Abstract
BACKGROUND AND OBJECTIVES:
Nontherapeutic medication ingestions continue to be a major pediatric health problem, with recent increases in ingestions despite a number of public health interventions. It is unknown how changes in adult prescription drug use relate to pediatric medication poisonings. The objective of the study was to measure the association between changing adult prescription drug patterns and pediatric medication exposures and poisonings and identify high-risk classes of medications and pediatric age groups.
METHODS:
We measured monthly pediatric exposures and poisonings using the National Poison Data System and prescriptions written for adults using the National Ambulatory Medical Care Surveys for 2000 through 2009. Associations between adult prescriptions for oral hypoglycemics, antihyperlipidemics, β-blockers, and opioids and exposures and poisonings among children 0 to 5, 6 to 12, and 13 to 19 years were analyzed by using multiple time-series analysis. Emergency department visits, serious injuries, and hospitalizations stemming from these associations were described.
RESULTS:
Adult medication prescriptions were statistically significantly associated with exposures and poisonings in children of all ages, with the strongest association observed for opioids. Across medications, the greatest risk was among children 0 to 5 years old, followed by 13- to 19-year-olds. Rates of emergency department visits were highest for events related to hypoglycemics (60.1%) and β-blockers (59.7%), whereas serious injuries and hospitalizations occurred most frequently with opioids (26.8% and 35.2%, respectively) and hypoglycemics (19.5% and 49.4%, respectively).
CONCLUSIONS:
Increasing adult drug prescriptions are strongly associated with rising pediatric exposures and poisonings, particularly for opioids and among children 0 to 5 years old. These associations have sizable impacts, including high rates of serious injury and health care use.
Keywords: prescription drugs, poisoning, epidemiology, prevention and control, drug therapy
What’s Known on This Subject:
Medication ingestions are increasing among children despite a number of public health interventions. The majority of these poisonings are related to prescription as opposed to over-the-counter medications.
What This Study Adds:
Rising rates of poisonings in children are strongly correlated with rising use of hypoglycemics, antihyperlipidemics, β-blockers, and opioids among adults. These events are associated with considerable health care utilization, both in terms of emergency department visits and hospital admissions.
Despite a number of public health interventions over the past 40 years, poisonings from prescription medications continue to be a major cause of morbidity among children.1–5 Interventions have included educational campaigns on safe medication storage and the introduction of child-resistant closures on adult prescription medication bottles. Although these measures are credited in part with reducing overall deaths from medication poisoning in children, visits to emergency departments (EDs) for medication exposures are increasing.6 More than 70 000 children are evaluated in EDs for unintentional medication exposures and poisonings each year.6,7 Of children presenting to EDs, at least 12% are hospitalized.7,8 Exposures and poisonings are most frequently linked to prescription medications compared with over-the-counter medications, with children aged ≤5 years at particularly high risk and comprising the majority of calls received by US Poison Centers.9,10 Many exposures among younger children occur in the child’s own home, with medications often belonging to an adult relative.11
After a brief decline in the 1990s, the number of medication exposures and poisonings is again increasing among children.7,12 Between 2001 and 2008, rates of pediatric ED visits and hospitalizations resulting from medication exposures and poisonings increased by 30% and 36%, respectively.7 At the same time, adult prescription rates are increasing.13 However, population changes in adult medication prescriptions have not been linked with population changes in pediatric exposures and poisonings. We aimed to estimate the association of drug prescriptions in adults with patterns of exposures and poisonings in children, and any subsequent health care utilization and morbidity. In addition, we aimed to identify high-risk classes of medications and pediatric age groups by measuring drug- and age-specific associations.
Methods
Study Design
We analyzed exposures and poisonings reported to US Poison Centers from 2000 through 2009 for the medications most commonly prescribed to adults. These were analgesics (11.4% of all prescriptions), antihyperlipidemics (6.2% of all prescriptions), antidepressants (4.5% of all prescriptions), antidiabetic agents (4.2% of all prescriptions), and β-blockers (4% of all prescriptions).13,14 Antidepressants were excluded to focus on drugs that are primarily prescribed in adults to avoid events related to therapeutic use in children. Analgesics were also limited to opioids to exclude over-the-counter medications (eg, acetaminophen). Finally, we limited our analysis to single-ingredient medications and excluded nonoral medications (eg, insulin). The study was deemed exempt from review by the Institutional Review Board at Boston Children’s Hospital.
Pediatric Exposures and Poisonings
Data on pediatric exposures and poisonings were obtained from the American Association of Poison Control Centers’ National Poison Data System (NPDS). NPDS is a comprehensive data repository with information on calls to all 57 US Poison Centers. Detailed information is recorded for every exposure or poisoning, including patient demographics, names of implicated medications, and specifics surrounding the circumstances of the ingestion. Case follow-up is performed to obtain additional information on clinical effects, healthcare requirements, and medical outcome. All NPDS reports for January 1, 2000, through December 31, 2009 were analyzed for medication implicated, clinical effects, and associated ED use or hospitalization among children 0 to 5, 6 to 12, and 13 to 19 years old. Cases were excluded if the description of the exposure or poisoning indicated that the event was related to a pediatric prescription, such as a parent making a dosing error or giving medication doses too closely together.
