Abstract
BACKGROUND AND OBJECTIVE:
Although many treatments have been studied in children with autism spectrum disorders (ASDs), less attention has focused on interventions that may be helpful in adolescents and young adults with ASD. The goal of this study was to systematically review evidence regarding medication treatments for individuals between the ages of 13 and 30 years with ASD.
METHODS:
The Medline, PsycINFO, and ERIC databases were searched (1980–December 2011), as were reference lists of included articles. Two investigators independently assessed studies against predetermined inclusion/exclusion criteria. Two investigators independently extracted data regarding participant and intervention characteristics, assessment techniques, and outcomes and assigned overall quality and strength of evidence ratings on the basis of predetermined criteria.
RESULTS:
Eight studies of medications were identified that focused on 13- to 30-year-olds with ASD; 4 of the studies were of fair quality. The strength of evidence was insufficient for all outcomes associated with medications tested in this population; however, the 2 available studies of the atypical antipsychotic medication risperidone in this age range were consistent with the moderate evidence in children with ASD for treating problem behavior, including aggression, and high strength of evidence for adverse events, including sedation and weight gain.
CONCLUSIONS:
There is a marked lack of data on use of medication treatments for adolescents and young adults with ASD. The evidence on the use of risperidone in this age range is insufficient when considered alone but is consistent with the data in the population of children with ASD.
Keywords: autism spectrum disorders, antipsychotics, risperidone, serotonin reuptake inhibitors
Core symptoms of autism spectrum disorders (ASDs), including impairments related to social communication and restricted/repetitive behaviors and interests, typically affect individuals throughout their life span, although developmental expression may vary.1 Given the lifelong nature of ASD, most individuals are exposed to multiple interventions addressing different target symptoms. In contrast to behavioral treatments, which may target core symptoms, medication treatments are primarily directed toward associated symptoms in individuals with ASD. As part of a systematic review of therapies for adolescents and young adults (ages 13–30 years) with ASD, we reviewed the literature on medication treatments used to target associated symptoms. Information on other therapies (eg, behavioral, educational, allied health) addressed in the full review can be found at http://www.effectivehealthcare.ahrq.gov.
Methods
Search Strategy
We searched Medline via the PubMed interface, PsycINFO (psychology and psychiatry literature), and ERIC (educational literature) from 1980 to December 2011 by using relevant controlled vocabulary terms and key terms related to ASD (eg, autistic disorder) and therapy (eg, therapeutics). We also hand-searched the reference lists of all included articles and of recent narrative and systematic reviews related to therapies for ASD to identify potentially relevant articles.
Study Selection
Study inclusion and exclusion criteria were developed in consultation with an expert panel of clinicians and researchers involved in ASD. All study designs were included, and we required that studies include at least 20 participants with ASD between 13 and 30 years of age. Two investigators independently reviewed each study against the inclusion criteria (Table 1) with disagreements resolved through adjudication by a senior investigator.
TABLE 1.
Inclusion and Exclusion Criteria
| Category | Criteria |
|---|---|
| Study population | Adolescents or young adults (ages 13–30 years) with ASD (autistic disorder, Asperger syndrome, PDD-NOS) or families/caregivers of individuals with ASD between the ages of 13 and 30 years |
| Interventions | Interventions aimed at ameliorating core symptoms of ASD, affecting independent functioning, adaptive behavior, or the transition process, or targeting family outcomes |
| Comparators | Placebo |
| Other intervention | |
| Outcomes | Social skills/interaction, language and communication, repetitive and other maladaptive behaviors, motor outcomes, psychological distress, adaptive skills development, academic skills development, and family outcomes, including family distress and family satisfaction |
| Time period | Studies published from 1980–present with no limits on timing of outcomes |
| Setting | Any setting, including educational, residential, and clinic |
| Publication languages | English only |
| Admissible evidence (study design and other criteria) | |
| Admissible design | Controlled trials, observational studies including prospective and retrospective cohort studies, prospective and retrospective case series |
| Study size | At least 20 total individuals between 13 and 30 years of age with ASD or family members of such individuals |
| Other criteria | Original research studies that provide sufficient detail regarding methods and results to enable use and adjustment of the data and results |
| Patient populations must include adolescents or young adults (13–30 years of age) with ASD or families/caregivers of individuals with ASD between the ages of 13 and 30 years | |
| Studies must address 1 or more of the following: (1) treatment modality aimed at modifying ASD core symptoms, common comorbidities, family-related outcomes, or assisting with transitional issues; and (2) outcomes (including harms) related to interventions for ASD | |
| Studies must include extractable data on relevant outcomes, including data presented in text or tables (versus solely in figures) | |
| Studies must present aggregate data (versus only data for each individual participant) |
PDD-NOS, pervasive developmental disorder–not otherwise specified.
