Introduction
Of the estimated 525,000 new cases of cervical cancer and 275,000 deaths from cervical cancer in 2008, over 85% were in developing countries (1). Cervical cancer accounts for over 2.7 million years of life lost among women between the ages of 25 and 64 worldwide, 2.4 million of which occur in resource poor countries (2). Over 20% of the annual deaths from cervical cancer occur in countries in sub-Saharan Africa, such as Zambia, where it is the most common cause of cancer death among women (1). Of the women in developing countries who die of cervical cancer, fewer than 5% have ever had a screening exam for the disease (3).
Zambia has one of the highest age-standardized incidence rates of cervical cancer in Africa, estimated at 54 cases/100,000 women per year, and the associated mortality rate is 44 deaths/100,000 women per year (4). In 2006 the government implemented a cervical cancer screening program which utilizes the “see-and-treat” and visual inspection with acetic acid (VIA) model of evaluation. It has been successful, screening approximately 10,000 women per year since its inception (5). The program is expanding from the capital Lusaka into more rural provinces of Zambia. Neither of the two approved vaccines for human papillomavirus (HPV) is currently available in Zambia. Although a recent study explored common myths about cervical cancer among Zambian women, no published studies have examined their attitudes regarding HPV vaccination (6).
The objectives of this study were to better understand knowledge regarding cancer and cervical cancer, to assess exposure to vaccines, and evaluate the acceptability of HPV vaccination among women in Zambia.
Methods
This cross-sectional investigation was conducted from August through September 2009 in Lusaka, Zambia. All women from the ages of 18 to 65 years of age presenting to two clinics providing general medical, antenatal, and gynecology services were eligible to participate. Every 10th woman who presented was approached by a trained research nurse to ask whether she was interested in completing a nurse-administered questionnaire. No personal identifiers were collected with the questionnaires to ensure confidentiality.
The questions were based on modified versions of questionnaires from several other studies on attitudes towards HPV vaccination (7-17). Prior to the start of the study, we conducted two focus groups with adult women presenting to both clinics to assess general knowledge about vaccines, cancer, and cervical cancer. The questionnaire was further modified and made culturally appropriate based on results from these focus groups and discussion with local healthcare providers (unpublished results).
The questionnaire collected demographic information and addressed knowledge of cancer and cervical cancer, as well as exposure to vaccines. Women were asked to answer questions regarding the vaccination of their children. If they did not have children, women answered in the hypothetical. If a participant was not aware of vaccines, subsequent questions regarding vaccination were skipped. If a participant was not aware of cancer as a diagnosis or condition, subsequent questions regarding cancer and cervical cancer were skipped. Before asking questions about attitudes toward and acceptability of HPV vaccination, all participants received a standard educational script on HPV and cervical cancer risk factors and prevention. Participants were also asked how much they would be willing to pay for HPV vaccination.
After obtaining written informed consent, research nurses administered the questionnaire. The questionnaire was created in English, translated into Nyanja, the vernacular Bantu language in Lusaka, and back-translated for accuracy. Both the questionnaire and informed consent documents were available in both English and Nyanja. Each participant received a small monetary reimbursement for her time and participation.
The study was approved by the institutional review boards at Beth Israel Deaconess Medical Center and the University of Zambia.
Results
A total of 319 women completed the survey. Nine of these women did not meet eligibility criteria for age and were excluded from the analysis. The mean age was 33.2±8.2 years and 86.7% were married. Most (86.7%) women had children, and the mean number of children was 2.9±2.1. The mean amount of education completed was 8.3± 3.6 years, and 3.9% received no education. Forty-nine percent reported they were unemployed. Demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics of women surveyed in Lusaka, Zambia
| Characteristic | n = 310 |
|---|---|
| Mean ± SD | |
| Age in years | 33.2 ± 8.2 |
| Years of education | 8.3 ± 3.6 |
| Number of children | 2.9 ± 2.1 |
| Female children | 1.5 ± 1.3 |
| Male children | 1.4 ± 1.3 |
| n (%) | |
| Have children | 269 (86.7) |
| Occupation | |
| Unemployed | 144 (46.5) |
| Business woman | 64 (20.6) |
| Manual laborer | 35 (11.3) |
| Education | 13 (4.2) |
| Healthcare | 10 (3.3) |
| Public sector | 7 (2.2) |
| Other | 19 (6.1) |
| Marital status | |
| Married | 264 (85.1) |
| Single | 20 (6.5) |
| Widowed | 18 (5.8) |
| Separated/divorced | 8 (2.6) |
Almost all women reported knowledge of other vaccines and wanted their children to be vaccinated (Table 2). Of women who knew of vaccines, 98% wanted their children vaccinated. Of women who had their children vaccinated, 13.9% reported adverse vaccine reactions among their children.
