Abstract
Although several potential mechanosensors/mechanotransducers have been proposed, the precise mechanisms by which ECs sense and respond to mechanical forces and translate them into biochemical signals remains unclear. Here, we report that two major ligand-dependent tyrosine autophosphorylation sites of VEGFR2, Y1175 and Y1214, are rapidly activated by shear stress in human coronary artery endothelial cells (HCAECs). Neutralizing antibody against VEGFR2 not only abrogates flow-induced phosphorylation of these tyrosine residues, but also has a marked inhibitory effect on downstream eNOS activation. In situ proximity ligation assay revealed that VEGF and VEGFR2 are closely associated in HCAECs, and more importantly, this association is increased with flow. Finally, we show that flow-induced VEGFR2 activation is attenuated in the presence of the broad spectrum matrix metalloproteinase (MMP) inhibitor, GM6001. Taken together, our results suggest that a ligand-dependent mechanism involving the activity of MMPs plays a key role in the early, shear stress-induced activation of VEGFR2.
Keywords: Shear stress, VEGF, VEGFR2, Matrix metalloproteinase
1. Introduction
Mechanotransduction is the process by which mechanical forces are converted into biochemical responses by cells. Fluid shear stress, the tangential force generated by blood flow, acts primarily on vascular endothelial cells (ECs) and has been shown to activate numerous signaling pathways and gene expression programs that are important in normal physiological processes as well as in certain pathological conditions, such as atherosclerosis and cancer. The mechanisms by which ECs sense and transduce mechanical stimulation into biochemical signals are still unclear, although several mechanosensors/mechanotransducers have been proposed, which include the cell–cell junction [1–3], the junctional adhesion molecule, platelet endothelial cell adhesion molecule-1 (PECAM-1) [4], heterotrimeric G proteins [5,6], receptor tyrosine kinases (RTKs), such as vascular endothelial growth factor receptor 2 (VEGFR2) [7], integrins [8], the glycocalyx [9], ion channels [10], and the actin cytoskeleton [11]. Since a single mechanosensor/ mechanotransducer is unlikely to exist, more attention has been focused on understanding the interactions and potential crosstalk that these different molecular components have with one another. Interestingly, a mechanosensory complex comprised of PECAM-1, VE-cadherin, and VEGFR2 has also been described and shown to be critical for the activation of a subset of known signaling pathways in response to flow [3].
VEGFR2 is a member of a superfamily of RTKs that is expressed predominantly on ECs and whose activation is critical for both normal cardiovascular development and pathological angiogenesis [12,13]. Binding of VEGFR2 by its ligand, VEGF-A (VEGF), is known to induce dimerization and activation of its receptor kinase activity,which subsequently leads to autophosphorylation on tyrosine residues. Studies by Shibuya and colleagues revealed that tyrosine residues 1175 and 1214 are the two major VEGF-dependent autophosphorylation sites of VEGFR2 [14]. The activity of VEGFR2 is primarily regulated by the bioavailability of VEGF, which in turn depends on alternative splicing of VEGF mRNA into different isoforms and also by processing of secreted VEGF and/or the extracellular matrix (ECM) to which it is bound.
The major human isoforms are VEGF121, VEGF165, and VEGF189, which differ in their binding to VEGFR2 and to various ECM molecules, including glycosaminoglycans (GAGs) found in heparan sulfate proteoglycans (HSPGs). Release of cell-associated VEGF is known to occur either through proteolytic cleavage of ECM [15] or by direct VEGF cleavage [16] by proteases such as plasmin, elastase, and a subset of matrix metalloproteases (MMPs). MMPs are a family of zinc-dependent proteases that are produced by a variety of cells, including ECs, and function to degrade components of the ECM. Several members of the family have been shown to bind to HSPGs in the ECM and/or at the cell surface where they are thought to be anchored to prevent their rapid diffusion and to position them for interaction with cell surface adhesion molecules or receptors [17].
Recent studies have revealed that endothelial cells, specifically of arterial origin, express VEGF in vivo and can activate VEGFR2 through either an intracrine and/or autocrine–juxtacrine signaling loop [18–20]. VEGFR2 is also known to be activated by shear stress with tyrosine phosphorylation detected as early as 1 min [7,21]. Since shear stress-induced tyrosine phosphorylation of VEGFR2 was not inhibited by pre-treatment with anti-VEGF antibody, it was concluded that the effect of shear stress was not due to release of VEGF and is therefore ligand-independent. However, it has been argued that autocrine VEFGR2 activation may occur intracellularly [19] and therefore is not affected by treatment with large, cell-impermeable antibodies. It has also been suggested that confluent ECs signal efficiently through a juxtacrine mechanism, which makes VEGF inaccessible to antibody neutralization [18].
