). Inhibiting the formation of this pericellular coat blocks the proliferation of ASMCs in response to PDGF [7, 8]. Although PDGF stimulates the proliferation of ASMCs, TGF-β1 which also stimulates versican synthesis [41] inhibits ASMC proliferation in vitro, suggesting that versican synthesis is not directly causatively linked to the proliferative phenotype. However, interference with versican synthesis in ASMCs, fibroblasts, and in some cancer cells inhibits their proliferation, suggesting that versican synthesis and accumulation is necessary, but not sufficient to cause changes in mitotic cell activity [12, 14, 31, 34]. Thus, the versican–HA complex that surrounds cells serves as an important, but infrequently considered, mechanism for controlling cell shape and cell division.
Figure 5.
ECM transitions required for cell proliferation and migration. In order for cells to change shape during division and migration, they must modify their pericellular environment by first degrading the existing ECM and replacing it with components that allow the cell to change shape. Two ECM molecules that are produced during these events and allow this to happen are hyaluronan (red) and versican (blue). These changes lead to expansion of the pericellular matrix and changes in the mechanical properties of the ECM that influence cell phenotype. [Permission will be obtained; Wight et al, 2011, Am J Physiol Gastrointest Liver Physiol.]
Another mechanism by which versican could influence proliferation is by acting as a mitogen itself, by binding to growth factor receptors via epidermal growth factor (EGF) sequences in the G3 domain of the molecule [15]. For example, expression of G3 mini-genes in NIH/3T3 cells enhances cell proliferation, and the effect can be blocked by deletion of the EGF domains in the G3 construct [94]. This same construct exerts a dominant-negative effect on cell proliferation through inhibiting the binding of G3 to the cell surface, via the lectin domain in G3 [15, 95]. The concentration of versican associated with the cell surface appears to be a critical factor, and loss of versican from the cell surface is associated with decreased cell proliferation. Maximal growth-promoting activity is achieved in NIH/3T3 cells and chondrocytes with both G1 and G3 mini-gene constructs, supporting the concept that versican regulates proliferation by binding directly to a growth factor receptor and by interfering with cell adhesion [94, 96]. Work in NIH/3T3 cells in vitro suggests that V1 and V2 isoforms may have opposing activities. For example, the V1 isoform enhances the proliferation of NIH/3T3 cells and protects these cells from apoptosis, while V2 decreases their proliferation and has no activity on apoptosis [97]. V2 has recently been shown to enhance angiogenesis by endothelial cells by slowing cell proliferation and enhancing fibronectin synthesis [98]. Additional recent studies indicate that the V3 isoform may regulate cell proliferation as well. The capacity of V3 to promote a specific phenotype opposite to what is seen with V0/V1 is manifested when ASMCs are transduced with V3 [32]. Forced expression of V3 causes decreases in cell growth [32]. Interestingly, suppression of V0/V1 synthesis had the same effect as V3 expression on ASMC and skin fibroblast proliferation [30, 31].
Thus, versican expression is associated with a proliferative cell phenotype and is often found in tissues exhibiting elevated proliferation, such as in tumors (see reviews, [12, 14]). Using a mouse model of spontaneous breast cancer, Gao and colleagues demonstrated that bone marrow-derived myeloid progenitor cells in the pre-metastatic mouse lung secrete versican, which promotes a mesenchymal-to-epithelial transition of tumor cells and subsequent metastasis [69, 70]. Versican derived from myeloid cells in this model appears to promote tumor growth by enhancing tumor cell proliferation, possibly by blocking the TGFβ-smad2/3 pathway. Earlier studies demonstrated that versican accumulation in Lewis lung carcinoma can interact with macrophage TLR2 to induce secretion of inflammatory cytokines, such as tumor necrosis factor- α (TNFα) and IL-1–6 [99, 100] promoting tumor expansion and metastasis. It is of interest that highly sulfated CS GAG chains on versican may be critical to promote this activity [45, 101]. Li and colleagues recently demonstrated that in co-culture experiments of macrophages and ovarian cancer cells, versican produced by the tumor cells promoted the production of an antimicrobial protein through interacting with toll-like receptor 2 (TLR2) on macrophages that, in turn, promoted ovarian tumor cell proliferation[102]. Evidence is accumulating that versican, either directly or indirectly, is functioning as a matrikine or agonist in promoting tumor cell proliferation. Whether similar activity for versican exists during inflammation in non-cancerous tissue awaits further investigations. It may be that ligation of immune receptors, such as TLR2, by versican is responsible for the activation of multiple cell types and the induction of inflammatory cytokine secretion in many disease situations [100, 103]. Along with its effects on the proliferation of cancer cells, versican enhances cell survival and apoptotic resistance of these cells [104, 105], and protects them through inhibiting cytotoxic drug action and Fas-dependent programmed cell death [105].
There is also evidence that specific isoforms of versican may affect the proliferative activity of tumor cells. For example, expression of V0/V1 isoforms of versican is increased in malignant melanoma, which contributes to the increased proliferation rate and decreased adhesion of the tumor cells [106, 107]. Overexpression of V3 by melanoma tumor cells reverses this phenotype by decreasing proliferation and increasing adhesion [108]. The decrease in proliferation was accompanied by a decrease in the activation of ERK1/2 in response to EGF [38]. Recent studies by this group now show that V3 interferes with the CD44-EGFR/ErbB2 pathway in the regulation of cell proliferation and migration [109, 110].
5. Versican and Cell Migration
Versican also affects the migration of a number of cells [111]. For example, PDGF not only promotes versican expression and ASMC proliferation, but can also affect ASMC migration primarily through inhibition of miRNA-143 and regulation of versican synthesis [54]. Versican is expressed also along neural crest pathways and influences neural cell migration [58, 112]. A number of studies suggest that versican blocks neural crest migration because cells do not enter tissues that express versican [113–115]. Pax3 is a transcription factor associated with defective neural cell migration. Splotch mice are characterized by mutations in the Pax3 gene and exhibit neural crest-related abnormalities, including the failure of neural crest cells to colonize target tissues. However, neural crest cells derived from these mutant mice migrate as controls in vitro, so it has been suggested that the defect may not reside in the neural crest cells themselves, but rather in the ECM environment through which they migrate. Indeed, earlier studies [113] demonstrated that versican was markedly overexpressed in Splotch mutants in neural crest cell migration pathways, suggesting that versican may be responsible for defective cell migration in this species. Other studies show that overexpression of Pax3 in a medulloblastoma cell line causes upregulation of the V2 splice variant of versican and a downregulation of the V3 variant [116]. Such differential regulation of the versican isoforms may explain, in part, the migratory defect in the Splotch mouse. It is of interest that the V3 isoform lacks CS chains, which should reduce the exclusionary properties of the ECM. Clearly, expression of V3 in ASMCs decreases their migratory activity [32]. Versican can also influence the migration of lymphoid cells. Recent experiments indicate that T cells do not migrate when they are added to an ECM enriched in versican and HA, but this inhibition of migration can be reversed if this ECM is first pretreated with blocking antibodies to versican [117].