Adult Drug Prescriptions
Adult drug prescriptions were measured using the National Ambulatory Medical Care Survey,15 an annual national survey of patient visits to US non–federally employed outpatient medical providers conducted by the National Center for Health Statistics. Physician offices are randomly chosen for participation and are trained by National Center for Health Statistics staff on survey completion. Patient visits are randomly chosen in 1-week reporting periods to provide representative visits throughout the year. Data collected include patient demographics, details of the clinical presentation, and care provided, including drug therapy. Physicians record up to 8 administered medications (only 6 for the years 2000–2002) that were prescribed or continued at the time of the visit. These “drug mentions” provide a representative view of US medication use.14,16,17 We identified all drug mentions for the drug classes of interest between January 1, 2000, and December 31, 2009, among patients aged ≥20 years. Using the unique drug codes, we excluded combination medications and nonoral formulations to match the drugs implicated in exposures and poisonings among children.
Health Care Utilization and Morbidity Related to Exposures
Health care utilization is recorded in NPDS according to 7 levels: received no treatment at a health care facility, treated and released, admitted to a noncritical care unit, admitted to a critical care unit, admitted to a psychiatric facility, failed to arrive at a health care facility despite referral, and lost to follow-up.1 For our analysis, we combined admissions to noncritical care and critical care units and considered failure to arrive at a health care facility as lost to follow-up.
Morbidity related to medication exposures and poisonings was measured using the NPDS medical outcome categories. We combined “moderate effect” (more prolonged or serious symptoms that require clinical attention), “major effect” (life-threatening signs or symptoms or a residual disability resulting from the exposure), and death (fatality that was a direct result of the medication exposure) and considered these outcomes as serious injuries.7
Statistical Analysis
The mean monthly number of exposures and poisonings related to the 4 drug classes of interest was calculated for each of the pediatric age groups. Similarly, the mean monthly number of drug mentions among adults was measured for medications in these drug classes. Both the number of pediatric poisonings and of adult drug mentions were normalized using US Census estimates to prevent confounding of the results by changes in population composition over the study period.18
Associations
Trends in pediatric exposures and poisonings were described by using multiple time-series analysis.19 In principle, the statistical analysis involved the specification of time-series models where exposures and poisonings at any given month (t) were a function of the previous months’ adult mentions of 1 of the 4 adult studied medications (t – i) after adjusting for seasonality (exposure and poisoning trends may vary similarly year to year and month to month) and autocorrelation (measurements occurring closer in time are more similar than those farther apart in time). Monthly dummy indicators were included as covariates to adjust for potential biases related to seasonality. This method is assumption-free, allowing seasonality to follow nonlinear patterns.20 Newey-West standard errors were used so the error variance estimates would be valid under autocorrelation violation of regression assumptions, yielding conservative estimates of the confidence intervals regardless of any specific model adjustment.21,22 This analysis strategy produced valid estimates insensitive to possible seasonal and autocorrelational confounding.
Causality
The causal significance of associations were judged by using a Granger causality model in which trends from the previous 1 to 6 months for adult medication mentions were used to predict exposure or poisonings after controlling for trends in exposure and poisonings from the previous 1 to 6 months (Exposure and poisonings t = Adult Mentionst–1 +Adult Mentionst-2 . . . +Adult Mentionst–6 + Exposure and poisoningst–1 + Exposure and poisoningst-2 . . . + Exposure and poisoningst–6). A statistically significant result for the combination of adult mention coefficients using a Wald test is interpreted to mean that adult mentions is a “Granger cause of” exposure or poisoning.22–24 This allowed us to make robust inferences regarding the significance of any association.
Results
There were 38 485 pediatric exposures and poisonings to oral hypoglycemics, 39 693 to antihyperlipidemics, 49 075 to β-blockers, and 62 416 to opioids over the 10-year study period. The mean yearly numbers of exposures and poisonings for each of the age groups and medication classes are shown in Table 1. Children 0 to 5 years of age experienced the greatest mean number of events per year for each of the 4 drug classes.
TABLE 1.
Exposures and Poisonings in Children, 2000–2009, From the NPDSa
| Medication Class | Mean Yearly Exposures and Poisonings, n | ||
|---|---|---|---|
| 0–5 y | 6–12 y | 13–19 y | |
| Oral hypoglycemics | 3195 | 206 | 440 |
| Antihyperlipidemics | 3486 | 232 | 244 |
| β-blockers | 3858 | 443 | 597 |
| Opioids | 3293 | 590 | 2330 |
There was missing age information for 86 children with an exposure or poisoning to oral hypoglycemics, 72 related to antihyperlipidemics, 106 related to β-blockers, and 287 related to opioids. These cases were excluded in the age-group analyses.