In this study, we hypothesized that shear stress-induced VEGFR2 activation occurs early during EC mechanotransduction and is dependent on binding by VEGF. Furthermore, we proposed that heparan sulfates of a putative heparan sulfate proteoglycan (HSPG) act as a reservoir for VEGF, which in turn activates its receptor either through flow-induced conformational changes that bring the ligand and its receptor into closer physical proximity or proteolytic release of ligand from heparan sulfates by MMPs and instantaneous binding to its receptor.
2. Materials and methods
2.1. Cell culture
Human coronary artery endothelial cells (HCAECs) were obtained from either Lonza (Walkersville, MD) or Cell Applications, Inc. (San Diego, CA) and maintained in complete endothelial growth medium (EGM-2; Lonza) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and penicillin–streptomycin. HCAECs within six passages were used for all experiments.
2.2. Reagents
Antibodies for Western blot analysis directed against phospho-VEGFR2 (Y1175), VEGFR2, phospho-Akt (S473), and phospho-eNOS (S1177) were from Cell Signaling Technology (Danvers, MA). Antibody against phospho-VEGFR2 (Y1214) was from R&D Systems (Minneapolis, MN). Anti-Akt antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-eNOS antibody was from BD Biosciences (San Jose, CA). Neutralizing antibody against VEGFR2 (MAB3571) was also purchased from R&D Systems. Purified mouse IgG was from Invitrogen (Carlsbad, CA). Recombinant human VEGF165 was from BioLegend (San Diego, CA). GM6001 and the respective negative control, GM6001NC, were from EMD Chemicals (San Diego, CA), and reconstituted in DMSO.
2.3. Shear stress
Cells were seeded onto glass microscope slides and grown into confluent monolayers. Prior to all experimental procedures, cells were serum-starved overnight in endothelial basal medium (EBM-2, Lonza) supplemented with 1% FBS and penicillin–streptomycin to establish quiescence. Slides were mounted on a conventional parallel- plate flow chamber [22] and cells were subjected to a steady fluid shear stress of 14 dyne/cm2 by perfusion with CO2-equilibrated EBM-2 containing 0.5% bovine serum albumin (Roche, Indianapolis, IN) using a PHD 2000 syringe pump (Harvard Apparatus, Holliston, MA). Cells on slides that were mounted but not subjected to shear stress, denoted “Sham”, served as controls.
2.4. Preparation of cell lysates
Cells were scraped into ice cold DPBS containing 2 mM sodium orthovanadate and collected by centrifugation. Pellets were resuspended in lysis buffer (50 mM Tris–HCl, pH 7.5; 125 mM NaCl; 60 mM octyl-glucoside) containing protease (Complete; Roche) and phosphatase (PhosSTOP; Roche) inhibitors, which were added fresh immediately prior to cell lysis. Lysates were incubated for 30 min on ice and then centrifuged at 14,000g for 15 min at 4 °C to remove insoluble material.
2.5. Western blot analysis
Proteins were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) in MOPS SDS running buffer (Invitrogen) and transferred to PVDF membranes (Immobilon-P; Millipore, Temecula, CA). Membranes were blocked for 1 h with 3% BSA in Tris-buffered saline (TBS) and then incubated with a primary antibody for 2 h or overnight in 3% BSA–TBST (TBS with 0.1% Tween 20) at 4 °C. After washing and incubating with horseradish peroxidase-conjugated secondary antibodies for 1 h, the membranes were incubated with chemiluminescence substrate (SuperSignal West Pico or West Femto; Thermo Scientific, Rockford, IL) and exposed to X-ray film. Unsaturated films were digitally scanned and band intensities were quanitifed using ImageJ (NIH).
