Abstract
OBJECTIVE:
To characterize the atypical cutaneous presentations in the coxsackievirus A6 (CVA6)–associated North American enterovirus outbreak of 2011–2012.
METHODS:
We performed a retrospective case series of pediatric patients who presented with atypical cases of hand, foot, and mouth disease (HFMD) from July 2011 to June 2012 at 7 academic pediatric dermatology centers. Patients were included if they tested positive for CVA6 or if they met clinical criteria for atypical HFMD (an enanthem or exanthem characteristic of HFMD with unusual morphology or extent of cutaneous findings). We collected demographic, epidemiologic, and clinical data including history of skin conditions, morphology and extent of exanthem, systemic symptoms, and diagnostic test results.
RESULTS:
Eighty patients were included in this study (median age 1.5 years, range 4 months–16 years). Seventeen patients were CVA6-positive, and 63 met clinical inclusion criteria. Ninety-nine percent of patients exhibited a vesiculobullous and erosive eruption; 61% of patients had rash involving >10% body surface area. The exanthem had a perioral, extremity, and truncal distribution in addition to involving classic HFMD areas such as palms, soles, and buttocks. In 55% of patients, the eruption was accentuated in areas of eczematous dermatitis, termed “eczema coxsackium.” Other morphologies included Gianotti-Crosti–like (37%), petechial/purpuric (17%) eruptions, and delayed onychomadesis and palm and sole desquamation. There were no patients with serious systemic complications.
CONCLUSIONS:
The CVA6-associated enterovirus outbreak was responsible for an exanthem potentially more widespread, severe, and varied than classic HFMD that could be confused with bullous impetigo, eczema herpeticum, vasculitis, and primary immunobullous disease.
Keywords: hand, foot, and mouth disease; coxsackievirus; atopic dermatitis; exanthem
What’s Known on This Subject:
Coxsackievirus A6 (CVA6) was identified as an important cause of “severe” hand, foot, and mouth disease (HFMD) during the 2011–2012 outbreak in North America. The atypical cutaneous features in this outbreak have not been well documented.
What This Study Adds:
The cutaneous manifestations of CVA6-associated HFMD may be more extensive and variable than classic HFMD. Four distinct morphologies characterize this exanthem: (1) widespread vesiculobullous and erosive lesions, (2) “eczema coxsackium,” (3) an eruption similar to Gianotti-Crosti, and (4) purpuric lesions.
In March 2012, the Centers for Disease Control and Prevention (CDC) reported a growing number of “severe and extensive” cases of hand, foot, and mouth disease (HFMD) attributed to coxsackievirus A6 (CVA6).1 Over the past 5 years, CVA6 has also been implicated in HFMD outbreaks in Finland, France, Spain, India, Taiwan, Japan, Singapore, China, and Boston, Massachusetts.2–11 The atypical cutaneous features characterizing the 2011–2012 North American CVA6-associated HFMD outbreak have not been clearly defined.
HFMD was first described by C.R. Robinson as a self-limited febrile illness characterized by “pharyngeal lesions and vesicular exanthem” affecting young children in a Toronto suburb in the summer of 1957.12 Since that report, HFMD has become a widely recognized childhood exanthem, representing a frequent and characteristic manifestation of enterovirus infections. Classic HFMD consists of fever, oral erosions, and gray-white, oval vesicles on the hands, feet, and buttocks.13,14 Children aged <5 years are most often affected with transmission occurring via fecal-oral route, vesicle fluid, or respiratory secretions.15 Coxsackievirus A16 (CVA16) and enterovirus 71 are the 2 most commonly reported causes of HFMD.16,17 CVA16 has traditionally been considered the most frequent cause of HFMD in the United States.17 Enterovirus 71 has been responsible for a number of outbreaks of HFMD associated with severe neurologic complications in Asia.18,19
The purpose of this multi-institutional, retrospective study was to better define the more severe, varied, and extensive dermatologic manifestations of the CVA6-associated outbreak in North America.