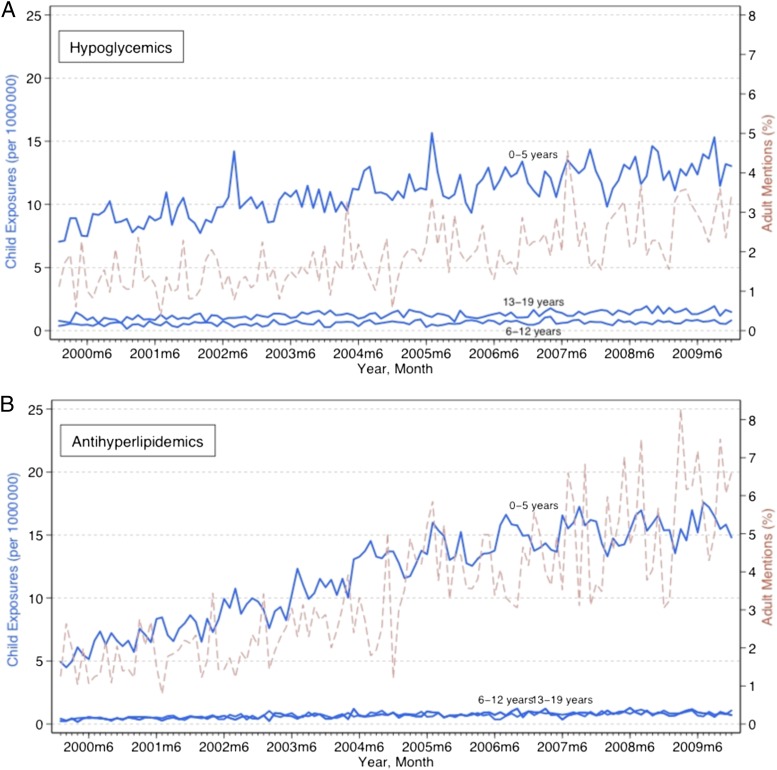
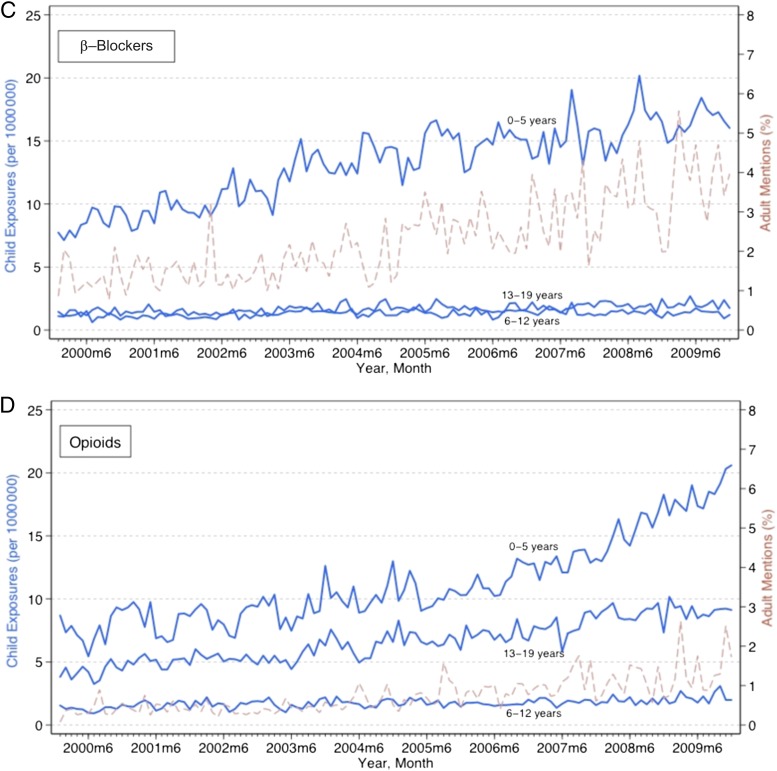
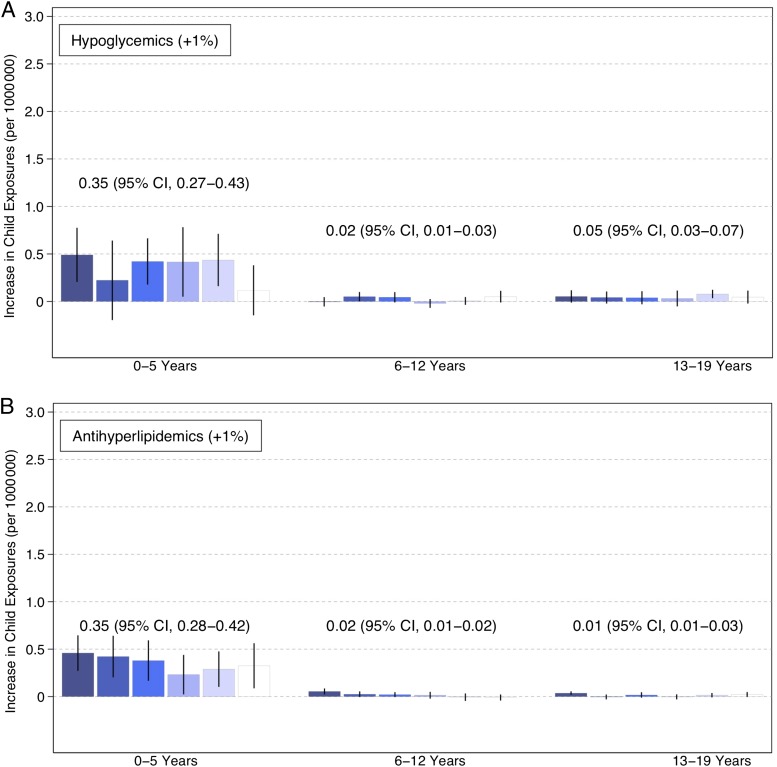
Figure 1 displays time trends for pediatric exposures and poisonings by each of the 3 age groups. Population-adjusted pediatric medication exposures and poisonings increased statistically significantly for all the drug classes among all age groups over the study period, based on a linear trend (P < .001). These increases varied substantially across age groups and drug classes. Among children 0 to 5 years old, opioid exposures and poisonings increased 0.09 (95% confidence interval [CI], 0.07–0.11) per 1 000 000 children each month, compared with 0.006 (95% CI, 0.004–0.008) among 6- to 12-year-olds, and 0.04 (95% CI, 0.04–0.05) among 13- to 19-year-olds. Increases were generally larger among children 0 to 5 years of age for all drugs. Among 0- to 5-year-olds, hypoglycemic exposures and poisonings increased 0.04 (95% CI, 0.04–0.05), antihyperlipidemics increased 0.10 (95% CI, 0.08–0.11), and β-blockers increased 0.08 (95% CI, 0.07–0.09) all per million children each month.
