Abstract
OBJECTIVE:
Febrile infants in the first 90 days may have life-threatening serious bacterial infection (SBI). Well-appearing febrile infants with SBI cannot be distinguished from those without by examination alone. Variation in care resulting in both undertreatment and overtreatment is common.
METHODS:
We developed and implemented an evidence-based care process model (EB-CPM) for the management of well-appearing febrile infants in the Intermountain Healthcare System. We report an observational study describing changes in (1) care delivery, (2) outcomes of febrile infants, and (3) costs before and after implementation of the EB-CPM in a children’s hospital and in regional medical centers.
RESULTS:
From 2004 through 2009, 8044 infants had 8431 febrile episodes, resulting in medical evaluation. After implementation of the EB-CPM in 2008, infants in all facilities were more likely to receive evidence-based care including appropriate diagnostic testing, determination of risk for SBI, antibiotic selection, decreased antibiotic duration, and shorter hospital stays (P < .001 for all). In addition, more infants had a definitive diagnosis of urinary tract infection or viral illness (P < .001 for both). Infant outcomes improved with more admitted infants positive for SBI (P = .011), and infants at low risk for SBI were more often managed without antibiotics (P < .001). Although hospital admissions were shortened by 27%, there were no cases of missed SBI. Health Care costs were also reduced, with the mean cost per admitted infant decreasing from $7178 in 2007 to $5979 in 2009 (−17%, P < .001).
CONCLUSIONS:
The EB-CPM increased evidence-based care in all facilities. Infant outcomes improved and costs were reduced, substantially improving value.
KEY WORDS: fever, infant, outcomes, cost
What’s Known on This Subject:
Febrile infants in the first 90 days may have life-threatening serious bacterial infection. Well-appearing febrile infants with serious bacterial infections cannot be distinguished from those without by examination alone. Variation in care resulting in both undertreatment and overtreatment is common.
What This Study Adds:
The systemwide implementation of an evidence-based care process model for the care of febrile infants in Intermountain Healthcare was associated with increased delivery of evidence-based care, improved infant outcomes, and lower costs. This model adopted nationally can improve value.
Evaluation of fever in infants aged 1 to 90 days is common, yet there are no national guidelines addressing management. Approximately 10% will have serious bacterial infection (SBI), which can be life threatening.1,2 However, the majority of infants have viral infections, and infants with laboratory and clinically confirmed viral infections are less likely to have SBI.1,3–5 Independent recommendations for care of the febrile infant published in 1993 and revised in 2000 did not address viral infections.6,7 Compliance with these recommendations is limited, and variation in care is substantial.8–11
In the Intermountain Healthcare system, we noted variation in care delivered at regional medical centers (RMCs) compared with Primary Children’s Medical Center (PCMC, Salt Lake City, UT), a tertiary children’s hospital. For example, in 2004, the proportion of febrile infants who had urinalysis ranged from 19% in 1 RMC to 70% at PCMC, although urinary tract infection (UTI) is the most common SBI in febrile infants.
The Intermountain Pediatric Clinical Program (IPCP) undertook a quality improvement initiative to address care of febrile infants. The IPCP has administrative, laboratory, nursing, and physician representatives from all Intermountain Healthcare regions and includes pediatricians from the University of Utah. The IPCP used Six Sigma methodology (Table 1) to develop an evidence-based care process model (EB-CPM) for febrile infants.12–14 CPMs are designed to decrease variation, improve quality, and support local preferences.15
TABLE 1.
Modified Six Sigma Process Used to Develop and Implement the EB-CPM for Febrile Infants
| Six Sigma Steps | Actions of the Pediatric Clinical Programs Guidance Council |
|---|---|
| Define | Identify febrile infants |
| • Developed method for identification using electronic administrative data | |
| • Map the process flow to be improved | |
| • Created flow diagrams from patient, nursing, laboratory, and physician perspectives | |
| Measure | Develop a data collection plan |
| • Developed through iterative process | |
| Collect data from Intermountain Healthcare facilities | |
| • Baseline data for all quality measures generated | |
| • Reports generated for individual facilities and combined target facilities | |
| Analyze | Analyze data collected to determine root causes for defects and sources of variation |
| • Baseline data demonstrated poor compliance with laboratory testing with root causes including lack of equipment, laboratory schedules, and courier services | |
| • Identify and prioritize opportunities for improvement | |
| • Identify gap between current performance and goal | |
| Improve | Design creative solutions using technology |
| • Web-based tools including care algorithms, standard order sets, parent information | |
| • In-person and web-based training modules | |
| Develop and deploy implementation plan | |
| • All target hospitals participated in 2007 | |
| Control | Develop and document an ongoing monitoring plan |
| • Monitor all quality and balance measures monthly | |
| • Monitor critical outcomes of febrile infants including deaths and missed SBI | |
| • Address equipment, laboratory, and courier schedules | |
| Institutionalize performance by modification of systems and structures | |
| • Care of febrile infant uses web-based tools available to all providers | |
| • All hospital representatives receive institutional feedback monthly | |
| • Hospital representatives inform providers of performance and outcome data | |
| • Individual provider data for those participating in maintenance of certification |
The EB-CPM for febrile infants incorporated evidence derived from local institutions1,4,5,16–23 and others.2,7,8,24–42 We defined 6 quality measures by consensus of representatives serving on the IPCP and their constituents. Quality measures targeted laboratory testing, SBI risk determination, antibiotic selection, hospital admission, and discharge. After in-person and web-based training, education, and feedback with clinical personnel at PCMC and 3 RMCs during 2007, the EB-CPM was implemented at all Intermoun-tain Healthcare facilities on January 1, 2008. Web-accessible tools including algorithms, orders, antibiotic recommendations, and references were available at the points of care in all facilities. All facilities received monthly performance feedback from the IPCP. The objectives of this article are to describe the changes in (1) care delivery, (2) outcomes of febrile infants, and (3) costs before and after the implementation of the EB-CPM.