2.6. In situ proximity ligation assay (PLA)
PLA was performed on Methanol/Acetone-fixed HCAEC monolayers that were re-hydrated in PBS, then blocked and treated according to the manufacturer’s protocol (Olink Biosciences, Uppsala, Sweden). Primary antibodies used were a goat anti-VEGFR2 antibody (R&D Systems) and a mouse monoclonal anti-VEGF antibody (Abcam, Cambridge, MA). Images were acquired on a LSM5 PASCAL confocal fluorescence microscope (Carl Zeiss, Germany) equipped with a Plan Apochromatic 63/1.4 numerical aperture oil immersion objective and both the PLA signal and cell nuclei were quantified using ImageJ. A minimum of ten fields of acquisition were acquired for each of the four individual experiment.
2.7. Statistical analysis
All experimental data are expressed as means ± SE from at least three independent experiments. Single comparisons between groups were performed using paired, two-tailed Student’s t-test. P-values of <0.05 were considered statistically significant.
3. Results
3.1. Phosphorylation of VEGFR2 at Y1175 and Y1214 is rapidly induced by flow in HCAECs
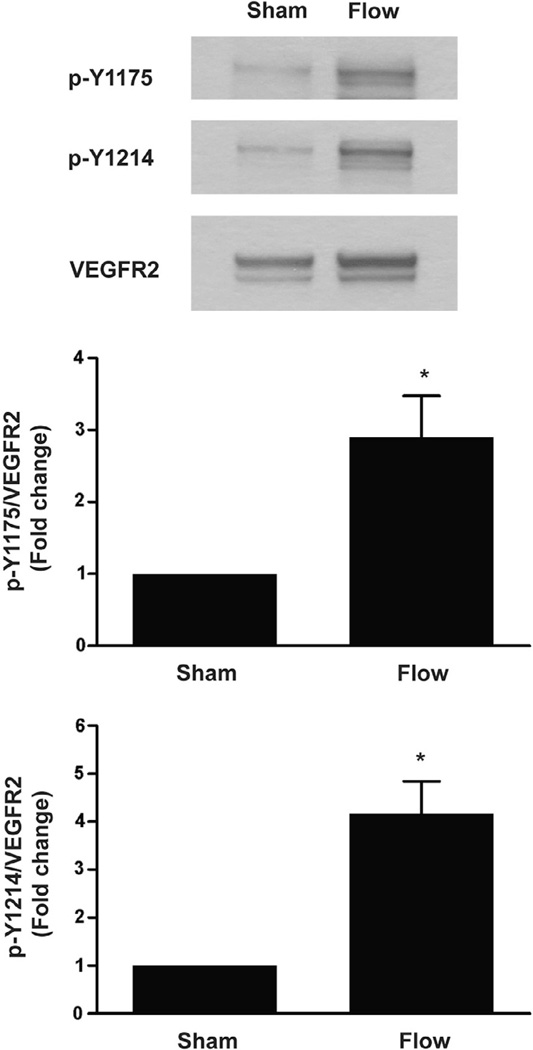
It has been previously shown that VEGFR2 phosphorylation can be induced by fluid shear stress as early as 1 min in bovine aortic endothelial cells (BAECs) [7,21]. In both studies, flow-induced VEGFR2 activation was shown by immunoprecipitation of lysates with anti-VEGFR2 followed by immunoblotting with a pan-phosphotyrosine antibody. Information regarding the phosphorylation of specific VEGFR2 tyrosine residues in response to shear stress is largely limited. To determine whether the two major VEGF-dependent autophosphorylation sites of VEGFR2, tyrosine 1175 (Y1175) and tyrosine 1214 (Y1214), are specifically activated by early flow onset, we subjected human coronary artery endothelial cells (HCAEC) to shear stress and examined their phosphorylation by immuno- blotting using phosphotyrosine-specific antibodies. As shown in Fig. 1, both Y1175 and Y1214 phosphorlyation were markedly increased (~3-fold and ~4-fold, respectively) in response to a 15 s step increase in fluid shear stress as compared to control. Quantification revealed that these increases were statistically significant (P = 0.0458 and P = 0.0187, respectively). These findings show that flow induces an early phosphorylation of two major ligand-dependent tyrosine residues within VEGFR2 in human ECs, suggesting that flow-induced inVEGFR2 activation may be ligand-dependent.
Fig. 1.
