Abstract
OBJECTIVE:
Vitamin D influences cardiovascular and immune function. We aimed to establish the prevalence of vitamin D deficiency in critically ill children and identify factors influencing admission 25-hydroxy vitamin D (25(OH)D) levels. We hypothesized that levels would be lower with increased illness severity and in children with serious infections.
METHODS:
Participants were 511 severely or critically ill children admitted to the PICU from November 2009 to November 2010. Blood was collected near PICU admission and analyzed for 25(OH)D concentration by using Diasorin radioimmunoassay.
RESULTS:
We enrolled 511 of 818 (62.5%) eligible children. The median 25(OH)D level was 22.5 ng/mL; 40.1% were 25(OH)D deficient (level <20 ng/mL). In multivariate analysis, age and race were associated with 25(OH)D deficiency; summer season, vitamin D supplementation, and formula intake were protective; 25(OH)D levels were not lower in the 238 children (46.6%) admitted with a life-threatening infection, unless they had septic shock (n = 51, 10.0%) (median 25(OH)D level 19.2 ng/mL; P = .0008). After adjusting for factors associated with deficiency, lower levels were associated with higher admission day illness severity (odds ratio 1.19 for a 1-quartile increase in Pediatric Risk of Mortality III score per 5 ng/mL decrease in 25(OH)D, 95% confidence interval 1.10–1.28; P < .0001).
CONCLUSIONS:
We found a high rate of vitamin D deficiency in critically ill children. Given the roles of vitamin D in bone development and immunity, we recommend screening of those critically ill children with risk factors for vitamin D deficiency and implementation of effective repletion strategies.
KEY WORDS: critical care, vitamin D, septic shock
What’s Known on This Subject:
Vitamin D is essential for bone health and for cardiovascular and immune function. In critically ill adults, vitamin D deficiency is common and associated with sepsis and with higher critical illness severity. The influence on pediatric critical illness is unclear.
What This Study Adds:
We found a high prevalence of vitamin D deficiency in critically ill children, which was associated with higher critical illness severity. Vitamin D deficiency was less common in younger patients, in non-Hispanic white patients, in patients admitted over the summer, and in children taking supplemental vitamin D, with increasing amounts being more protective.
Vitamin D is essential for bone health1–4 and optimal cardiovascular5–10 and innate immune11–15 function. Levels of 25-hydroxy vitamin D (25(OH)D) are most often used to assess adequacy of vitamin D stores. Patients with levels <20 ng/mL are commonly categorized as vitamin D deficient,1,16,17 and treatment is initiated in children to prevent rickets.16 Influenced by decreased vitamin D intake and decreased sun exposure, >60% of US children have insufficient levels of 25(OH)D (<30 ng/mL), with 15% being deficient.16–19 The rising rate of vitamin D insufficiency and deficiency has alarmed some experts,4,16,18–20 although others have questioned the clinical importance of 25(OH)D insufficiency.21
The level of 25(OH)D needed for adequate immune and cardiovascular function is unclear. Vitamin D deficiency has been associated with increased viral respiratory infections22–26 and sepsis27–29 in children and adults. Supplementing Japanese schoolchildren with 1200 IU of vitamin D over winter months decreased influenza infections.30 This could be because 25(OH)D influences production of cathelicidin hCAP-18, an anti-microbial peptide.14,31 Critically ill adult patients with sepsis have lower vitamin D levels, associated with lower cathelicidin levels.28
Vitamin D deficiency has been associated with higher levels of admission illness severity in adult ICU patients.32–34 Low 25(OH)D levels prehospitalization and at ICU admission have been associated with short- and long-term all-cause mortality and bacteremia in critically ill adults.27,34,35 The prevalence of vitamin D deficiency and its influence on critical illness severity in children is unknown. We aimed to establish the prevalence of vitamin D deficiency in a cohort of critically ill children and identify factors influencing 25(OH)D levels on admission to the pediatric ICU. We hypothesized that children with lower 25(OH)D levels would have higher illness severity and that levels would be lowest in PICU patients admitted for a life-threatening infection.
Methods
We screened all children admitted to the medical-surgical PICUs from November 9, 2009 to November 9, 2010. Eligibility criteria included the following: (1) age <21 years and (2) estimated PICU stay of ≥48 hours (excluding short-term monitoring patients) or admission due to a probable infection. Patients admitted to the cardiac ICU were excluded because of high incidence of cardiac bypass, which can lower 25(OH)D levels.36 The Children’s Hospital Boston institutional review board approved the study. After obtaining informed consent, parents or guardians were interviewed about their child’s racial and ethnic background, sun exposure, and intake of vitamin D–containing foods and supplements by using a questionnaire adapted from a previous vitamin D study.16 Dose of vitamin D supplements (ergocalciferol or cholecalciferol) was obtained from parent report. Formula intake in diet was dichotomized as yes–no to account for inconsistency in daily intake amount between patients.
