Abstract
Background
The number of circulating blood monocytes impacts atherosclerotic lesion size, and in mouse models, elevated levels of high-density lipoprotein cholesterol suppress blood monocyte counts and atherosclerosis. We hypothesized that individuals with mild renal dysfunction at increased cardiovascular risk would have reduced high-density lipoprotein levels, high blood monocyte counts, and accelerated atherosclerosis.
Methods and Results
To test whether mild renal dysfunction is associated with an increase in a leukocyte subpopulation rich in monocytes that has a known association with future coronary events, we divided individuals from the Malmö Diet and Cancer study (MDC) into baseline cystatin C quintiles (n=4757). Lower levels of renal function were accompanied by higher monocyte counts, and monocytes were independently associated with carotid bulb intima-media thickness cross-sectionally (P=0.02). Cystatin C levels were positively and plasma high-density lipoprotein cholesterol levels negatively associated with monocyte counts at baseline, after adjustment for traditional risk factors. Several amino acid metabolites tied to low levels of high-density lipoprotein cholesterol and insulin resistance measured in a subset of individuals (n=752) by use of liquid chromatography–mass spectrometry were independently associated with a 22% to 34% increased risk of being in the top quartile of monocytes (P<0.05).
Conclusions
A low high-density lipoprotein cholesterol, insulin resistance phenotype occurs in subjects with mild renal dysfunction and is associated with elevated monocytes and atherosclerosis. High blood monocyte counts may represent a previously unrecognized mechanism underlying the strong relationship between cystatin C and cardiovascular risk.
Keywords: atherosclerosis, immunology, kidney, metabolomics, risk factors
Patients with chronic kidney disease (CKD) have a markedly increased risk of atherosclerotic cardiovascular disease.1 The risk of cardiovascular events increases as the estimated glomerular filtration rate declines.2 Although CKD may be associated with several well-known atherosclerosis risk factors, such as diabetes, hypertension, and elevated blood cholesterol, the atherosclerosis in patients with CKD is not fully explained by traditional risk factors.3 The recent SHARP trial (Study of Heart and Renal Protection)4 demonstrated a 17% relative risk reduction in first major atherosclerotic event in 9270 CKD patients receiving simvastatin plus ezetimibe versus placebo; however, event rates remained high in treated patients, and similar to 2 previous trials in hemodialysis patients, there was no significant reduction in mortality or nonfatal myocardial infarction from low-density lipoprotein cholesterol lowering.4–6 Therefore, low-density lipoprotein cholesterol reduction deals with only a portion of cardiovascular risk in CKD, and additional causes of accelerated atherosclerosis must be explored to devise new treatments.
Multiple risk factors for coronary heart disease have been identified in CKD,3 beginning with mild impairments in renal function above an estimated glomerular filtration rate of 60 mL·min−1·1.73 m−2; however, the significance and interrelationships among these risk factors are not well understood. Patients with mild renal dysfunction develop an unfavorable lipid profile characterized by rising triglyceride and declining high-density lipoprotein cholesterol (HDL-C) concentrations,3,7 each of which is independently associated with coronary heart disease.3 Atherogenic remnants of triglyceriderich lipoproteins accumulate as renal function deteriorates,8 and patients with CKD9 and end stage renal disease (ESRD)10 are at increased risk for cardiovascular events9 and cardiovascular death10 from increasing atherosclerosis as shown by carotid intima-media thickness (IMT) measurements.9,10
The number of circulating monocytes and their differentiation into lipid-laden macrophages in the arterial wall are fundamental factors involved in plaque formation,11,12 and in recent years, peripheral monocyte count has emerged as a strong and independent predictor of cross-sectional and future atherosclerosis in large population-based cohorts.13,14 In patients with ESRD who are undergoing hemodialysis, total monocyte counts and certain monocyte subsets are increased cross-sectionally compared with healthy control subjects,15 and small studies have shown that specific subsets are associated with cardiovascular events and mortality in ESRD16 and CKD.17 In addition, spikes in monocyte count to >11% of total leukocytes over time are associated with a composite end point of ESRD and death.18 However, in predialysis stages of CKD, these small studies have not shown increased total monocyte counts at baseline compared with subjects without CKD,17,18 and to the best of our knowledge, a large-scale, detailed analysis of monocyte count as a marker of atherosclerosis in the setting of mild renal dysfunction has never been undertaken.
The ability of high-density lipoprotein (HDL) to stimulate removal of cholesterol from macrophages, cholesterol efflux, is thought to be central to its antiatherogenic mechanism.19 In mouse models, we recently discovered that the absence of ABCA1 and ABCG1, 2 ATP-binding cassette transporters that promote HDL-mediated cholesterol efflux,20 leads to proliferation of hematopoietic stem and progenitor cells, myeloid progenitor cells, and blood monocytes in association with accelerated atherosclerosis, and that transplantation of knockout bone marrow into apolipoprotein A1 (apoA1) transgenic mice with high HDL-C levels dramatically reverses this phenotype.20 We therefore hypothesized that individuals with mild renal dysfunction measured by elevated cystatin C (cysC) concentrations at increased cardiovascular risk21,22 might have reduced HDL-C levels, contributing to elevated monocyte counts. In addition, given recent studies showing that certain plasma metabolites predict characteristics of the metabolic syndrome23 and future diabetes,24 we explored whether several metabolic markers associated with low HDL-C23 would also be associated with the monocytosis of mild renal dysfunction.
Methods
Study Population
All human study protocols were approved by the Institutional Review Board of Lund University (Sweden). All study participants provided written informed consent. The Malmö Diet and Cancer Study (MDC) is a prospective, population-based cohort that included 28 449 randomly selected men (born between 1923 and 1945) and women (born between 1923 and 1950) who underwent a baseline examination between 1991 and 1996. From this cohort, 6103 persons enrolled in 1991 to 1994 were randomly selected to participate in the MDC cardiovascular cohort (MDC-CC), which was designed to investigate the epidemiology of carotid artery disease. We excluded participants with prior myocardial infarction or stroke at baseline (n=143). Of the remaining participants, fasting plasma samples at baseline were available for 5400.25 Among these, complete data on conventional cardiovascular risk factors were available for 5220. To assess the cross-sectional clinical end points below, we divided 4757 MDC-CC individuals who had cysC measured at baseline into cysC quintiles.