Shear stress induces early onset VEGFR2 activation. Confluent monolayers of HCAECs were exposed to flow at 14 dynes/cm2 for 15 s. Cell lysates were prepared and analyzed by SDS–PAGE followed by immunoblotting with anti-VEGFR2 and anti-phospho-VEGFR2 antibodies (p-Y1175 and p-Y1214). Sham corresponds to control cells that were mounted on a flow chamber but not subjected to flow. A representative blot from at least three independent experiments is shown. Densitometric analyses of tyrosine-specific VEGFR2 phosphorylation is depicted in bar graphs as the fold change relative to sham control with sham mean value set to 1. Error bars indicate the standard error of the mean (SEM; N = 4). *P < 0.05.
3.2. Flow-induced eNOS activation is attenuated by VEGFR2 neutralization
It had previously been concluded that the activation of VEGFR2 by shear stress is ligand-independent based on the findings that flow-induced Akt and eNOS activation were not affected by a neutralizing antibody against its ligand VEGF, despite their decrease by down regulation of VEGFR2 or pharmacological inhibition of its tyrosine kinase activity [21]. Recently, it has been demonstrated using antibody neutralization of VEGFR2 in human EC that VEGF/ VEGFR2 signaling is important for EC mechanoprotection during chronic shear stress [18]. Therefore, we sought to re-evaluate the role that VEGF ligand may play in the shear response by taking this same approach and examining its effect first, on the specific phosphorylation of Y1175 and Y1214, and then secondly, on signaling pathways known to be mediated by flow.
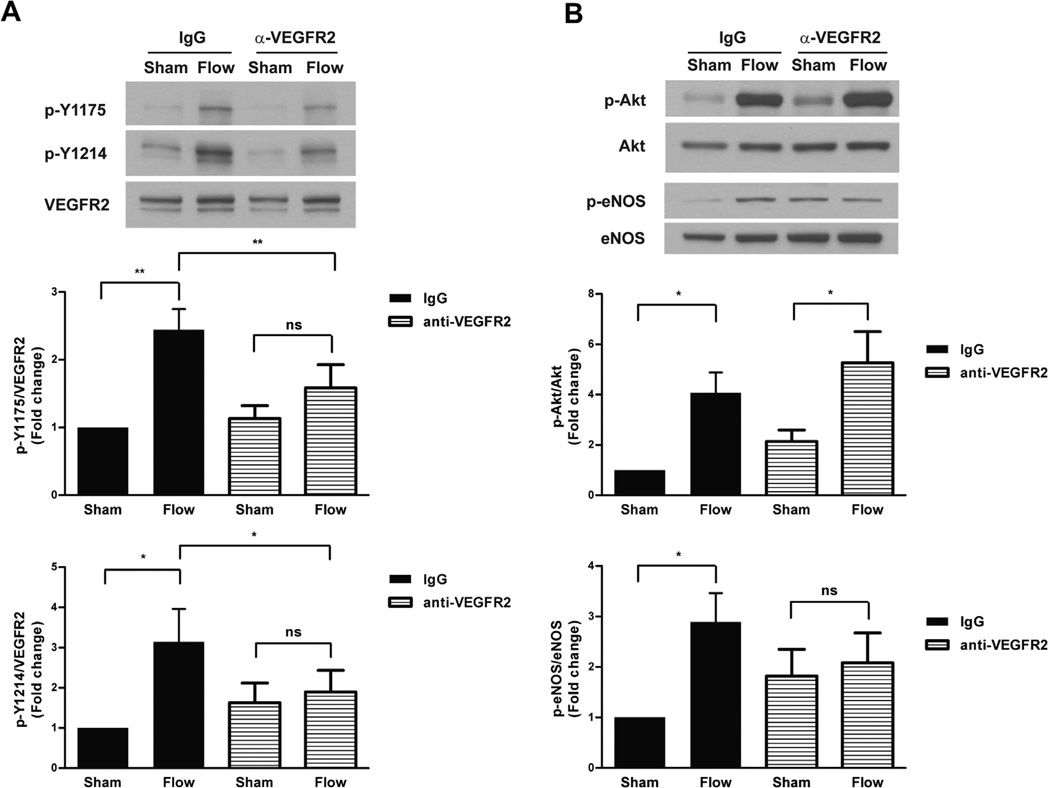
HCAECs were treated with a neutralizing antibody against the extracellular domain of VEGFR2 prior to exposure to shear stress to block the binding of ligand to its receptor. As shown in Fig. 2A, flow-induced phosphorylation of both Y1175 and Y1214 of VEGFR2 after 15 s was abrogated by the presence of anti-VEGFR2 when compared to that in cells treated with pre-immune IgG. To examine the effects of VEGFR2 neutralization on downstream signaling pathways known to be activated by flow, we next analyzed the activation of Akt and eNOS (Fig. 2B). As expected, phosphorylation of Akt was markedly increased (4-fold) in HCAECs pre-treated with IgG and subjected to flow (5 min). However, pretreatment with anti-VEGFR2 IgG had no statistically significant effect on shear-induced Akt phosphorylation indicating that flow-induced Akt phosphorylation was not mediated by ligand activation of VEGFR2. eNOS phosphorylation was significantly increased in control experiments in which cells were pre-incubated with IgG. Interestingly, in the presence of the VEGFR2 neutralizing antibody, there was no significant increase in eNOS phosphorylation compared to the sham condition. Altogether, these findings suggest that early VEGFR2 activation in response to flow is ligand-dependent. Furthermore, flow-induced eNOS, but not Akt activation, is mediated by this ligand-dependent activation of VEGFR2.