Methods
We performed a multi-institutional retrospective case series of pediatric patients between the ages of 0 and 18 years evaluated by pediatric dermatologists at 7 institutions (University of California, San Francisco [UCSF]; New York University; Washington University in St Louis; The Hospital for Sick Children, Toronto; Children’s Hospital Boston; Stony Brook University in collaboration with North Shore-Long Island Jewish Health System Laboratories; and Yale University) from July 2011 to June 2012 with atypical presentations of HFMD. Patients were identified through a search of electronic health records and photo archives at each institution. Patients were included if infection with CVA6 was confirmed by nucleotide sequencing of samples from oropharynx, skin, blood, or stool or if they met clinical case criteria for atypical or severe HFMD. The clinical inclusion criteria were predetermined by the lead authors based on initial experience with this outbreak. Two categories of clinical case criteria needed to be fulfilled for inclusion: (1) features suggestive of HFMD and (2) unusual extent or morphology. Features suggestive of HFMD were defined as an enanthem characteristic of HFMD (small vesicles and erosions on the oral mucosa), exanthem with features suggestive of HFMD (gray-white, oval vesicles on the hands, feet, and buttocks), or history of exposure to HFMD 2 to 14 days before disease onset. To meet the criteria for unusual morphology or extent of dermatologic findings, patients needed to have at least 1 of the following: HFMD exanthem involving >5% of the body surface area (BSA); erosions, vesicles (fluid filled blister <1 cm) and/or bullae (fluid filled blister >1cm) with acrofacial accentuation; purpuric, petechial, or hemorrhagic lesions; coalescing papules symmetrically distributed on the face, arms, and legs with relative sparing of the trunk similar to Gianotti-Crosti; or large bullae (>2 cm). Patients were excluded if the presentation could be explained by another specific illness such as, but not limited to, varicella or herpes simplex virus (HSV) infection, bullous impetigo, uncomplicated atopic dermatitis (AD), contact dermatitis, or other bullous diseases, or if the exanthem and enanthem were consistent with classic HFMD with <5% BSA involved. Two investigators (EM, IJF) reviewed data collection forms and available photographs for all cases. Institutional review board approval was obtained from UCSF as the coordinating board and from each participating institution as required.
A standardized data collection form was used to record epidemiologic and clinical data including age, gender, ethnicity, race, school or day-care participation, sick contacts, travel history, history of skin conditions, disease onset and duration, systemic symptoms, relevant laboratory results, treatment received, and whether they were managed as an inpatient or outpatient. The morphology and distribution of the eruption were assessed in detail including primary skin lesion types and location, extent (BSA), secondary features, nail changes, and oropharyngeal involvement. We also recorded whether the eruption was concentrated in areas previously or currently affected by AD (similar to eczema herpeticum), localized to an area of previous skin injury, or appeared similar to Gianotti-Crosti. Enterovirus detection was done by either 1-step real-time reverse transcriptase polymerase chain reaction (PCR) or nucleic acid sequence–based amplification using the NucliSens EasyQ EV assay (bioMerieux, Durham, NC). For the enterovirus-positive samples, when available, enterovirus type was determined by sequencing at the CDC or the California Department of Public Health.20
Data were compiled and analyzed by using Excel and SAS version 9.2 (SAS Institute, Cary, NC). Characteristics were examined for 3 subsets of patients: (1) patients with and without AD; (2) patients PCR-positive for CVA6 versus patients not tested for CVA6; and (3) patients classified into 3 age groups (<1, 1–5, >5 years). The Mann-Whitney test was used to compare differences in medians of a continuous characteristic between groups, and the Fisher’s exact test was used to compare differences in percentages of a categorical characteristic between groups.
Results
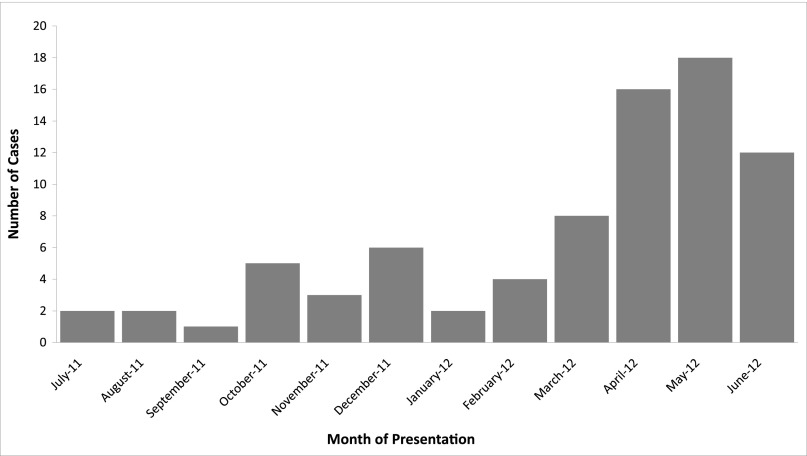
Eighty patients from 7 institutions were included in the analysis (14 from UCSF, 13 from New York University, 13 from Washington University, 13 from The Hospital for Sick Children, 11 from Children’s Hospital Boston, 10 from Stony Brook University, and 6 from Yale University). Seventeen patients met the virologic case criteria, and 63 met the clinical case criteria. The median age of the patients was 1.5 years (range 4 months–16 years; Table 1). Males and females were equally represented. The majority of patients were evaluated and managed in the outpatient setting, with only 13% hospitalized. Most patients presented in the spring and early summer of 2012 (Fig 1). Forty-three (54%) patients had a recorded exposure (21 day care, 11 family, 8 school, and 3 other) to HFMD.
TABLE 1.
Demographic Data, N = 80
| Age | |
| Median | 1.5 y |
| Range | 4 mo–16 y |
| Gender | |
| Male | 38 (47%) |
| Female | 43 (53%) |
| Race/ethnicitya | |
| White | 46 (57%) |
| African American | 15 (19%) |
| Hispanic/Latino | 9 (11%) |
| Asian | 8 (10%) |
| Unknown | 3 (4%) |
Percentages were rounded and do not add up to 100%.