Clinical Examination and Laboratory Assays
MDC participants underwent baseline history, examination, and laboratory assessment. Fasting EDTA plasma was frozen at -80°C immediately after collection. CysC, an endogenous substance freely filtered by the kidney, captures the association of mild renal dysfunction with cardiovascular risk better than creatinine-based glomerular filtration rate equations and is often preferred for use in assessment of cardiovascular end points in these individuals.21,22 CysC, fasting levels of HDL-C and triglyceride, the homeostasis model assessment of insulin resistance (HOMA-IR),26 and total and differential peripheral leukocytes were measured as described in the Methods section of the online-only Data Supplement.
Metabolite Profiling
Metabolites were profiled from EDTA plasma collected at the baseline examination in 759 MDC-CC individuals by use of previously described methodology23,24 (see the Methods section in the online-only Data Supplement for details). These subjects were derived from a nested incident cardiovascular disease case-control study (n=506)27 with case and control subjects matched by sex, age, and Framingham risk score28 and a nested incident diabetes case-control study (n=326).24 From this pool of 832, subjects were excluded who had cardiovascular disease before the baseline examination or incomplete data on cysC or who had been in both studies above, which left 759 individuals. There were 752 individuals with complete data on all covariates (metabolite cohort).
Clinical End Points
We primarily examined the surrogate cardiovascular end point, top quartile of monocytes, measured at the time of the screening examination and defined in the online-only Data Supplement, which notably has been associated with future coronary events in an adjusted analysis of >25 000 individuals from MDC.29 In MDC-CC, the top quartile of monocytes contained 0.70 to 1.80 million cells/mL, or a mean of 11% of total white blood cells. In addition, we examined a secondary end point at baseline: maximal carotid bulb IMT (IMTmaxBulb), measured in millimeters (further details provided in the online-only Data Supplement).
Statistical Analysis
All analyses were performed cross-sectionally at the time of the baseline visit. We divided 4757 MDC-CC subjects with baseline cysC into cysC quintiles and initially hypothesized (1) that subjects in quintile 5 for cysC would have the lowest HDL-C level and the highest monocyte count and (2) that quintile 5 of cysC and HDL-C would each independently be associated with the categorical primary outcome of top quartile of monocytes after multivariable adjustment for age, sex, quintiles 1 to 4 cysC, HOMA-IR, and current smoking in a logistic regression model. Next, in 752 of these subjects, we explored the relationship of various candidate amino acid (AA) metabolites previously shown to have inverse associations with HDL-C23 to top quartile monocytes. Each metabolite was examined in a separate multivariable logistic regression model adjusted for age, sex, continuous (standardized) cysC, HDL-C, and HOMA-IR. All metabolite values were subjected to natural logarithm transformations because of their nonnormal distribution and then standardized (to mean=0, SD=1). Finally, using a multivariable linear regression model adjusted for age, sex, quintiles 1 to 4 cysC, HOMA-IR, and current smoking, we hypothesized that quintile 5 of cysC, HDL-C, and continuous monocytes would each independently be associated with the continuous secondary outcome IMTmax Bulb (log transformed because of its skewed distribution). In all analyses, HOMA-IR was also log transformed because of its skewed distribution.
All analyses were performed with SAS version 9.1.3 (SAS Institute, Cary, NC). Continuous variables are summarized as mean±SD. ANOVA was used to test for a difference in HDL-C, triglyceride, monocyte, HOMA-IR, and AA means (respectively) across cysC quintiles. Results of logistic regression analyses are reported as odds ratio (ORs) with 95% confidence interval (CI). Results of linear regression analyses are reported as standardized regression coefficients (β, SE). A 2-tailed probability value of <0.05 was considered statistically significant. Given the exploratory nature of the AA analyses, nominal significance testing (P<0.05) was used without correction of probability values for multiple comparisons.
Results
Baseline characteristics of the MDC-CC human study sample and the metabolite cohort are shown in Table 1. Mean (±SD) age of subjects with complete data on conventional cardiovascular risk factors (n=5220) was 58±6 years, and 60% were women. Comparable age, sex distribution, and level of traditional risk factors were seen in the metabolite cohort (n=752).
Table 1. MDC-CC: Baseline Characteristics.
| Complete Data on Conventional Cardiovascular Risk Factors* (n=5220) | Metabolite Cohort† (n=752) | |
|---|---|---|
| Age, y | 58±6 | 59±6 |
| Men | 2044 (40) | 373 (50) |
| SBP, mm Hg | 141±19 | 147±19 |
| DBP, mm Hg | 87±9 | 90±9 |
| Antihypertensive treatment | 866 (17) | 186 (25) |
| Hypertension | 3224 (64) | 577 (77) |
| BMI, kg/m2 | 25.7±3.9 | 27±4 |
| LDL-C, mg/dL | 162.4±38.7 | 166.3±38.7 |
| HDL-C, mg/dL | 54.1±15.5 | 50.3±11.6 |
| Diabetes mellitus | 415 (8) | 55 (7) |
| HOMA-IR, mean±SD | 1.9±2.7 | 2.4±3.1 |
| Current smoking | 1365 (26) | 230 (31) |
Values are n (%) or mean±SD.
BMI indicates body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; MDC-CC, cardiovascular cohort of the Malmö Diet and Cancer Study; and SBP, systolic blood pressure.
Complete data on conventional cardiovascular risk factors at baseline were available in 5220 individuals.
Baseline plasma metabolite Profiling was performed in 752 individuals with complete covariate data.