We also required that studies be published in the year 1980 or later (after the publication of standardized diagnostic criteria for ASD in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition).
Data Extraction
Using standardized forms, 2 investigators independently extracted data regarding study design; descriptions of the study populations, intervention, and comparison groups; and baseline and outcome data, as well as data about adverse events. Data on the conduct and timing of assessments were also captured to inform the assessment of quality. Principal outcomes of interest included effects on core symptoms of ASD and comorbid symptoms and conditions, including sleep, anxiety, hyperactivity, and challenging behavior(eg, irritability/agitation), as well as effects on family-related outcomes.
Study Quality Assessment
Two investigators independently assessed each study by using a quality assessment form developed by the review team with input from experts in the field and adapted from a previous Agency for Healthcare Research and Quality review of therapies for children with ASD.2 We evaluated the following elements with a series of yes/no questions in each domain (eg, “Were outcomes coded and assessed by individuals blinded to the intervention status of the participants?”): study design, diagnostic approach, participant ascertainment and characterization, intervention description, outcomes measurement, and statistical analysis.
Disagreements between assessors were resolved through discussion to reach consensus. Overall assessment of quality was determined with a prespecified algorithm that is available in the full report.
The strength of evidence of the current research was assessed by using methods established in the Agency for Healthcare Research and Quality Effective Health Care Program’s Methods Guide for Effectiveness and Comparative Effectiveness Reviews.3 Assessments are based on consideration of 4 domains: risk of bias, consistency in direction of the effect, directness in measuring intended outcomes, and precision of effect (Table 2). We determined the strength of evidence separately for major intervention-outcome pairs by using a prespecified approach described in detail in the full review.
TABLE 2.
Domains Used to Assess Strength of Evidence
| Domain | Description |
|---|---|
| Risk of bias | Reflects issues in study design and conduct that could result in biased estimates of effect |
| Consistency | Reflects similarity of effect sizes seen across studies. Consistency cannot be assessed when only 1 study is available |
| Directness | Reflects the relationship between the intervention and the ultimate health outcome of interest |
| Precision | Reflects the level of certainty around the effect observed |
Data Synthesis
Given considerable heterogeneity in the interventions and outcome measures used in studies meeting our inclusion criteria, we did not conduct any meta-analyses. Characteristics of study populations and interventions were summarized, and descriptive statistics were used to report study outcomes.
Results
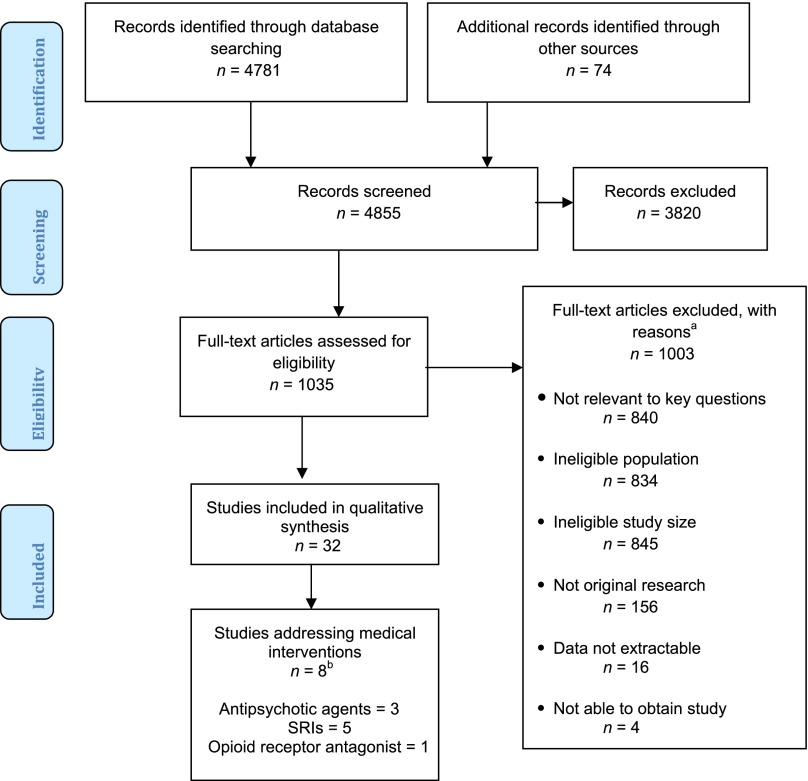
Figure 1 outlines the flow of articles retrieved for the review. The original literature search was conducted for a broad review of all interventions for adolescents and young adults with ASD, although only studies of medications are included in this analysis. As noted in Fig 1, studies were most commonly excluded based on sample size, population, and lack of relevance to the questions addressed in the review. Eight unique studies addressed medications in adolescents and young adults with ASD. Of these, 5 were randomized controlled trials (RCTs) and 3 were case series. We located 3 studies addressing antipsychotic medications4–6; 5 studies addressing serotonin reuptake inhibitor (SRI) medications5,7–10; and 1 study addressing the opioid receptor antagonist naltrexone.11 Tables 3 and 4 summarize study characteristics and key results.