Blood was obtained as close as possible to PICU admission either by drawing fresh blood or retrieving samples of leftover plasma refrigerated in the hospital laboratory. All plasma was stored refrigerated, frozen at −80°C within 7 days, then shipped frozen in batch for analysis. A direct radioimmunoassay developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN) was used to measure total circulating 25(OH)D and 1,25(OH)2D concentrations (inter- and intraassay coefficient of variation of 10%).37
Height was not routinely collected to calculate BMI, so probable obesity was defined as admission weight >2 SD above the 50th percentile for age. Severity of illness in the first 24 hours was measured by using the Pediatric Risk of Mortality III (PRISM III) score.38 Maximum level of vasopressor use during PICU stay was assessed by using the Sequential Organ Failure Assessment cardiovascular (CV-SOFA) score with 0–1: no vasopressors, 2: dopamine <5 mcg/kg/min, 3: dopamine 5 to 15 mcg/kg/min or norepinephrine/epinephrine <0.1 mcg/kg/min, and 4: dopamine >15 mcg/kg/min or norepinephrine/epinephrine >0.1 mcg/kg/min.39
To determine infection status on admission, patients who had any cultures or viral testing performed on the PICU admission day, or with a diagnosis of a confirmed or suspected infection within 7 days before PICU admission, were reviewed by a critical care physician. Confirmed infection was defined as having a (1) culture of a pathogenic bacteria from blood, cerebrospinal fluid, or lung plus receipt of antibiotics; (2) positive fungal culture plus antifungal treatment; or (3) viral pathogen detected. Suspected infection included all patients meeting systemic inflammatory response syndrome or community acquired pneumonia criteria with negative microbial testing who received a course of antibiotic treatment. Severe septic shock was defined as confirmed or suspected infection with vasopressor therapy (CV-SOFA score ≥3) on PICU admission day. Levels of 25(OH)D were categorized as normal (≥30 ng/mL), insufficient (<30 ng/mL),26,28 and deficient (<20 ng/mL).17,40,41 Data were managed by using REDCap (Research Electronic Data Capture) tools hosted at Children’s Hospital Boston.42
In the most critically ill children, it was not possible to obtain prospective consent before or at PICU admission, so an accurate measurement of the child’s fluid status at the exact time when the blood was sampled during fluid resuscitation was determined not to be feasible. When we were not able to obtain consent at the time of ICU admission, previously obtained and stored excess laboratory samples were retrieved from the clinical laboratory. Therefore, in a post hoc analysis, we tested whether timing of the blood sample relative to PICU admission was related to 25(OH)D level. In a second post hoc analysis, we evaluated the influence of total fluid bolus volume within ≤12 hours before PICU admission on 25(OH)D level in subjects admitted from the emergency department, operating room, or inpatient ward at our institution.
To allow for the skewed distribution of 25(OH)D levels, we used the Spearman correlation coefficient to assess the association of 25(OH)D with continuous variables, the Mann-Whitney test for dichotomous variables, and the Kruskal-Wallis test for multicategory variables. Patient characteristics associated with 25(OH)D in univariate analysis (P ≤ .10) were included in the multivariable model. We used multiple logistic regression to assess the influence of these risk factors on vitamin D deficiency, dichotomized at <20 ng/mL. To model quartiles of PRISM-III score and the 4-level CV-SOFA score during PICU stay, we used ordinal multinomial logistic regression. SAS was used for all computations (version 9.2, SAS Institute, Cary, NC).
Results
We screened 2366 patients admitted to the PICU between November 9, 2009, and November 9, 2010, and enrolled 511/818 (62.5%) eligible patients with a plasma specimen available close to PICU admission. Reasons for nonenrollment of eligible subjects included the following: (1) consent refusal (12.3%), (2) unavailable parents or guardians (14.7%), and (3) no acceptable plasma specimen available (10.6%). Nonenrolled eligible patients were less likely to be receiving mechanical ventilation than those enrolled (59/307 [19.2%] vs 337/511 [66%], P < .0001).
The baseline characteristics of the cohort are shown in Table 1. The median patient age was 5.3 years (interquartile range [IQR] 1.4–12.9 years). The median 25(OH)D level of enrolled patients was 22.5 ng/mL (IQR 16.4–31.3); 71.2% had 25(OH)D insufficiency (<30 ng/mL), and 40.1% were 25(OH)D deficient (10–19.9 ng/mL in 33.1% and <10 ng/mL in 7%). Thirteen (2.5%) patients died during hospitalization (12 died while in the PICU), with a median 25(OH)D level of 19.4 ng/mL (IQR 16.6–31.4).
TABLE 1.