MDC-CC Renal Demographics by CysC Quintiles
We divided 4757 MDC-CC subjects with baseline plasma cysC into cysC quintiles (Table 2). Quintile 5, which represented the highest cysC levels, contained 992 individuals with a cysC range from 0.88 to 3.29 mg/L. Given the mean cysC of 0.99±0.18 mg/L in this quintile and the corresponding mean estimated glomerular filtration rate of 69±15 mL·min−1·1.73 m−2 (Table 2), we designated quintile 5 of cysC as “mild renal dysfunction.” Only 38 subjects had cysC ≥1.23 mg/L, which approximates an estimated glomerular filtration rate <60 mL·min−1·1.73 m−2,30 commonly accepted as CKD.2
Table 2. MDC-CC: Renal Demographics by Cystatin C Quintiles*.
| CysC Quintile | CysC Range, mg/L | CysC, Mean±SD, mg/L | eGFR (MDRD), Mean±SD, mL·min−1·1.73 m−2 | No. of Subjects |
|---|---|---|---|---|
| 1 | 0.28–0.67 | 0.62±0.05 | 80.54±15.75 | 1002 |
| 2 | 0.68–0.73 | 0.71±0.02 | 77.99±14.02 | 927 |
| 3 | 0.74–0.79 | 0.77±0.02 | 74.93±14.49 | 932 |
| 4 | 0.80–0.87 | 0.83±0.02 | 72.96±13.68 | 904 |
| 5 | 0.88–3.29 | 0.99±0.18 | 68.84±14.57 | 992 |
CysC indicates cystatin C; eGFR, estimated glomerular filtration rate; MDC-CC, cardiovascular cohort of the Malmö Diet and Cancer Study; and MDRD, Modification of Diet in Renal Disease.
Baseline plasma cysC data were available in 4757 individuals, who were divided into cysC quintiles. eGFR was calculated using the MDRD Study equation.
Mild Renal Dysfunction Is Linked to Low HDL-C Level and High Monocyte Count
In cross-sectional analysis of 4581 to 4757 MDC-CC individuals with available covariate data, lower levels of renal function marked by higher cysC concentrations were associated with lower fasting plasma HDL-C levels and higher monocyte counts (Table 3; Figure, A and C; P<0.001 [ANOVA]). The percentage decrease in HDL-C and increase in monocytes with lower levels of renal function across cysC quintiles 1 to 5 were similar: 14% and 11%, respectively. Consistent with our primary hypothesis, individuals in quintile 5 of cysC with mild renal dysfunction had the lowest plasma HDL-C level (49.4±13.7 mg/dL) and the highest monocyte count (0.54±0.19 million cells/mL). Because HDL-C levels are typically inversely correlated with plasma triglyceride levels,7 we also explored the relationship of triglyceride levels to cysC and indeed observed that individuals in quintile 5 of cysC had the highest plasma triglyceride level (139.7±70.6 mg/dL; Table 3; Figure, B) and the greatest insulin resistance (HOMA-IR score; Table 3).
Table 3. MDC-CC: Metabolic Risk and Monocyte Count by Cystatin C Quintiles.
| CysC Quintile | Three AA Score* (Isoleucine+Phenylalanine+Tyrosine) | HOMA-IR† | HDL-C Level,† mg/dL | TG Level,† mg/dL | Monocytes,† Million Cells/mL |
|---|---|---|---|---|---|
| 1 | −0.19±0.98 | 1.7±1.9 | 57.6±14.6 | 101.5±57.8 | 0.48±0.17 |
| 2 | −0.07±0.97 | 1.7±1.9 | 54.7±14.4 | 115.5±64.6 | 0.50±0.17 |
| 3 | −0.03±0.97 | 2.0±2.4 | 52.8±13.8 | 122.8±69.6 | 0.51±0.18 |
| 4 | 0.15±1.08 | 2.0±2.2 | 51.1±13.1 | 131.7±85.5 | 0.51±0.16 |
| 5 | 0.13±0.96 | 2.6±4.7 | 49.4±13.7 | 139.7±70.6 | 0.54±0.19 |
| P | 0.017 | <0.001 | <0.001 | <0.001 | <0.001 |
Values are mean±SD.
AA indicates amino acid; CysC, cystatin C; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; MDC-CC, cardiovascular cohort of the Malmö Diet and Cancer Study; and TG, triglycerides.
Baseline plasma metabolite Profiling was performed in 759 individuals with complete data on cysC. All AA metabolite variables are log-transformed and standardized continuous variables.
From 4757 subjects with baseline plasma cysC, the HOMA-IR (n=4581), plasma HDL-C (n=4662), TG (n=4709), and monocytes (n=4757) were measured. Monocytes were derived by an automatic cell counter 3-part differential method that distinguished cells on the basis of their size (lymphocytes, monocytes plus rare basophils/eosinophils, and neutrophils).
Figure.
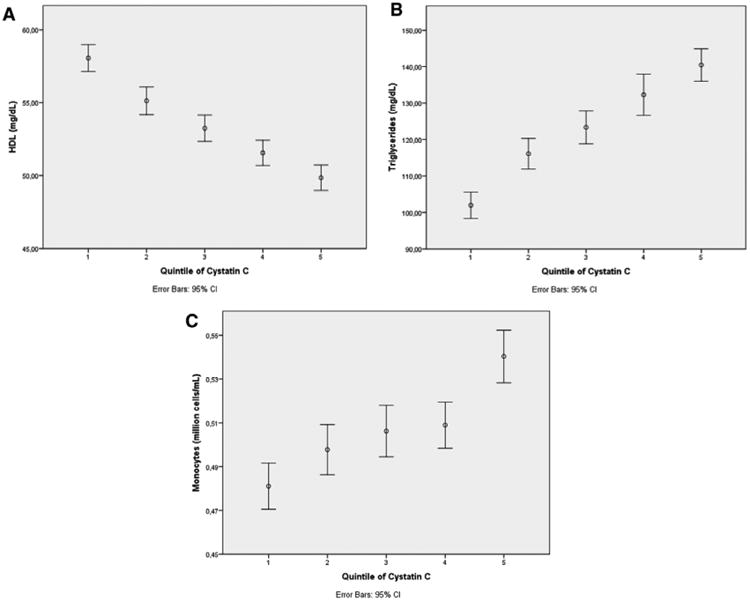
Mild renal dysfunction is linked to a low level of high-density lipoprotein (HDL) cholesterol, a high triglyceride level, and high numbers of monocytes. In cross-sectional analysis of 4662 to 4757 individuals in the cardiovascular cohort of the Malmö Diet and Cancer Study who had available covariate data, lower levels of renal function marked by higher cystatin C concentrations were associated with lower fasting plasma HDL levels (A), higher fasting plasma triglyceride levels (B), and higher monocyte counts (C) (P<0.001 for each, ANOVA). Results are shown as the mean of each variable by cystatin C quintile, with error bars representing the 95% confidence interval (CI). Monocytes were derived by an automatic cell counter 3-part differential method that distinguished cells on the basis of their size (lymphocytes, monocytes plus rare basophils/eosinophils, and neutrophils).