FIGURE 1.
Disposition of articles identified for review. aNumbers do not tally because studies could be excluded for multiple reasons. bOne study4 addresses an antipsychotic and an SRI.
TABLE 3.
Overview of Studies
| Characteristic | RCTs (n = 5) | Prospective Case Series (n = 2) | Retrospective Case Series (n = 1) | Total Literature (N = 8a) |
|---|---|---|---|---|
| Intervention | ||||
| Antipsychoticsa | 3 | 0 | 0 | 3 |
| SRIsa | 2 | 2 | 1 | 5 |
| Opioid receptor antagonist | 1 | 0 | 0 | 1 |
| Treatment duration, mo | ||||
| >1 to ≤3 | 4 | 2 | 0 | 6 |
| >3 to ≤6 | 1 | 0 | 0 | 1 |
| >6 to ≤12 | 0 | 0 | 1 | 1 |
| Study population | ||||
| United States | 3 | 2 | 1 | 6 |
| Europe | 1 | 0 | 0 | 1 |
| Other | 1 | 0 | 0 | 1 |
| Total participants | 172 | 77 | 23 | 272 |
One study assessed both an antipsychotic agent and an SRI.
TABLE 4.
Key Outcomes of Studies Assessing Medication Treatments in Adolescents and Young Adults With ASD
| Study (Author, Year, Country Groups, N; Enrollment/N Final; Study, Quality) | Age, y, Mean ± SD | IQ, Mean ± SD | Outcome Measure/ Baseline Scores, Mean ± SD | Outcome Measure/Posttreatment Scores, Mean ± SD |
|---|---|---|---|---|
| Antipsychotic agents | ||||
| Hellings et al,4 2006; United States; G1 + G2: placebo phase, then dose risperidone, followed by crossover to the other risperidone dose, then another placebo phase; placebo I phase: 3–5 wk of placebo, n = 40; acute-dose phase: G1 low-dose (n = 39) or G2 high-dose risperidone (n = 36); placebo II phase: 3–5 wk of placebo, n = 33; maintenance phase: optimal dose risperidone, n = 32; poor quality | G1 + G2: 22 ± 13.1 | NR, 40/40 with intellectual disability | ABC Irritability: G1 + G2, placebo I phase: 19.16 ± 9.96; G1 + G2, placebo II phase: 18.23 ± 12.36 | ABC Irritability: G1, Low-dose acute phase: 11.15 ± 9.28; G2, High-dose acute phase: 13.31 ± 8.92; P = .13 G1 versus G2, P = .0002 G1 + G2 acute phase versus G1 + G2 placebo II; maintenance phase scores only reported graphically |
| Remington et al,5 2001; Canada; G1: clomipramine; G2: haloperidol; G3: placebo; Overall N: 37/36; fair quality | Overall: 16.3 (SD NR) | NR | CARS: overall, 41.8 ± 7.1 | CARS: G1: 37.8 ± 8.7; G2: 36.7 ± 6.1; G3: 39.4 ± 7.0; P < .05, G2 versus baseline; ABC reported only graphically |
| McDougle et al,6 1998; United States; G1: risperidone, 15/12; G2: placebo,16/12; G2a: open-label risperidone after placebo, n = 15; fair quality | G1 + G2: 28.1 ± 7.3 | G1 + G2: 54.6 ± 23.9 | Y-BOCS, compulsion: G1: 16.5 ± 3.58; G2: 14.29 ± 3.50; G2a: 14.27 ± 2.92; SIB-Q: G1: 47.8 ± 19.5; G2: 37.7 ± 11.9; G2a: 32.43 ± 15.89 | Y-BOCS, compulsion: G1: 12.77 ± 3.63; G2: 14.35 ± 3.02; P < .001, G1 versus G2; G2a: 11.47 ± 3.64; P < .03, G2a versus baseline; SIB-Q: G1: 24.2 ± 9.5; G2: 32.8 ± 15.0; P < .001, G2 versus G1; G2a: 23.07 ± 13.45; P < .05, G2a versus baseline |
| SRIs | ||||
| Remington et al,5 2001; Canada; G1: clomipramine; G2: haloperidol; G3: placebo; Overall N: 37/36; fair quality | Overall: 16.3 (SD NR) | NR | CARS; Overall: 41.8±7.1 | CARS: G1: 37.8 ± 8.7; G2: 36.7 ± 6.1; G3: 39.4 ± 7.0; P < .05, G2 versus baseline; ABC reported only graphically |
| McDougle et al,8 1998; United States; G1: sertraline, n = 42/37; G1a: AD; G1b: AS; G1c: PDD-NOS; poor quality | 26.1 ± 5.8 | 60.5 ± 22.7 (28 with intellectual disability) | Y-BOCS, total score: G1a: 16.5 ± 6.7; G1b: 25.7 ± 4.1; G1c: 18.2 ± 4.8; Vineland Maladaptive Behavior: G1a: 27.0 ± 9.4; G1b: 19.8 ± 8.6; G1c: 28.3 ± 10.8; SIB-Q: G1a: 32.7 ± 16.5; G1b: 17.5 ± 7.7; G1c: 36.2 ± 16.4; | Y-BOCS, total score: G1a: 11.5 ± 5.8; G1b: 27.8 ± 5.3; G1c: 14.8 ± 5.7; P = .005, G1 versus baseline; Vineland Maladaptive Behavior Scale: G1a: 13.8 ± 6.0; G1b: 20.2 ± 8.2; G1c: 19.5 ± 9.1; P = .0001, G1 versus baseline SIB-Q: G1a: 15.5 ± 9.5; G1b: 18.8 ± 7.7; G1c: 20.2 ± 12.8; P = .0001, G1 versus baseline |