FIGURE 1.
Number of atypical HFMD cases by month of presentation (N = 79).
The primary dermatologic finding was an eruption consisting of vesicles, bullae, or erosions. This presentation was seen in all but 1 patient who presented with papules concentrated on the extremities, including palms and soles, perioral face, and buttocks. The eruption affected the extremities in all patients and usually involved the hands and feet; lesions were also frequently observed on the face (62/79; 79%), torso (45/80; 56%), and buttocks/groin (55/72; 76%; Table 2, Figs 2, 3, 4, 5, and 6). Forty-two (61%) of 69 children with available data had >10% BSA involved. Cutaneous morphologies included relatively monomorphous erosions and vesicles concentrated in areas previously or currently affected by AD, similar to eczema herpeticum (44/80; 55%; Fig 3), hemorrhagic or purpuric lesions (13/78; 17%; Fig 4), and an eruption similar to Gianotti-Crosti (28/76; 37%). Fourteen patients had lesions in areas of skin injury including sunburn (Fig 5), irritant dermatitis (diaper, thumb-sucking, perioral, and medication induced), tinea pedis, and lacerations/scars. Two patients had large bullae (Fig 6). Intraoral erosions were identified in 39 of 77 (51%) patients. Of patients with available specific follow-up data, 9/38 (24%) had nail changes 4 to 6 weeks after initial presentation and 14 of 27 (52%) had desquamation of their palms or soles 1 to 3 weeks after initial presentation.
TABLE 2.
Comparison of CVA6-Positive Cases to Cases Without CVA6 Confirmation, No. Positive / Total No. Reported (%)a
| All Cases | CVA6+ Cases | Cases Without CVA6 Confirmation | P | |
|---|---|---|---|---|
| (n = 80) | (n = 17) | (n = 63) | ||
| Age, y, median (range) | 1.5 (0.33–16) | 1.25 (0.33–16) | 1.58 (0.33–16) | .59 |
| Sex | ||||
| Female | 42/80 (53%) | 12/17 (71%) | 30/63 (48%) | .11 |
| Male | 38/80 (48%) | 5/17 (30%) | 33/63 (52%) | |
| Body surface area | ||||
| <10% | 27/69 (39%) | 6/12 (50%) | 21/57 (37%) | .34 |
| 11%–25% | 18/69 (26%) | 4/12 (33%) | 14/57 (25%) | |
| >26% | 25/69 (35%) | 2/12 (17%) | 23/57 (39%) | |
| Distribution | ||||
| Palm/soles | 67/80 (84%) | 15/17 (88%) | 52/63 (83%) | .72 |
| Extremities | 79/79 (100%) | 17/17 (100%) | 62/62 (100%) | — |
| Face | 62/79 (79%) | 12/17 (71%) | 50/62 (81%) | .51 |
| Torso | 45/80 (56%) | 12/17 (70%) | 33/63 (52%) | .27 |
| Buttocks, groin, perineum | 55/72 (76%) | 12/17 (71%) | 43/55 (78%) | .53 |
| Morphology | ||||
| Vesicle, bullae, or erosions | 79/80 (99%) | 17/17 (100%) | 62/63 (98%) | 1.00 |
| Eczema herpeticum-like | 44/80 (55%) | 6/17 (35%) | 38/63 (60%) | .10 |
| Gianotti-Crosti-like | 28/76 (37%) | 8/16 (50%) | 20/60 (33%) | .25 |
| Purpuric/petechial | 13/78 (17%) | 3/17(18%) | 10/61 (16%) | 1.00 |
| Accentuation in areas of skin injury | 14/76 (18%) | 4/17 (24%) | 10/59 (17%) | .50 |
| Oral erosions/ulcerations | 39/77 (51%) | 9/17 (53%) | 30/60 (50%) | 1.00 |
| Nail changes | 9/38 (24%) | 2/8 (25.0%) | 7/30 (23%) | 1.00 |
| Hospitalized | 10/80 (13%) | 2/17 (12%) | 8/63 (13%) | 1.00 |
| Fever | 56/75 (75.0%) | 11/14 (79%) | 45/61 (74%) | 1.00 |
| Illness duration, d; (mean ± SD) | 12.2 ± 7.0 (n = 39) | 8.8 ± 3.6 (n = 12) | 13.7 ± 7.7 (n = 27) | .07 |
Not all features were known for each patient. Patients could be reported as having multiple morphologies and distributions. Percentages were rounded and do not add up to 100%. —, indicates P value can not be calculated when the condition is present in 100% of patients.
FIGURE 2.