Methods
Protection of Human Subjects
The Institutional Review Boards of Intermountain Healthcare and the University of Utah approved this study. Informed consent was waived. Provider use of the EB-CPM was voluntary.
Setting
This observational study was performed at Intermountain Healthcare, a not-for-profit, integrated health care system that provides care for ∼85% of Utah children and a higher proportion of infants. The 21 Intermountain Healthcare hospitals include PCMC and 3 RMCs located in Ogden, Provo, and St George, Utah. The RMCs and PCMC provide care for most febrile infants and were designated target facilities. Midlevel providers and resident and attending physicians practicing family medicine, pediatrics, and adult and pediatric emergency medicine staff target facilities. All facilities had the same viral diagnostic technology and electronic record system throughout the study.
Identification of Febrile Infants
Febrile infants were identified from the Intermountain enterprise data warehouse (EDW). The EDW contains clinical, laboratory, and administrative data for all facilities. We developed a definition for febrile infants based on age, reason for visit, admitting diagnosis, International Classification of Diseases, Ninth Revision, and All Patient Refined DIagnosis Related Groups (APR-DRGs) coding and validated it against a prospectively collected sample.1 The definition has a sensitivity and specificity of 93% and 90%, respectively.43 SBI was identified through the EDW and was defined as culture-confirmed bacteremia, meningitis, or UTI. UTI was defined as ≥50 000 colony forming units/mL of a single pathogen.44 An infant with missed SBI was defined as having SBI and treatment either only in the emergency department (ED) or hospital admission within 5 days of ED discharge.
EB-CPM Recommendations
The full EB-CPM for outpatients and inpatients is available in the Supplemental Information. The EB-CPM is for well-appearing febrile infants aged 1 to 90 days. Separate CPMs are available for early-onset neonatal sepsis in the nursery45 and for infants and children with findings consistent with sepsis or septic shock.46 Providers determine well appearance and whether use of a CPM is appropriate.
The febrile infant EB-CPM includes a history and physical examination and recommends obtaining a complete blood count (CBC) and urinalysis for all infants. Infants are classified as high-risk for SBI using a modification of the Rochester criteria.31,47 A recent review demonstrated the Rochester criteria and Philadelphia criteria have similar diagnostic accuracy in predicting SBI, and the Rochester criteria were more accurate in neonates.48 High-risk infants are those aged ≤28 days or with history of preterm birth (<37 weeks), chronic medical conditions, abnormal CBC (<5000 or >15 000 white blood cells per mm3) or urinalysis results (>10 white blood cells/high power field).47 The electronic record and orders capture risk designation.
Management without antibiotics is recommended for infants not identified as high risk and thus considered low risk for SBI. The EB-CPM, consistent with other expert guidance,6,7 recommends admission and antibiotic treatment of high-risk infants. Viral diagnostic testing is recommended for all admitted infants, including testing for enteroviruses by polymerase chain reaction between June and November or if cerebral spinal fluid pleocytosis18 is present and testing for respiratory viruses by direct fluorescent assay or polymerase chain reaction year-round. Antibiotic recommendations reflect the epidemiology and resistance of SBI pathogens at Intermountain Healthcare.
For admitted infants, duration of antibiotic therapy and length of stay (LOS) are based on results of bacterial and viral diagnostic testing at 24 hours. Admitted culture-negative infants at high risk for SBI and who test positive for a viral pathogen or who are at low-risk for SBI are eligible for discontinuation of antibiotics and discharge at 24 hours. All other culture-negative infants are eligible for the same at 36 hours. Given the distance between RMCs and the central laboratory (Salt Lake City, UT), we allowed 6 hours for specimen transport and measured the proportion of infants discharged within 42 hours of specimen collection.
Statistical Analysis
We identified 6 quality measures and 4 balancing measures to assess unintended consequences of EB-CPM implementation. We compared performance on these measures at the target facilities during baseline (July 1, 2004–December 31, 2007) and implementation (January 1, 2008–December 31, 2009) by using general linear models for continuous measures (with log transformations when the data had a skewed distribution) and logistic regression models for binary outcomes. A temporal analysis for performance changes during the baseline period was performed and did not yield significant year-to-year differences in individual facilities or the system.