Demographic and Other Characteristics of the Subjects Known Previous to PICU Admission and Association With 25(OH)D Levels
| Characteristic | N (%) | 25(OH)D, ng/mLa | P Valueb |
|---|---|---|---|
| Total sample | 511 (100) | 22.5 (16.4–31.3) | |
| Gender | |||
| Female | 251 (49.1) | 23.0 (16.7–30.8) | .68 |
| Male | 260 (50.9) | 21.6 (16.0–31.7) | |
| Age, y | |||
| <1 | 98 (19.2) | 26.6 (16.7–37.0) | <.0001c |
| 1–4 | 149 (29.2) | 24.3 (19.4–31.8) | |
| 5–12 | 137 (26.8) | 20.6 (14.2–29.6) | |
| 13–17 | 101 (19.8) | 18.7 (13.7–26.6) | |
| 18–21 | 26 (5.1) | 24.8 (19.4–27.5) | |
| Race | |||
| White non-Hispanic | 321 (62.8) | 23.2 (16.9–31.5) | .04d |
| White Hispanic | 55 (10.8) | 19.1 (13.7–29.6) | |
| Black | 67 (13.1) | 20.8 (13.7–29.8) | |
| Other | 68 (13.3) | 23.5 (17.7–32.4) | |
| Insurance | |||
| Private | 210 (41.1) | 21.3 (15.3–28.9) | .01 |
| Government | 300 (58.7) | 24.2 (17.3–32.4) | |
| Season | |||
| Fall or winter | 296 (57.9) | 21.1 (15.4–28.9) | <.0001 |
| Spring | 127 (24.9) | 22.4 (15.6–30.3) | |
| Summer | 88 (17.2) | 29.9 (20.6–41.8) | |
| Supplements | |||
| Vitamin D or multivitamin | 182 (35.6) | 25.4 (17.6–35.9) | <.0001 |
| Both | 31 (6.1) | 31.1 (24.7–41.8) | |
| None | 298 (58.3) | 20.4 (14.8–27.7) | |
| Enteral formula | |||
| Any | 208 (40.7) | 27.8 (20.0–38.0) | <.0001 |
| None | 303 (59.3) | 19.9 (13.8–27.1) | |
| Underlying chronic conditions | |||
| Any | 421 (82.4) | 23.4 (17.1–31.6) | .009 |
| None | 90 (17.6) | 20.5 (14.3–27.1) | |
| Underlying chronic conditionse | |||
| Respiratory | 213 (50.6) | 24.3 (17.0–34.5) | .30 |
| Asthma | 106 (25.2) | 21.1 (16.0–31.0) | .33 |
| Neurologic | 200 (47.5) | 25.3 (17.7–35.2) | .03 |
| Seizure | 107 (25.4) | 26.6 (19.3–39.2) | .001 |
| Oncologic | 55 (13.1) | 19.6 (14.0–26.2) | .003 |
| Immunodeficiency | 31 (7.4) | 24.3 (19.0–31.1) | .64 |
| Renal | 22 (5.2) | 23.0 (11.7–29.7) | .44 |
| Gastrointestinal | 44 (10.5) | 22.5 (16.7–32.5) | .98 |
| Nutritional | 49 (11.6) | 29.8 (20.0–33.0) | .05 |
| Endocrine | 42 (10.0) | 24.6 (18.6–32.6) | .54 |
Median (quartile limits).
Testing for association with serum 25(OH)D level by Mann-Whitney test (dichotomy) or Kruskal-Wallis test (multicategory characteristic).
Spearman correlation between age and 25(OH)D level: −0.18, P < .0001.
“Other” category omitted from comparison.
Compared with those with other chronic conditions.
Table 1 shows the results of the univariate analyses of baseline factors present before PICU admission and their association with admission 25(OH)D levels. Children who were previously healthy and older children had lower 25(OH)D levels. History of vitamin D supplementation, intake of enteral formula (which contained 30–134 IU vitamin D/cup), and admission during summer were associated with higher 25(OH)D levels. We had reliable parental report on home dose in 48 of the 64 patients taking uni-vitamin D supplements in which the mean daily intake was 1320 IU. Although many multivitamins contain the recommended daily allowance of vitamin D (400 IU), some contain 100 to 200 IU per tablet, preventing accurate determination of daily vitamin D. The 29 children with probable obesity had lower 25(OH)D levels than the children with normal or low body weight for age (mean 17.7 vs 22.7 ng/mL, P = .009). History of renal disease before PICU admission was not associated with 25(OH)D level; patients with an elevated creatinine level near PICU admission actually had higher 25(OH)D levels (median 24.6 vs 20 ng/mL, P < .0001).
In the multivariate analysis of 25(OH)D deficiency (Table 3, model 1), past medical history was aggregated into 4 categories based on results of the univariate analyses: previously healthy, oncologic disorder, seizure disorder, and other chronic conditions. These categories were not significantly associated with 25(OH)D deficiency (P = .21). Independent factors associated with decreased risk of 25(OH)D deficiency were younger age, white race with non-Hispanic ethnicity, summer season, vitamin D supplementation, and formula intake.