Mild Renal Dysfunction Is Linked to a High-Risk Metabolic Profile
In 759 MDC-CC individuals derived from the nested incident cardiovascular disease case-control study27 and nested incident diabetes case-control study24 described above, we used a liquid chromatography–mass spectrometry–based platform23,24 to investigate whether branched-chain and aromatic AAs previously associated with insulin resistance and diabetes risk23,24 would be associated with worsening renal function. Higher cysC concentrations were associated with higher levels of the 3 AA score (isoleucine+phenylalanine+tyrosine) and the 5 AA score (isoleucine+phenylalanine+tyrosine+val ine+leucine), which predict onset of future diabetes24 (Table 3; P=0.017 and P=0.048, respectively [ANOVA]). Of note, in previously published linear regression analyses adjusted for age and sex, each individual AA listed above has a highly significant inverse relationship with plasma HDL-C, a significant positive relationship with plasma triglycerides, and various associations with other metabolic traits and insulin resistance phenotypes, which highlights the connection of the AAs we measured to metabolic risk.23 Individuals in quintile 5 of cysC with mild renal dysfunction exhibited a high-risk profile, with elevated branched-chain and aromatic AAs, low HDL-C, high triglycerides, high HOMA-IR score, and high monocytes (Table 3).
Mild Renal Dysfunction and Low HDL-C Level Are Associated With Increased Risk of Being in the Top Quartile of Monocytes
In MDC, the top quartile of monocytes, measured at the time of the baseline examination and defined above, was associated with future coronary events in an adjusted analysis of >25 000 individuals.29 Therefore, we cross-sectionally examined the top quartile of monocytes as a surrogate cardiovascular end point in a multivariable logistic regression model that contained age, sex, log-transformed HOMA-IR, HDL-C, and quintiles 1 to 5 of cysC (n=4574; Table 4). Compared with quintile 1 of cysC, quintile 5 of cysC (mild renal dysfunction) was independently associated with 57% increased odds of being in the top quartile of monocytes (OR, 1.570; 95% CI, 1.215–2.029; P=0.001). Quintiles 2 to 4 of cysC were not independently associated with the top quartile of monocytes. CysC remained associated with the top quartile of monocytes when entered into the multivariable model as a standardized continuous variable instead of being divided into quintiles; however, the odds were attenuated (OR, 1.155; 95% CI, 1.072–1.246; P<0.001), which confirmed that individuals at greatest risk for increased monocytes were in quintile 5 of cysC. HDL-C level was independently associated with 42% decreased odds of being in the top quartile of monocytes (OR, 0.576; 95% CI, 0.444–0.746; P<0.001). Female sex was also independently associated with 38% decreased odds (OR, 0.624; 95% CI, 0.528–0.737; P<0.001). Age and baseline HOMA-IR were not independently associated with the top quartile of monocytes (Table 4). Given the known strong association between cigarette smoking and monocyte count,31 we then further adjusted our logistic regression model for this variable. We discovered that both the increased odds of monocytosis associated with quintile 5 of cysC (mild renal dysfunction) and the decreased odds of monocytosis associated with HDL-C level were independent of current smoking and all other variables in the model (Table 4).
Table 4. MDC-CC: Independent Associations With Top Quartile of Monocytes*.
| With Further Adjustment for Smoking | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | OR | 95% CI | P | OR | 95% CI | P |
| Quintile 5 CysC | 1.570 | 1.215–2.029 | 0.001 | 1.443 | 1.115–1.868 | 0.005 |
| Quintile 4 CysC | 1.120 | 0.861–1.458 | 0.397 | 1.069 | 0.821–1.393 | 0.620 |
| Quintile 3 CysC | 1.242 | 0.962–1.604 | 0.097 | 1.214 | 0.939–1.569 | 0.140 |
| Quintile 2 CysC | 1.097 | 0.846–1.422 | 0.486 | 1.074 | 0.827–1.394 | 0.592 |
| Quintile 1 CysC | 1.000 | Referent | … | 1.000 | Referent | … |
| HDL-C | 0.576 | 0.444–0.746 | <0.001 | 0.599 | 0.462–0.776 | <0.001 |
| Sex (female) | 0.624 | 0.528–0.737 | <0.001 | 0.618 | 0.523–0.731 | <0.001 |
| Age | 1.003 | 0.989–1.017 | 0.665 | 1.009 | 0.995–1.023 | 0.216 |
| HOMA-IR | 0.970 | 0.734–1.282 | 0.830 | 1.030 | 0.780–1.361 | 0.834 |
| Current smoking | … | … | … | 1.713 | 1.450–2.023 | <0.001 |
CI indicates confidence interval; CysC, cystatin C; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance (log-transformed); MDC-CC, cardiovascular cohort of the Malmö Diet and Cancer Study; and OR, odds ratio.
All variables above were analyzed together in a multivariable logistic regression model (n=4574) to evaluate the outcome of top quartile of monocytes.