Fig. 2.
Antibody neutralization abrogates the VEGFR2 shear response. (A) HCAECs were pre-incubated with purified mouse IgG or mouse anti-VEGFR2 IgG (5 lg/ml) for 5 min prior to exposure to 15 s of shear stress. Cell lysates were analyzed by SDS–PAGE followed by immunoblotting for VEGFR2, p-Y1175, and p-Y1214. A representative blot from at least three independent experiments is shown. The bar graphs show the quantification of VEGFR2 phosphorylation as the fold change relative to IgG-treated sham control with its mean value set to 1. Error bars indicate SEM (N = 5 and N = 6 for p-Y1175 and p-Y1214, respectively). *P < 0.05, **P < 0.01. (B) HCAECs were subjected to 5 min of shear stress after pre-incubation (5 min) with either control IgG or anti-VEGFR2 IgG. Cell lysates were analyzed for Akt and eNOS phosphorylation by immunoblotting for phospho- Akt (S473) and phospho-eNOS (S1177). Total Akt and eNOS expression levels were used for normalization. A representative blot from at least three independent experiments is shown. Bar graphs show the quantification as the fold change relative to control sham and error bars indicating SEM (N = 5 and N = 4, respectively). *P < 0.05.
3.3. Flow causes an increase in VEGF-VEGFR2 association
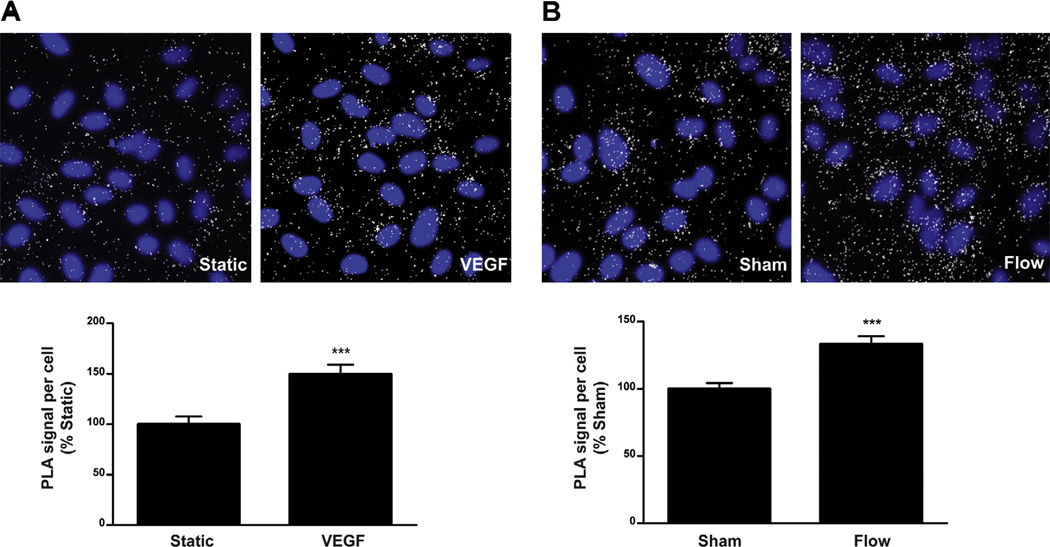
To gain insight into the mechanism of flow-induced VEGFR2 phosphorylation, we examined the physical associations between VEGF and VEGFR2 in response to shear stress using in situ proximity ligation assay. Consistent with findings from a recent report [23], a complex of endogenous VEGF and VEGFR2 also exists in quiescent HCAECs. More importantly, a significant increase (33.5%; P < 0.0001) in VEGF-VEGFR2 association is detected as early as 15 s after the onset of flow (Fig. 3B), the same time point we observed phosphorylation of Y1175 and Y1214. As a control, PLA was performed on cells with and without treatment with exogenous VEGF165 (Fig. 3A), which similarly showed increased VEGF-VEGFR2 association. Taken together, these results strongly support the notion that VEGFR2 activation induced by flow is ligand-dependent.