TABLE 3.
Multiple Logistic Regression Models Assessing Joint Influence of Factors Associated With Vitamin D Deficiency, Influence of Vitamin D Levels and Illness Severity (PRISM III Raw Score) After Adjusting for Model 1 Factors, and the Association Between Vitamin D Levels and Vasopressor Use (CV-SOFA Score), Adjusting for PRISM III and Model 1 Factors
| Predictor | Contrast | OR (95% CI)a | pb | pc |
|---|---|---|---|---|
| 1. Vitamin D <20 ng/mL | ||||
| Age | per 5 y | 1.43 (1.21–1.70) | <.0001 | |
| Race | Non-Hispanic white versus other | 0.51 (0.33–0.79) | .003 | |
| Insurance | Private versus government | 0.84 (0.54–1.30) | .43 | |
| Season | Summer versus fall/winter | 0.27 (0.14–0.51) | <.0001 | .0002 |
| Summer versus spring | 0.25 (0.12–0.50) | .0001 | ||
| Supplements | D or multivitamin versus none | 0.54 (0.35–0.84) | .006 | .001 |
| Both versus none | 0.20 (0.06–0.62) | .006 | ||
| Formula | Any versus none | 0.38 (0.24–0.61) | <.0001 | |
| Previous medical history | — | — | — | .08 |
| 2. Increasing PRISM III score (quartiles) | ||||
| Serum 25(OH)D | −5 ng/mL | 1.19 (1.10–1.28) | <.0001 | |
| Age | per 5 y | 1.20 (1.05–1.38) | .008 | |
| Race | Non-Hispanic white versus other | 0.95 (0.68–1.32) | .75 | |
| Season | — | — | — | .40 |
| Supplements | — | — | — | .66 |
| Formula | Any versus none | 1.56 (1.08–2.27) | .02 | |
| Previous medical history | Oncologic versus none | 2.64 (1.42–4.91) | .002 | .01 |
| Oncologic versus seizure | 2.45 (1.28–4.7) | .007 | ||
| Oncologic versus other | 2.37 (1.36–4.13) | .002 | ||
| 3. Increasing CV-SOFA score (0/1, 2, 3, or 4) | ||||
| Serum 25(OH)D | −5 ng/mL | 1.13 (1.01–1.27) | .03 | |
| Age | per 5 y | 1.02 (0.85–1.22) | .85 | |
| Race | Non-Hispanic white versus other | 1.51 (0.94–2.44) | .09 | |
| Season | — | — | — | .97 |
| Supplements | — | — | — | .86 |
| Formula | Any versus none | 1.21 (0.73–2.02) | .46 | |
| Previous medical history | Oncological versus none | 0.22 (0.09–0.52) | .0005 | .001 |
| Seizure versus none | 0.37 (0.17–0.78) | .009 | ||
| Other versus none | 0.36 (0.20–0.65) | .0007 | ||
| PRISM III score | per 1 unit | 1.26 (1.20–1.32) | <.0001 |
OR with 95% CI, adjusted for all other predictors. For binary end point, OR is the multiplicative increase in odds of outcome associated with the indicated change in predictor. For ordinal end points, OR is the multiplicative increase in odds of outcome falling above any given division of categories associated with the indicated change in predictor.
Testing hypothesis OR = 1. Any pairwise contrasts not listed were nonsignificant by Bonferroni criterion, P > (.05 ÷ number of contrasts).
For multicategory predictors, testing equal likelihood of outcome in all categories.
As shown in Table 2, median 25(OH)D levels were not associated with the underlying reason for PICU admission which included confirmed or suspected life-threatening infection, although 25(OH)D levels were markedly lower in the 51 patients with severe septic shock. Each subgroup of infection was added to the regression model for 25(OH)D deficiency, including viral respiratory and septic shock; none had a significant effect alone or in aggregate. The 94 subjects diagnosed with lower respiratory tract infection (LRTI) had the same median 25-(OH)D level (22.5 ng/mL) as those without, and addition of LRTI to the regression model of vitamin D deficiency revealed no significant relationship (P = .62).
TABLE 2.