Versican influences the migration of a variety of other cell types, and this activity appears to be mostly associated with the anti-adhesive activities involving the G1 domain of the molecule. In the nervous system and in axonal growth, the V2 splice variant inhibits axonal outgrowth and migration [60, 118–122]. This inhibiting activity of versican can be reduced, but not eliminated, by removing CS chains, indicating that multiple domains of versican are involved in controlling axon regeneration. Although the V2 isoform is widely present in the CNS [123], it is predominately localized to the myelinated fiber tracts. Oligodendrocytes are the likely source of V2 [124, 125]. The finding that both the GAGs and core protein domains of the molecule are involved in the inhibitory activity suggests a direct interaction with the cells, or modification of the surrounding matrix to form exclusionary boundaries. The fact that versican plays a fundamental role in axonal migration is highlighted by studies that show upregulation of versican along with other hyalectins) following CNS injury [124]. These changes have been associated with the failure of nerves to regenerate. The importance of the hyalectins in preventing nerve regeneration is further highlighted by studies that show that degradation of CS chains by chondroitinase ABC lyase treatment following spinal cord injury in experimental animals promotes regeneration of both ascending and descending corticospinal-tract axons [126]. Such results suggest that manipulating versican synthesis in spinal cord injury may be a useful intervention for therapeutic treatment of this condition. No doubt that versican does not act alone in creating these exclusionary boundaries and it will be important to identify other key players with which versican interacts. Failure of axons to regenerate is also characteristic of multiple sclerosis, and versican appears to increase in plaques present in the white matter of the brain from patients with multiple sclerosis [127].
Versican also appears to be involved in the motility of cancer cells. A number of studies suggest that tumor stromal-derived versican can influence tumor cell motility and invasiveness to promote metastasis in several different cancers [12, 107, 128–133].
6. Versican and Cell Adhesion
Early studies showed that versican is anti-adhesive [9, 134–136], and this activity appears to reside in the G1 domain of versican [96, 134]. However, the carboxyl-terminal domain of versican interacts with the β1 integrin of glioma cells, activating focal adhesion kinase (FAK), promoting cell adhesion and preventing apoptosis in this cell type [96, 134, 137]. The pro-adhesive property of the G3 domain of versican raises the possibility that different breakdown products of versican might affect cell adhesion in different ways. Interestingly, overexpression of V3 which lacks the CS chains, but contains the G1 and G3 domains, leads to extreme cell spreading and increased adhesion in ASMCs [32].
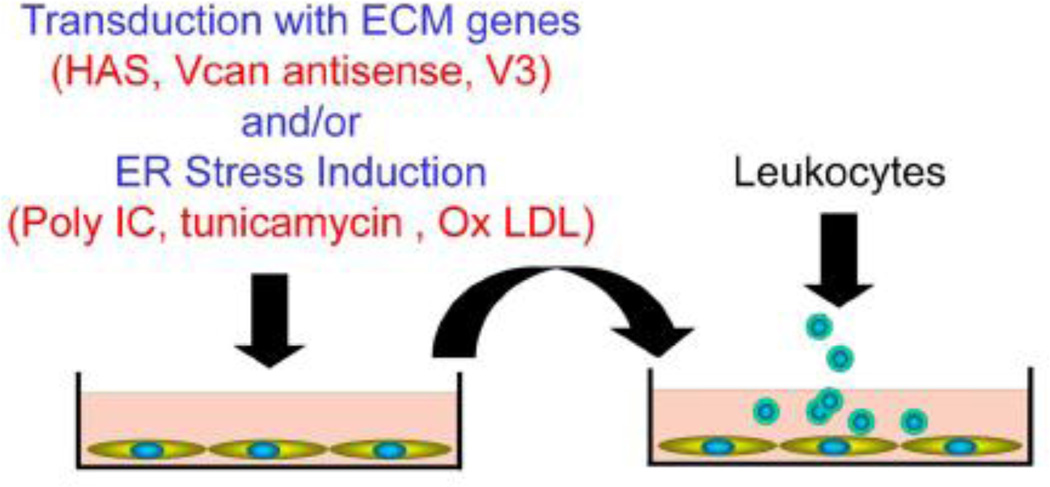
We and others have recently shown that versican can influence the adhesion of myeloid and lymphoid cells as part of the inflammatory response see reviews [23, 39]. As myeloid and lymphoid cells enter tissues, they come into contact with specific components of the ECM, such as HA and versican, together with a number of other proteins including TSG-6 and inter-alphatrypsin inhibitor (IαI) which promotes their adhesion [39, 138–142]. These components interact to form filamentous cable-like structures that serve as scaffolds for the infiltrating cells. Using in vitro models and stimulating stromal cells such as fibroblasts and ASMCs with agonists that promote endoplasmic reticulum (ER) stress, such as poly I:C or tunicamycin [139, 141, 143, 144] (Figure 6), we and others have shown that versican is a critical player in leukocyte adhesion to the ECM (Figure 6) [39, 142, 145]. In addition to monocyte adhesion, humanactivated T lymphocytes adhere to this ECM as well (Figure 7) [117]. Not only do T cells adhere, but versican also significantly reduces their ability to migrate and produce IL-10 when in contact with the versican-enriched ECM. Such studies indicate that specific components of the ECM such as versican can have dramatic effects on the phenotype of immune cells. Thus, versican should be considered a potential therapeutic target in the control of the immune response associated with inflammation in disease [27–29]. The fact that the production of this leukocyte adherent ECM is formed by cells stimulated by ER stress agonists is highly significant and more attention needs to be given to the key pathways involved in the generation of this ECM.
Figure 6.
An in vitro protocol to examine the generation of ECMs that bind leukocytes. Stromal cells such as ASMCs, lung fibroblasts, or synoviocytes are transduced with matrix genes or exposed to agents that promote ER stress, and the ECM generated and assessed for ability to support leukocyte adhesion. HAS = hyaluronan synthase; Vcan = versican; V3 = versican isoform V3; Ox LDL = oxidized low density lipoprotein.
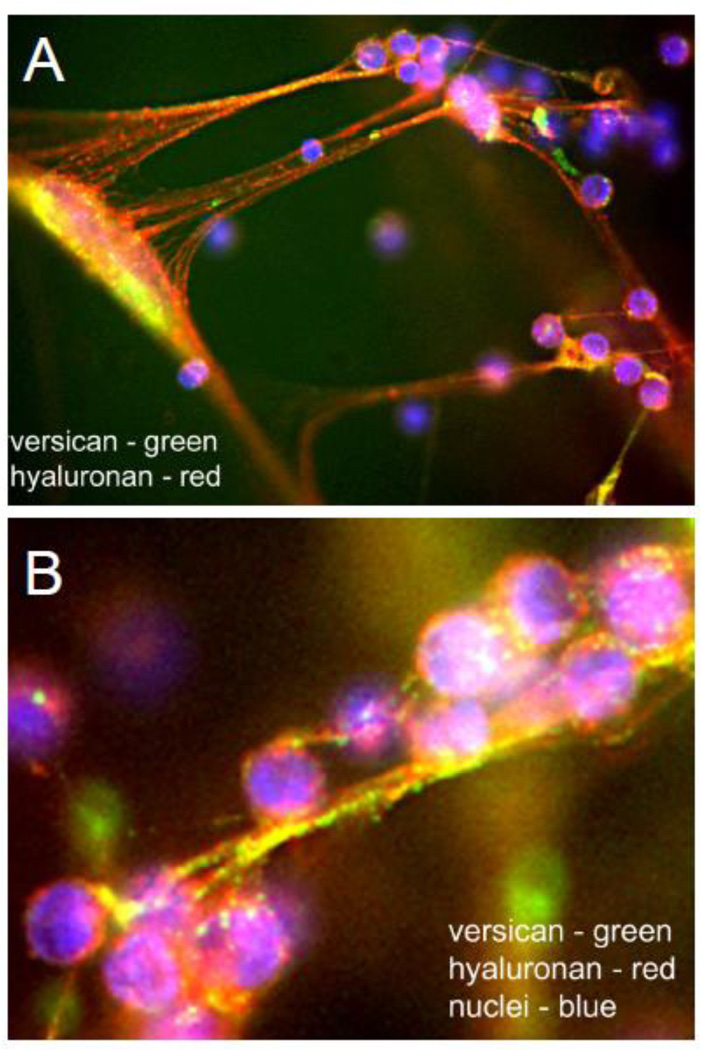
Figure 7.