Elevated Levels of Metabolites Tied to Low HDL-C Are Associated With Increased Risk of Being in the Top Quartile of Monocytes
In 752 MDC-CC individuals, we used a liquid chromatography– mass spectrometry–based platform23,24 to assess whether certain AA metabolites tied to low plasma HDL-C23 were also independently associated with increased odds of being in the top quartile of monocytes at baseline (Table 5). Individual multivariable logistic regression models contained the log-transformed and standardized candidate metabolite of interest, as well as age, sex, log-transformed HOMA-IR, cysC, and HDL-C. Tyrosine, glutamate, carnitine, alanine, n-carbamoyl-β-alanine, allantoin, and dimethylglycine, each of which has a known inverse association with HDL-C,23 were independently associated with 22% to 34% increased odds of being in the top quartile of monocytes (P<0.05 for all). a-Glycerophosphocholine, which is positively associated with plasma triglycerides,23 was independently associated with 23% increased odds of being in the top quartile of monocytes (P=0.02). Interestingly, the 3 AA score (isoleucine+ phenylalanine+tyrosine), the components of which are inversely associated with plasma HDL-C and positively associated with plasma triglycerides,23 predicts onset of future diabetes24 and cardiovascular events27 and increases as renal function declines (Table 3). Each 1-SD increase of the 3 AA score was independently associated with a 28% increased odds of being in the top quartile of monocytes (OR, 1.281; 95% CI, 1.039–1.581; P=0.02). Glutamine, which is correlated with high plasma HDL-C and negatively associates with insulin resistance phenotypes,23 was nearly significantly associated with 14% reduced odds of being in the top quartile of monocytes (OR, 0.858; 95% CI, 0.714–1.030; P=0.1; Table 5).
Table 5. Independent Metabolite Associations With Top Quartile of Monocytes*.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Three AA score (isoleucine+phenyl alanine+tyrosine) | 1.281 | 1.039–1.581 | 0.021 |
| Five AA score (isoleucine+phenylal anine+tyrosine+valine+leucine) | 1.239 | 1.004–1.530 | 0.046 |
| Tyrosine | 1.335 | 1.098–1.624 | 0.004 |
| Glutamate | 1.295 | 1.072–1.563 | 0.007 |
| Glutamine | 0.858 | 0.714–1.030 | 0.100 |
| Carnitine | 1.241 | 1.025–1.502 | 0.027 |
| Alanine | 1.245 | 1.024–1.513 | 0.028 |
| n-carbamoyl-β-alanine | 1.228 | 1.014–1.486 | 0.035 |
| Allantoin | 1.250 | 1.033–1.514 | 0.022 |
| Dimethylglycine | 1.220 | 1.013–1.470 | 0.036 |
| a-Glycerophosphocholine | 1.232 | 1.030–1.473 | 0.023 |
AA indicates amino acid; CI, confidence interval; and OR, odds ratio.
Each AA variable above was analyzed in a separate multivariable logistic regression model that evaluated the outcome of top quartile of monocytes, adjusted for sex, age, log-transformed homeostasis model assessment of insulin resistance, high-density lipoprotein cholesterol, and continuous standardized cystatin C (n=752). All metabolite variables are log-transformed and standardized continuous variables. Ten other individual metabolites were analyzed that did not reach statistical significance: proline, adenosine, choline, serotonin, taurine, trimethylamine N-oxide, phenylalanine, isoleucine, leucine, and valine.
Mild Renal Dysfunction, Low HDL-C Level, and Monocytes Are Independently Associated With Carotid Atherosclerosis
We examined the cross-sectional atherosclerosis end point IMTmaxBulb (n=3134), measured in millimeters and log-transformed. A multivariable linear regression model to evaluate this atherosclerosis outcome contained age, sex, log-transformed HOMA-IR, HDL-C, quintiles 1 to 5 of cysC, and monocyte count (Table 6). Age was independently and strongly associated with IMTmaxBulb (β=0.24; P<0.001). In addition, monocytes were independently and significantly associated with increased IMTmaxBulb (P=0.02), which confirmed the results of other large studies linking monocytes to carotid IMT and atherosclerotic plaque formation.13,14 Mild renal dysfunction (quintile 5 of cysC), compared with quintile 1 of cysC, was also independently and significantly associated with increased IMTmaxBulb (P<0.01). Quintiles 2 to 4 of cysC were not independently associated with IMTmaxBulb. When cysC was entered into the multivariable model (which contained monocytes and the other variables above) as a standardized continuous variable instead of divided into quintiles, it remained strongly and independently associated with IMTmaxBulb (P<0.01). Finally, low HDL-C level was independently associated with an increase (P=0.02) and female sex with a decrease (P<0.001) in IMTmaxBulb (Table 6). On further adjustment of our linear regression model for current smoking, low HDL-C and mild renal dysfunction (quintile 5 of cysC) remained significantly and independently associated with IMTmaxBulb. The association of monocytes with atherosclerosis in this expanded model was overshadowed by the strong effect of smoking (Table 6); however, in a published analysis of >25 000 MDC individuals, the top quartile of monocytes measured at the time of the baseline examination was associated with future coronary events independent of smoking.29
Table 6. MDC-CC: Independent Associations With Carotid Bulb Intima-Media Thickness*.
| With Further Adjustment for Smoking | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Standardized β-Coefficient (SE) | P | Standardized β-Coefficient (SE) | P |
| Age | 0.243 (0.001) | <0.001 | 0.260 (0.001) | <0.001 |
| Sex (female) | −0.082 (0.006) | <0.001 | −0.085 (0.006) | <0.001 |
| Monocytes | 0.039 (0.016) | 0.024 | 0.024 (0.016) | 0.173 |
| HDL-C | −0.047 (0.009) | 0.019 | −0.040 (0.009) | 0.044 |
| Quintile 5 CysC | 0.072 (0.009) | 0.002 | 0.055 (0.009) | 0.018 |
| Quintile 4 CysC | 0.017 (0.009) | 0.455 | 0.007 (0.009) | 0.761 |
| Quintile 3 CysC | −0.002 (0.009) | 0.915 | −0.008 (0.009) | 0.722 |
| Quintile 2 CysC | 0.008 (0.009) | 0.725 | 0.003 (0.009) | 0.894 |
| Quintile 1 CysC | Referent | … | Referent | … |
| HOMA-IR | 0.002 (0.011) | 0.895 | 0.012 (0.010) | 0.508 |
| Current smoking | … | … | 0.122 (0.006) | <0.001 |
CysC indicates cystatin C; HDL-C, high-density lipoprotein cholesterol; and HOMA-IR, the homeostasis model assessment of insulin resistance (log-transformed).