Table 2.
Knowledge and awareness of vaccines among women in Lusaka, Zambia
| Characteristic | n = 310 (%) |
|---|---|
| Ever heard of vaccines | |
| Yes | 306 (98.7) |
| No | 4 (1.3) |
| Among women who had ever heard of vaccines (n = 306) | |
| Want children to receive vaccinations | 300 (98.0) |
| Had children vaccinated | 265 (86.6) |
| Among women who had their children vaccinated (n = 265) | |
| Child experienced reaction to vaccine | 37 (13.9) |
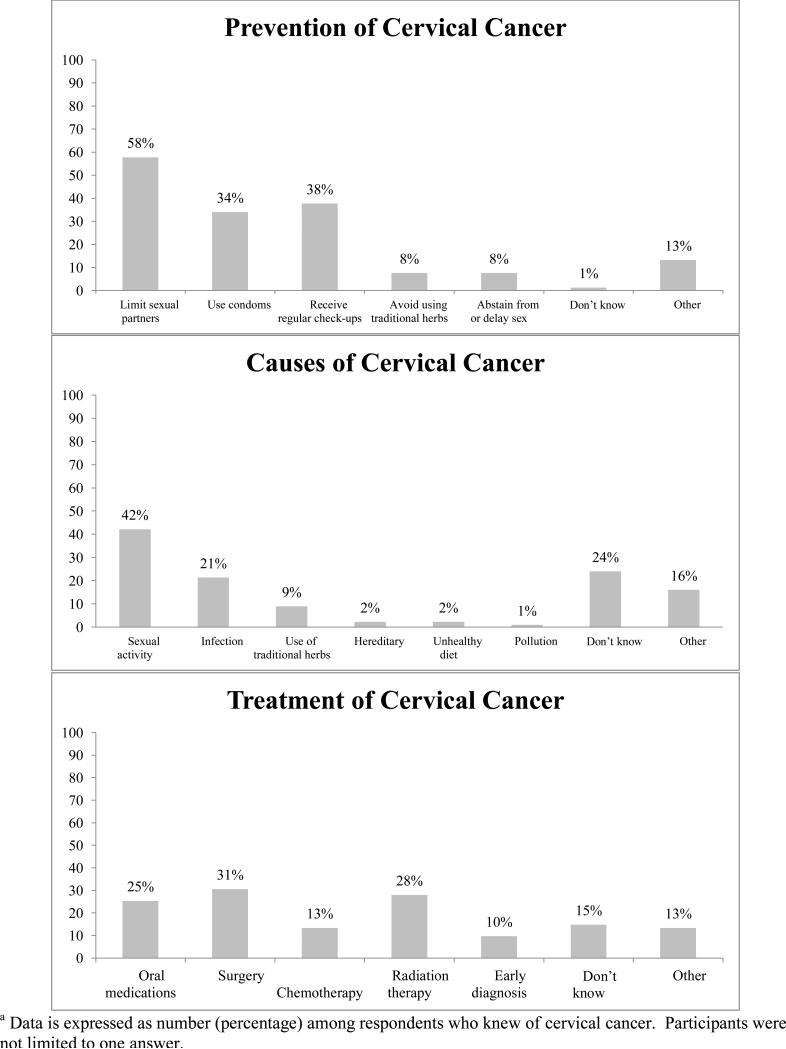
Three hundred women (96.8%) had heard of cancer and of them, 224 women (74.7%) had heard of cervical cancer. Of women who knew of cervical cancer, 42.2% sited sexual activity or multiple partners as a cause, 73.3% believed it to be preventable, and 57.8% sited limiting sexual partners as a way to prevent cervical cancer (Figure 1). The majority of women (62.3%) aware of cervical cancer believe that there is a treatment for it. Seventy percent of women reported ever having a pelvic or speculum exam. Among women who had heard of cervical cancer, 33.3% believed that there was a relationship between cervical cancer and AIDS.
Figure 1.
Cervical cancer prevention methods, causes, and treatment option sited by women in Lusaka, Zambia (n=227)a
Following the standardized education component, 47.4% of women would pay something for the HPV vaccine. Almost all women (96.1%) would vaccinate themselves and 98.1% would allow their children to be vaccinated. Ninety-three percent of women believed that their families would allow their children to be vaccinated for a sexually transmitted infection (Table 3).