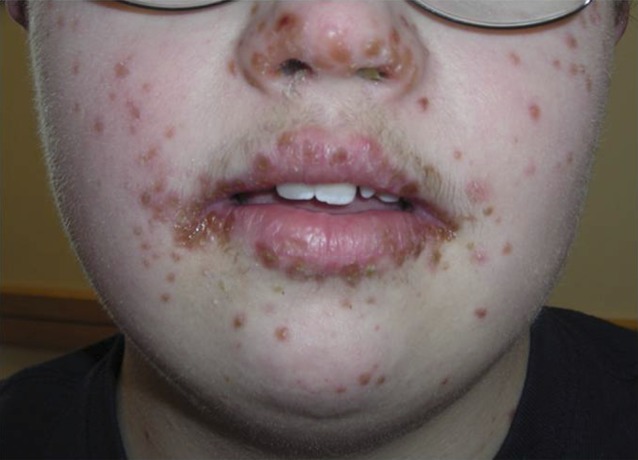
A 13-year-old with facial erosions and vesicles who had confirmed CVA6 infection.
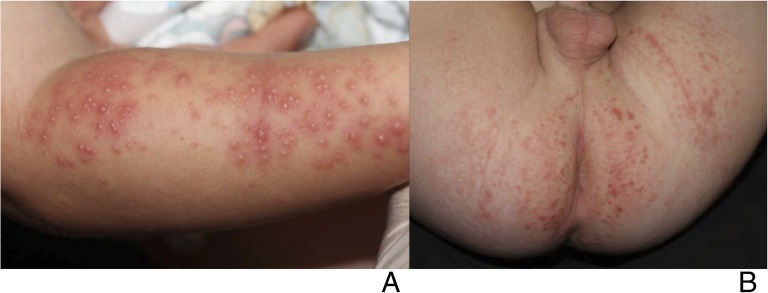
FIGURE 3.

Eczema coxsackium: A toddler with confirmed CVA6 infection who had erosions localized to areas of AD.
FIGURE 4.

A 16-year-old with confirmed CVA6 infection who had purpuric papules and vesicles on the feet.
FIGURE 5.
A toddler with confirmed CVA6 infection who had vesicles localized to areas of sunburn (a) in addition perianal and buttocks erosions (b).
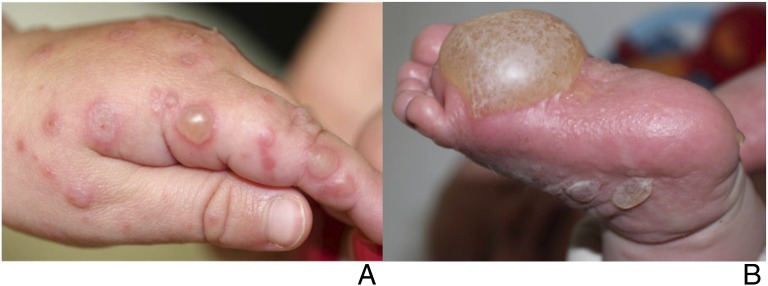
FIGURE 6.
A 4-month-old (a) and 15-month-old (b) with confirmed CVA6 infection who presented with acral bullae.
Infants <1 year old were significantly more likely to have bullae (Fig 6) than older children (38% of patients aged <1 year vs 7% of patients aged 1–5 years vs 18% of patients aged >5 years; P = .039). Older children were more likely to have hemorrhagic or purpuric lesions (Fig 4; 8% < 1 year vs 12% 1–5 years vs 43% >5 years; P = .021) and nail changes (33% <1 year vs 14% 1–5 years vs 75% >5 years, P = .022). There were no other significant morphologic differences between age groups.
Forty-nine of 79 (62%) patients had a preexisting skin condition, 40 (82%) of whom had a history of AD. Patients with AD were significantly more likely to have an eczema herpeticum-like presentation (81% of patients with AD, versus 24% of patients without AD; P < .0001) (Figure 3). There were no other significant differences between patients with and without AD.
Table 3 lists the results of the most common diagnostic tests that were performed. Seventeen patients were confirmed to have CVA6 by nucleotide sequencing. Confirmed CVA6 cases were compared with cases without confirmation of CVA6 infection (Table 2). The 2 groups were similar with regard to demographics, morphology, and distribution of skin lesions, and systemic symptoms. Four patients had skin biopsies of representative papulovesicles, vesicles, or bullae with variable findings including spongiotic dermatitis, focal interface dermatitis with areas of subepidermal separation, papillary dermal edema, and dermal inflammation.
TABLE 3.
Outcomes of Diagnostic Tests
| Diagnostic test | No. Positive | No. Performed |
|---|---|---|
| Coxsackievirus A6 nucleotide sequencing | 17 | 17a |
| Enterovirus PCR and/or NucliSens nucleic acid sequence based amplification | 24 | 27b |
| Enteroviral culture | 1 | 13 |
| HSV (DFA, culture, or PCR) | 0 | 27 |
| VZV (DFA or PCR) | 0 | 3 |
| Bacterial culture | 2 (MSSA, MRSA) | 14 |
| Skin biopsy | —c | 4 |
DFA, direct fluorescent antibody; MRSA, methicillin resistant staphylococcus aureus; MSSA, methicillin senstitive staphylococcus aureus; VZV, varicella zoster virus.