A. Lung fibroblasts stimulated with poly I:C produce a copious amount of ECM enriched in HA (green) and versican (red) in the form of long cables that bind added CD4+ activated T cells (blue). B. Higher magnification showing the co-localization of versican and hyaluronan cables and the HA-rich surface of the T cells. [Permission will be obtained. Evanko, Matrix Biol, 2012.]
7. Versican and ECM Assembly
ECM remodeling takes place throughout different phases of disease progression as part of an injury and/or inflammatory response. These phases involve breakdown and disassembly of various ECM components and reassembly of particular components as part of the pathogenesis of these diseases. The sequence of changes is not unlike what is seen during wound repair in which the early ECM changes are characterized by ECM deposits which create a loose, open, and watery matrix (referred to as a “provisional ECM”) [146–148] which allows for cellular invasion and repair. This provisional matrix is then replaced by a more fibrous ECM enriched in collagens and assorted glycoproteins. Versican interacts with several different ECM molecules and, in part, plays a central role in ECM assembly. The domain structure of versican lends itself to multiple types of interactions through either protein–protein or protein–carbohydrate interactions. Perhaps the best known of these interactions involves a specific interaction between the amino-terminal domain of versican (G1) and HA [149]. This interaction is stabilized by another protein — link protein — which exhibits selective binding specificity for both HA and versican [150].
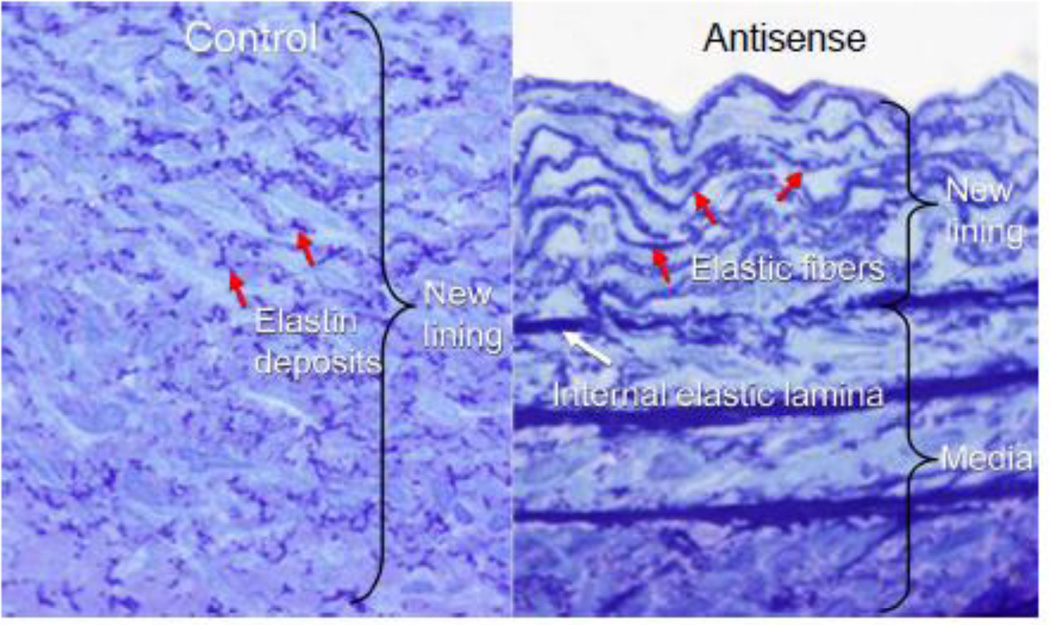
In addition to HA, versican interacts with other ECM molecules and controls their organization. Versican interacts with tenascin-R through the lectin-binding domain of versican and involves protein–carbohydrate interactions [17, 151, 152]. The lectin-binding domain participates in other ligand interactions as well. For example, versican interacts with fibulin-1 and fibulin-2 [153, 154], a growing family of ECM proteins that are expressed in particularly high levels in the developing heart valve. In adults, however, fibulin-1 and -2 are found associated with microfibrils that are part of elastic fibers. Versican also can interact with proteins associated with elastin in elastic fibers. For example, versican interacts with the elastic fiberassociated protein fibrillin [19, 153–155],and versican has been shown to co-localize with elastic fibers in skin [19]. Furthermore, fibrillins bind fibulin 2, and fibulin is preferentially localized to the elastin/microfibril interface in some tissues, but not in others [156]. It may be that fibulin serves as a bridge between versican and fibrillin, forming high-ordered multi-molecular structures important in the assembly of elastic fibers. The relationship of versican to elastic fiber assembly is interesting and unusual. What is conspicuously absent in newly remodeled ECM is elastic fibers. In fact, elastic fibers are conspicuously absent from atherosclerotic and restenotic lesions. The importance of elastic fibers in regulating vascular disease is highlighted by studies of the elastin KO mouse. Disruption of elastin synthesis by targeting the promoter and first exon of the tropoelastin gene [157] leads to subendothelial proliferation of ASMCs and obstructive vascular closure. In fact, cells from the elastin KO animals have been shown to proliferate more rapidly than their normal littermate cells in vitro, but normal proliferation is restored upon addition of elastin to the KO cells [158, 159]. Elastin peptides have been shown also to regulate ASMC proliferation and migration [160] and elastin has been used to dampen the restenotic response in experimental animals [159]. Thus, factors regulating elastic fiber formation may be critical to controlling vascular lesion formation. One factor that appears to inhibit elastic fiber assembly is CS, which is part of the versican molecule [161]. We have found that blocking versican expression by antisense (Figure 8) or using forced expression of the versican variant that lacks CS, V3, in ASMCs leads to changes in tropoelastin expression and accumulation of elastic fibers in long-term ASMC cultures [34]. When these V3-transduced ASMCs are seeded into balloon injured rat carotid arteries, a compact and highly structured neointima enriched in elastic lamellae develops [34]. Furthermore, in a recent study, Merrilees and colleagues demonstrated that injecting rabbit V3-transduced ASMCs into injured rabbit carotid arteries in animals placed on a lipid rich diet prevented lipid build up and monocyte ingress over an 8-week period [33] (Figure 9). Previous studies had shown that monocytes do not adhere well to elastin, but adhere avidly to collagen [162], suggesting that elastin is a poor adhesive substrate for monocytes. Thus, V3 may promote the formation of an ECM that resists monocyte adhesion and accumulation and may be an effective anti-inflammatory treatment in the prevention of cardiovascular disease. The impact of V3 on elastogenesis is further highlighted by studies that show that V3 transduction of skin fibroblasts taken from patients exhibiting defective elastogenesis, such as in Costello Syndrome and Hurler’s Disease, reverses their phenotype and corrects impaired elastogenesis that characterizes these cells [30].
Figure 8.
Light micrographs of a 28-day neointima from a balloon-injured rabbit carotid artery seeded with ASMCs containing the LXSN empty retroviral vector (left panel) or seeded with ASMCs transduced with a retroviral vector containing antisense to versican (right panel). The neointima seeded with the antisense-expressing cells contains multiple lamellae of elastic fibers. [From Huang, R. et al, 2006. Permission will be obtained.]
Figure 9.