All variables above were analyzed together in a multivariable linear regression model (n=3134 for the outcome log-transformed maximal carotid bulb intima-media thickness).
Discussion
It is well established that individuals with early decrements in renal function, measured by cysC, are at increased risk for cardiovascular events and death21,22; however, the mechanism underlying the strong relationship of cysC to cardiovascular risk has remained a matter of considerable debate.32–35 Because of the lack of independent association between cysC levels and carotid IMT in recent population-based studies,32,33 it has been suggested that in contrast to patients with CKD9 and ESRD,10 accelerated atherosclerosis may not be the primary mechanism explaining the independent relationship between cysC level and cardiovascular risk in individuals with early kidney disease.32,33 However, other studies demonstrated that in individuals with an estimated glomerular filtration rate >60 mL·min−1·1.73 m−2, cysC is associated with early-stage coronary atherosclerotic plaque morphology on multidetector computed tomography,34 as well as with coronary atherosclerosis extent by angiography, 35 after adjustment for traditional risk factors. Because of the well-known association between cysC and cardiovascular events21,22 and the fact that cysC concentrations perform better than creatinine-based equations in predicting glomerular filtration rate in individuals at higher levels of renal function,36 we hypothesized that mild renal dysfunction measured by cysC would be associated with elevated monocyte count at baseline, an important marker and likely mediator of atherosclerotic plaque formation13,14 that is increased in ESRD.15
We now report in a cohort of >4500 individuals that even mild levels of renal dysfunction are accompanied by higher levels of circulating monocytes, and compared with the first quintile cysC, the fifth quintile cysC in the present study was strongly and independently associated with 44% to 57% increased odds of monocytosis at baseline, after adjustment for traditional risk factors. Consistent with our findings, a recent publication associated cysC with peripheral monocyte count in a small population sample (490 subjects)37 but did not relate monocytes to HDL-C levels or carotid IMT measurements. The present study, which was nearly 10 times larger, showed elevated monocyte counts in subjects with mild renal dysfunction and also demonstrated that low HDL-C and AA metabolites tied to low HDL-C23 were independently associated with the monocytosis of mild renal dysfunction. Moreover, we have shown a strong relationship of monocyte count with IMT at the carotid bifurcation, an area of low sheer stress prone to early plaque formation,38 which suggests a direct mechanism of accelerated atherogenesis in mild renal dysfunction. We propose that the largely unelucidated mechanisms underlying the relationship of cysC to cardiovascular risk21,22 involve increased circulating monocytes and low HDL-C level, which leads to accelerated atherosclerosis. Importantly, although ESRD patients undergoing hemodialysis have elevated total monocyte counts compared with control subjects,15 small studies to date have not shown an increase in total monocyte counts at baseline in individuals with predialysis stages of CKD.17,18 The present new findings raise the possibility that low HDL-C may be causally related to defective cholesterol efflux in hematopoietic stem and progenitor cells and myeloid cells of patients with mild renal dysfunction, promoting increased monocyte formation.
We made the novel discovery that individuals in the fifth quintile of cysC exhibited a high-risk metabolic profile with elevated branched-chain and aromatic AAs and that the combination of isoleucine+phenylalanine+tyrosine not only forecasts diabetes24 and cardiovascular events27 but is independently associated with a 28% increased odds of being in the top quartile of monocyte count at baseline. Multiple other AA metabolites tied to low plasma HDL-C level and various other insulin resistance phenotypes (tyrosine, glutamate, carnitine, alanine, n-carbamoyl-β-alanine, allantoin, dimethylglycine)23 were also independently associated with increased odds of being in the top quartile of monocytes. Insulin resistance emerges with incipient renal disease,39 and the combination of CKD plus the metabolic syndrome is associated with cardiovascular events.40 The present findings suggest that new sensitive markers of insulin resistance and the metabolic syndrome23 may represent key underlying factors that contribute to increased monocytes in mild renal dysfunction.
One possible explanation for the relationship between AA metabolites and monocytosis relates to the mechanism by which nutritional factors contribute to insulin resistance.41 Exposure of cells to high physiological concentrations of branched-chain AAs activates mammalian target of rapamycin (mTORC1) signaling pathways important for protein synthesis and inhibits early steps in insulin action, which leads to decreased glucose use in skeletal muscle.41 mTORC1 is an evolutionarily conserved protein kinase that enhances cell growth and proliferation and suppresses autophagy, a degradative process in which intracellular contents are broken down in lysosomes to provide nutrients during periods of starvation.42 Interestingly, it was very recently discovered that autophagy is required for cholesterol efflux to HDL and apolipoprotein A1 from murine macrophage foam cells,43 which suggests that major pathways that suppress autophagy, such as mTORC1, may be involved in defective cholesterol efflux and its downstream effects, including monocytosis. We therefore propose that AA-mediated activation of mTORC141 may be involved in impaired HDL-mediated cholesterol efflux via suppression of autophagy,43 and consistent with this hypothesis, we discovered that AA metabolites tied to low HDL-C23 were independently associated with increased odds of monocytosis.