Coxsackievirus A6 nucleotide sequencing was not available to all patients who had enterovirus PCR and/or NucliSens nucleic acid sequence–based amplification. For specimens tested at the CDC or the California Department of Public Health, enterovirus PCR was performed first: if positive, CVA6 nucleotide sequencing was performed; if negative, no additional testing was done.
The 3 cases with negative enterovirus PCR were included based on clinical criteria. One specimen was collected 10 d after the acute phase of the illness. Two of the 3 specimens were skin swabs, which are less sensitive and more dependent on collection technique.
For skin histopathology, see discussion in text.
The most commonly reported symptoms were fever (75%) and sore throat/mouth (36%). Other symptoms such as cough, vomiting, diarrhea, or headache were reported in ≤10 patients. No patients had serious systemic or neurologic complications. Total illness duration (onset of first symptom to clearance of rash and all other symptoms) ranged from 3 to 35 days (mean days 12.2, SD 7.1).
Discussion
HFMD is classically defined as an enterovirus-associated exanthem characterized by fever; stomatitis of the oral mucosa; and a vesicular rash affecting the hands, feet, and occasionally the buttocks.12,13,21 This study helps characterize the wide-ranging and severe cutaneous features observed in the CVA6-associated HFMD outbreak first reported by the Centers for Disease Control and Prevention in March 2012.1 We identified 4 morphologies that characterize the severe end of the spectrum of disease associated with this atypical exanthem and distinguish it from classic HFMD: (1) widespread vesiculobullous and erosive lesions extending beyond the palms and soles, (2) an eczema herpeticum-like eruption termed “eczema coxsackium,” (3) an eruption similar to Gianotti-Crosti, and (4) a petechial or purpuric eruption (Table 4).
TABLE 4.
Clinical Features and Differential Diagnosis of Severe CVA6-Associated HFMD
| Findings Suggestive of HFMD a: 1) Fever, 2) Oral erosions, 3) Mild gastrointestinal symptoms, 4) Oval vesicles on hands and feet, 5) Known sick contacts | ||
|---|---|---|
| Atypical Cutaneous Morphology | Clinical Differential Diagnosis | |
| Vesiculobullous and erosive eruption | • Widespread (>5% BSA distribution) | • Bullous impetigo |
| • Perioral, acral, buttock predilection | • Varicella | |
| • Bullae more common aged <1year | • Primary immunobullous disorders | |
| Eczema coxsackium | • Vesicles and erosions in areas of eczematous dermatitis | • Eczema herpeticum |
| • Secondary bacterial infection in setting of AD | ||
| Gianotti Crosti-like eruption | • Acrofacial papulovesicles and erosions with relative sparing of the trunk similar to Gianotti-Crosti syndrome | • Gianotti Crosti syndrome |
| • Other viral exanthems | ||
| • Urticaria multiforme | ||
| Petechial and purpuric rash | • Most often seen in patients > 5 years of age | • Leukocytoclastic vasculitis |
| • Often acral | • Glove and stocking purpura (parvovirus infection) | |
| Delayed cutaneous findings | • Onychomadesis (nail shedding) and Beau’s lines (tranverse grooves) | • Onychomadesis: Medication induced (tetracyclines), after severe systemic illness |
| • Acral desquamation | • Acral Desquamation: after toxin or superantigen-mediated disease (Group A Streptococcus infection, Kawasaki disease, or toxic shock syndrome) | |
Diagnosis can be confirmed by enterovirus PCR (serum; oropharyngeal and skin swab as available). As indicated, rule out other entities with viral and bacterial cultures, viral DFA or PCR, and skin biopsy.
May be variably present.
The most common dermatologic presentation in our sample was widespread vesicles, bullae, and/or erosions. Classically, the vesicular exanthem of HFMD is restricted to the hands, feet, and occasionally the buttocks.10,13,14,19,21,22 The accompanying enanthem consists of small vesicles and erosions on the oral mucosa.12,13 The exanthem in our series differed by commonly involving the perioral area, extremities, and torso in addition to more classic HFMD locations. Other recent studies have also emphasized the perioral distribution of CVA6-associated disease.10,23 Vesicles, variably sized bullae, and/or erosions involved >10% BSA in the majority of our patients. To the best of our knowledge, widespread vesiculobullous exanthems have not been reported in previous HFMD outbreaks. Intraoral erosions were less common than in classic HFMD where the rate of intraoral erosions ranges from 75% to 100%.9,12,24–26
A large percentage of patients with underlying AD presented with vesicles and erosions within areas affected by AD that we term “eczema coxsackium.” This morphology was strikingly similar to eczema herpeticum caused by HSV1. CVA16 is the only enterovirus known to cause a similar eruption, with only 3 cases reported in the literature (1 case involving an adult patient with Darier’s disease27 and 2 in children with AD28). Why only certain viruses such as HSV1, vaccinia, and now CVA6 more commonly lead to vesicles and erosions in areas of dermatitis is not understood. Enteroviral infections, particularly CVA6, should now be considered in the differential diagnosis of patients presenting with new-onset vesicles and extensive erosions in preexisting areas of eczema.