| Brodkin et al,9 1997; United States; G1: clomipramine, 35/33; G1a: responders, n = 18; G1b: nonresponders, n = 15; poor quality | G1: 30.2 ± 7.0 | G1: 64.6 ± 27.2 | Y-BOCS, total score: G1a: 18.7 ± 6.8; G1b: 17.9 ± 6.2; Y-BOCS, compulsion subscale: G1a: 13.7 ± 3.3; G1b: 13.9 ± 2.5; Y-BOCS, obsession subscale: G1a: 10 ± 6.8; G1b: 6.7 ± 6.2; Brown Aggression Scale: G1a: 10.6 ± 7.4; G1b: 6.5 ± 4.1 | Y-BOCS, total score: G1a: 9.1 ± 3.0; G1b: 17.3 ± 7.8; P < .001, G1 versus baseline; P < .001, G1a versus G1b; Y-BOCS, compulsion subscale: G1a: 6.9 ± 2.1; G1b: 12.5 ± 3.3; P < .001, G1 versus baseline; P < .001, G1a versus G1b; Y-BOCS, obsession subscale: G1a: 4.4 ± 2.8; G1b: 8 ± 6.6; P < .001, G1 versus baseline; P < .001, G1a versus G1b; Brown Aggression Scale: G1a: 3.7 ± 3.6; G1b: 6.4 ± 4.6; P < .001, G1 versus baseline; P < .001, G1a versus G1b |
| McDougle et al,7 1996; United States; G1: fluvoxamine, 15/15; G2: placebo, 15/15; fair quality | G1: 30.1 ± 7.1; G2: 30.1 ± 8.4 | G1: 82.5 ± 26.8; G2: 77.3 ± 33.1 | Y-BOCS, total score: G1: 21.4 ± 7.3; G2: 21.5 ± 6.8 | Y-BOCS, total score: G1: 13.7 ± 9.1; G2: 21.9 ± 6.7; P < .003, G1 versus G2; Data for Vineland Maladaptive Behavior and Brown Aggression Scale were not reported, although statistically significant improvements were noted |
| Cook et al,10 1992; United States; G1: fluoxetine, 23/23; poor quality | 15.9 ± 6.2 | NR, 19 with intellectual disability | CGI-S, total: 5.7 ± 0.8; CGI-S, compulsion: 5.5 ± 1.5 | CGI-S, total: 4.9 ± 1.1; P < .002, G1 versus baseline; CGI-S, compulsion: 4.7 ± 1.6; P < .005, G1 versus baseline |
| Opioid receptor antagonist | ||||
| Willemsen-Swinkels et al,11 1995; the Netherlands; G1 + G2: 4-week naltrexone phase for cohorts 1 (50 mg daily) and 2 (150 mg daily) (ASD patients only); G3 + G4: 4-week placebo phase for cohorts 1 and 2 (ASD patients only); overall N: 33/31; fair quality | Overall: 29 ± 6.0 | NR | ABC Stereotypy: G1 + G2: 9.7 ± 4.7; G3 + G4: 8.3 ± 5.2 | ABC Stereotypy: G1 + G2: 10.0 ± 4.7; G3 + G4: 9.0 ± 4.8; P = .018, G1 + G2 versus G3 + G4 |
AD, autistic disorder; AS, Asperger syndrome; CARS, Childhood Autism Rating Scale; CGI-S, Clinical Global Impression of Severity; G, group; NR, not reported; PDD-NOS, pervasive developmental disorder–not otherwise specified; SIB-Q, Self-Injurious Behavior Questionnaire; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Antipsychotic Medications
One fair-quality RCT of risperidone6 included adults with autistic disorder or pervasive developmental disorder–not otherwise specified. The mean ± SD age of the 31 individuals who began the trial was 28.1 ± 7.3 years, and mean full-scale IQ was 54.6 ± 23.9. Only 24 participants completed the trial. The experimental design was a 12-week, randomized, double-blind, placebo-controlled phase followed by a 12-week, open-label risperidone treatment phase for individuals from the placebo group. Participants discontinued all psychiatric medications for >4 weeks before the trial started. Risperidone dosing could have ranged from 1 mg to 10 mg/day over the course of the study; the highest end dose was 6 mg/day. The primary outcomes were global improvement, repetitive behavior, aggression, and social relatedness. Participants with a Clinical Global Impression of Improvement (CGI-I) score of “much improved” or “very much improved” were considered responders.