Cost data were derived from the Intermountain Healthcare cost-accounting program, an activity-based microcosting system that identifies and aggregates the variable and fixed-cost components of hospital services and products according to the date of service.15 Because of the nonnormality of cost data, we used the Wilcoxon-Mann-Whitney test to compare the mean cost per infant during the 2 periods. Costs were adjusted for inflation to 2009 dollars.
Results
Participants
There were 8044 infants with 8431 febrile episodes resulting in evaluation at Intermountain Healthcare facilities (Table 2); 6991 (83%) occurred in target facilities. Infants at evaluation had a mean age of 45 days; 54% were boys, and 62% were white, 26% Latino, 2% African American, 2% Pacific Islander, 1% Asian, 1% Native American, and 6% unknown. In 3781 (45%) episodes, infants were classified as high risk for SBI.
TABLE 2.
Characteristics of Febrile Infant Episodes Across All Intermountain Healthcare Facilities
| Variable | Base and Training, n = 5444a (%) | Implementation, n = 2987a (%) | P Value |
|---|---|---|---|
| Episode at Target Facility | 4524 (83) | 2467 (83) | .600 |
| Age | .001 | ||
| ≤28 d | 1617 (30) | 787 (26) | |
| 29–90 d | 3827 (70) | 2200 (74) | |
| Inpatient admission | 2516 (46) | 1424 (48) | .200 |
| Observation unit admission | 372 (7) | 284 (10) | <.001 |
| Any admission | 2888 (53) | 1708 (57) | <.001 |
| Infants with any SBIb | 440 (8) | 295 (10) | .006 |
| UTI | 360 (7) | 257 (9) | <.001 |
| Bacteremia | 112 (2) | 60 (2) | .328 |
| Meningitis | 16 (0.3) | 7 (0.2) | .670 |
| Infants with SBI admitted at first encounter | 378/440 (86) | 267/295 (91) | .070 |
| Infants with bacteremia or meningitis admitted at first encounter | 117/128 (91) | 66/67 (99) | .060 |
| Death | 2 (0.04) | 1 (0.03) | 1.000 |
Unless otherwise indicated.
Infants may have ≥1 type of SBI.
Of all febrile episodes, 735 of 8431 (9%) had culture-confirmed SBI. Among infants with bacterial cultures of blood, urine, or cerebrospinal fluid (n = 6363), 735 (12%) had SBI. Infants were more likely to be diagnosed with SBI during the implementation period because of an increase in the diagnosis of UTI (+29%, P < .001; Table 2). The proportion of admitted infants with SBI increased from 13% to 16% after implementation (+23%, P = .011).
Performance of Quality Measures in Target Facilities
Laboratory Testing
The proportion of infants with recommended laboratory testing increased in all target facilities during the implementation period (Table 3). By 2009, almost all admitted infants had CBC (93%) and urinalysis (99%), and there was little variation between facilities. Infants were more likely to have blood (73% vs 79%) and urine cultures (74% vs 79%) after implementation (P < .001 for both). Infants were also more likely to have viral testing during the implementation and the proportion of admitted infants diagnosed with an enterovirus or respiratory virus increased from 25% to 36% (+40%, P < .001).
TABLE 3.
Comparison of Key Quality and Balancing Measures for the EB-CPM in Target Facilities
| Target Facilities Base and Training Periods, N = 4524,a n (%) | Target Facilities Implementation Period, N = 2467,a n(%) | Absolute Difference in Propositions (95% CI, P value) | |
|---|---|---|---|
| Quality measure | |||
| Obtain both CBC and urinalysis | 3040 (67) | 1975 (80) | 13% (11% to 15%, <.001) |
| Determine risk status for SBI | 3057 (68) | 1836 (74) | 7% (5% to 9%, <.001) |
| Perform viral testing for admitted Infants | 1992/2620 (76) | 1296/1540 (84) | 8% (6% to 11%, <.001) |
| Administer only formulary antibiotic therapy for admitted infants receiving antibiotics | 1167/1511 (77) | 826/901 (92) | 15% (12% to 17%, <.001) |
| Discontinue antibiotics within 36 h for infants with negative bacterial cultures | 547/1172 (47) | 415/658 (63) | 16% (12% to 21%, <.001) |
| Discharge eligible infants by 42 h | 682/1418 (48) | 586/777 (75) | 27% (23% to 31%, <.001) |
| Balancing measure | |||
| Lumbar Puncture | 2261 (50) | 1281 (52) | 2% (−0.5% to 4.4%, .120) |
| Infants admitted within 72 h post ED discharge | 92/1904 (5) | 45/927 (5) | 0.02% (−1.7% to 1.7%, 1.000) |
| Readmission within 72 h after discharge inpatient or observation unit | 21/2620 (0.8) | 10/1540 (0.6) | −0.2% (−0.7% to 0.4%, .710) |
| Missed SBI after admission | 0 | 0 | NA |
CI, confidence interval; NA, not applicable.
Unless otherwise denoted.