Several limitations of the present study warrant consideration. First, the automatic cell counter that we used did not differentiate among monocytes and basophils/eosinophils; however, as published previously, the latter 2 leukocyte classes are rare compared with monocytes, and the same outcome that we examined was associated with future coronary events in a large adjusted analysis.29 This finding, as well as the significant and independent association we found between this count and carotid IMT, agrees with literature linking monocytes to carotid atherosclerosis13,14 and cardiovascular events17 and strengthens our results. HDL-C has been associated with monocyte count in studies one third to one fifth the size of the present study, both in healthy individuals and in subjects with the metabolic syndrome.44,45 The present much larger study extends this primary observation to a new population, demonstrating that HDL-C plays an important role in the monocytosis of individuals with mild renal dysfunction measured by cysC. We additionally showed that AA metabolites tied to low HDL-C and insulin resistance23 were associated with monocytosis and demonstrated a strong relationship of monocyte count with carotid IMT, which substantially implicates the present findings in atherogenesis. The failure of HDL-elevating therapies in recent clinical trials,46,47 as well as lack of a strong, direct relationship between HDL-elevating single-nucleotide polymorphisms and cardiovascular disease in human genome-wide association studies,48 has led to the suggestion that low HDL-C represents a risk marker only, possibly integrating the effects of insulin resistance, hypertriglyceridemia, remnant accumulation, and other factors, without a direct causal relationship to atherogenesis.48 Whether low HDL-C levels contribute directly to monocytosis and atherosclerosis risk or represent a biomarker of metabolic risk cannot be discerned from our studies.
In conclusion, we provide important evidence that cysC is significantly and independently associated with monocytosis and that the fifth quintile of cysC and monocytes were each independently associated with carotid IMT, which strongly suggests that accelerated atherosclerosis is responsible at least in part for increased cardiovascular disease risk in mild renal dysfunction. Increased monocyte counts at baseline in individuals with mild renal dysfunction may arise from low HDL-C levels, possibly reflecting defective cholesterol efflux pathways, and monocytosis and HDL-C levels are related to a high-risk AA signature that forecasts diabetes24 and cardiovascular events.27 Elevated monocytes may provide a previously unrecognized and key mechanism for the strong link between cysC and cardiovascular risk.21,22
Supplementary Material
Clinical Perspective.
Patients with chronic kidney disease have a markedly increased risk of atherosclerotic cardiovascular disease. The number of circulating monocytes and their differentiation into lipid-laden macrophages in the arterial wall are fundamental factors involved in plaque formation. In small studies of patients with end-stage renal disease, total monocyte counts and certain monocyte subsets are increased cross-sectionally compared with healthy control subjects, and specific subsets are associated with cardiovascular events and mortality in end-stage renal disease and chronic kidney disease. In a large population-based cohort, we now report in >4500 individuals the novel finding that mild renal dysfunction measured by cystatin C is strongly and independently associated with monocytosis at baseline. We also demonstrate that low high-density lipoprotein cholesterol and amino acid metabolites tied to low high-density lipoprotein cholesterol are independently associated with the monocytosis of mild renal dysfunction, and we show a strong relationship of monocyte count with carotid intima-media thickness, which suggests a direct mechanism of accelerated atherogenesis in mild renal dysfunction. The ability of high-density lipoprotein to stimulate removal of cholesterol from macrophages, “cholesterol efflux,” is thought to be central to its antiatherogenic mechanism, and in mouse models, the absence of ATP-binding cassette transporters that promote cholesterol efflux leads to proliferation of hematopoietic stem and progenitor cells, myeloid progenitor cells, and blood monocytes in association with accelerated atherosclerosis. High levels of high-density lipoprotein cholesterol suppress myelopoiesis in animal models. In individuals with mild renal dysfunction, low high-density lipoprotein cholesterol may be causally related to defective cholesterol efflux in hematopoietic stem and progenitor cells and myeloid cells, promoting increased monocyte formation and accelerated atherosclerosis.
Acknowledgments
Sources of Funding: Dr Ganda was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000040, formerly the National Center for Research Resources, grant number UL1 RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was additionally supported by the Leducq Foundation. Drs Magnusson and Melander were supported by grants from the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, the Medical Faculty of Lund University, Skåne University Hospital, the Albert Påhlsson Research Foundation, the Crafoord Foundation, the Ernhold Lundströms Research Foundation, the Region Skåne, the Hulda and Conrad Mossfelt Foundation, the Southwest Skanes Diabetes Foundation, the King Gustaf V and Queen Victoria Foundation, the Lennart Hanssons Memorial Fund, Knut and Alice Wallenberg Foundation, and the Marianne and Marcus Wallenberg Foundation. Dr Gerszten was supported by National Institutes of Health R01 grants HL 98280 and DK 081572.
Footnotes
Disclosures: Drs Gerszten and Wang are named as coinventors on a patent application to the US Patent Office pertaining to metabolite predictors of diabetes mellitus. The remaining authors report no conflicts.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.112.000682/-/DC1.