FIGURE 1.
Trends in pediatric exposures and poisonings related to oral hypoglycemics, antihyperlipidemics, β-blockers, and opioids and adult mentions of these medications. Exposures and poisonings increased statistically significantly for all age groups related to each of the drug classes (P < .001). Adult drug mentions increased statistically significantly for all drug classes (P < .001).
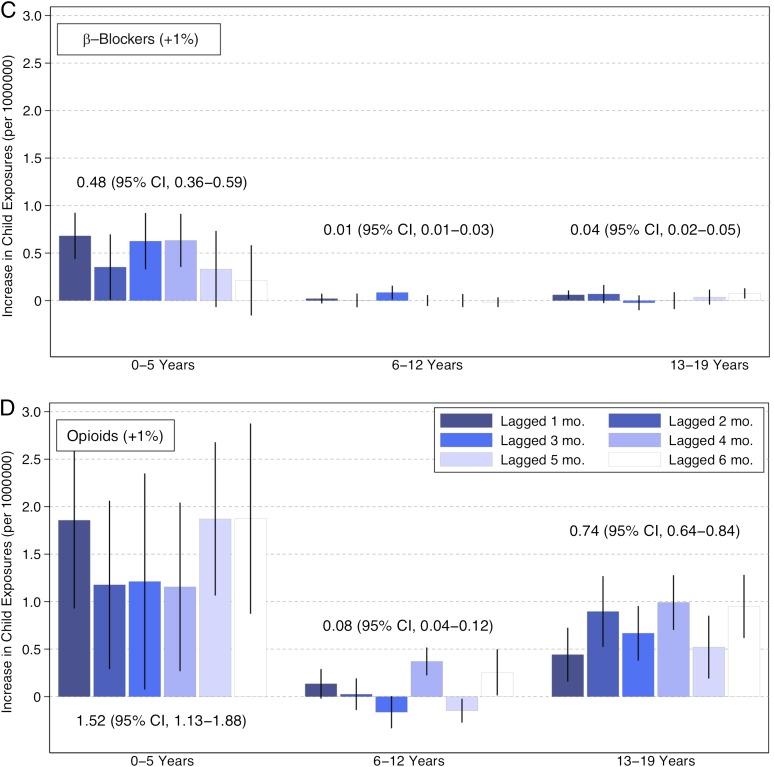
There were significant associations between adult medication use and exposures and poisonings in children averaging across projected increases 1 to 6 months into the future. These associations were generally twice as strong for opioids as other drug classes and strongest among children 0 to 5 years of age across drug classes. A 1% increase in adults taking opioids was associated with 1.53 (95% CI, 1.13–1.88), 0.08 (95% CI, 0.04–0.12), and 0.74 (95% CI, 0.64–0.84) more exposures and poisonings per 1 000 000 children among 0- to 5-, 6- to 12-, and 13- to 19-year-olds, respectively (Fig 2). Hypoglycemic adult mentions were associated with a 0.35 (95% CI, 0.27–0.43), 0.02 (95% CI, 0.01–0.03), and 0.05 (95% CI, 0.03–0.07) increase in exposures and poisonings per 1 000 000 children among the 0- to 5-, 6- to 12-, and 13- to 19-year-olds, respectively. Antihyperlipidemic adult mentions were associated with a 0.35 (95% CI, 0.28–0.42), 0.02 (95% CI, 0.01–0.02) and 0.01 (95% CI, 0.01–0.03) increase in exposures and poisonings per 1 000 000 children, among 0–5, 6–12 and 13–19 year olds, respectively. β-blocker adult mentions were associated with a 0.48 (95% CI, 0.36–0.59), 0.01 (95% CI, 0.01–0.03), and 0.04 (95% CI, 0.02–0.05) increase in exposures and poisonings per 1 000 000 children among 0- to 5-, 6- to 12-, and 13- to 19-year-olds, respectively.