In addition to localizing to areas of AD, the eruption in this recent outbreak demonstrated a predilection for areas of previous trauma or inflammation. Examples of this phenomenon in our series included vesicles, bullae, and erosions that developed in areas of preexisting sunburn, diaper dermatitis, irritant dermatitis, healing lacerations, and tinea pedis. This predilection for areas of trauma or injury may explain in part why classic HFMD and this more severe eruption are commonly seen on the buttocks, palms and soles, all of which are areas of increased trauma and friction in children. Other viral exanthems, such as varicella, have also been reported to occur in areas of sunburn and diaper dermatitis.28,29
A distribution similar to Gianotti-Crosti was documented in one-third of the patients in our study, with lesions involving the cheeks, extensor surfaces of the extremities, and buttocks, but sparing the trunk.30 Classic Gianotti-Crosti is characterized by monomorphous lichenoid papules and/or papulovesicles, whereas the eruption associated with this outbreak was more often papulovesicular with prominent erosions. Epstein-Barr virus and hepatitis B virus are the most commonly reported causes of Gianotti-Crosti,30 but enteroviruses such as coxsackieviruses A16, B4, and B5 have also been implicated.31
A petechial and purpuric rash was documented in 17% of our patients, most often in those aged >5 years and most frequently found on acral sites. Petechial and purpuric eruptions are a known cutaneous manifestation of enteroviral infections,32,33 and CVA6 infection should be added to the differential diagnosis of acral purpura, particularly in the setting of a community outbreak.
Delayed cutaneous features of this exanthem include nail changes and desquamation of the palms and soles, which typically occurred weeks after the resolution of the vesiculobullous eruption. Onychomadesis (separation of the proximal nail plate from the nail matrix and nail bed), and Beau’s lines (horizontal ridging of the nail plate) were a common feature of CVA6 infection in previous reports23,34 and were documented in 9 of 38 cases with available follow-up data in this series. However, this may be an underestimate because only a minority of patients was followed beyond their acute illness. Onychomadesis associated with HFMD most often occurs 3 to 8 weeks after HFMD is diagnosed.35,36 It is generally asymptomatic, and the nails typically regrow normally within several months.
The demographics and systemic manifestations observed in our patients are representative of previous reports of HFMD outbreaks, with a predilection for preschool-age children of either gender, occurring primarily during the late spring to early summer.3,6 Fever and oropharyngeal pain were the 2 most commonly reported symptoms. The low rate of gastrointestinal and respiratory symptoms is also consistent with past reports of CVA6 HFMD.3,6 Overall, the extracutaneous features of the 2011–2012 HFMD outbreak appear similar to those of the most common cause of HFMD, CVA16, rather than more virulent strains such as enterovirus 71.37 Only 10 patients in our sample were hospitalized, primarily because of the unusual nature of the skin disease. These children often received empriric antivirals and antibiotics and a diagnostic evaluation for extensive vesicles and bullae. Although a few patients did have dehydration in the setting of oral ulcerations, no patients in our study developed serious systemic complications that sometimes occur with enterovirus infections, such as myocarditis, pneumonia/pneumonitis, aseptic meningitis, or meningoencephalitis. Our report suggests that the extensive and varied cutaneous features seen in this outbreak do not portend an increased risk for severe systemic illness.
The phenotypic variability and unusual nature of the skin eruptions documented in this HFMD outbreak mimic and may be confused with other infectious and inflammatory skin diseases, emphasizing the need for accurate diagnostic testing. Enterovirus PCR testing requirements vary by laboratory, but testing can be performed from swabs of skin vesicle fluid, oropharynx, perirectal skin, stool, or blood. Viral culture for CVA6 is not recommended because CVA6 does not grow well in culture.38 However, HSV culture and/or direct fluorescent antibody testing should be considered to rule out herpesvirus infections in patients presenting with vesiculobullous disease.
Our study has several limitations. The retrospective nature of this study resulted in incomplete data capture. CVA6 was confirmed in only 17 of our cases, raising the possibility that not all of the patients in this report had CVA6 and that other enterovirus strains may have coexisted during this outbreak. However, no substantial differences in clinical presentation were noted when the confirmed CVA6 patients were compared with the patients meeting clinical inclusion criteria. Three patients had negative enterovirus PCR and were included based on clinical criteria. It is possible that these patients were not CVA6 infected, but it is also possible that their tests were negative because of inadequate skin specimen collection technique or because they were collected after the acute phase of the illness. Not all patients were tested for HSV or bacterial infections; therefore, we cannot rule out the possibility that other viral or bacterial infections could have contributed to some of the eruptions. Lastly, because all patients included in our sample were referred to an academic pediatric dermatology center, a referral bias toward more severe manifestations likely exists. Approximately half of the patients in this series had exposure to contacts with clinical HFMD who were not tested for enterovirus, or, more specifically, for CVA6. Therefore, it is possible that some patients infected with CVA6 demonstrate a more typical HFMD course. However, our study does not attempt to characterize all cutaneous eruptions associated with CVA6. Instead, our goal was to capture the more severe and unusual dermatologic features seen with this outbreak. Larger, prospective studies are needed to provide comprehensive epidemiologic data on the full clinical spectrum of disease and demographic risk factors of CVA6 infections.