Fig. 3.
VEGF and VEGFR2 proximity is increased with flow. In situ proximity ligation assay (PLA) was performed using antibodies directed against VEGF and VEGFR2 on HCAECs that were left untreated (Static) or stimulated with VEGF165 (200 ng/ml) for 5 min, (A) and on HCAECs that were subjected to no flow (Sham) or step flow for 15 s (B). Representative confocal images of cells with the indicated treatments are shown. White dots indicate close proximity of VEGF and VEGFR2. Nuclei are shown in blue. Bar graphs show the quantification as PLA signal per cell relative to control, which is set to 100%. Error bars indicate SEM (Sham, N = 40; Flow, N = 41). ***P < 0.0001.
3.4. MMP activity is important for flow-induced VEGFR2 activation
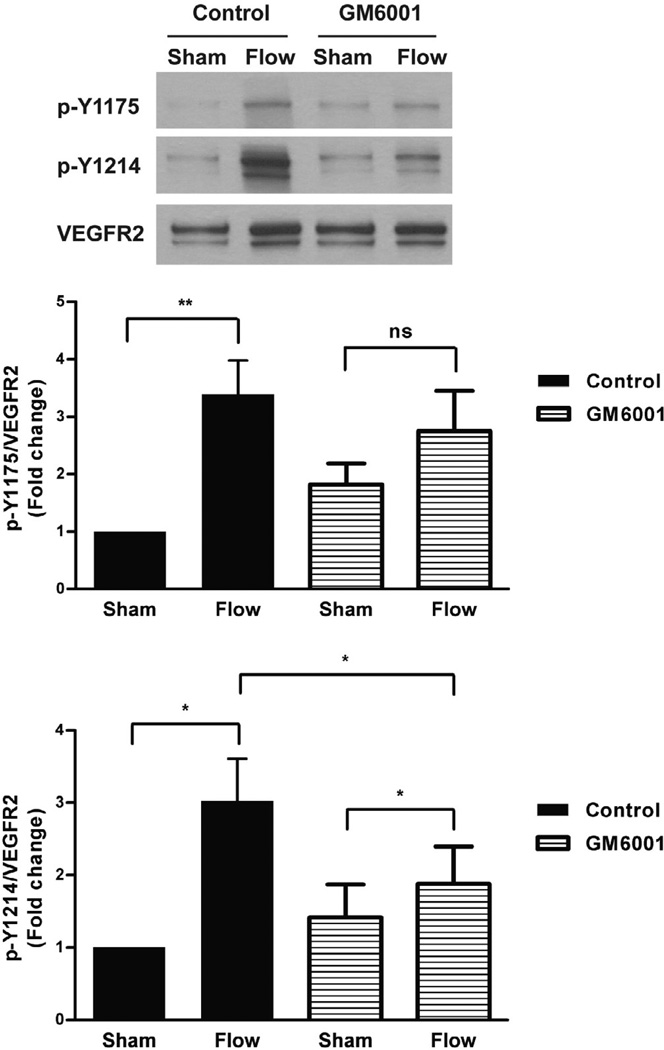
It is well-established that transactivation of the epidermal growth-factor receptor (EGFR), another member of the RTK family of cell surface receptors, by G protein-coupled receptor (GPCR) stimulation involves the release of its ligand, heparin-binding EGF (HB-EGF), from the cell surface via an MMP-dependent mechanism [24]. To determine whether ligand-dependent VEGFR2 activation in response to flow is controlled by a similar MMP-mediated mechanism, we treated HCAECs with the broad spectrum MMP inhibitor, GM6001 (2 µM), prior to exposure to shear stress (Fig. 4). As a negative control, we used its inactive analogue, GM6001, Negative control (GM6001NC; 2 µM). As expected, shear stress caused a marked increase in both phospho-Y1175 and phospho- Y1214 (~3.4-fold and ~3-fold, respectively) in cells pre-treated with GM6001NC for 10 min, which is similar to the levels we typically observe in untreated cell (Fig. 1). In cells pre-treated with GM6001, however, Y1175 phosphorylation was no longer increased by shear stress when compared to sham. Additionally, flow-induced Y1214 phosphorylation was reduced significantly in cells pre-treated with GM6001 compared to control cells. Taken together, our findings suggest that ligand-dependent VEGFR2 activation in response to shear stress is mediated by the activity of MMPs.