FIGURE 2.
Increases in pediatric exposures and poisonings associated with adult drug mentions of opioids, antihyperlipidemics, oral hypoglycemics, and β-blockers. Each bar indicates a month-specific mean predicted change in pediatric exposures and poisonings for a 1 percentage point increase in adult medication mentions. Lines show the corresponding confidence intervals estimated by using 1000 draws from the mean value’s variance-covariance matrix for the maximum likelihood estimate. Displayed numeric values represent the mean association averaging over 1 to 6 months.
The month-specific patterns suggest a 1% increase in adult drug mentions was followed by larger increases in child exposures and poisonings in the next immediate month than 2 to 6 months later. This pattern was most evident among children 0 to 5 years old where exposures and poisonings increased 0.49 (95% CI 0.20–0.78), 0.46 (95% CI 0.27–0.65), 0.68 (95% CI 0.44–0.93), and 1.86 (95% CI 0.93–2.79) per 1 000 000 children for hypoglycemics, antihyperlipidemics, β-blockers, and opioids, respectively. On the basis of 2011 population estimates, this translates into an additional 11.2 hypoglycemic, 10.5 antihyperlipidemic, 15.6 β-blocker, and 42.6 opioid exposures and poisonings.
Granger causality analysis suggested adult medication mentions were a Granger cause of child exposures and poisonings for 7 of the 12 combinations of medication and pediatric age group. For instance, adult opioid mentions were a Granger cause of child exposures and poisonings for all age groups (0–5 years: F = 2.20, P < .05; 6–12 years: F = 6.42; P < .001; 13–19 years: F = 4.31, P < .001). Among children 0 to 5 years, adult mentions of all medications were Granger causes of increased exposures and poisonings for the same medication (P < .05). Opioids and antihyperlipidemic adult mentions were also Granger causes of increased exposures and poisonings among children 6 to 12 years old (P < .05). Only opioid adult mentions were Granger causes of increased exposure or poisoning among children 13 to 19 years old. These results suggest that the associations described in Fig 2 are indicative of a causal relationship in 7 cases.
Health Care Utilization and Morbidity Related to Pediatric Exposures and Poisonings
A total of 92 715 (61.3%) exposures and poisonings resulted in an ED evaluation. Hypoglycemics and β-blockers were associated with the highest rates of ED visits, 60.3% (n = 23 205) and 59.6% (n = 29 233) of exposures and poisonings, respectively (Table 2). Visit rates for opioids were 46.4% (n = 30 277) and for antihyperlipidemics 25.3% (n = 10 050). Children 0 to 5 years old experienced the highest rates of visits for exposures and poisonings related to β-blockers (62.7%) and hypoglycemics (61.2%). Among children 13 to 19 years old, the majority of exposures and poisonings resulted in an ED visit for every drug class.
TABLE 2.
ED Utilization Among Children With Exposures and Poisonings Related to Adult Prescription Drugs, 2000–2009a
| Medication Class | ED Visit, n (%) | No ED Visit, n (%) | Lost to Follow- up, n (%) |
|---|---|---|---|
| Hypoglycemics | 23 205 (60.3) | 12 242 (31.8) | 3038 (7.9) |
| 0–5 y | 19 540 (61.2) | 9985 (31.3) | 2421 (7.6) |
| 6–12 y | 885 (43.1) | 991 (48.2) | 179 (8.7) |
| 13–19 y | 2762 (62.8) | 1230 (28.0) | 406 (9.2) |
| Antihyperlipidemics | 10 050 (25.3) | 28 610 (72.1) | 1033 (2.6) |
| 0–5 y | 8414 (24.1) | 25 670 (73.6) | 773 (2.2) |
| 6–12 y | 267 (11.5) | 2000 (86.1) | 57 (2.5) |
| 13–19 y | 1364 (55.9) | 881 (36.1) | 195 (8.0) |
| β-blockers | 29 233 (59.6) | 16 040 (32.7) | 3802 (7.7) |
| 0–5 y | 24 187 (62.7) | 11 504 (29.8) | 2884 (7.5) |
| 6–12 y | 1518 (34.3) | 2610 (59.0) | 297 (6.7) |
| 13–19 y | 3508 (58.8) | 1890 (31.7) | 571 (9.6) |
| Opioids | 30 227 (48.4) | 21 793 (34.9) | 10 396 (16.7) |
| 0–5 y | 15 280 (46.4) | 13 619 (41.4) | 4034 (12.3) |
| 6–12 y | 1359 (23.0) | 3495 (59.3) | 1044 (17.7) |
| 13–19 y | 13 559 (58.2) | 4579 (19.7) | 5160 (22.2) |
There was missing age information for 86 children with an exposure or poisoning to oral hypoglycemics, 72 related to antihyperlipidemics, 106 related to β-blockers, and 287 related to opioids. These cases were excluded in the age-group analyses.