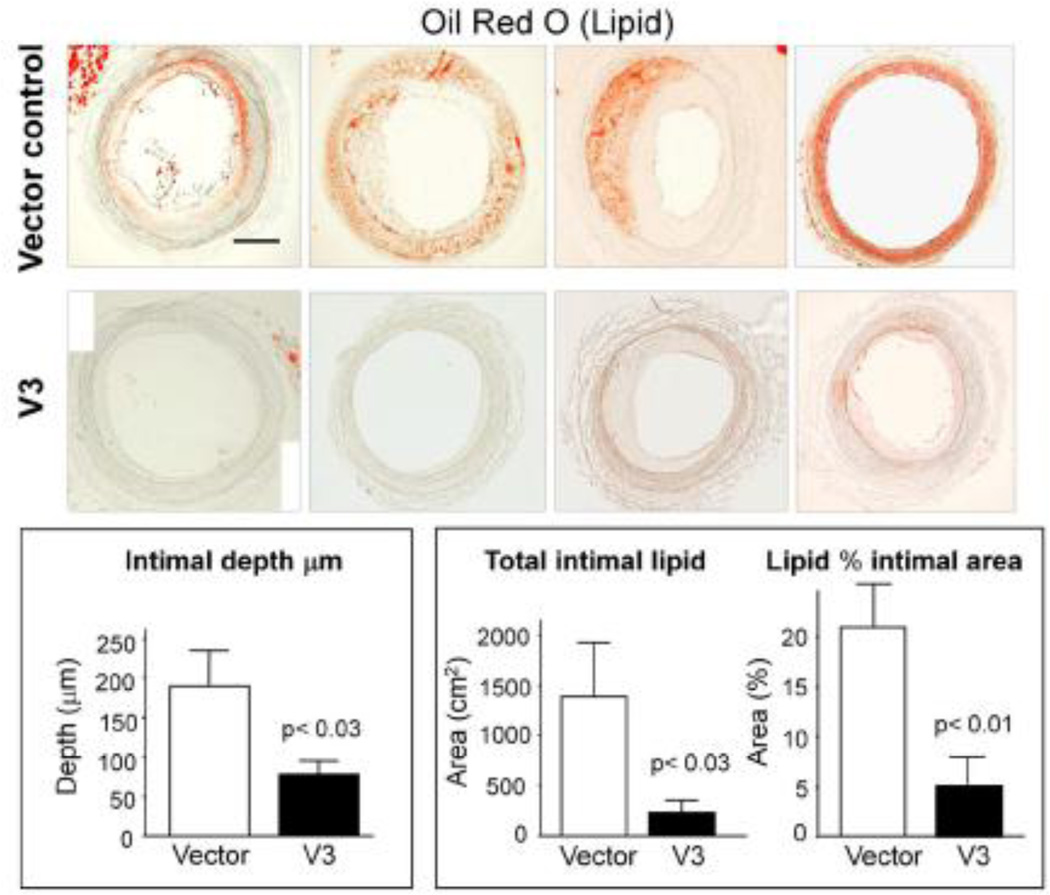
Lipid accumulation in balloon-injured rabbit carotid arteries seeded with ASMCs transduced with an empty retroviral vector (top row) or with ASMCs transduced with a retroviral vector containing the V3 gene (middle row) in animals fed a high fat diet for 8 weeks. Lipid is excluded from the arteries expressing the V3 gene. Bottom row is the quantitation of lipid using the oil red O stain and morphometric analyses. [From Merrilees et al, 2001.]
8. Conclusion
Versican is an ECM macromolecule that is critical to maintaining tissue integrity and homeostasis in the living organism. As reviewed in studies cited above, not only is versican a structural component of the ECM, but also a molecule that interacts with cells to control their behavior in part by influencing cell shape and thus their phenotype in the pathogenesis of a number of diseases.
Versican can impact cell phenotype from the outside of the cell in different ways and it will be important in the future to decipher the molecular mechanisms(s) either direct and/or indirect involved in versican determining cell fate. What is emerging is that not only does versican possess unique biological activity as intact molecules or as part of complexes, but also it is likely that versican fragments generated as part of disease pathogenesis will affect cell phenotype in very unique ways. The fact that versican plays a central role in controlling cell behavior in disease suggests that it could become a potential selective target promising wide therapeutic benefits.
Highlights.
Versican is an ECM component that increases in disease.
Versican interacts with stromal cells and leukocytes to control their phenotype.
Versican degradation is associated with tissue atrophy and apoptosis.
Agonists that control cell phenotype regulate versican synthesis and accumulation.
Targeting versican in disease is a promising approach for wide therapeutic benefits.
Acknowledgements
The authors wish to thank Dr. Virginia M. Green for editing, careful reading, and preparation of the manuscript. The authors are also indebted to all past and present trainees, collaborators, and technicians, who have worked tirelessly to define the importance of this ECM macromolecule in the pathogenesis of disease. The authors also apologize to those authors whose original contributions were not cited in lieu of reviews to fulfill space requirements.
This study was supported by grants from the National Institutes of Health P01 HL030086, P01 HL18645, and U01 AI101984 (T.N.W); the American Heart Association Postdoctoral Fellowship 09 POST 20500065 (I.K.); and from the Health Research Council of New Zealand, The Auckland Medical Research Foundation, and the National Heart Foundation of New Zealand (M.J.M.).
Abbreviations
- ECM
extracellular matrix
- HA
hyaluronan
- GAG
glycosaminoglycan
- CS
chondroitin sulfate
- ASMCs
arterial smooth muscle cells
- PDGF
platelet-derived growth factor
- KO
knockout
- miRNA
microRNA
- UTR
untranslated region
- CNS
central nervous system
- MMPs
matrix metalloproteinases
- PSGL-1
P-selectin glycoprotein-1
- ADAMTS
A Disintegrin And Metalloproteinase with Thrombospondin Motifs
- SNPs
single nucleotide polymorphisms
- EGF
epidermal growth factor
- TLR2
toll-like receptor 2
- FAK
focal adhesion kinase
- IαI
inter-alpha-trypsin inhibitor
- ER
endoplasmic reticulum.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO, Yamada KM, editors. Extracellular Matrix Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2012. [Google Scholar]
- 2.Karamanos N, editor. Extracellular Matrix: Pathobiology and Signaling. Berlin/Boston: Walter de Gruyter; 2012. [Google Scholar]
- 3.Mecham RP, editor. The Extracellular Matrix: an Overview. Berlin: Springer-Verlag; 2011. [Google Scholar]
- 4.Hay ED. Cell biology of extracellular matrix. New York: Plenum Press; 1991. [Google Scholar]
- 5.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann D. Versican. In: Iozzo R, editor. Proteoglycans: Structure, Biology and Molecular Interactions. New York: Marcel Dekker, Inc; 2000. pp. 327–341. [Google Scholar]
- 7.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 8.Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv. Drug Deliv. Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagata M, Saga S, Kato M, Bernfield M, Kimata K. Selective distributions of proteoglycans and their ligands in pericellular matrix of cultured fibroblasts. Implications for their roles in cell-substratum adhesion. J. Cell Sci. 1993;106:55–65. doi: 10.1242/jcs.106.1.55. [DOI] [PubMed] [Google Scholar]
- 10.Wight TN. The pathobiology of versican. In: Karamanos N, editor. Extracellular Matrix: Pathobiology and Signaling. KG, Berlin: Walter De Gruyter GMBH & Co.; 2012. pp. 154–170. [Google Scholar]
- 11.Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ. Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 12.Du WW, Yang W, Yee AJ. Roles of versican in cancer biology--tumorigenesis, progression and metastasis. Histol. Histopathol. 2013;28:701–713. doi: 10.14670/HH-28.701. [DOI] [PubMed] [Google Scholar]
- 13.Ricciardelli C, Brooks JH, Suwiwat S, Sakko AJ, Mayne K, Raymond WA, Seshadri R, LeBaron RG, Horsfall DJ. Regulation of stromal versican expression by breast cancer cells and importance to relapse-free survival in patients with node-negative primary breast cancer. Clin. Cancer Res. 2002;8:1054–1060. [PubMed] [Google Scholar]
- 14.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 15.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsova SA, Issa P, Perruccio EM, Zeng B, Sipes JM, Ward Y, Seyfried NT, Fielder HL, Day AJ, Wight TN, Roberts DD. Versican-thrombospondin-1 binding in vitro and colocalization in microfibrils induced by inflammation on vascular smooth muscle cells. J. Cell Sci. 2006;119:4499–4509. doi: 10.1242/jcs.03171. [DOI] [PubMed] [Google Scholar]
- 17.Lundell A, Olin AI, Morgelin M, al-Karadaghi S, Aspberg A, Logan DT. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure (Camb.) 2004;12:1495–1506. doi: 10.1016/j.str.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Yamagata M, Yamada KM, Yoneda M, Suzuki S, Kimata K. Chondroitin sulfate proteoglycan (PG-M-like proteoglycan) is involved in the binding of hyaluronic acid to cellular fibronectin. J. Biol. Chem. 1986;261:13526–13535. [PubMed] [Google Scholar]
- 19.Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J. Biol. Chem. 2002;277:4565–4572. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- 20.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werfel J, Krause S, Bischof AG, Mannix RJ, Tobin H, Bar-Yam Y, Bellin RM, Ingber DE. How changes in extracellular matrix mechanics and gene expression variability might combine to drive cancer progression. PLoS ONE. 2013;8:e76122. doi: 10.1371/journal.pone.0076122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat. Rec. (Hoboken) 2010;293:968–981. doi: 10.1002/ar.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv. Drug Deliv. Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Theocharis AD. Versican in health and disease. Connect. Tissue Res. 2008;49:230–234. doi: 10.1080/03008200802147571. [DOI] [PubMed] [Google Scholar]