Despite these limitations, this report highlights several important clinical findings not previously reported in outbreaks of HFMD. Awareness of the potential extent and variability of this condition should help to avoid confusion with other skin conditions such as eczema herpeticum, vasculitis, impetigo, and primary immunobullous disease, as well as to avoid errors in diagnosis and management in future outbreaks. We recommend enterovirus PCR testing in cases in which diagnosis is in doubt.
Acknowledgments
The authors thank the following people for technical support: Shannon Rogers, Centers for Disease Control and Prevention, Atlanta, GA; Tasha Padilla and the staff of the Viral and Rickettsial Disease Laboratory, California Department of Public Health, Richmond, CA.
Glossary
- AD
atopic dermatitis
- BSA
body surface area
- CDC
Centers for Disease Control and Prevention
- CVA6
coxsackievirus A6
- CVA16
coxsackievirus A16
- HFMD
hand, foot, and mouth disease
- HSV
herpes simplex virus
- MRSA
methicillin resistant staphylococcus aureus
- MSSA
methicillin senstitive staphylococcus aureus
- PCR
polymerase chain reaction
- UCSF
University of California, San Francisco
Footnotes
Dr Mathes conceptualized and designed the study, designed the data collection instruments, coordinated the 7 sites, supervised data collection at 1 site, carried out the initial analyses, interpreted the data, and reviewed and revised the initial manuscript; Dr Oza drafted the initial manuscript and supervised the initial analysis; Dr Frieden conceptualized and designed the study, designed the data collection instruments, and reviewed and revised the initial manuscript; Drs Yagi and Oberste coordinated acquisition of data, assisted in interpretation of data, and critically revised the initial manuscript; Dr Cordoro critically reviewed and revised the data collection instruments, and reviewed and revised the initial manuscript; Dr Howard conceptualized and designed the study, and reviewed and revised the initial manuscript; Dr Kristal conceptualized and designed the study, coordinated and supervised data collection at 1 site, and critically revised the initial manuscript; Dr Ginocchio coordinated acquisition of data at 1 site and critically revised the initial manuscript; Drs Schaffer, Maguiness, Bayliss, Lara-Corrales, and Garcia-Romero coordinated and supervised data collection at 1 site, and critically revised the initial manuscript; Dr Kelly conceptualized the study and critically revised the initial manuscript; Ms Salas conceptualized the study, coordinated acquisition of data at 1 site, and critically revised the initial manuscript; Mr Nix coordinated acquisition of data, assisted in interpretation of data, and critically revised the initial manuscript; Dr Glaser conceptualized and designed the study, designed the data collection instruments, assisted with collection and interpretation of data, and reviewed and revised the initial manuscript; Dr Antaya conceptualized and designed the study, designed the data collection instruments, coordinated collection of data at 1 site and interpretation of data, and reviewed and revised the initial manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Christine C. Ginocchio is a consultant, received payment for lectures, and has a grant/pending grant from bioMerieux. bioMerieux makes the enterovirus assay that was used in the diagnosis of enterovirus at North Shore-Long Island Jewish Health System Laboratories. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The data analysis for this publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through University of California, San Francisco-Clinical and Transitional Science Institute grant UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. No additional external funding was secured for this study.
References
- 1.Centers for Disease Control and Prevention (CDC) . Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep. 2012;61(12):213–214 [PubMed] [Google Scholar]
- 2.Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15(9):1485–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirand A, Henquell C, Archimbaud C, et al. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 and A10 infections in 2010, France: a large citywide, prospective observational study. Clin Microbiol Infect. 2012;18(5):E110–E118 [DOI] [PubMed] [Google Scholar]
- 4.Gopalkrishna V, Patil PR, Patil GP, Chitambar SD. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J Med Microbiol. 2012;61(pt 3):420–425 [DOI] [PubMed] [Google Scholar]
- 5.Bracho MA, González-Candelas F, Valero A, Córdoba J, Salazar A. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17(12):2223–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo SH, Huang YC, Huang CG, et al. Clinical and epidemiologic features of Coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44(4):252–257 [DOI] [PubMed] [Google Scholar]