Table 3.
Attitudes towards HPV vaccination among women in Lusaka, Zambia
| Attitude | n=310 (%) |
|---|---|
| Would vaccinate self against HPV | |
| Yes | 298 (96.1) |
| No | 11 (3.6) |
| Don't know | 1 (0.3) |
| Would allow children to be vaccinated against HPV | 304 (98.1) |
| Family would approve of children being vaccinated against HPV | |
| Yes | 291 (93.9) |
| No | 12 (3.9) |
| Don't know | 7 (2.3) |
| Amount willing to pay for HPV vaccine (Zambian kwacha) | |
| Nothing | 163 (52.6) |
| 5,000 – 10,000 (US$ 1-2) | 88 (28.4) |
| 20,000 – 50,000 (US$ 4-10) | 31 (10.0) |
| 50,000 – 100,000 (US$ 10-20) | 20 (6.5) |
| More than 100,000 (>US$ 20) | 8 (2.6) |
Discussion
Our data demonstrate widespread acceptance of HPV vaccination among adult women seen in medical clinics in Lusaka, Zambia, both for themselves and for their children. The potential impact of HPV vaccination on deaths from cervical cancer in Zambia is substantial given the high prevalence of disease.
Since its introduction in 2006, the HPV vaccine has been highly debated because it is the first vaccine aimed at the primary prevention of cancer, targeted at school-age children, and caused by a sexually transmitted infection. Numerous studies have explored the attitudes of men and women on HPV vaccination, both for themselves and their children (7-17). Interestingly, the majority of these studies were conducted in countries where cervical cancer rates have dwindled in the last few decades due to widespread screening measures. In countries where cervical cancer remains a leading cause of death for women, relatively few studies have been done. Furthermore, only a handful of studies have explored knowledge and attitudes in sub-Saharan Africa (18-22), where cervical cancer, in the setting of high HIV/AIDS prevalence, creates an even greater burden on women's health.
In our study, familiarity with vaccines and trust of vaccination was high, findings confirmed by recent data published by UNICEF reporting vaccination rates from 77-92% of childhood vaccines in Zambia (23). This undoubtedly contributes to the nearly universal acceptability of HPV vaccination in our study. Women sited health clinics most often as their source of information regarding vaccination, making clinics a logical venue for cervical cancer education.
Our findings are similar to that of other studies in Africa (18, 20-22). In Ghana, 94% of 264 women surveyed were willing to vaccinate themselves or their daughters, and a study in Botswana showed that 88% of 376 women would allow their adolescent daughters to receive the HPV vaccine (21, 22). Similarly, a survey of 147 Kenyan women reported that 95% of them were willing to have their daughters vaccinated to prevent cervical cancer. Willingness decreased when women learned that 3 shots were required (18). Our study did not assess acceptability based on doses in vaccine regimen; however, this could be explored in a follow-up survey.
There are several limitations of this study. Participants were a self-selected group of care seekers as they were recruited at health clinics, thereby potentially limiting the generalizability of these findings to women who access health care. In addition, a nurse-administered questionnaire may lead to social desirability bias that may explain the positive response to vaccines among our study participants. While vaccination rates estimated by UNICEF are subject to measurement error, the high familiarity with vaccines can be explained by the high uptake of vaccines in Zambia. The cross-sectional nature of the study also limits the amount of causal inference that can be made and restricts associations to the time period studied.
The cost of the HPV vaccines has largely been prohibitive for low-resource countries. Mathematical models predict that the vaccine has the potential to prevent approximately 3 million deaths in the world's poorest countries if 70% of adolescent girls receive the vaccine over ten consecutive years (24). In addition to the cost, other characteristics of HPV vaccination (cold storage requirement, target age in adolescence) make its implementation difficult. Without external monetary assistance, developing countries are unlikely to sustain an HPV vaccine program; however, studies like this one that document high acceptance of the vaccine provide data to support initiating life-saving immunization against cervical cancer.
The Zambian Ministry of Health and Centre for Infectious Disease Research in Zambia have implemented a successful cervical cancer screening program (5). They have demonstrated efficacy and safety of a screening program adapted to a resource-poor setting (25). By training nurses to perform initial screening and treatment of abnormal cervical lesions, the program has screened over 57,000 women and over 6,000 women have been treated for pre-cancerous lesions since its inception. The program has trained over 70 healthcare professionals worldwide on their method and model of screening (26). Studies like this one help demonstrate that women are receptive to HPV vaccination. Our study also provides information that may be used to develop educational messages when HPV vaccination becomes available in Zambia.