References
- 1.Yerkey MW, Kernis SJ, Franklin BA, Sandberg KR, McCullough PA. Renal dysfunction and acceleration of coronary disease. Heart. 2004;90:961–966. doi: 10.1136/hrt.2003.015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and non-traditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 4.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 6.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 7.de Boer IH, Astor BC, Kramer H, Palmas W, Seliger SL, Shlipak MG, Siscovick DS, Tsai MY, Kestenbaum B. Lipoprotein abnormalities associated with mild impairment of kidney function in the Multi-Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol. 2008;3:125–132. doi: 10.2215/CJN.03390807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nestel PJ, Fidge NH, Tan MH. Increased lipoprotein-remnant formation in chronic renal failure. N Engl J Med. 1982;307:329–333. doi: 10.1056/NEJM198208053070601. [DOI] [PubMed] [Google Scholar]
- 9.Szeto CC, Chow KM, Woo KS, Chook P, Ching-Ha Kwan B, Leung CB, Kam-Tao Li P. Carotid intima media thickness predicts cardiovascular diseases in Chinese predialysis patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1966–1972. doi: 10.1681/ASN.2006101184. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Takita T, Maruyama Y, Kumagai H, Hishida A. Impact of carotid atherosclerosis on long-term mortality in chronic hemodialysis patients. Kidney Int. 2003;64:1472–1479. doi: 10.1046/j.1523-1755.2003.00205.x. [DOI] [PubMed] [Google Scholar]
- 11.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 12.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35:1619–1624. doi: 10.1161/01.STR.0000130857.19423.ad. [DOI] [PubMed] [Google Scholar]
- 14.Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njølstad I, Arnesen E. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromsø Study. Stroke. 2005;36:715–719. doi: 10.1161/01.STR.0000158909.07634.83. [DOI] [PubMed] [Google Scholar]
- 15.Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte sub-population in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782–2790. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, Krause M, Schlitt A, Köhler H, Girndt M. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 17.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Light RP. Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1393–1399. doi: 10.2215/CJN.10521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 20.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 22.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, Deik AA, Magnusson M, Fox CS, O'Donnell CJ, Vasan RS, Melander O, Clish CB, Gerszten RE, Wang TJ. Metabolite Profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125:2222–2231. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engström G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 27.Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engstrom G, Ostling G, Clish C, Wang TJ, Gerszten RE, Melander O. A diabetes-predictive amino acid score and future cardiovascular disease. [Accessed January 1, 2013];Eur Heart J. doi: 10.1093/eurheartj/ehs424. Published online before print December13, 2012. http://eurheartj.oxfordjournals.org/content/early/2012/12/12/eurheartj.ehs424.long. [DOI] [PMC free article] [PubMed]
- 28.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 29.Adamsson Eryd S, Smith JG, Melander O, Hedblad B, Engström G. Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophil counts. Arterioscler Thromb Vasc Biol. 2012;32:533–539. doi: 10.1161/ATVBAHA.111.240416. [DOI] [PubMed] [Google Scholar]
- 30.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MR, Kinmonth AL, Luben RN, Bingham S, Day NE, Wareham NJ, Welch A, Khaw KT. Smoking status and differential white cell count in men and women in the EPIC-Norfolk population. Atherosclerosis. 2003;169:331–337. doi: 10.1016/s0021-9150(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 32.Bui AL, Katz R, Kestenbaum B, de Boer IH, Fried LF, Polak JF, Wasserman BA, Sarnak MJ, Siscovick D, Shlipak MG. Cystatin C and carotid intima-media thickness in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2009;53:389–398. doi: 10.1053/j.ajkd.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han L, Bai X, Lin H, Sun X, Chen XM. Lack of independent relationship between age-related kidney function decline and carotid intima-media thickness in a healthy Chinese population. Nephrol Dial Transplant. 2010;25:1859–1865. doi: 10.1093/ndt/gfp718. [DOI] [PubMed] [Google Scholar]
- 34.Imai A, Komatsu S, Ohara T, Kamata T, Yoshida J, Miyaji K, Shimizu Y, Takewa M, Hirayama A, Deshpande GA, Takahashi O, Kodama K. Serum cystatin C is associated with early stage coronary atherosclerotic plaque morphology on multidetector computed tomography. Atherosclerosis. 2011;218:350–355. doi: 10.1016/j.atherosclerosis.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 35.Niccoli G, Conte M, Della Bona R, Altamura L, Siviglia M, Dato I, Ferrante G, Leone AM, Porto I, Burzotta F, Brugaletta S, Biasucci LM, Crea F. Cystatin C is associated with an increased coronary atherosclerotic burden and a stable plaque phenotype in patients with ischemic heart disease and normal glomerular filtration rate. Atherosclerosis. 2008;198:373–380. doi: 10.1016/j.atherosclerosis.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 36.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evangelopoulos AA, Vallianou NG, Bountziouka V, Katsagoni C, Bathrellou E, Vogiatzakis ED, Bonou MS, Barbetseas J, Avgerinos PC, Panagiotakos DB. Association between serum cystatin C, monocytes and other Inflammatory markers. Intern Med J. 2012;42:517–522. doi: 10.1111/j.1445-5994.2011.02500.x. [DOI] [PubMed] [Google Scholar]
- 38.Hulthe J, Wikstrand J, Emanuelsson H, Wiklund O, de Feyter PJ, Wendelhag I. Atherosclerotic changes in the carotid artery bulb as measured by B-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997;28:1189–1194. doi: 10.1161/01.str.28.6.1189. [DOI] [PubMed] [Google Scholar]
- 39.Fliser D, Pacini G, Engelleiter R, Kautzky-Willer A, Prager R, Franek E, Ritz E. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53:1343–1347. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal S, Shlipak MG, Kramer H, Jain A, Herrington DM. The association of chronic kidney disease and metabolic syndrome with incident cardiovascular events: Multiethnic Study of Atherosclerosis. Cardiol Res Pract. 2012;2012:806102. doi: 10.1155/2012/806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101:1519–1529. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang YY, Juhász G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, Neufeld TP. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem Soc Trans. 2009;37(pt 1):232–236. doi: 10.1042/BST0370232. [DOI] [PubMed] [Google Scholar]
- 43.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterhouse DF, Cahill RA, Sheehan F, McCreery C. Prediction of calculated future cardiovascular disease by monocyte count in an asymptomatic population. Vasc Health Risk Manag. 2008;4:177–187. doi: 10.2147/vhrm.2008.04.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shim WS, Kim HJ, Kang ES, Ahn CW, Lim SK, Lee HC, Cha BS. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;73:284–291. doi: 10.1016/j.diabres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Boden WE, Probstfeld JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz GG, Olsson AG, Ballantyne CM, Barter PJ, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Shah PK, Tardif JC, Chaitman BR, Duttlinger-Maddux R, Mathieson J dal-OUTCOMES Committees and Investigators. Rationale and design of the dal-OUTCOMES trial: effcacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158:896–901. e3. doi: 10.1016/j.ahj.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