Fig. 4.
Flow-Induced VEGFR2 phosphorylation is attenuated by MMP inhibition. HCAECs were subjected to no flow (Sham) or flow for 15 s after pre-incubation (10 min) with the MMP inhibitor GM6001 or the inactive analogue GM6001NC (negative control). Western blot analyses were performed on cell lysates to examine VEGFR2 phosphorylation. A representative blot from at least three independent experiments is shown. Phospho-Y1175 and phospho-Y1214 were quantified and depicted in the bar graphs as fold change relative to control sham with error bars indicating SEM (N = 7). *P < 0.05, **P < 0.01.
4. Discussion
This study provides the first evidence that two major ligand-dependent autophosphorylation sites of VEGFR2, Y1175 and Y1214, are activated as early as 15 s after onset of fluid shear stress in human endothelial cells. Furthermore, we show that by blocking the ability of VEGF to bind to its receptor with a neutralizing antibody against VEGFR2, activation of these two major sites is significantly reduced. Our results obtained using in situ PLA not only verify the existence of a complex between endogenous VEGF and VEGFR2, but also indicates that their association is increased under flow conditions. Altogether, these results argue in favor of a ligand-dependent mechanism of VEGFR2 activation by shear stress in human ECs.
It has long been thought that activation of VEGFR2 by shear stress is a ligand-independent event based on the fact that anti-VEGF antibody had no inhibitory effect [7,21]. However, two recent studies using alternative approaches for inhibiting VEGF-VEGFR2 signaling, such as cell-specific genetic deletion of VEGF, small molecule antagonists against VEGFR2, siRNA knockdown of VEGF, and VEGFR2 neutralization in addition to VEGF neutralization showed that whereas extracellular blockade of VEGF was insufficient in inhibiting downstream VEGF signaling by endogenous VEGF, i.e. vascular EC homeostasis/survival, the other methods proved successful [18,19]. Despite their different conclusions, intracrine vs. juxtacrine, both studies support the idea that VEGF is inaccessible to anti-VEGF antibody neutralization.
There may be other reasons for the conflicting results between the published literature and those we report here. First, it is unclear at least from the data presented by Jin et al. whether the neutralizing antibody against human VEGF (577B11) [25] that was used is cross-reactive with bovine VEGF or even functional for that matter as they did not show its effect directly on VEGFR2 phosphorylation. Secondly, it is possible that neutralizing antibody directed against VEGF can only bind soluble VEGF, i.e. VEGF121, and can not readily access cell-associated VEGF, i.e. VEGF165 and VEGF189, especially when sequestered and protected by heparan sulfates. In the case of exogenously added VEGF165, as was used in [21] as a positive control, there is no issue of accessibility since there was a 30 min pre-incubation period with anti-VEGF. Finally, there is the obvious difference in experimental model systems used between the two studies. Specifically, our studies were performed in HCAECs, which we selected based on the observation that all three major VEGF isoforms are expressed (unpublished results). In contrast, Jin et al. used BAECs, which have been shown not to express VEGF transcripts [26]. It is possible that bovine and human ECs respond differently to shear stress and have different mechanisms for eliciting the same biological pathways.
Another major difference between our findings and the aforementioned study is with regards to the dependency of downstream signaling on VEGFR2 activation. Whereas Jin et al. demonstrated that both eNOS and Akt phosphorylation require VEGFR2 activation, we observed that only eNOS phosphorylation is affected. The inability of VEGFR2 neutralization to affect flow-induced Akt phosphorylation despite attenuating eNOS phosphorylation is consistent with the findings by Jo and colleagues, who demonstrated that shear stress stimulates phosphorylation of eNOS by an Akt-independent mechanism [27]. The fact that flow-induced eNOS activation is not completely inhibited by VEGFR2 neutralization indicates that there is still some contribution from an Akt-dependent pathway [28].