In conclusion, this study provides one of the first questionnaire-based assessments of women in Zambia regarding their knowledge and attitudes on cervical cancer and HPV vaccination. Our findings confirm the acceptance of vaccination in Lusaka and suggest that awareness of cervical cancer and both utilization and acceptability of vaccines are high among women in these two health clinics in Lusaka, Zambia. Zambia has one of the highest incidence and mortality rates due to cervical cancer in the world. The addition of HPV vaccination in the country will help create a comprehensive preventative strategy to help alleviate significant suffering and death from this malignancy.
Acknowledgments
Role of the funding source
Funding for this project was provided by the Charles Engelhard Foundation. The Foundation played no role in the study design; the collection, analysis, and interpretation of data; the writing of the manuscript; or the decision to submit the paper for publication.
Footnotes
Disclosure
The authors declare that they have nothing to disclose.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. Epub 2011/02/08. [DOI] [PubMed] [Google Scholar]
- 2.Yang BH, Bray FI, Parkin DM, Sellors JW, Zhang ZF. Cervical cancer as a priority for prevention in different world regions: an evaluation using years of life lost. International journal of cancer Journal international du cancer. 2004;109(3):418–24. doi: 10.1002/ijc.11719. Epub 2004/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006;24(Suppl 3):S3/71–7. doi: 10.1016/j.vaccine.2006.05.121. Epub 2006/09/05. [DOI] [PubMed] [Google Scholar]
- 4.Castellsagué X, dSS, Aguado T, Louie KS, Bruni L, Muñoz J, Diaz M, Irwin K, Gacic M, Beauvais O, Albero G, Ferrer E, Byrne S, Bosch FX. HPV and Cervical Cancer in the World. 2007 Report. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre); 2007. [December 14, 2011]. Available from: www.who.int/hpvcentre. [Google Scholar]
- 5.Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, Pfaendler KS, Chibwesha C, Mkumba G, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med. 2011;8(5):e1001032. doi: 10.1371/journal.pmed.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirwa S, Mwanahamuntu M, Kapambwe S, Mkumba G, Stringer J, Sahasrabuddhe V, et al. Myths and misconceptions about cervical cancer among Zambian women: rapid assessment by peer educators. Global health promotion. 2010;17(2 Suppl):47–50. doi: 10.1177/1757975910363938. Epub 2010/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slomovitz BM, Sun CC, Frumovitz M, Soliman PT, Schmeler KM, Pearson HC, et al. Are women ready for the HPV vaccine? Gynecologic oncology. 2006;103(1):151–4. doi: 10.1016/j.ygyno.2006.02.003. Epub 2006/03/23. [DOI] [PubMed] [Google Scholar]
- 8.Kahn JA, Rosenthal SL, Hamann T, Bernstein DI. Attitudes about human papillomavirus vaccine in young women. International journal of STD & AIDS. 2003;14(5):300–6. doi: 10.1258/095646203321605486. Epub 2003/06/14. [DOI] [PubMed] [Google Scholar]
- 9.McClelland A, Liamputtong P. Knowledge and acceptance of human papillomavirus vaccination: perspectives of young Australians living in Melbourne, Australia. Sexual health. 2006;3(2):95–101. doi: 10.1071/sh05035. Epub 2006/06/28. [DOI] [PubMed] [Google Scholar]
- 10.Giles M, Garland S. A study of women's knowledge regarding human papillomavirus infection, cervical cancer and human papillomavirus vaccines. The Australian & New Zealand journal of obstetrics & gynaecology. 2006;46(4):311–5. doi: 10.1111/j.1479-828X.2006.00598.x. Epub 2006/07/27. [DOI] [PubMed] [Google Scholar]
- 11.Moreira ED, Jr., Oliveira BG, Ferraz FM, Costa S, Costa Filho JO, Karic G. Knowledge and attitudes about human papillomavirus, Pap smears, and cervical cancer among young women in Brazil: implications for health education and prevention. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2006;16(2):599–603. doi: 10.1111/j.1525-1438.2006.00377.x. Epub 2006/05/10. [DOI] [PubMed] [Google Scholar]