Among children of all ages, serious injuries were associated with 17.5% (n = 16 196) of exposures and poisonings evaluated and treated in EDs. Approximately 19.5% (n = 4518) of exposures to hypoglycemics, 8.8% (n = 885) of exposures to antihyperlipidemics, 9.8% (n = 2857) of exposures to β-blockers, and 26.3% (n = 7966) of exposures to opioids resulted in serious injury (Table 3). For each of the drug classes, children experienced increasing rates of serious injury with increasing age. Among patients 13 to 19 years old, rates of injuries ranged from 29.5% for events related to antihyperlipidemics to 40.5% for those resulting from opioids. Opioid exposures were the most likely to suffer a serious clinical effect and were also most likely to be admitted to a medical unit.
TABLE 3.
Injuries and Admissions Among Children Treated in EDs for Exposures and Poisonings Related to Adult Prescription Drugs, 2000–2009a
| Medication Class | Significant Injury, n (%)b | Medical Admission, n (%) | Psychiatric Admission, n (%) |
|---|---|---|---|
| Hypoglycemics | 4518 (19.5) | 11 462 (49.4) | 401 (1.7) |
| 0–5 y | 3317 (17.0) | 9565 (49.0) | 5 (0.03) |
| 6–12 y | 195 (22.0) | 375 (42.4) | 20 (2.3) |
| 13–19 y | 1002 (36.3) | 1514 (55.0) | 376 (13.6) |
| Antihyperlipidemics | 885 (8.8) | 2629 (26.2) | 319 (3.2) |
| 0–5 y | 446 (5.3) | 1997 (23.7) | 3 (0.04) |
| 6–12 y | 37 (13.9) | 67 (25.1) | 11 (4.1) |
| 13–19 y | 402 (29.5) | 564 (41.4) | 305 (22.4) |
| β-blockers | 2857(9.8) | 8408 (28.8) | 519 (1.8) |
| 0–5 y | 1305 (5.4) | 6344 (25.8) | 6 (0.02) |
| 6–12 y | 201 (13.3) | 325 (21.4) | 18 (1.2) |
| 13–19 y | 13 850(38.5) | 1830 (52.2) | 494 (14.1) |
| Opioids | 7966 (26.3) | 10 560 (35.0) | 1965 (6.5) |
| 0–5 y | 2177 (14.3) | 4268 (27.9) | 8 (0.05) |
| 6–12 y | 300 (22.1) | 427 (31.4) | 39 (2.9) |
| 13–19 y | 5485 (40.5) | 5857 (43.2) | 1916 (14.1) |
There was missing age information for 86 children with an exposure or poisoning to oral hypoglycemics, 72 related to antihyperlipidemics, 106 related to β-blockers, and 287 related to opioids. These cases were excluded in the age-group analyses.
Defined as children meeting outcome criteria for moderate effect, major effect, or death.
Over a third of all pediatric exposures and poisoning patients evaluated in the ED were hospitalized (35.7%; n = 33 059). Admission rates were highest among patients ingesting hypoglycemics (49.4%) and opioids (35.0%).
Discussion
Increasing rates of adult drug prescriptions are strongly associated with increases in drug exposures and poisonings among children and appear to be a direct cause of exposures and poisonings. Exposures to opioids are most closely related to trends in adult use, and children aged 0 to 5 years old are at greatest risk for exposure among all 4 adult drug classes studied. There is substantial health care utilization and morbidity associated with these events among children of all ages,
The Poisoning Prevention Packaging Act of 1970 mandated the use of child-resistant packaging for prescription medications and is credited with making pediatric fatalities from medication exposures and poisonings relatively uncommon in the United States.6,25,26 The 2008 Preventing Overdoses and Treatment Exposures Task Force has further promoted the development of a new generation of safety packaging.6,27 Our work suggests that even though these programs may be effective, child exposures and poisonings continue to be a significant and increasing problem, and interventions need to take into account the increases in adult prescriptions available to children. Pediatricians should consult parents of patients on storing medications, focusing on how exposures vary based on the child’s age and intention. Physicians prescribing drugs to adults should also be aware of the potential risk of exposures to children and provide guidance accordingly. Finally, certain drugs may require additional packaging modification. For example, in 2012, the manufacturers of Suboxone, in response to an analysis from the US Poison Centers, announced that they would voluntarily discontinue the supply of Suboxone tablets and sell only unit-dose packaged Suboxone film.28
Increased adult prescription medication availability is likely to persist. The NHANES found that between 1999–2000 and 2007–2008, the percentage of US adults who took ≥1 prescription drug in the preceding month increased by 10%.14 Moreover, the rising rate of obesity in the United States suggests increases in pediatric exposure and poisonings will continue because prescriptions for antihyperlipidemics, oral hypoglycemics, and β-blockers are all commonly used to treat obesity-related complications.29–31 Rates of polypharmacy are also on the rise.14 Without greater emphasis on interventions focusing on the changed context of increased availability of prescription drugs in households, the number of medication exposures and poisonings in children will likely continue to increase.