Admission of Infants With SBI at Initial Evaluation
Admission of infants subsequently proven to have SBI was associated with increased laboratory evaluation during the implementation period. The proportion of infants with SBI who were admitted at the initial evaluation increased from 86% to 91% and those with bacteremia or meningitis increased from 91% to 99%. Of the 28 infants with SBI discharged from the ED during the implementation period, 27 had UTI and received antibiotic treatment as outpatients. There were no missed cases of meningitis during the implementation period. This compares with the preimplementation period when there were 68 infants subsequently identified with SBI discharged from the ED including 8 with bacteremia and 3 with meningitis.
Antibiotic Selection and Treatment
Febrile infants in target facilities received antibiotic therapy in 4229 of 6991 (61%) of episodes. Infants classified as high risk for SBI were more likely to receive antibiotics than those classified as low risk (85% vs 63% P < .001). Infants classified as low risk were less likely to receive antibiotics in the inpatient (91% vs 85%, −7%, P = .005) or outpatient setting 43% vs 34%, −26%, P = .002) after implementation.
The recommended antibiotics are ampicillin, gentamicin, cefotaxime, and ceftriaxone. Infants admitted after the introduction of the EB-CPM were more likely to receive only recommended antibiotics (77% vs 92%, +15%) and to have antibiotics discontinued by 36 hours (47% vs 63%, +16%, P < .001 for both). In 94% of all episodes of SBI and 99% of meningitis episodes, the recommended antibiotics were active against the recovered pathogens.
Hospital Length of Stay
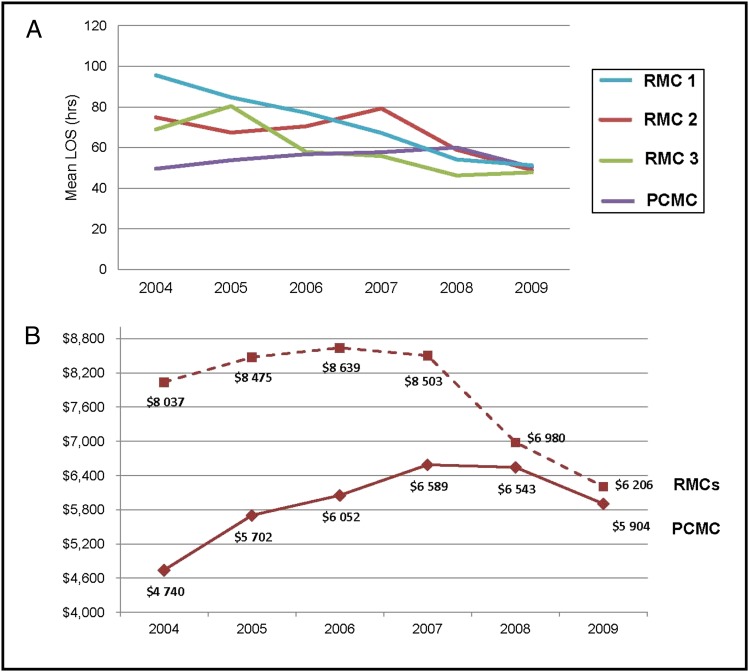
The mean hospital LOS for infants without SBI decreased from 60 to 44 hours after implementation (−27%, P < .001), resulting in 1644 fewer hospital days. The LOS at PCMC was increasing 2.4% annually in 2004–2008 (Fig 1). After implementation, there was a 12.0% decrease in LOS in 2009 compared with 2008 (P = .001). The LOS in all RMCs decreased significantly, and all target facilities achieved a common baseline for LOS by 2009 (Fig 1).
FIGURE 1.
A, LOS for inpatient or observation unit episodes at target facilities. B, Cost for RMCs and Primary Children’s Medical Center.
Balancing Measures and Cost
The performance of lumbar puncture remained stable, and there were no cases of missed meningitis after implementation. Although LOS was significantly decreased, there was no increase in readmissions and no readmissions with SBI after hospital discharge.
In our cohort, 91% of the costs for febrile infants occurred in the inpatient setting. Thus, although mean laboratory costs in the ED increased at PCMC ($153 vs $184, P < .001) and in the RMCs ($44 vs $69, P < .001), these costs were more than offset by the decreased costs for admitted infants. After implementation, the mean cost per admitted infant fell from $7178 in 2007 to $5979 in 2009 (−17%, P < .001).
Implementation was associated with reduced inpatient costs in all RMCs ($8037 vs $6206, −23%, P < .001). At PCMC, baseline inpatient costs were lower than at the RMCs (−$1914 in 2007) but were increasing at a faster annual rate ($233 vs −$366). After implementation of the EB-CPM, the mean inpatient cost per infant at PCMC declined 11.6% (P < .001). Variation in the LOS and costs between the RMCs and PCMC were virtually eliminated by 2009.