- 26.Wight TN. The biomatrix of the vascular system and the control of cell phenotype. In: Balazs EA, editor. Structure and Function of Biomatrix: Control of Cell Behavior and Gene Expression. Vol. 5. Edgewater, NJ: Matrix Biology Institute; 2012. pp. 315–340. [Google Scholar]
- 27.Merrilees MJ, Wight TN. Targeting the matrix: potential benefits for versican therapeutics. Elsevier: Current Comments; 2012. [Google Scholar]
- 28.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol. Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 30.Hinek A, Braun KR, Liu K, Wang Y, Wight TN. Retrovirally mediated overexpression of versican v3 reverses impaired elastogenesis and heightened proliferation exhibited by fibroblasts from Costello syndrome and Hurler disease patients. Am. J. Pathol. 2004;164:119–131. doi: 10.1016/S0002-9440(10)63103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ. Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- 32.Lemire JM, Merrilees MJ, Braun KR, Wight TN. Overexpression of the V3 variant of versican alters arterial smooth muscle cell adhesion, migration, and proliferation in vitro. J. Cell Physiol. 2002;190:38–45. doi: 10.1002/jcp.10043. [DOI] [PubMed] [Google Scholar]
- 33.Merrilees MJ, Beaumont BW, Braun KR, Thomas AC, Kang I, Hinek A, Passi A, Wight TN. Neointima formed by arterial smooth muscle cells expressing versican variant v3 is resistant to lipid and macrophage accumulation. Arterioscler. Thromb. Vasc. Biol. 2011;31:1309–1316. doi: 10.1161/ATVBAHA.111.225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merrilees MJ, Lemire JM, Fischer JW, Kinsella MG, Braun KR, Clowes AW, Wight TN. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ. Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. [DOI] [PubMed] [Google Scholar]
- 35.Kamiya N, Watanabe H, Habuchi H, Takagi H, Shinomura T, Shimizu K, Kimata K. Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J. Biol. Chem. 2006;281:2390–2400. doi: 10.1074/jbc.M509341200. [DOI] [PubMed] [Google Scholar]
- 36.Kern CB, Norris RA, Thompson RP, Argraves WS, Fairey SE, Reyes L, Hoffman S, Markwald RR, Mjaatvedt CH. Versican proteolysis mediates myocardial regression during outflow tract development. Dev. Dyn. 2007;236:671–683. doi: 10.1002/dvdy.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010;29:304–316. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miquel-Serra L, Serra M, Hernández D, Domenzain C, Docampo MJ, Rabanal R, de Torres I, Wight TN, Fabra A, Bassols A. V3 versican isoform expression has a dual role in human melanoma tumor growth and metastasis. Lab. Invest. 2006;86:889–901. doi: 10.1038/labinvest.3700449. [DOI] [PubMed] [Google Scholar]
- 39.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014 doi: 10.1016/j.matbio.2014.01.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardoso LE, Little PJ, Ballinger ML, Chan CK, Braun KR, Potter-Perigo S, Bornfeldt KE, Kinsella MG, Wight TN. Platelet-derived growth factor differentially regulates the expression and post-translational modification of versican by arterial smooth muscle cells through distinct protein kinase C and extracellular signal-regulated kinase pathways. J. Biol. Chem. 2010;285:6987–6995. doi: 10.1074/jbc.M109.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schönherr E, Järveläinen HT, Sandell LJ, Wight TN. Effects of platelet-derived growth factor and transforming growth factor-β 1 on the synthesis of a large versican-like chondroitin sulfate proteoglycan by arterial smooth muscle cells. J. Biol. Chem. 1991;266:17640–17647. [PubMed] [Google Scholar]
- 42.Little PJ, Ballinger ML, Burch ML, Osman N. Biosynthesis of natural and hyperelongated chondroitin sulfate glycosaminoglycans: new insights into an elusive process. Open Biochem. J. 2008;2:135–142. doi: 10.2174/1874091X00802010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Little PJ, Tannock L, Olin KL, Chait A, Wight TN. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-beta1 exhibit increased binding to LDLs. Arterioscler. Thromb. Vasc. Biol. 2002;22:55–60. doi: 10.1161/hq0102.101100. [DOI] [PubMed] [Google Scholar]
- 44.Chait A, Wight TN. Interaction of native and modified low-density lipoproteins with extracellular matrix. Curr. Opin. Lipidol. 2000;11:457–463. doi: 10.1097/00041433-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Ten Dam GB, Murugan S, Yamada S, Hashiguchi T, Mizumoto S, Oguri K, Okayama M, van Kuppevelt TH, Sugahara K. Involvement of highly sulfated chondroitin sulfate in the metastasis of the lewis lung carcinoma cells. J. Biol. Chem. 2008;283:34294–34304. doi: 10.1074/jbc.M806015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta. 2013;1830:4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev. Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- 48.Hatano S, Kimata K, Hiraiwa N, Kusakabe M, Isogai Z, Adachi E, Shinomura T, Watanabe H. Versican/PG-M is essential for ventricular septal formation subsequent to cardiac atrioventricular cushion development. Glycobiology. 2012;22:1268–1277. doi: 10.1093/glycob/cws095. [DOI] [PubMed] [Google Scholar]
- 49.Suwan K, Choocheep K, Hatano S, Kongtawelert P, Kimata K, Watanabe H. Versican/PG-M assembles hyaluronan into extracellular matrix and inhibits CD44-mediated signaling toward premature senescence in embryonic fibroblasts. J. Biol. Chem. 2009;284:8596–8604. doi: 10.1074/jbc.M806927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit. Rev. Eukaryot. Gene Expr. 2004;14:203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 51.Rahmani M, Carthy JM, McManus BM. Mapping of the Wnt/beta-catenin/TCF response elements in the human versican promoter. Methods Mol. Biol. 2012;836:35–52. doi: 10.1007/978-1-61779-498-8_3. [DOI] [PubMed] [Google Scholar]
- 52.Rahmani M, Wong BW, Ang L, Cheung CC, Carthy JM, Walinski H, McManus BM. Versican: signaling to transcriptional control pathways. Can. J. Physiol. Pharmacol. 2006;84:77–92. doi: 10.1139/y05-154. [DOI] [PubMed] [Google Scholar]
- 53.Domenzain-Reyna C, Hernandez D, Miquel-Serra L, Docampo MJ, Badenas C, Fabra A, Bassols A. Structure and regulation of the versican promoter: the versican promoter is regulated by AP-1 and TCF transcription factors in invasive human melanoma cells. J. Biol. Chem. 2009;284:12306–12317. doi: 10.1074/jbc.M807108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J. Biol. Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee DY, Shatseva T, Jeyapalan Z, Du WW, Deng Z, Yang BB. A 3'-untranslated region (3'UTR) induces organ adhesion by regulating miR-199a* functions. PLoS ONE. 2009;4:e4527. doi: 10.1371/journal.pone.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee DY, Jeyapalan Z, Fang L, Yang J, Zhang Y, Yee AY, Li M, Du WW, Shatseva T, Yang BB. Expression of versican 3'-untranslated region modulates endogenous microRNA functions. PLoS ONE. 2010;5:e13599. doi: 10.1371/journal.pone.0013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutnam ZJ, Wight TN, Yang BB. miRNAs regulate expression and function of extracellular matrix molecules. Matrix Biol. 2013;32:74–85. doi: 10.1016/j.matbio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, Bronner-Fraser M, Shinomura T, Kimata K, Morgelin M, Lofberg J, Perris R. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development. 2000;127:2823–2842. doi: 10.1242/dev.127.13.2823. [DOI] [PubMed] [Google Scholar]