The observed differences in medication exposures and poisonings across age groups may be related to age-specific behaviors that lead to these events.1,13 Among younger children, poisonings tend to be related to exploratory behavior and result in unintentional exposures to a medication.1 Children aged ≤5 years are most susceptible to this type of ingestion and experience higher rates of exposures and poisonings. Among teenagers, ingestions are more likely to be intentional, including for recreational purposes or with the intention of self-harm.1,32 As a result, the morbidity and health care utilization (including psychiatric admissions, as our findings demonstrate) are higher in this older patient population.1,13
Opioid exposures far exceeded events related to the other drug classes among teenagers. This class of drugs differs from the others in its potential for abuse. Opioid diversion is a well-recognized public health problem among adults and has also been identified among teenagers.33–37 Drug overdose death rates have more than tripled in the United States since 1990, with >75% of these deaths attributable to opioids.36 The rise in deaths and health care use related to opioid diversion has been paralleled by a 300% increase in the sales of opioids since 1999.36 Our findings support the need for further research to delineate the factors surrounding adolescent access to and abuse of these drugs.
One of the limitations of our study is that NPDS relies in part on patient-reported information, and we could not consistently determine the amount of drug that was ingested. However, the health care facility utilization and medical outcome data provide a measure of the extent of serious ingestions. Because we limited our data to single-ingredient medications, we were unable to include certain combination medications (eg, acetaminophen-hydrocodone), resulting in potential underestimation of the number of exposures and poisonings for the drug classes of interest. It is also possible that some patients ingested >1 medication, and we were unable to ascertain whether the clinical effects experienced were related solely to the medications of interest. In addition, it is important to note that reporting to Poison Control Centers is voluntary, and physicians may be more likely to report exposures related to certain medications. Poison center data therefore provide an approximation of real-work poisoning epidemiology and are subject to underestimates of certain types of exposures. Finally, additional information on the circumstances surrounding ingestions is not available in NPDS, and additional information describing whose medication was taken, why it was prescribed, and the person’s relationship to the exposed child would be particularly useful in developing future safety interventions.
Conclusions
The rising use of opioids, antihyperlipidemics, oral hypoglycemics, and β-blockers among adults is associated with a corresponding rise in exposures and poisonings related to these drugs in children. These events are associated with considerable health care utilization, both in terms of ED visits and hospital admissions. Our findings support the need to strengthen intervention efforts around prescription drug ingestions and to develop prevention strategies that are both age- and medication-specific.
Glossary
- CI
confidence interval
- ED
emergency department
- NPDS
National Poison Database System
Footnotes
Dr Burghardt contributed to study conception and design, data acquisition, analysis and interpretation of data, drafting, and revising the article; Dr Ayers contributed to data acquisition, analysis and interpretation of data, and revising the article; Dr Brownstein contributed to study conception and design, data acquisition and analysis, and revision; Dr Bronstein contributed to study conception and design and revision; Dr Burns Ewald contributed to study conception and design, data acquisition, and revision; and Dr Bourgeois contributed to study conception and design, data acquisition, analysis and interpretation of data, drafting, and revision; all authors provided final approval of the article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Ayers was supported by a grant from the National Institute of Child Health and Human Development (T32-5T32HD040128-10).
References
- 1.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila). 2010;48(10):979–1178 [DOI] [PubMed] [Google Scholar]
- 2.Lifshitz M, Gavrilov V. Acute poisoning in children. Isr Med Assoc J. 2000;2(7):504–506 [PubMed] [Google Scholar]
- 3.Olguin HJ, Garduño LB, Pérez JF, Pérez CF. Unintentional poisoning with drugs in a Mexican pediatric population. J Popul Ther Clin Pharmacol. 2011;18:e156–e160 [PubMed] [Google Scholar]
- 4.Shannon M. Ingestion of toxic substances by children. N Engl J Med. 2000;342(3):186–191 [DOI] [PubMed] [Google Scholar]
- 5.Riordan M, Rylance G, Berry K. Poisoning in children 2: painkillers. Arch Dis Child. 2002;87(5):397–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budnitz DS, Salis S. Preventing medication overdoses in young children: an opportunity for harm elimination. Pediatrics 2011;127(6). Available at: www.pediatrics.org/cgi/content/full/127/6/e1597 [DOI] [PubMed]
- 7.Bond GR, Woodward RW, Ho M. The growing impact of pediatric pharmaceutical poisoning. J Pediatr. 2012;160(2):265–270.e1 [DOI] [PubMed] [Google Scholar]