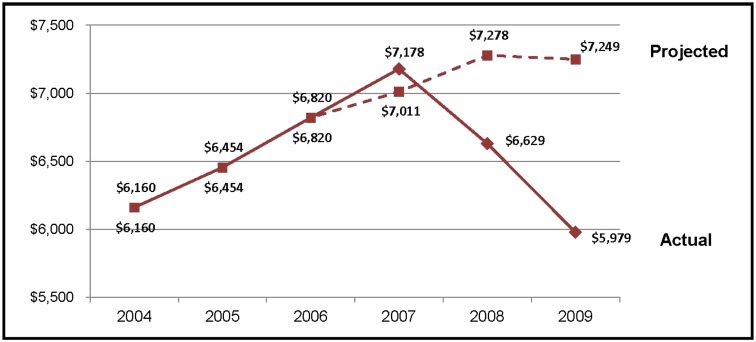
The mean cost per admitted infant was lower in 2009 than in 2004 (Fig 2). Savings were realized through decreased LOS and reductions in antibiotic prescribing and ancillary testing not recommended by the EB-CPM. Using a model based on a rate of inflation equal to the CPI, costs in 2009 were predicted to be 18% greater than in 2004. In contrast, our data demonstrated that costs in 2009 were 3% lower than in 2004 (Fig 2). In 2009, the cost per admitted infant was $1270 less than predicted resulting in an estimated savings of ∼$1.9 million.
FIGURE 2.
Average total cost per admitted infant at target facilities.
Discussion
We report the successful implementation of an EB-CPM for the care of febrile infants. Implementation was associated with an increase in evidence-based care delivered by a diverse group of providers at a children’s hospital and in community RMCs. Following implementation, there was a slight increase in the admission rate of febrile infants, an increased documentation of UTIs and viral infections, a higher percentage of patients with SBI who were admitted after UTI detection, a decreased length of stay, a more appropriate use of recommended antibiotics, and a similar rate (with trends toward improvement) in admission of patients with meningitis and bacteremia compared with the preimplementation period. Implementation was also associated with a considerable reduction in costs. Although the infrastructure and resources devoted to quality improvement in Intermountain Healthcare may not be available in all settings, the creation of facility specific care process models with internal process control and performance evaluation is a flexible tool that can be adapted by other health care systems to improve care and outcomes while reducing costs.49
The care of the febrile infant is controversial, and there are variations in care associated with site of care and type of provider.8–11,50–54 Strategies for classification of infants at risk for SBI, admission of high-risk infants, and treatment of low-risk infants as outpatients have been extensively evaluated and discussed.7,24,26,36,37,55 Variation in practice continues, perhaps because of the lack of an accepted guideline and the absence of research comparing different care processes to determine if any are associated with better outcomes or lower costs.
The EB-CPM was created to define best practices for Intermountain Healthcare and to create a common process for delivering quality care across many hospitals. Intermountain hospitals, although widely separated geographically and using different provider staffing models, are all committed to quality improvement, have representation through the IPCP, and share common laboratory and electronic medical record resources.15 These elements were vital to the development and dissemination of the EB-CPM.
Implementation of the EB-CPM resulted in increased evidence-based care delivery as measured by 6 indicators. The investigators worked with target facilities to ensure that the indicators were relevant to the medical providers and that processes were in place to support delivering recommended care without interrupting workflow. The increase in delivery of evidence-based care demonstrates the value of providing decision support at the point-of-care to guide clinicians. Parents of febrile infants anywhere in Utah can now be assured that their infant will receive high-quality care anywhere in the Intermountain Healthcare system, whether evaluated in RMCs by nonpediatric providers or at PCMC by pediatricians and pediatric subspecialists.
Increased evidence-based care was associated with improved infant outcomes. On the basis of studies demonstrating the low rate of SBI among infants with viral infection1,3–5,41 and data demonstrating that the majority (∼85%) of all positive blood cultures in this population are detected within 24 hours,33,38 the EB-CPM recommends discharge for admitted infants with positive viral testing and negative bacterial cultures at 24 hours. Diagnoses of viral illnesses increased by 40% after implementation resulting in opportunities for earlier discharge and discontinuation of antibiotics for many infants.
Implementation of the EB-CPM improved recognition and treatment of SBI. The increasing proportion of admitted febrile infants with SBI in the postimplementation period supports the use of screening criteria to identify well-appearing infants at high-risk for SBI. The increase in urinalysis testing identified infants with UTI who may have been missed before implementation. Bacteremia and meningitis are rare but potentially life-threatening occurrences. After implementation, 99% of infants with bacteremia or meningitis were admitted at the initial evaluation compared with 91% before implementation. Although this difference did not reach statistical significance, the value of early recognition and treatment of bacteremia and meningitis in nearly all infected infants cannot be discounted. Finally, the EB-CPM improved antibiotic treatment decisions with infants benefitting from the selection of antibiotics appropriate for SBI pathogens and reductions in antibiotic use in low-risk and culture-negative infants.
We detected no adverse consequences after implementation of the EB-CPM. The performance of lumbar puncture, considered invasive by many parents and clinicians, did not increase, and yet there were no cases of missed meningitis. There was a modest increase in the proportion of infants admitted because of an increase in admissions of <24 hours in observation units, and 75% of all admitted infants were discharged from the hospital by 42 hours. Although the mean hospital LOS was 16 hours shorter than before implementation, the readmission rate was stable at <0.5%, and there were no cases of missed SBI after hospital discharge.