Association of 25(OH)D Levels With Reason for PICU Admission and Admission to the PICU for a Life-Threatening Confirmed or Suspected Infection
| Characteristic | N (%) | 25(OH)D, ng/mLa | P Valueb |
|---|---|---|---|
| Reasons for PICU admission | |||
| Planned surgical | 115 (22.6) | 22.9 (17.3–31.4) | .40 |
| Orthopedic | 43 (8.4) | 19.4 (14.4–29.6) | .12 |
| Neurosurgical | 18 (3.5) | 24.9 (17.3–31.9) | .65 |
| General surgery | 20 (3.9) | 19.5 (17.2–26.4) | .49 |
| Other | 97 (19.0) | 24.4 (17.7–31.6) | .34 |
| Emergent | 23 (4.5) | 20.5 (13.6–31.9) | .54 |
| Trauma | 11 (2.2) | 27.2 (14.6–39.4) | .42 |
| Status epilepticus/neurologic monitoring | 87 (17.0) | 26.1 (18.1–32.6) | .05 |
| Electrolyte disturbance/diabetic ketoacidosis | 31 (6.1) | 19.9 (12.0–33.3) | .57 |
| Asthma exacerbation | 44 (8.6) | 22.3 (18.1–30.0) | .51 |
| Respiratory failure | 117 (22.9) | 22.7 (17.0–31.2) | .87 |
| Infection on PICU admission | |||
| Any confirmed or suspected infectionc | 238 (46.6) | 21.9 (15.8–30.1) | .19 |
| Positive viral testd | 47 (9.2) | 23.9 (14.8–33.0) | .72 |
| Positive bacterial teste | 97 (19.0) | 20.0 (15.3–31.9) | .28 |
| No microbiologic confirmation | 94 (18.4) | 21.8 (16.1–29.0) | .40 |
| Severe septic shockf | 51 (10.0) | 19.2 (12.6–24.8) | .0008 |
Median (quartile limits).
Testing for association with serum 25(OH) D level by Mann-Whitney test (dichotomy) or Kruskal-Wallis test (multicategory characteristic).
Includes confirmed (viral, bacterial, fungal, multiple) or suspected infection; excludes confirmed but non-life-threating infection.
Respiratory syncytial virus, influenza, parainfluenza, without confirmed bacterial infection.
Sepsis, positive cerebrospinal fluid; includes fungal (5) and multiple (12).
Cardiovascular sequential organ failure score ≥3 plus confirmed or suspected infection.
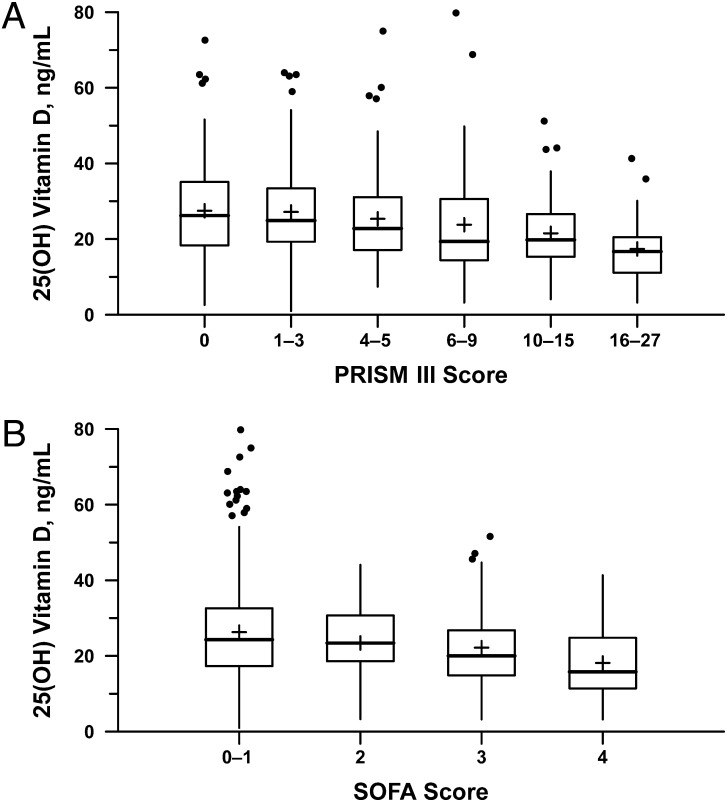
Duration of mechanical ventilation during the PICU stay was not significantly associated with 25(OH)D (r = –0.1007, P = .10). The median PRISM-III raw score on admission day was 5 (IQR 0–11.5), and it was inversely correlated with 25(OH)D level (r = −0.23, P < .0001; see Fig 1A). A multinomial logistic regression model was created with PRISM-III quartiles as the outcome, adjusted for factors known previous to PICU admission associated with 25(OH)D admission levels. As shown in Table 3 (model 2), lower admission 25(OH)D was inversely associated with PRISM-III, with a 5 ng/mL decrease corresponding to a 1.19-fold increase in a patient’s odds of belonging to the next higher PRISM-III quartile (95% confidence interval [CI] 1.10–1.28; P < .0001). This was not explained by the cohort with septic shock, as addition of severe septic shock to this model altered the effect estimate minimally (odds ratio [OR] 1.18; P < .0001).
FIGURE 1.