- 8.Franklin RL, Rodgers GB. Unintentional child poisonings treated in United States hospital emergency departments: national estimates of incident cases, population-based poisoning rates, and product involvement. Pediatrics. 2008;122(6):1244–1251 [DOI] [PubMed] [Google Scholar]
- 9.Rodgers GB, Franklin RL, Midgett JD. Unintentional paediatric ingestion poisonings and the role of imitative behaviour. Inj Prev. 2012;18(2):103–108 [DOI] [PubMed] [Google Scholar]
- 10.Schillie SF, Shehab N, Thomas KE, Budnitz DS. Medication overdoses leading to emergency department visits among children. Am J Prev Med. 2009;37(3):181–187 [DOI] [PubMed] [Google Scholar]
- 11.Bailey JE, Campagna E, Dart RC, RADARS System Poison Center Investigators . The underrecognized toll of prescription opioid abuse on young children. Ann Emerg Med. 2009;53(4):419–424 [DOI] [PubMed] [Google Scholar]
- 12.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2008 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (Phila). 2009;47(10):911–1084 [DOI] [PubMed] [Google Scholar]
- 13.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief. 2010(42):1–8 [PubMed]
- 14.National Ambulatory Medical Care Survey: NAMCS and NHAMCS Web Tables, 2009. Available at: www.cdc.gov/nchs/data/ahcd/namcs_summary/2009_namcs_web_tables.pdf. Accessed January 7, 2013
- 15.National Ambulatory Medical Care Survey: NAMCS and NHAMCS Web Tables, 2008. www.cdc.gov/nchs/ahcd/web_tables.htm#2008. Accessed November 1, 2011
- 16.Copp HL, Shapiro DJ, Hersh AL. National ambulatory antibiotic prescribing patterns for pediatric urinary tract infection, 1998–2007. Pediatrics. 2011;127(6):1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisniewski AM, Purdy CH, Blondell RD. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis. 2008;27(1):1–11 [DOI] [PubMed] [Google Scholar]
- 18.US Census Bureau. National intercensal estimates (1990–2000): Intercensal estimates of the United States population by age and sex, 1990–2000: All months. Available at: www.census.gov/popest/data/intercensal/national/files/US-EST00INT-ALLDATA.csv. Accessed November 14, 2011
- 19.Lutkepohl H. New introduction to multiple time series analysis. Berlin: Birkhauser; 2006 [Google Scholar]
- 20.Barnett AGDA. Analysing seasonal health data. Berlin: Springer; 2010 [Google Scholar]
- 21.Newey WK, West K. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica. 1987;55(3):703–708 [Google Scholar]
- 22.Chen YRG, Feng J, Ding M. Analyzing multiple nonlinear time series with extended Granger causality. Phys Lett A. 2004;324(1):26–35 [Google Scholar]
- 23.Hesse WME, Möller E, Arnold M, Schack B. The use of time-variant EEG Granger causality for inspecting directed interdependencies of neural assemblies. J Neurosci Methods. 2003;124(1):27–44 [DOI] [PubMed] [Google Scholar]
- 24.Granger CWJ. Causality, cointegration, and control. J Econ Dyn Control. 1988;12:551–559 [Google Scholar]
- 25.Liebelt EL, DeAngelis CD. Evolving trends and treatment advances in pediatric poisoning. JAMA. 1999;282(12):1113–1115 [DOI] [PubMed] [Google Scholar]
- 26.Budnitz DS, Lovegrove MC. The last mile: taking the final steps in preventing pediatric pharmaceutical poisonings. J Pediatr. 2012;160(2):190–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. The PROTECT Initiative: Advancing Children’s Medication Safety, 2012. Available at: www.cdc.gov/medicationsafety/protect/protect_initiative.html. Accessed June 30, 2012
- 28.RB Press release, September 25, 2012. Reckitt Benckiser Pharmaceuticals Inc. to Voluntarily Discontinue the Supply of Suboxone Tablets (buprenorphine and naloxone sublingual tablets [CIIII]. Available at www.rb.com/site/RKBR/Templates/MediaInvestorsGeneral2.aspx?pageid=1328&cc=GB. Accessed January 16, 2013
- 29.Ogden CL, Carroll, MD, Kit BK, Flegal, KM. Prevalence of Obesity in the United States, 2009–2010 (NCHS Data Brief 82). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/nchs/data/databriefs/db82.pdf. Accessed August 1, 2012
- 30.Freedmam D.S. Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report. Obesity—United States, 1988–2008. Available at: cdc.gov/mmwr/preview/mmwrhtml/su6001a15.htm?s_cid=su6001a15_w. Accessed August 1, 9, 2012
- 31.Centers for Disease Control and Prevention. Adult obesity facts. Available at: www.cdc.gov/obesity/data/adult.html. Accessed July 12, 2012
- 32.Mintegi S, Fernández A, Alustiza J, et al. Emergency visits for childhood poisoning: a 2-year prospective multicenter survey in Spain. Pediatr Emerg Care. 2006;22(5):334–338 [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention . CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–13 [PubMed] [Google Scholar]
- 34.Tormoehlen LM, Mowry JB, Bodle JD, Rusyniak DE. Increased adolescent opioid use and complications reported to a poison control center following the 2000 JCAHO pain initiative. Clin Toxicol (Phila). 2011;49(6):492–498 [DOI] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. Lock It Up: Medicine Safety in Your Home Available at: www.fda.gov/ForConsuers/ConsumerUpdates/ucm272905.htm. Accessed January 16, 2013
- 36.Centers for Disease Control and Prevention. Injury Prevention and Control. Policy Impact: Prescription Painkiller Overdoses Available at: www.cdc.gov/homeandrecreationalsafety/rxbrief. Accessed August 2, 2012
- 37.Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346–1347 [DOI] [PubMed] [Google Scholar]