The implementation of the EB-CPM reduced costs and increased the value of the health care delivered. Variations in care can unnecessarily increase cost through overtreatment, including excess testing, inappropriate antibiotic use, or prolonged LOS, and through undertreatment, resulting in delayed recognition and treatment of SBI. By 2009, the target facilities all had similar LOS and costs, indicating adoption of similar process for the evaluation and management of febrile infants. Infants and families benefitted from improv-ed health outcomes, shorter hospital stays, and lower cost. Savings for the hospital system were realized through lower direct care costs, improvements in care that may reduce medical liability, and reduction in hospital days, which can delay the need for new bed construction and reduce long-term capital outlay.
This study has several strengths and limitations. Strengths include the size of the febrile infant cohort, the largest ever reported. The results are also strengthened by the quality of the shared EDW, which allowed us to evaluate outcomes across the system including readmissions and missed SBI. The study is limited to a single health care system; however, we examined multiple hospital facilities with different characteristics, suggesting that an EB-CPM could be successfully implemented in other settings. Documentation of training was not required for providers. Since 2009, pediatricians have been able to use the EB-CPM for maintenance of certification (MOC). Evaluation of performance of trained providers using the EB-CPM for maintenance of certification compared with all providers is ongoing. The observation period after implementation was only 2 years; however, we continue to monitor the quality measures monthly through the IPCP and have seen either maintenance or additional improvement in all measures through 2011. For example, in 2011, 90% of admitted infants with negative bacterial cultures were discharged by 42 hours compared with 75% in 2008–2009. The changes observed may have been due to factors other than the introduction of the EB-CPM. However, there were no significant changes in the environment such as availability of diagnostic testing or new external guidelines over the entire study period. Furthermore, the fact that there were no significant changes observed during the 4-year baseline period and the sustained monthly improvements in the 6 quality measures that occurred in all facilities after the implementation of the EB-CPM makes this unlikely. Finally, there were likely unmeasured sources of variation that may have resulted in failure to achieve universal compliance with the EB-CPM. We seek to identify and address these factors through our monthly IPCP meetings and update the EB-CPM and available support as new data become available.
Conclusions
The introduction of an EB-CPM changed the culture of caring for febrile infants across a large health care system. Variation in care was substantially reduced. Infant outcomes were exceptional, and significant savings were realized. The EB-CPM for febrile infants is an example of value-driven health care that addresses a common problem and can be used to inform guidelines disseminated nationally.
Supplementary Material
Acknowledgments
We thank Drs Mandy Allison, Anne Blaschke, Chuck Norlin, and Paul C. Young for their careful review of the manuscript and for their support of this research.
Glossary
- CBC
complete blood count
- EB-CPM
evidence-based care process model
- ED
emergency department
- EDW
enterprise data warehouse
- IPCP
Intermountain Pediatric Clinical Program
- LOS
length of stay
- PCMC
Primary Children’s Medical Center
- RMC
regional medical center
- SBI
serious bacterial infection
- UTI
urinary tract infection
Footnotes
All authors are responsible for the research within the article and have participated in the concept and design, acquisition of data, and analysis and interpretation of data of the manuscript. Dr Byington, Ms Reynolds, and Drs Nelson, Sheng, Osguthorpe, Pavia, Clark were responsible for drafting the article or revising it critically for important intellectual content. All authors provided final approval of the version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported in part by a Public Health Services research grant UL1RR025764 from the National Center for Research Resources (CLB, XS, and LS), the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K24HD047249 (CLB, XS, and KK), the Agency for Health Research and Quality grant AHRQ R18HS018034 (CLB, XS, KK, REN, and LS), the National Institutes of Health/National Cancer Institute grant KM1CA156723 (CLB, REN), and the HA and Edna Benning Presidential Endowment (CLB).
COMPANION PAPER: A companion to this article can be found on page e199, online at www.pediatrics.org/cgi/doi/10.1542/peds.2012-1178.