Correlation between 25(OH) vitamin D level and illness severity. A, 25(OH)D level is inversely correlated with PRISM-III score on PICU admission day. B, 25(OH)D level is inversely correlated with maximum cardiovascular SOFA (CV-SOFA) during ICU stay. The CV-SOFA score is calculated as follows: 0–1 = no vasopressor requirement; 2 = dopamine ≤5 mcg/kg/min; 3 = dopamine >5 mcg/kg/min or epinephrine/norepinephrine ≤0.1; 4 = dopamine >15 mcg/kg/min or epinephrine/norepinephrine >0.1.
There were 134 patients (26.2%) who received vasopressors (CV-SOFA ≥3) during their PICU stay. Patients receiving vasopressors had lower 25(OH)D levels (median 19.8 vs 24.3 ng/mL, P < .0001). Increasing vasopressor use (CV-SOFA score) was correlated with decreasing 25(OH)D levels (r = −.19, P < .0001; Fig 1B). In the multinomial logistic regression model (Table 3, model 3), lower admission 25(OH)D levels were associated with higher CV-SOFA scores, a 5 ng/mL decrease corresponding to a 1.13-fold increase in odds of belonging to the next higher category of CV-SOFA score (95% CI 1.01–1.27; P = .03). This effect remained after adjusting for severe septic shock (OR 1.16, 95% CI 1.02–1.31; P = .02).
The median timing of the first vitamin D sampling was 0.6 hours after PICU admission (IQR −5.3 to 9.7 hours). Timing of sampling was weakly inversely correlated with 25(OH)D level, r = −.12, P = .009. Fluid bolus data were available on 374/511 patients (73.1%) who were transferred from the inpatient ward, operating room, or the emergency department at our hospital. The median fluid bolus before PICU admission was 22.2 mL/kg, which had a weak inverse correlation with 25(OH)D level (r = −.12, P = .01).43 Inclusion of the fluid resuscitation data did not markedly influence the association between any of the risk factors for vitamin D deficiency on PICU admission; all remained significant. Inclusion of fluid resuscitation volume into the model for predicting PRISM-III score showed that although increased fluid bolus amounts were associated with rising admission illness severity (OR 1.11, 95% CI 1.07–1.16, P < .0001), 25(OH)D levels remained inversely associated with PRISM-III (OR 1.10, 95% CI 1.01–1.20, P = .03). Fluid bolus volume before PICU admission was not a significant predictor of maximal CV-SOFA score during the PICU stay (OR 1.04, 95% CI 0.99–1.09, P = .11) and was not tested as a potential confounder.
A post hoc comparison of 1,25(OH)2D levels between 17 cases with CV-SOFA ≥3 to 18 children with CV-SOFA = 0 revealed a strong positive correlation between 25(OH)D and 1,25(OH)D levels, r = .60, P < .0001 with no respective differences in median 1,25(OH)2D levels (31.4 vs 45.1 pg/mL, P = .29). Ionized calcium was measured on 245 subjects at admission, and was modestly correlated with 25(OH)D, r = .22, P = .0006. However, only 30 patients had clinically low ionized calcium, and their 25(OH)D levels were similar to the 215 patients with normal levels (median 22.7 vs 24.5 ng/mL, respectively, P = .47); the addition of this group had no effect on the regression models for PRISM-III or CV-SOFA scores.
Discussion
We identified a high prevalence of vitamin D deficiency and insufficiency in critically ill children in this prospective cohort study. Previously healthy children had lower 25(OH)D levels than those with underlying chronic illnesses, probably because parents of chronically ill children were more likely to report supplementing their children with vitamin D by using vitamins and/or enteral formula. Vitamin D supplementation before PICU admission was strongly protective against 25(OH)D deficiency. School-age children, those with darker skin, and children not receiving vitamin D supplementation were more likely to have low 25(OH)D levels, especially during colder months when sun exposure was limited. These risk factors for vitamin D deficiency we identified in these critically ill children have repeatedly been described as risk factors for vitamin D deficiency in outpatients,3,16,18,19,44,45 which supports our belief that the 25(OH)D levels drawn around PICU admission reflect preadmission status. As has been reported in critically ill adults, we found lower 25(OH)D levels associated with higher PICU admission day illness severity after adjusting for related pre-ICU factors.27,34
The 40% prevalence of vitamin D deficiency (31% deficiency in infants and toddlers, 46% in school-age children, 57% in adolescents) in our cohort of critically ill children is higher than was reported in the healthy US pediatric population (9% in toddlers,18 17% in school age,18 and 14% deficiency in adolescents17) and in studies of pediatric outpatients in Boston (12.1% deficiency in infants and toddlers; 42% in healthy adolescents).19,35 Our high prevalence of vitamin D deficiency in critically ill children is more similar to that reported in adult ICU patients in France and Boston where ≥26% were reported as deficient.27,46
In contrast to our expectations, we did not find critically ill children with confirmed or suspected infections to have lower 25(OH)D levels than other critically ill patient groups, with the exception of children presenting in severe septic shock. Studies have shown a relationship between vitamin D levels and illness severity in infected children. Children with LRTI had a higher risk of hospital admission if their 25(OH)D level was in the severely deficient range.24 Children with acute LRTIs admitted to the PICU had lower 25(OH)D levels compared with children admitted to the ward.25 Unfortunately, we did not have a control group of children with infections who were not admitted to the PICU and were unable to test the relationship between hospitalization or PICU admission and 25(OH)D level in infected children.