- 59.Cattaruzza S, Schiappacassi M, Ljungberg-Rose A, Spessotto P, Perissinotto D, Morgelin M, Mucignat MT, Colombatti A, Perris R. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J. Biol. Chem. 2002;277:47626–47635. doi: 10.1074/jbc.M206521200. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 61.Kischel P, Waltregny D, Dumont B, Turtoi A, Greffe Y, Kirsch S, De Pauw E, Castronovo V. Versican overexpression in human breast cancer lesions: known and new isoforms for stromal tumor targeting. Int. J. Cancer. 2010;126:640–650. doi: 10.1002/ijc.24812. [DOI] [PubMed] [Google Scholar]
- 62.Uhlin-Hansen L, Wik T, Kjellen L, Berg E, Forsdahl F, Kolset SO. Proteoglycan metabolism in normal and inflammatory human macrophages. Blood. 1993;82:2880–2889. [PubMed] [Google Scholar]
- 63.Chang MY, Chan CK, Braun KR, Green PS, O'Brien KD, Chait A, Day AJ, Wight TN. Monocyte-to-macrophage differentiation: synthesis and secretion of a complex extracellular matrix. J. Biol. Chem. 2012;287:14122–14135. doi: 10.1074/jbc.M111.324988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asplund A, Friden V, Stillemark-Billton P, Camejo G, Bondjers G. Macrophages exposed to hypoxia secrete proteoglycans for which LDL has higher affinity. Atherosclerosis. 2011;215:77–81. doi: 10.1016/j.atherosclerosis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 66.Malla N, Berg E, Theocharis AD, Svineng G, Uhlin-Hansen L, Winberg JO. In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. FEBS J. 2013;280:2870–2887. doi: 10.1111/febs.12291. [DOI] [PubMed] [Google Scholar]
- 67.Masuda A, Yasuoka H, Satoh T, Okazaki Y, Yamaguchi Y, Kuwana M. Versican is upregulated in circulating monocytes in patients with systemic sclerosis and amplifies a CCL2-mediated pathogenic loop. Arthritis Res. Ther. 2013;15:R74. doi: 10.1186/ar4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, Port JL, Stiles B, Sukumar S, Altorki NK, Rafii S, Mittal V. Myeloid progenitor cells in the premetastatic lung promote metastases by Inducing mesenchymal to epithelial transition. Cancer Res. 2012;72:1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72:4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sorokin L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 72.Schor H, Vaday GG, Lider O. Modulation of leukocyte behavior by an inflamed extracellular matrix. Dev. Immunol. 2000;7:227–238. doi: 10.1155/2000/51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 2000;67:149–159. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- 74.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng PS, Wen J, Ang LC, Sheng W, Viloria-Petit A, Wang Y, Wu Y, Kerbel RS, Yang BB. Versican/PG-M G3 domain promotes tumor growth and angiogenesis. FASEB J. 2004;18:754–756. doi: 10.1096/fj.03-0545fje. [DOI] [PubMed] [Google Scholar]
- 77.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J. Biol. Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kenagy RD, Plaas AH, Wight TN. Versican degradation and vascular disease. Trends Cardiovasc. Med. 2006;16:209–215. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J. Biol. Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- 80.Hattori N, Carrino DA, Lauer ME, Vasanji A, Wylie JD, Nelson CM, Apte SS. Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis. J. Biol. Chem. 2011;286:34298–34310. doi: 10.1074/jbc.M111.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kenagy RD, Fischer JW, Lara S, Sandy JD, Clowes AW, Wight TN. Accumulation and loss of extracellular matrix during shear stress-mediated intimal growth and regression in baboon vascular grafts. J. Histochem. Cytochem. 2005;53:131–140. doi: 10.1369/jhc.4A6493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kenagy RD, Min SK, Clowes AW, Sandy JD. Cell death-associated ADAMTS4 and versican degradation in vascular tissue. J. Histochem. Cytochem. 2009;57:889–897. doi: 10.1369/jhc.2009.953901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell. 2009;17:687–698. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Formato M, Farina M, Spirito R, Maggioni M, Guarino A, Cherchi GM, Biglioli P, Edelstein C, Scanu AM. Evidence for a proinflammatory and proteolytic environment in plaques from endarterectomy segments of human carotid arteries. Arterioscler. Thromb. Vasc. Biol. 2004;24:129–135. doi: 10.1161/01.ATV.0000104013.71118.53. [DOI] [PubMed] [Google Scholar]
- 85.Barascuk N, Genovese F, Larsen L, Byrjalsen I, Zheng Q, Sun S, Hosbond S, Poulsen TS, Diederichsen A, Jensen JM, Mickley H, Register TC, Rasmussen LM, Leeming DJ, Christiansen C, Karsdal MA. A MMP derived versican neo-epitope is elevated in plasma from patients with atherosclerotic heart disease. Int J Clin Exp Med. 2013;6:174–184. [PMC free article] [PubMed] [Google Scholar]
- 86.Jonsson-Rylander AC, Nilsson T, Fritsche-Danielson R, Hammarstrom A, Behrendt M, Andersson JO, Lindgren K, Andersson AK, Wallbrandt P, Rosengren B, Brodin P, Thelin A, Westin A, Hurt-Camejo E, Lee-Sogaard CH. Role of ADAMTS-1 in atherosclerosis: remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler. Thromb. Vasc. Biol. 2005;25:180–185. doi: 10.1161/01.ATV.0000150045.27127.37. [DOI] [PubMed] [Google Scholar]
- 87.Morrison AC, Bare LA, Chambless LE, Ellis SG, Malloy M, Kane JP, Pankow JS, Devlin JJ, Willerson JT, Boerwinkle E. Prediction of coronary heart disease risk using a genetic risk score: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2007;166:28–35. doi: 10.1093/aje/kwm060. [DOI] [PubMed] [Google Scholar]
- 88.Sabatine MS, Ploughman L, Simonsen KL, Iakoubova OA, Kirchgessner TG, Ranade K, Tsuchihashi Z, Zerba KE, Long DU, Tong CH, Packard CJ, Pfeffer MA, Devlin JJ, Shepherd J, Campos H, Sacks FM, Braunwald E. Association between ADAMTS1 matrix metalloproteinase gene variation, coronary heart disease, and benefit of statin therapy. Arterioscler. Thromb. Vasc. Biol. 2008;28:562–567. doi: 10.1161/ATVBAHA.107.156653. [DOI] [PubMed] [Google Scholar]
- 89.Miguel RF, Pollak A, Lubec G. Metalloproteinase ADAMTS-1 but not ADAMTS-5 is manifold overexpressed in neurodegenerative disorders as Down syndrome, Alzheimer's and Pick's disease. Brain Res. Mol. Brain Res. 2005;133:1–5. doi: 10.1016/j.molbrainres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Ruigrok YM, Rinkel GJ, Wijmenga C. The versican gene and the risk of intracranial aneurysms. Stroke. 2006;37:2372–2374. doi: 10.1161/01.STR.0000236499.55301.09. [DOI] [PubMed] [Google Scholar]
- 91.Zhu X, Shi Y, Lu F, Huang GF, Hu LJ. [Association of single nucleotide polymorphisms of CSPG2 and HSPG2 genes with intracranial aneurysm in ethnic Han Chinese population] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013;30:218–221. doi: 10.3760/cma.j.issn.1003-9406.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 92.Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch. Biochem. Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- 93.Schönherr E, Kinsella MG, Wight TN. Genistein selectively inhibits platelet-derived growth factor stimulated versican biosynthesis in monkey arterial smooth muscle cells. Arch. Biochem. Biophys. 1997;339:353–361. doi: 10.1006/abbi.1996.9854. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y, Cao L, Kiani C, Yang BL, Hu W, Yang BB. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J. Cell Biochem. 1999;73:445–457. [PubMed] [Google Scholar]