References
- 1.Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662–1666 [DOI] [PubMed] [Google Scholar]
- 2.Baraff LJ, Oslund SA, Schriger DL, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11(4):257–264 [DOI] [PubMed] [Google Scholar]
- 3.Greenes DS, Harper MB. Low risk of bacteremia in febrile children with recognizable viral syndromes. Pediatr Infect Dis J. 1999;18(3):258–261 [DOI] [PubMed] [Google Scholar]
- 4.Bender JM, Ampofo K, Gesteland P, et al. Influenza virus infection in infants less than three months of age. Pediatr Infect Dis J. 2010;29(1):6–9 [DOI] [PubMed] [Google Scholar]
- 5.Rittichier KR, Bryan PA, Bassett KE, et al. Diagnosis and outcomes of enterovirus infections in young infants. Pediatr Infect Dis J. 2005;24(6):546–550 [DOI] [PubMed] [Google Scholar]
- 6.Baraff LJ, Bass JW, Fleisher GR, et al. Agency for Health Care Policy and Research . Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med. 1993;22(7):1198–1210 [DOI] [PubMed] [Google Scholar]
- 7.Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. 2000;36(6):602–614 [DOI] [PubMed] [Google Scholar]
- 8.Pantell RH, Newman TB, Bernzweig J, et al. Management and outcomes of care of fever in early infancy. JAMA. 2004;291(10):1203–1212 [DOI] [PubMed] [Google Scholar]
- 9.Zerr DM, Del Beccaro MA, Cummings P. Predictors of physician compliance with a published guideline on management of febrile infants. Pediatr Infect Dis J. 1999;18(3):232–238 [DOI] [PubMed] [Google Scholar]
- 10.Young PC. The management of febrile infants by primary-care pediatricians in Utah: comparison with published practice guidelines. Pediatrics. 1995;95(5):623–627 [PubMed] [Google Scholar]
- 11.Wittler RR, Cain KK, Bass JW. A survey about management of febrile children without source by primary care physicians. Pediatr Infect Dis J. 1998;17(4):271–277, discussion 277–279 [DOI] [PubMed] [Google Scholar]
- 12.Chassin MR. Is health care ready for Six Sigma quality? Milbank Q. 1998;76(4):565–591, 510 [DOI] [PMC free article] [PubMed]
- 13.Pelletier L. Interview with Beth Langham on Six Sigma in healthcare. J Healthc Qual. 2003;25(2):26–27, 37 [DOI] [PubMed]
- 14.Scalise D. Six Sigma. The quest for quality. Hosp Health Netw. 2001;75(12):41–46 [PubMed]
- 15.James B, Savitz L. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1–7 [DOI] [PubMed] [Google Scholar]
- 16.Antonow JA, Hansen K, McKinstry CA, Byington CL. Sepsis evaluations in hospitalized infants with bronchiolitis. Pediatr Infect Dis J. 1998;17(3):231–236 [DOI] [PubMed] [Google Scholar]
- 17.Byington CL, Castillo H, Gerber K, et al. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children’s hospital. Arch Pediatr Adolesc Med. 2002;156(12):1230–1234 [DOI] [PubMed] [Google Scholar]
- 18.Byington CL, Kendrick J, Sheng X. Normative cerebrospinal fluid profiles in febrile infants. J Pediatr. 2011;158(1):130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964–968 [DOI] [PubMed] [Google Scholar]
- 20.Byington CL, Taggart EW, Carroll KC, Hillyard DR. A polymerase chain reaction-based epidemiologic investigation of the incidence of nonpolio enteroviral infections in febrile and afebrile infants 90 days and younger. Pediatrics. 1999;103(3). Available at: www.pediatrics.org/cgi/content/full/103/3/E27. [DOI] [PubMed] [Google Scholar]
- 21.Carroll KC, Taggart B, Robison J, Byington C, Hillyard D. Evaluation of the roche AMPLICOR enterovirus PCR assay in the diagnosis of enteroviral central nervous system infections. J Clin Virol. 2000;19(3):149–156 [DOI] [PubMed] [Google Scholar]
- 22.Kadish HA, Loveridge B, Tobey J, Bolte RG, Corneli HM. Applying outpatient protocols in febrile infants 1–28 days of age: can the threshold be lowered? Clin Pediatr (Phila). 2000;39(2):81–88 [DOI] [PubMed] [Google Scholar]
- 23.Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71–77 [DOI] [PubMed] [Google Scholar]
- 24.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437–1441 [DOI] [PubMed] [Google Scholar]
- 25.Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics. 1999;103(3):627–631 [DOI] [PubMed] [Google Scholar]
- 26.Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120(1):22–27 [DOI] [PubMed] [Google Scholar]
- 27.Dagan R. Nonpolio enteroviruses and the febrile young infant: epidemiologic, clinical and diagnostic aspects. Pediatr Infect Dis J. 1996;15(1):67–71 [DOI] [PubMed] [Google Scholar]
- 28.DeAngelis C, Joffe A, Wilson M, Willis E. Iatrogenic risks and financial costs of hospitalizing febrile infants. Am J Dis Child. 1983;137(12):1146–1149 [DOI] [PubMed] [Google Scholar]
- 29.Ferrera PC, Bartfield JM, Snyder HS. Neonatal fever: utility of the Rochester criteria in determining low risk for serious bacterial infections. Am J Emerg Med. 1997;15(3):299–302 [DOI] [PubMed] [Google Scholar]
- 30.Garra G, Cunningham SJ, Crain EF. Reappraisal of criteria used to predict serious bacterial illness in febrile infants less than 8 weeks of age. Acad Emerg Med. 2005;12(10):921–925 [DOI] [PubMed] [Google Scholar]