We identified a relationship between illness severity, as defined by PRISM-III score on admission, and 25(OH)D level. The association between vitamin D level and both severity of illness and vasopressor use may be due to its role in innate immune function and inflammation, its role in calcium homeostasis, or influenced by fluid shifts and dilution. Because of interhospital transfers and poor documentation, we do not have reliable and complete data on fluid resuscitation before PICU admission for every subject. Even in those who were in-hospital, where fluid bolus total amount was documented, it was usually not possible to accurately determine when the blood was sampled in relation to the fluid resuscitation. Although we were reassured that the inverse relationship between critical illness severity and 25(OH)D levels remained after adjusting for pre-PICU fluid bolus volume in patients where these data were available, we cannot accurately estimate the role of fluid shifts because this would require knowledge of 25(OH)D status in the preillness state.
Fluid resuscitation may explain the decreased vitamin D levels in children in septic shock. Studies in critically ill adults have shown an inverse relationship between outpatient vitamin D levels and illness severity and mortality in large adult cohorts.27 Vitamin D levels have been shown to be stable over the hospital course in patients with malaria47 and with acute myocardial infarction.48 However, they have also been shown to decrease by 40% in adults patients with an inflammatory response after knee surgery without significant fluid resuscitation and to remain decreased by 20% after hospitalization.49 Cardiac bypass has also been shown to markedly reduce 25(OH)D levels,36 which is why we excluded cardiac bypass patients and levels done during extracorporeal membrane oxygenation support. It remains unclear what portion of this relationship is due to fluid shifts, inflammation, or stores before illness onset.
Although we did not enroll 36.4% of the eligible patients, we believe that these nonenrolled patients were less likely to be critically ill because they were less likely than the enrolled group to have laboratory specimens drawn at admission and to receive mechanical ventilator support. We were also unable to thoroughly assess the longitudinal trend in 25(OH)D levels over the course of the PICU admission, and we believe the trend of 25(OH)D levels over time in critically ill children is an important goal for future investigations.
Conclusions
We have identified a high prevalence of vitamin D insufficiency, deficiency, and severe deficiency in critically ill children admitted to the PICU and an inverse association between 25(OH)D levels and illness severity on admission. Pre-PICU dietary intake of vitamin D in the form of vitamins or formula protected against deficiency. We hypothesize that higher 25(OH)D levels may decrease the severity of critical illness brought on by an overwhelming insult such as infection or injury. Whether aggressive vitamin D supplementation in the early stages of critical illness improves clinical outcomes merits additional testing. Given the high rate of vitamin D deficiency in critically ill children and the essential role of vitamin D in healthy bone development, we recommend screening critically ill children with risk factors for vitamin D deficiency and identifying effective repletion strategies.
Acknowledgments
We thank the children and parents/guardians who participated in this study. We also acknowledge the staff of the medical and surgical PICUs for their support. The Division of Critical Care and the Department of Anesthesia supported Drs Randolph and Madden throughout the project. The work of Ying Feng, MS, at the Clinical Research Program was helpful in the initial stages of the analysis.
Glossary
- 25(OH)D
25-hydroxy vitamin D
- CI
confidence interval
- CV-SOFA
Sequential Organ Failure Assessment cardiovascular
- IQR
interquartile range
- LRTI
lower respiratory tract infection
- OR
odds ratio
- PRISM-III
Pediatric Risk of Mortality III
Footnotes
Drs Madden and Randolph participated in protocol conception and design, obtaining funding, subject enrollment, data collection, management analysis and interpretation, and manuscript preparation. Drs Feldman and Gordon participated in the study design, data analysis and interpretation, and critical revision of the article. Ms Smith, Ms Agan, Ms Keisling, and Mr Sullivan participated in the acquisition of data, data management, subject enrollment, laboratory sample management, data analysis, and manuscript revision. Dr Hollis participated in the study design and sample analysis and critically revised the manuscript.
FINANCIAL DISCLOSURE: Dr Hollis serves as a consultant for Diasorin Inc, Stillwater, MN; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a grant from the Clinical Research Program (CRP) at Children’s Hospital Boston. Dr Feldman was supported in part by Harvard Clinical and Translational Science Center, National Institutes of Health grant UL1 RR-025758. Dr Madden participated with support from Harvard Catalyst (National Institutes of Health award UL1 RR-025758 and financial contributions from Harvard University and its affiliated academic health care centers).