- 95.Wu Y, Zhang Y, Cao L, Chen L, Lee V, Zheng PS, Kiani C, Adams ME, Ang LC, Paiwand F, Yang BB. Identification of the motif in versican G3 domain that plays a dominant-negative effect on astrocytoma cell proliferation through inhibiting versican secretion and binding. J. Biol. Chem. 2001;276:14178–14186. doi: 10.1074/jbc.M100618200. [DOI] [PubMed] [Google Scholar]
- 96.Yang BL, Zhang Y, Cao L, Yang BB. Cell adhesion and proliferation mediated through the G1 domain of versican. J. Cell Biochem. 1999;72:210–220. doi: 10.1002/(sici)1097-4644(19990201)72:2<210::aid-jcb5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 97.Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, Wu Y, Lee V, Slingerland J, Dumont D, Yang BB. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol. Biol. Cell. 2005;16:1330–1340. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang W, Yee AJ. Versican V2 isoform enhances angiogenesis by regulating endothelial cell activities and fibronectin expression. FEBS Lett. 2013;587:185–192. doi: 10.1016/j.febslet.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 99.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang W, Xu GL, Jia WD, Ma JL, Li JS, Ge YS, Ren WH, Yu JH, Liu WB. Ligation of TLR2 by versican: a link between inflammation and metastasis. Arch. Med. Res. 2009;40:321–323. doi: 10.1016/j.arcmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 101.Kawashima H, Atarashi K, Hirose M, Hirose J, Yamada S, Sugahara K, Miyasaka M. Oversulfated chondroitin/dermatan sulfates containing GlcAbeta1/IdoAalpha1-3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J. Biol. Chem. 2002;277:12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- 102.Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang F, Wu KY, Wan HY. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS ONE. 2013;8:e56616. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Z, Miao L, Wang L. Inflammation amplification by versican: the first mediator. Int J Mol Sci. 2012;13:6873–6882. doi: 10.3390/ijms13066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.LaPierre DP, Lee DY, Li SZ, Xie YZ, Zhong L, Sheng W, Deng Z, Yang BB. The ability of versican to simultaneously cause apoptotic resistance and sensitivity. Cancer Res. 2007;67:4742–4750. doi: 10.1158/0008-5472.CAN-06-3610. [DOI] [PubMed] [Google Scholar]
- 105.Cattaruzza S, Schiappacassi M, Kimata K, Colombatti A, Perris R. The globular domains of PG-M/versican modulate the proliferation-apoptosis equilibrium and invasive capabilities of tumor cells. FASEB J. 2004;18:779–781. doi: 10.1096/fj.03-0660fje. [DOI] [PubMed] [Google Scholar]
- 106.Touab M, Arumi-Uria M, Barranco C, Bassols A. Expression of the proteoglycans versican and mel-CSPG in dysplastic nevi. Am. J. Clin. Pathol. 2003;119:587–593. doi: 10.1309/ME25-J1G5-ENE5-7LM3. [DOI] [PubMed] [Google Scholar]
- 107.Touab M, Villena J, Barranco C, Arumi-Uria M, Bassols A. Versican is differentially expressed in human melanoma and may play a role in tumor development. Am. J. Pathol. 2002;160:549–557. doi: 10.1016/S0002-9440(10)64874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Serra M, Miquel L, Domenzain C, Docampo MJ, Fabra A, Wight TN, Bassols A. V3 versican isoform expression alters the phenotype of melanoma cells and their tumorigenic potential. Int. J. Cancer. 2005;114:879–886. doi: 10.1002/ijc.20813. [DOI] [PubMed] [Google Scholar]
- 109.Hernandez D, Miquel-Serra L, Docampo MJ, Marco-Ramell A, Bassols A. Role of versican V0/V1 and CD44 in the regulation of human melanoma cell behavior. Int J Mol Med. 2011;27:269–275. doi: 10.3892/ijmm.2010.577. [DOI] [PubMed] [Google Scholar]
- 110.Hernandez D, Miquel-Serra L, Docampo MJ, Marco-Ramell A, Cabrera J, Fabra A, Bassols A. V3 versican isoform alters the behavior of human melanoma cells by interfering with CD44/ErbB-dependent signaling. J. Biol. Chem. 2011;286:1475–1485. doi: 10.1074/jbc.M110.127522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cattaruzza S, Perris R. Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biol. 2005;24:400–417. doi: 10.1016/j.matbio.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Perris R, Perissinotto D. Role of the extracellular matrix during neural crest cell migration. Mech. Dev. 2000;95:3–21. doi: 10.1016/s0925-4773(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 113.Henderson DJ, Copp AJ. Role of the extracellular matrix in neural crest cell migration. J. Anat. 1997;191:507–515. doi: 10.1046/j.1469-7580.1997.19140507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development. 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. [DOI] [PubMed] [Google Scholar]
- 115.Perris R, Perissinotto D, Pettway Z, Bronner-Fraser M, Morgelin M, Kimata K. Inhibitory effects of PG-H/aggrecan and PG-M/versican on avian neural crest cell migration. FASEB J. 1996;10:293–301. doi: 10.1096/fasebj.10.2.8641562. [DOI] [PubMed] [Google Scholar]
- 116.Mayanil CS, George D, Freilich L, Miljan EJ, Mania-Farnell B, McLone DG, Bremer EG. Microarray analysis detects novel Pax3 downstream target genes. J. Biol. Chem. 2001;276:49299–49309. doi: 10.1074/jbc.M107933200. [DOI] [PubMed] [Google Scholar]
- 117.Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, Muir E, Levine JM, Geller HM, Rogers JH, Faissner A, Fawcett JW. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axoninhibitory proteoglycan is NG2. J. Neurosci. 1999;19:8778–8788. doi: 10.1523/JNEUROSCI.19-20-08778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niederost BP, Zimmermann DR, Schwab ME, Bandtlow CE. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J. Neurosci. 1999;19:8979–8989. doi: 10.1523/JNEUROSCI.19-20-08979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Brain derived versican V2 is a potent inhibitor of axonal growth. J. Cell Sci. 2000;113:807–816. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- 121.Dutt S, Cassoly E, Dours-Zimmermann MT, Matasci M, Stoeckli ET, Zimmermann DR. Versican V0 and V1 direct the growth of peripheral axons in the developing chick hindlimb. J. Neurosci. 2011;31:5262–5270. doi: 10.1523/JNEUROSCI.4897-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dutt S, Kleber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J. Biol. Chem. 2006;281:12123–12131. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- 123.Yamaguchi Y. Chondroitin sulfate proteoglycans in the nervous system. In: Iozzo R, editor. Proteoglycans: Structure, Biology, and Molecular Interactions. New York: Marcel Dekker; 2000. pp. 379–402. [Google Scholar]
- 124.Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J. Neurosci. 2002;22:2225–2236. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milev P, Maurel P, Chiba A, Mevissen M, Popp S, Yamaguchi Y, Margolis RK, Margolis RU. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem. Biophys. Res. Commun. 1998;247:207–212. doi: 10.1006/bbrc.1998.8759. [DOI] [PubMed] [Google Scholar]
- 126.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 127.Sobel RA, Ahmed AS. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2001;60:1198–1207. doi: 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- 128.Yeung TL, Leung CS, Wong KK, Samimi G, Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ, Mok SC. TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013;73:5016–5028. doi: 10.1158/0008-5472.CAN-13-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ricciardelli C, Russell DL, Ween MP, Mayne K, Suwiwat S, Byers S, Marshall VR, Tilley WD, Horsfall DJ. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J. Biol. Chem. 2007;282:10814–10825. doi: 10.1074/jbc.M606991200. [DOI] [PubMed] [Google Scholar]