- 12.Donders GG, Gabrovska M, Bellen G, Van Keirsbilck J, Van Den Bosch T, Riphagen I, et al. Knowledge of cervix cancer, human papilloma virus (HPV) and HPV vaccination at the moment of introduction of the vaccine in women in Belgium. Archives of gynecology and obstetrics. 2008;277(4):291–8. doi: 10.1007/s00404-007-0487-1. Epub 2007/10/30. [DOI] [PubMed] [Google Scholar]
- 13.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Preventive medicine. 2007;45(2-3):107–14. doi: 10.1016/j.ypmed.2007.05.013. Epub 2007/07/14. [DOI] [PubMed] [Google Scholar]
- 14.Di Giuseppe G, Abbate R, Liguori G, Albano L, Angelillo IF. Human papillomavirus and vaccination: knowledge, attitudes, and behavioural intention in adolescents and young women in Italy. British journal of cancer. 2008;99(2):225–9. doi: 10.1038/sj.bjc.6604454. Epub 2008/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerend MA, Magloire ZF. Awareness, knowledge, and beliefs about human papillomavirus in a racially diverse sample of young adults. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;42(3):237–42. doi: 10.1016/j.jadohealth.2007.08.022. Epub 2008/02/26. [DOI] [PubMed] [Google Scholar]
- 16.Kahn JA, Rosenthal SL, Jin Y, Huang B, Namakydoust A, Zimet GD. Rates of human papillomavirus vaccination, attitudes about vaccination, and human papillomavirus prevalence in young women. Obstetrics and gynecology. 2008;111(5):1103–10. doi: 10.1097/AOG.0b013e31817051fa. Epub 2008/05/02. [DOI] [PubMed] [Google Scholar]
- 17.Dinh TA, Rosenthal SL, Doan ED, Trang T, Pham VH, Tran BD, et al. Attitudes of mothers in Da Nang, Vietnam toward a human papillomavirus vaccine. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2007;40(6):559–63. doi: 10.1016/j.jadohealth.2007.02.003. Epub 2007/05/29. [DOI] [PubMed] [Google Scholar]
- 18.Becker-Dreps S, Otieno WA, Brewer NT, Agot K, Smith JS. HPV vaccine acceptability among Kenyan women. Vaccine. 2010;28(31):4864–7. doi: 10.1016/j.vaccine.2010.05.034. Epub 2010/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harries J, Moodley J, Barone MA, Mall S, Sinanovic E. Preparing for HPV vaccination in South Africa: key challenges and opinions. Vaccine. 2009;27(1):38–44. doi: 10.1016/j.vaccine.2008.10.033. Epub 2008/11/04. [DOI] [PubMed] [Google Scholar]
- 20.Katahoire RA, Jitta J, Kivumbi G, Murokora D, Arube WJ, Siu G, et al. An assessment of the readiness for introduction of the HPV vaccine in Uganda. African journal of reproductive health. 2008;12(3):159–72. Epub 2009/05/14. [PubMed] [Google Scholar]
- 21.Coleman MA, Levison J, Sangi-Haghpeykar H. HPV vaccine acceptability in Ghana, West Africa. Vaccine. 2011;29(23):3945–50. doi: 10.1016/j.vaccine.2011.03.093. Epub 2011/04/13. [DOI] [PubMed] [Google Scholar]
- 22.DiAngi YT, Panozzo CA, Ramogola-Masire D, Steenhoff AP, Brewer NT. A cross- sectional study of HPV vaccine acceptability in Gaborone, Botswana. PloS one. 2011;6(10):e25481. doi: 10.1371/journal.pone.0025481. Epub 2011/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UNICEF [December 14, 2011];Information by Country: Zambia. 2010 Available from: http://www.unicef.org/infobycountry/zambia_statistics.html.
- 24.Goldie SJ, O'Shea M, Diaz M, Kim SY. Benefits, cost requirements and cost- effectiveness of the HPV16,18 vaccine for cervical cancer prevention in developing countries: policy implications. Reproductive health matters. 2008;16(32):86–96. doi: 10.1016/S0968-8080(08)32409-4. Epub 2008/11/26. [DOI] [PubMed] [Google Scholar]
- 25.Pfaendler KS, Mwanahamuntu MH, Sahasrabuddhe VV, Mudenda V, Stringer JS, Parham GP. Management of cryotherapy-ineligible women in a “screen-and-treat” cervical cancer prevention program targeting HIV-infected women in Zambia: lessons from the field. Gynecol Oncol. 2008;110(3):402–7. doi: 10.1016/j.ygyno.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zambia CfIDRi [May 17, 2012];Cervical Cancer Screening Program. 2011 Available from: http://www.cidrz.org/cervical_cancer_screening.