COMPANION PAPERS: Companions to this article can be found on pages 429 and 557, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2011-3059 and www.pediatrics.org/cgi/doi/10.1542/peds.
References
- 1.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society . Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417 [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88(10):4623–4632 [DOI] [PubMed] [Google Scholar]
- 3.Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding. American Academy of Pediatrics Committee on Nutrition . Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152 [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57(1):63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12(3):311–319 [DOI] [PMC free article] [PubMed]
- 7.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009;338(1):40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol. 2008;102(11):1540–1544 [DOI] [PubMed] [Google Scholar]
- 10.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–1637 [DOI] [PubMed] [Google Scholar]
- 11.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063 [DOI] [PubMed] [Google Scholar]
- 12.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76(9):3837–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65(5 pt 2):106R–113R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773 [DOI] [PubMed] [Google Scholar]
- 15.Enioutina EY, Bareyan D, Daynes RA. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J Immunol. 2009;182(7):4296–4305 [DOI] [PubMed] [Google Scholar]
- 16.Gordon CM, Feldman HA, Sinclair L, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics. 2009;123(3):797–803 [DOI] [PubMed] [Google Scholar]
- 18.Mansbach JM, Ginde AA, Camargo CA, Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537 [DOI] [PubMed] [Google Scholar]
- 20.Merewood A, Mehta SD, Grossman X, et al. Widespread vitamin D deficiency in urban Massachusetts newborns and their mothers. Pediatrics. 2010;125(4):640–647 [DOI] [PubMed] [Google Scholar]
- 21.Ross AC, Institute of Medicine (U. S.). Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes: Calcium Vitamin D. Washington, DC: National Academies Press; 2011 [PubMed] [Google Scholar]
- 22.Savitha MR, Nandeeshwara SB, Pradeep Kumar MJ, ul-Haque F, Raju CK. Modifiable risk factors for acute lower respiratory tract infections. Indian J Pediatr. 2007;74(5):477–482 [DOI] [PubMed] [Google Scholar]
- 23.Ginde AA, Mansbach JM, Camargo CA, Jr. Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9(1):81–87 [DOI] [PubMed] [Google Scholar]
- 24.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58(4):563–567 [DOI] [PubMed] [Google Scholar]
- 25.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44(10):981–988 [DOI] [PubMed] [Google Scholar]
- 26.Ginde AA, Mansbach JM, Camargo CA, Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39(4):671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginde AA, Camargo CA, Jr, Shapiro NI. Vitamin D insufficiency and sepsis severity in emergency department patients with suspected infection. Acad Emerg Med. 2011;18(5):551–554 [DOI] [PubMed] [Google Scholar]
- 30.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91(5):1255–1260 [DOI] [PubMed] [Google Scholar]
- 31.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–1077 [DOI] [PubMed] [Google Scholar]
- 32.McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc. 2011;12(3):208–211 [DOI] [PubMed] [Google Scholar]
- 33.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360(18):1912–1914 [DOI] [PubMed] [Google Scholar]
- 34.Braun AB, Gibbons FK, Litonjua AA, Giovannucci E, Christopher KB. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40(1):63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39(4):671–677 [DOI] [PMC free article] [PubMed]
- 36.Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care. 2010;14(6):R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752 [DOI] [PubMed] [Google Scholar]
- 39.Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800 [DOI] [PubMed] [Google Scholar]
- 40.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317–322 [DOI] [PubMed] [Google Scholar]
- 41.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58 [DOI] [PMC free article] [PubMed]
- 42.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg SL, Abramowitz SK. Statistics Using SPSS: An Integrative Approach. 2nd ed. New York, NY: Cambridge University Press; 2008 [Google Scholar]
- 44.Siddiqui TS, Rai MI. Presentation and predisposing factors of nutritional rickets in children of Hazara Division. J Ayub Med Coll Abbottabad. 2005;17(3):29–32 [PubMed] [Google Scholar]
- 45.Huh SY, Gordon CM. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord. 2008;9(2):161–170 [DOI] [PubMed] [Google Scholar]
- 46.Lucidarme O, Messai E, Mazzoni T, Arcade M, du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36(9):1609–1611 [DOI] [PubMed] [Google Scholar]
- 47.Newens K, Filteau S, Tomkins A. Plasma 25-hydroxyvitamin D does not vary over the course of a malarial infection. Trans R Soc Trop Med Hyg. 2006;100(1):41–44 [DOI] [PubMed] [Google Scholar]
- 48.Barth JH, Field HP, Mather AN, Plein S. Serum 25 hydroxy-vitamin D does not exhibit an acute phase reaction after acute myocardial infarction [published online ahead of print April 27, 2012]. Ann Clin Biochem.doi:10.1258/acb.2011.011195 [DOI] [PubMed] [Google Scholar]
- 49.Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93(5):1006–1011 [DOI] [PubMed] [Google Scholar]