- 130.Sakko AJ, Ricciardelli C, Mayne K, Suwiwat S, LeBaron RG, Marshall VR, Tilley WD, Horsfall DJ. Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res. 2003;63:4786–4791. [PubMed] [Google Scholar]
- 131.Skandalis SS, Kletsas D, Kyriakopoulou D, Stavropoulos M, Theocharis DA. The greatly increased amounts of accumulated versican and decorin with specific posttranslational modifications may be closely associated with the malignant phenotype of pancreatic cancer. Biochim. Biophys. Acta. 2006;1760:1217–1225. doi: 10.1016/j.bbagen.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 132.Wasa J, Nishida Y, Shinomura T, Isogai Z, Futamura N, Urakawa H, Arai E, Kozawa E, Tsukushi S, Ishiguro N. Versican V1 isoform regulates cell-associated matrix formation and cell behavior differentially from aggrecan in Swarm rat chondrosarcoma cells. Int. J. Cancer. 2012;130:2271–2281. doi: 10.1002/ijc.26230. [DOI] [PubMed] [Google Scholar]
- 133.Wu Y, Siadaty MS, Berens ME, Hampton GM, Theodorescu D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene. 2008;27:6679–6689. doi: 10.1038/onc.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ang LC, Zhang Y, Cao L, Yang BL, Young B, Kiani C, Lee V, Allan K, Yang BB. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J. Neuropathol. Exp. Neurol. 1999;58:597–605. doi: 10.1097/00005072-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 135.Yamagata M, Kimata K. Repression of a malignant cell-substratum adhesion phenotype by inhibiting the production of the anti-adhesive proteoglycan, PG-M/versican. J. Cell Sci. 1994;107:2581–2590. doi: 10.1242/jcs.107.9.2581. [DOI] [PubMed] [Google Scholar]
- 136.Yamagata M, Suzuki S, Akiyama SK, Yamada KM, Kimata K. Regulation of cell-substrate adhesion by proteoglycans immobilized on extracellular substrates. J. Biol. Chem. 1989;264:8012–8018. [PubMed] [Google Scholar]
- 137.Wu Y, Chen L, Zheng PS, Yang BB. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J. Biol. Chem. 2002;277:12294–12301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- 138.Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 139.de la Motte CA. Hyaluronan in intestinal homeostasis and inflammation: implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G945–G949. doi: 10.1152/ajpgi.00063.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.de la Motte CA, Drazba JA. Viewing hyaluronan: imaging contributes to imagining new roles for this amazing matrix polymer. J. Histochem. Cytochem. 2011;59:252–257. doi: 10.1369/0022155410397760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN. Intracellular hyaluronan: a new frontier for inflammation? Biochim. Biophys. Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 142.Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am. J. Respir. Cell Mol. Biol. 2010;43:109–120. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J. Biol. Chem. 2003;278:47223–47231. doi: 10.1074/jbc.M304871200. [DOI] [PubMed] [Google Scholar]
- 144.Wang A, de la Motte C, Lauer M, Hascall V. Hyaluronan matrices in pathobiological processes. FEBS J. 2011;278:1412–1418. doi: 10.1111/j.1742-4658.2011.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de la Motte C, Hascall VC, Drazba JA, Strong SA. Poly I:C induces mononuclear leukocyte-adhesive hyaluronan structures on colon smooth muscle cells: IaI and versican facilitate adhesion. In: Kennedy JF, Phillips GO, Williams PA, Hascall VC, editors. Hyaluronan: Chemical, Biochemical and Biological Aspects. Vol. 1. Cambridge, England: Woodhead Publishing Limited; 2002. pp. 381–388. [Google Scholar]
- 146.Clark RAF, Henson PM. The molecular and cellular biology of wound repair. New York: Plenum Press; 1988. [Google Scholar]
- 147.Clark RAF, Quinn JH, Winn HJ, Lanigan JM, Dellepella P, Colvin RB. Fibronectin is produced by blood vessels in response to injury. J. Exp. Med. 1982;156:646–651. doi: 10.1084/jem.156.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wight TN, Potter-Perigo S. The Extracellular Matrix: An Active or Passive Player in Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G950–G955. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J. Biol. Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- 150.Day AJ, Sheehan JK. Hyaluronan: polysaccharide chaos to protein organisation. Curr. Opin. Struct. Biol. 2001;11:617–622. doi: 10.1016/s0959-440x(00)00256-6. [DOI] [PubMed] [Google Scholar]
- 151.Aspberg A, Binkert C, Ruoslahti E. The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10590–10594. doi: 10.1073/pnas.92.23.10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aspberg A, Miura R, Bourdoulous S, Shimonaka M, Heinegård D, Schachner M, Ruoslahti E, Yamaguchi Y. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moeity. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10116–10121. doi: 10.1073/pnas.94.19.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aspberg A, Adam S, Kostka G, Timpl R, Heinegard D. Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. J. Biol. Chem. 1999;274:20444–20449. doi: 10.1074/jbc.274.29.20444. [DOI] [PubMed] [Google Scholar]
- 154.Olin AI, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. The proteoglycans aggrecan and Versican form networks with fibulin-2 through their lectin domain binding. J. Biol. Chem. 2001;276:1253–1261. doi: 10.1074/jbc.M006783200. [DOI] [PubMed] [Google Scholar]
- 155.Murasawa Y, Watanabe K, Yoneda M, Zako M, Kimata K, Sakai LY, Isogai Z. Homotypic versican G1 domain interactions enhance hyaluronan incorporation into fibrillin microfibrils. J. Biol. Chem. 2013;288:29170–29181. doi: 10.1074/jbc.M113.456947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reinhardt DP, Sasaki T, Dzamba BJ, Keene DR, Chu ML, Gohring W, Timpl R, Sakai LY. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J. Biol. Chem. 1996;271:19489–19496. doi: 10.1074/jbc.271.32.19489. [DOI] [PubMed] [Google Scholar]
- 157.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 158.Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 2003;13:51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 159.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 160.Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through elastin receptor facilitate proliferation and motility of arterial smooth muscle cells. J. Biol. Chem. 2002;277:44854–44863. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- 161.Hinek A, Mecham RP, Keeley F, Rabinovitch M. Impaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J. Clin. Invest. 1991;88:2083–2094. doi: 10.1172/JCI115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu SQ, Alkema PK, Tieche C, Tefft BJ, Liu DZ, Li YC, Sumpio BE, Caprini JA, Paniagua M. Negative regulation of monocyte adhesion to arterial elastic laminae by signal regulatory protein alpha and Src homology 2 domain-containing protein-tyrosine phosphatase-1. J. Biol. Chem. 2005;280:39294–39301. doi: 10.1074/jbc.M503866200. [DOI] [PubMed] [Google Scholar]