Fifty-seven percent (8 of 14 participants) were considered responders in the risperidone group, whereas none (0 of 16) in the placebo group were responders (P < .002).6 Aggressive behavior improved over time (P < .001) for the risperidone group compared with the placebo group. This result was consistent with improvements over time in the open-label phase (P < .05). Repetitive behavior improved over time (P < .001) for the risperidone group compared with the placebo group at each time point. This result was consistent with improvements over time in the open-label phase (P < .03). Symptomatic improvements for the risperidone group compared with placebo were significant over time for sensory motor (P < .004), affectual reactions (P < .001), and overall score (P < .05). Differences for social relationships, sensory responses, or language were not significant.
These results were largely consistent with the improvements over time in the open-label phase.6 Scores on clinician-rated visual analog scales were significantly decreased in the risperidone group compared with the placebo group for “anxious or nervous” (P < .02), “depressed” (P < .03), and “irritable” (P < .01). There were no significant differences for “calm,” “eye contact,” “happy,” “restless,” “social interaction,” “talkative,” or “tired.” Sedation was the most prominent adverse event. Seven participants did not complete the trial (3 in the risperidone arm and 4 in the placebo arm), with 6 participants dropping out due to lack of improvement or agitation, and 1 participant in the risperidone arm with abnormal gait.
A poor-quality crossover study addressed the safety and efficacy of risperidone in 40 children, adolescents, and adults with intellectual disability, 90% of whom had ASD.4 Twenty-three (57.5%) of participants responded fully (defined as a 50% reduction in the Aberrant Behavior Checklist [ABC] Irritability/Agitation subscale score). Symptoms on the Neuroleptic Side Effects Checklist that were the most significant (P < .001) with treatment included drowsiness, weight gain, and increased appetite. Mean weight gain during the entire study was 8.3 kg for adolescents and 6.0 kg for adults.
A fair-quality crossover study5 investigated the efficacy of haloperidol for the treatment of autism. The study design was a double-blind, placebo-controlled, crossover with random assignment to 7-week phases of haloperidol, clomipramine, and placebo (clomipramine results are summarized in the following SRI discussion). Haloperidol dosing started at 0.25 mg at bedtime and increased in 0.25-mg increments every 2 days until the dose was 0.50 mg twice daily; further 0.25-mg adjustments were then made every 3 to 4 days on the basis of clinical assessment. The dose was reduced to the last dose tolerated if adverse events were experienced. There was a dosage taper during week 7 of each phase, and 1-week placebo washout periods between each phase. No other psychotropic drugs were allowed except benztropine.
Of the 37 participants recruited, 36 (mean age: 16.3 years) were included in final analyses.5 The mean daily dose of haloperidol was 1.3 mg. The mean duration of haloperidol treatment was 5.8 weeks, with 23 (69.7%) of 33 participants completing the 7-week treatment phase. Seven of 10 participants who discontinued had adverse events, including fatigue (n = 5), dystonia (n = 1), and depression (n = 1). The mean duration of placebo treatment was 5.4 weeks, with 21 (65.6%) of 32 participants completing the 7-week phase; 1 of 9 participants who discontinued had adverse events (nosebleeds). The other 8 participants discontinued due to lack of improvement in symptoms. Haloperidol versus placebo was significant for reductions in ABC Hyperactivity/Defiance scores (P < .05) but not for the other ABC subscales. The investigators noted that carryover of effects between phases may have affected results in this crossover design, especially with the short 1-week washout. Other comparisons between haloperidol and placebo were not discussed.