- 7.Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48(1):49–54 [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto T, Iizuka S, Enomoto M, et al. Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18(2):337–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Yeo A, Phoon MC, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14(12):e1076–e1081 [DOI] [PubMed] [Google Scholar]
- 10.Flett K, Youngster I, Huang J, et al. Hand, foot, and mouth disease caused by coxsackievirus a6. Emerg Infect Dis. 2012;18(10):1702–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu QB, Zhang XA, Wo Y, et al. Circulation of Coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS ONE. 2012;7(12):e52073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson CR, Doane FW, Rhodes AJ. Report of an outbreak of febrile illness with pharyngeal lesions and exanthem: Toronto, summer 1957; isolation of group A Coxsackie virus. Can Med Assoc J. 1958;79(8):615–621 [PMC free article] [PubMed] [Google Scholar]
- 13.Cherry JD, Jahn CL. Hand, foot, and mouth syndrome. Report of six cases due to Coxsackie virus, group A, type 16. Pediatrics. 1966;37(4):637–643 [PubMed] [Google Scholar]
- 14.Lerner AM, Klein JO, Cherry JD, Finland M. New viral exanthems (concluded). N Engl J Med. 1963;269:736–740 [CONCL.] [DOI] [PubMed] [Google Scholar]
- 15.Ruan F, Yang T, Ma H, et al. Risk factors for hand, foot, and mouth disease and herpangina and the preventive effect of hand-washing. Pediatrics. 2011;127(4). Available at: www.pediatrics.org/cgi/content/full/127/4/e898 [DOI] [PubMed] [Google Scholar]
- 16.Ho M, Chen ER, Hsu KH, et al. Taiwan Enterovirus Epidemic Working Group . An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341(13):929–935 [DOI] [PubMed] [Google Scholar]
- 17.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA, Centers for Disease Control and Prevention . Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ. 2006;55(8):1–20 [PubMed] [Google Scholar]
- 18.Lee TC, Guo HR, Su HJ, Yang YC, Chang HL, Chen KT. Diseases caused by enterovirus 71 infection. Pediatr Infect Dis J. 2009;28(10):904–910 [DOI] [PubMed] [Google Scholar]
- 19.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10(11):778–790 [DOI] [PubMed] [Google Scholar]
- 20.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44(8):2698–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsop J, Flewett TH, Foster JR. “Hand-foot-and-mouth disease” in Birmingham in 1959. BMJ. 1960;2(5214):1708–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler JL, Mostow SR, Mellin H, Janney JH, Joseph JM. Epidemiologic investigation of hand, foot, and mouth disease. Infection caused by coxsackievirus A 16 in Baltimore, June through September 1968. Am J Dis Child. 1970;120(4):309–314 [DOI] [PubMed] [Google Scholar]
- 23.Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F, Zhang T, Hu Y, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J. 2011;8:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins PG, Warin RP. Hand, foot, and mouth disease. A clinically recognizable virus infection seen mainly in children. Clin Pediatr (Phila). 1967;6(6):373–376 [DOI] [PubMed] [Google Scholar]
- 26.Liu MY, Liu W, Luo J, et al. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS ONE. 2011;6(9):e25287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins PG, Crow KD. Recurrent Kaposi’s varicelliform eruption in Darier’s disease. Br J Dermatol. 1973;88(4):391–394 [DOI] [PubMed] [Google Scholar]
- 28.Messner J, Miller JJ, James WD, Honig PJ. Accentuated viral exanthems in areas of inflammation. J Am Acad Dermatol. 1999;40(2 pt 2):345–346 [DOI] [PubMed] [Google Scholar]
- 29.Belhorn TH, Lucky AW. Atypical varicella exanthems associated with skin injury. Pediatr Dermatol. 1994;11(2):129–132 [DOI] [PubMed] [Google Scholar]
- 30.Brandt O, Abeck D, Gianotti R, Burgdorf W. Gianotti-Crosti syndrome. J Am Acad Dermatol. 2006;54(1):136–145 [DOI] [PubMed] [Google Scholar]
- 31.James WD, Odom RB, Hatch MH. Gianotti-Crosti-like eruption associated with coxsackievirus A-16 infection. J Am Acad Dermatol. 1982;6(5):862–866 [DOI] [PubMed] [Google Scholar]
- 32.Fretzayas A, Douros K, Moustaki M, Nicolaidou P. Papular-purpuric gloves and socks syndrome in children and adolescents. Pediatr Infect Dis J. 2009;28(3):250–252 [DOI] [PubMed] [Google Scholar]
- 33.Nielsen HE, Andersen EA, Andersen J, et al. Diagnostic assessment of haemorrhagic rash and fever. Arch Dis Child. 2001;85(2):160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28(1):1–5 [DOI] [PubMed] [Google Scholar]
- 35.Bernier V, Labrèze C, Bury F, Taïeb A. Nail matrix arrest in the course of hand, foot and mouth disease. Eur J Pediatr. 2001;160(11):649–651 [DOI] [PubMed] [Google Scholar]
- 36.Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17(1):7–11 [DOI] [PubMed] [Google Scholar]
- 37.Chang LY, Lin TY, Huang YC, et al. Comparison of enterovirus 71 and coxsackie-virus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr Infect Dis J. 1999;18(12):1092–1096 [DOI] [PubMed] [Google Scholar]
- 38.Vuorinen T, Vainionpää R, Hyypiä T. Five years’ experience of reverse-transcriptase polymerase chain reaction in daily diagnosis of enterovirus and rhinovirus infections. Clin Infect Dis. 2003;37(3):452–455 [DOI] [PubMed] [Google Scholar]