- 31.Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile Infant Collaborative Study Group . Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390–396 [PubMed] [Google Scholar]
- 32.Kaplan RL, Harper MB, Baskin MN, Macone AB, Mandl KD. Time to detection of positive cultures in 28- to 90-day-old febrile infants. Pediatrics. 2000;106(6). Available at: www.pediatrics.org/cgi/content/full/106/6/E74. [DOI] [PubMed] [Google Scholar]
- 33.Kumar Y, Qunibi M, Neal TJ, Yoxall CW. Time to positivity of neonatal blood cultures. Arch Dis Child Fetal Neonatal Ed. 2001;85(3):F182–F186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuppermann N, Bank DE, Walton EA, Senac MO, Jr, McCaslin I. Risks for bacteremia and urinary tract infections in young febrile children with bronchiolitis. Arch Pediatr Adolesc Med. 1997;151(12):1207–1214 [DOI] [PubMed] [Google Scholar]
- 35.Liebelt EL, Qi K, Harvey K. Diagnostic testing for serious bacterial infections in infants aged 90 days or younger with bronchiolitis. Arch Pediatr Adolesc Med. 1999;153(5):525–530 [DOI] [PubMed] [Google Scholar]
- 36.Lieu TA, Baskin MN, Schwartz JS, Fleisher GR. Clinical and cost-effectiveness of outpatient strategies for management of febrile infants. Pediatrics. 1992;89(6 pt 2):1135–1144 [PubMed] [Google Scholar]
- 37.McCarthy CA, Powell KR, Jaskiewicz JA, et al. Outpatient management of selected infants younger than two months of age evaluated for possible sepsis. Pediatr Infect Dis J. 1990;9(6):385–389 [DOI] [PubMed] [Google Scholar]
- 38.McGowan KL, Foster JA, Coffin SE. Outpatient pediatric blood cultures: time to positivity. Pediatrics. 2000;106(2 pt 1):251–255 [DOI] [PubMed] [Google Scholar]
- 39.Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053–1056 [DOI] [PubMed] [Google Scholar]
- 40.Titus MO, Wright SW. Prevalence of serious bacterial infections in febrile infants with respiratory syncytial virus infection. Pediatrics. 2003;112(2):282–284 [DOI] [PubMed] [Google Scholar]
- 41.Verboon-Maciolek MA, Nijhuis M, van Loon AM, et al. Diagnosis of enterovirus infection in the first 2 months of life by real-time polymerase chain reaction. Clin Infect Dis. 2003;37(1):1–6 [DOI] [PubMed] [Google Scholar]
- 42.Wasserman GM, White CB. Evaluation of the necessity for hospitalization of the febrile infant less than three months of age. Pediatr Infect Dis J. 1990;9(3):163–169 [DOI] [PubMed] [Google Scholar]
- 43.Gesteland P, Valentine K, Korgenski EK, Raines B, Byington C. A Method for Identifying Febrile Infants Presenting to the Emergency Department Using Administrative Data. Toronto, Canada: Pediatric Academic Societies; 2007 [Google Scholar]
- 44.Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Pyuria and bacteriuria in urine specimens obtained by catheter from young children with fever. J Pediatr. 1994;124(4):513–519 [DOI] [PubMed] [Google Scholar]
- 45.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) . Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1–36 [PubMed] [Google Scholar]
- 46.Larsen GY, Mecham N, Greenberg R. An emergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics. 2011;127(6). Available at: www.pediatrics.org/cgi/content/full/127/6/e1585 [DOI] [PubMed]
- 47.Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr. 1985;107(6):855–860 [DOI] [PubMed] [Google Scholar]
- 48.Hui C, Neto G, Tsertvadez A, et al. Diagnosis and management of febrile infants (0–3 months). Evidence Report/Technology Assessment No. 205. Prepared by the University of Ottawa Evidence-based Practice Center under Contract No. HHSA290-2007-10059I. Publication No. 12-E004-EF. Rockville, MD: Agency for Healthcare Research and Quality; March 2012
- 49.Bohmer RM. The four habits of high-value health care organizations. N Engl J Med. 2011;365(22):2045–2047 [DOI] [PubMed] [Google Scholar]
- 50.Baraff LJ, Schriger DL, Bass JW, et al. Management of the young febrile child. Commentary on practice guidelines. Pediatrics. 1997;100(1):134–136 [DOI] [PubMed] [Google Scholar]
- 51.Bauchner H, Pelton SI. Management of the young febrile child: a continuing controversy. Pediatrics. 1997;100(1):137–138 [DOI] [PubMed] [Google Scholar]
- 52.Belfer RA, Gittelman MA, Muñiz AE. Management of febrile infants and children by pediatric emergency medicine and emergency medicine: comparison with practice guidelines. Pediatr Emerg Care. 2001;17(2):83–87 [DOI] [PubMed] [Google Scholar]
- 53.Berkowitz CD, Orr DP, Uchiyama N, et al. Variability in the management of the febrile infant under 2 months of age. J Emerg Med. 1985;3(5):345–351 [DOI] [PubMed] [Google Scholar]
- 54.Christakis DA, Rivara FP. Pediatricians’ awareness of and attitudes about four clinical practice guidelines. Pediatrics. 1998;101(5):825–830 [DOI] [PubMed] [Google Scholar]
- 55.Jhaveri R, Byington CL, Klein JO, Shapiro ED. Management of the non-toxic-appearing acutely febrile child: a 21st century approach. J Pediatr. 2011;159(2):181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.