SRI Medications
Five studies of SRIs met our criteria.5,7–10 One fair-quality RCT7 investigated the efficacy of fluvoxamine in adults with autistic disorder. Participants were not receiving any psychotropic medications for at least 6 weeks before starting the trial. The study randomized participants to receive placebo or fluvoxamine initiated at 50 mg daily and increased 50 mg every 3 to 4 days to maximum clinical response or a maximum dose of 300 mg/day. All 30 participants (15 fluvoxamine, 15 placebo) completed the 12-week trial. The mean age was 30.1 ± 7.1 years for the fluvoxamine group and 30.1 ± 8.4 years for the placebo group. The mean daily dose was 276.7 ± 41.7 mg/day for the fluvoxamine group and 283.3 ± 36.2 mg/day for the placebo group (difference not significant). Global improvement as measured by using CGI-I was higher for fluvoxamine compared with placebo (P < .001). Individuals were classified as responders if the CGI-I scores were very much improved or much improved. There were significantly more responders (P < .001) in the fluvoxamine group (8 of 15 participants) compared with the placebo group (0 of 15). On most measures of challenging behavior, the scores for the fluvoxamine group improved more than the scores for the placebo group. Adverse events in the fluvoxamine group included mild sedation (n = 2) and nausea (n = 3). There were no significant changes in anticholinergic effects, vital signs, routine laboratory analyses, or electrocardiogram results.
Another fair-quality study5 used a double-blind, placebo-controlled crossover design to investigate the efficacy of clomipramine and haloperidol for the treatment of autism. Investigators randomized participants to 7-week phases of haloperidol, clomipramine, and placebo. Clomipramine dosing started at 25 mg at bedtime and increased in 25-mg increments every 2 days until the dose was 50 mg twice daily; further 25-mg adjustments were then made every 3 to 4 days on the basis of clinical assessment. The dose was reduced to the last dose tolerated if adverse events were experienced. There was a dosage taper during week 7 of each phase and 1-week placebo washout periods between each phase. No other psychotropic drugs were allowed except benztropine. Of the 37 participants recruited, 36 (mean age: 16.3 years) were included in final analyses. The mean daily dose of clomipramine was 128.4 mg. The mean duration of clomipramine treatment was 4.5 weeks, with 12 (37.5%) of 32 participants completing the 7-week treatment phase; 12 of 20 participants who discontinued did so at least partially because of adverse events that included fatigue or lethargy (n = 4), tremor (n = 2), tachycardia (n = 1), insomnia (n = 1), diaphoresis (n = 1), nausea or vomiting (n = 1), decreased appetite (n = 1), and preexisting right bundle branch block (n = 1). The mean duration of placebo receipt was 5.4 weeks, with 21 (65.6%) of 32 participants completing the 7-week phase; 1 of 9 participants who discontinued had adverse events (nosebleeds). The investigators noted that carryover of effects between phases may have affected results in this crossover design, especially with the short 1-week washout period.
One poor-quality study9 assessed the efficacy and tolerability of clomipramine by using a prospective open-label case series design over 12 weeks. Of the 35 participants, 33 completed the study and were taking a mean dose of 139 ± 50 mg. Of the 33 participants completing the trial, 18 (55%) were responders as determined based on the CGI-I score of very much improved or much improved. Clomipramine treatment significantly reduced (P < .001) repetitive thoughts and behaviors and aggression. Adverse events, including seizures, constipation, weight gain, and sedation, were reported in 13 individuals.
Another poor-quality, 12-week, open-label prospective case series8 investigated the efficacy and tolerability of sertraline. Of the 42 participants, 37 completed the trial. The mean sertraline dose was 122.0 ± 60.5 mg. Of the 42 participants starting the trial, 24 (57%) were considered responders based on CGI-I score of very much improved or much improved. Five participants withdrew from the study: 3 due to anxiety/agitation, 1 due to syncope, and 1 because of noncompliance.
Finally, a poor-quality retrospective case series10 studied the therapeutic effects and tolerability of fluoxetine and included 23 individuals with ASD (mean age: 15.9 ± 6.2 years). CGI-S ratings of overall clinical severity improved in 15 participants, as did ratings of perseverative or compulsive behavior. Six of 23 participants experienced adverse events that “significantly” interfered with function or outweighed therapeutic benefits. Adverse events reported overall included agitation (n = 5), insomnia (n = 4), elated affect (n = 4), decreased appetite (n = 4), and increased screaming (n = 2).
Opioid Receptor Antagonist
One study of an opioid receptor antagonist met our review criteria.11 This fair-quality randomized, double-blind crossover study tested the efficacy and safety of naltrexone on self-injurious behavior and other autistic symptoms in intellectually disabled adults. Doses of concurrent medications, including antipsychotic agents, were held stable. The study randomized participants to receive naltrexone or placebo, with a 2-week, single-blinded placebo period followed by a single dose of naltrexone (100 mg) with placebo for the remainder of that week. This phase was followed by a 4-week treatment period, a 4-week washout period, and finally a crossover to the second 4-week treatment period. The first cohort received naltrexone 50 mg/day, but the dose for the second cohort was changed to naltrexone 150 mg/day. The primary outcome was self-injurious behavior.