Taken together, our results suggest that flow rapidly induces MMP activity, which in turn releases bound VEGF from an unidentified heparan sulfate proteoglycan(s) either through direct cleavage of VEGF [16] or cleavage of heparan sulfates [15]. This cleavage likely occurs in close proximity to VEGFR2, which allows capture of ligand and subsequent activation. The proenzyme forms of MMP-7, -9, and -13 as well as the proenzyme and active forms of MMP-2 have all been shown to bind to heparin [17]. Of these four proteases, MMP-9, but not MMP-2, is capable of liberating VEGF from heparan sulfates [15], whereas MMP-13 has been shown to degrade the protein core of a specific HSPG, perlecan [29]. MMP-7 and -9, but not MMP-2 and -13, have been reported to directly cleave murine VEGF164 [16]. Interestingly, human VEGF seems to be resistant to cleavage by any of the four heparin-binding MMPs [30], suggesting that direct cleavage of VEGF in our system is improbable.
We cannot rule out the possibility that flow causes an increased physical association between VEGFR2 and a putative HSPG that is decorated with growth factors, such as VEGF, and active protease( s) that function to release them. We would predict an initial increased association of VEGFR2 with an HSPG in response to flow followed by dissociation after VEGFR2 activation. Whatever the case may be, future studies specifically targeting MMP-9 and/or MMP-13 and HSPGs may provide further insights into understanding the mechanism(s) of VEGF-dependent VEGFR2 activation in response to early flow onset.
Acknowledgments
We thank Jiunn-Chern Yeh for preliminary studies. This work was supported by National Institutes of Health MERIT Award 5R37HL040696-26 and 3R37HL040696-26S1.
References
- 1.Melchior B, Frangos JA. Shear-induced endothelial cell-cell junction inclination. Am. J. Physiol. Cell Physiol. 2010;299:C621–C629. doi: 10.1152/ajpcell.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J. Physiol. 2009;587:2365–2373. doi: 10.1113/jphysiol.2009.172643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 4.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ. Res. 1996;79:834–839. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- 6.Melchior B, Frangos JA. Galphaq/11-mediated intracellular calcium responses to retrograde flow in endothelial cells. Am. J. Physiol. Cell Physiol. 2012;303:C467–C473. doi: 10.1152/ajpcell.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases integrins, and Shc . J. Biol. Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 8.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. USA. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 10.Barakat AI, Leaver EV, Pappone PA, Davies PF. A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ. Res. 1999;85:820–828. doi: 10.1161/01.res.85.9.820. [DOI] [PubMed] [Google Scholar]
- 11.Osborn EA, Rabodzey A, Dewey Jr. CF, Hartwig JH. Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am. J. Physiol. Cell Physiol. 2006;290:C444–C452. doi: 10.1152/ajpcell.00218.2005. [DOI] [PubMed] [Google Scholar]
- 12.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 13.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G, Hommes DW, Lamers CB, Sier CF. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur. J. Cancer. 2008;44:1904–1913. doi: 10.1016/j.ejca.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu WH, Woessner JF., Jr Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7) J. Biol. Chem. 2000;275:4183–4191. doi: 10.1074/jbc.275.6.4183. [DOI] [PubMed] [Google Scholar]
- 18.dela Paz NG, Walshe TE, Leach LL, Saint-Geniez M, D’Amore PA. Role of shear-stress-induced VEGF expression in endothelial cell survival. J. Cell Sci. 2012;125:831–843. doi: 10.1242/jcs.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA. Vascular endothelial growth factor localization in the adult. Am. J. Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ. Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 22.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 23.E G, Cao Y, Bhattacharya S, Dutta S, Wang E, Mukhopadhyay D. Endogenous vascular endothelial growth factor-A (VEGF-A) maintains endothelial cell homeostasis by regulating VEGF receptor-2 transcription. J. Biol. Chem. 2012;287:3029–3041. doi: 10.1074/jbc.M111.293985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 25.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladoux A, Frelin C. Expression of vascular endothelial growth factor by cultured endothelial cells from brain microvessels. Biochem. Biophys. Res. Commun. 1993;194:799–803. doi: 10.1006/bbrc.1993.1892. [DOI] [PubMed] [Google Scholar]
- 27.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 28.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 29.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin and heparanases. J. Biol. Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J. Biol. Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]