Of the 33 participants, 24 had autistic disorder.11 Participants’ mean age was 29 years, and IQ was not reported. Eleven participants were taking antipsychotic agents, with the dose held steady during the study. The single dose had no effect on the clinician-rated questionnaire, direct observation, self-injurious behavior, or plasma β-endorphins. Plasma cortisol was significantly increased (P = .006) with naltrexone compared with placebo.
Longer-term treatment (4 weeks) with naltrexone resulted in a significant increase in stereotypy as measured by using the ABC Stereotypy subscale.11 No changes in any of the other outcome measures were significant. The study did not report comparative statistics, but the CGI-I scale indicated that placebo was superior to 50 mg/day of naltrexone in 12 of 18 participants. The CGI-I scale also found that 50 mg/day of naltrexone was better than placebo in only 4 of 18 participants, whereas placebo was superior in 12 of 18 participants. The CGI-I scale also showed that 150 mg/day of naltrexone was better than placebo in 5 of 14 participants, whereas placebo was superior in an equal number of participants (5 of 14). There were no significant correlations between behavioral changes after the single dose of 100-mg naltrexone and the 4-week treatments with naltrexone (50 or 150 mg).
Further analyses with groups divided into participants with concurrent antipsychotic use and participants without such use did not yield any significant effect for naltrexone versus placebo.11 Adverse events included 1 subject with an acute increase in self-injurious behavior, 1 subject with nausea and tiredness, and 3 participants with sedation. Liver function test results remained within normal ranges.
Discussion
Assessment of the Literature
The use of medications in adolescents and young adults with ASD is extremely common.12 However, few data address the effectiveness and harms of medications specifically in this population. Of the 8 studies identified for this review, most focused on the use of medications to address specific challenging behaviors (ie, aggression or irritability). Four studies were fair quality,5–7,11 and 4 were poor.4,8–10
The most consistent findings were identified for antipsychotic medications. An RCT studying risperidone found improvements in aggression, repetitive behavior, sensory motor behaviors, and overall behavioral symptoms.6 A crossover study of risperidone also showed a significant reduction of irritability/agitation ratings with risperidone treatment.4 A placebo-controlled crossover study found that haloperidol significantly improved hyperactivity/defiance ratings, but no significant difference was found for irritability/agitation or other symptoms.5 Although there is limited literature available on the use of risperidone in adolescents or young adults with ASD, the efficacy of risperidone in studies including mostly children was reported in our previous report to have moderate strength of evidence for the treatment of irritability/agitation,13 which is consistent with the results of the 1 fair-quality RCT and 1 poor-quality crossover study in adults with ASD. The available evidence across age groups therefore seems consistent in demonstrating positive effects of risperidone for irritability/agitation symptoms in ASD.
A number of studies of SRIs were identified but with limited consistency across studies as a whole. An RCT of fluvoxamine showed decreases in repetitive behavior, aggression, autistic symptoms, and language usage.7 In contrast, no significant differences were observed in a crossover study of clomipramine versus placebo.5 Three case series of SRIs were also identified, including sertraline, fluoxetine, and clomipramine, with each study reporting some benefit to treatment.8–10 A recent study not meeting criteria for this review also contributes to the limited data on SRIs. Hollander et al14 reported a placebo-controlled RCT of fluoxetine that included 37 individuals with ASD (mean age: 34.31 years). They found improvements in repetitive behavior and ASD symptoms in the treatment group and mild adverse events. This study used a different medication than the 1 fair-quality study in our age range, so it would be unlikely to influence the strength of evidence for a specific medication. It is possible, however, that a systematic review of SRIs in the broader age range of adults with ASD could provide data that might increase our confidence in the effect. A crossover study of the opioid receptor antagonist naltrexone found no significant improvements in problem behavior and showed worsening of stereotyped behavior with naltrexone treatment compared with placebo.11
Based on the included studies in adolescents and adults with ASD, the strength of evidence (confidence in the estimate) is insufficient regarding adverse events associated with medications tested in this population. As in the case of efficacy, the data on adverse events associated with risperidone, including sedation and weight gain, are consistent with the strong strength of evidence for the association of treatment with these adverse events in children with ASD.13 The available evidence therefore seems consistent in supporting our understanding of the risk of these adverse events in ASD without being limited to a specific age range. Of course, this does not mean that other medications tested in ASD are free of adverse events. It is reasonable to expect that, in contrast to efficacy, which is more likely to be specific to disorder and symptom, adverse events are more likely to extend across diverse groups of participants studied. Clinicians evaluating the evidence and sharing information with families routinely take this perspective, as does the US Food and Drug Administration, in mandating that all adverse events be listed for a drug, rather than just those for a particular indication.
As 1 example, the limited studies of adults with ASD treated with risperidone indicate weight gain as an adverse event but in too few studies to draw a clear conclusion about the strength of evidence. There is, however, high strength of evidence for weight gain in children with ASD treated with risperidone, as noted in a previous comparative effectiveness review.13 Similarly, Cochrane reviews found substantial evidence for weight gain in adults with schizophrenia or bipolar disorder treated with risperidone.15,16 When the broader evidence base is considered, the consistency of these findings supports an association of weight gain with risperidone in adults with ASD, just as is true in children with ASD and adults with other disorders. This approach to assessing the evidence for adverse events is outside of the scope of this review, but similar conclusions could be drawn with respect to sedation and extrapyramidal symptoms with risperidone or haloperidol.
Future Directions
Overall, there is a dearth of evidence in all areas of care for adolescents and young adults with ASD. Basic understanding of the effects of aging on health, cognitive skills, and other domains of functioning is absent, and the lack of RCTs of medications, in which substantial adverse events may be associated with medication use in adolescence, is notable. Medication studies conducted in adolescents and young adults have focused largely on problem behaviors, and additional data are needed on medical comorbidities in adolescents with ASD. Clear evidence from earlier studies of antipsychotic agents in children supports the use of risperidone and aripiprazole in children with ASD.13 The only fair-quality study of risperidone in adults is consistent with the findings in children, but the strength of evidence based on the adult literature alone is insufficient to draw firm conclusions. Population studies may be helpful to empirically group ASD patients according to age in a way that fosters more effective studies of treatments. Understanding the age-appropriateness of potential medication treatments as based on social, physiologic, pharmacologic, and functional characteristics of the population would help to prioritize future research, including the ways in which medical comorbidities arise or increase as children with ASD move into adolescence and adulthood. Increased use of standardized age groupings would facilitate comparisons of effectiveness within medication treatment categories as well as with nonmedical therapies. One way to support accomplishing this goal is by developing treatment networks with adequate numbers of patients of varying ages to participate in research.
Thus far, medication treatment research in adolescents and young adults with ASD has been limited to compounds that are already approved for other indications. As targeted treatments for ASD emerge, initial studies will need to focus on adult populations to establish safety before moving into studies of adolescents and finally children. Study of compounds not yet on the market could be facilitated with partnerships between the academic and pharmaceutical communities. It will be critical to consider the appropriate outcome measures and settings in which to study medication response in adults. The heterogeneity in settings for adults with ASD (eg, living at home versus a group home) is a significant impediment to assessing symptom response. Ideally, medications would be combined with an educational or psychosocial intervention that would mirror the school and therapeutic settings in which individuals with ASD show improvements in social, communication, or behavioral function. Without some level of educational or social challenge, it may be difficult to assess medication response. Furthermore, some medications may show benefit only during critical points of development, making pediatric studies critical to assessing efficacy, even when it is necessary to first establish safety in older populations.
Research is also needed to determine which outcomes should be used in future studies. The ABC is a widely used, easily repeatable, and highly sensitive outcome measure for behavioral symptoms in ASD, but it does not directly index core social communication symptoms or anxiety or mood symptoms, nor does it capture broader outcomes such as quality of life. More outcome measures are needed to allow assessment of a broader range of symptoms, particularly in individuals who may be higher functioning. No studies provide adequate information on longer-term outcomes and particularly on outcomes related to achieving goals for independence and quality of life. Substantial, foundational research should be conducted to identify and validate outcome measures in the adolescent and young adult population with ASD.
Finally, for all research in this area, we encourage greater transparency in reporting, particularly as it relates to reporting of randomization approaches, characterization of study participants, description of the intervention, and measures of fidelity and adherence. These are all necessary to understand correctly the potential impact of the interventions being reported.
Conclusions
Given the number of individuals affected by ASD, there is a dramatic lack of evidence on best approaches to therapies for adolescents and young adults with these conditions. Little evidence supports the use of medication treatments in the adolescent and young adult population. Although the studies that have been conducted focused on the use of medications to address specific challenging behaviors, the effectiveness in managing irritability and agitation in this age group remains largely unknown and can at best be inferred from studies including mostly younger children.
Acknowledgments
We are grateful for the contributions of our review team, technical expert panel members, and key informants. We also appreciate the input of the parents who participated as we formulated our approach.
Glossary
- ABC
Aberrant Behavior Checklist
- ASD
autism spectrum disorder
- CGI-I
Clinical Global Impression of Improvement
- RCT
randomized controlled trial
- SRI
serotonin reuptake inhibitor
Footnotes
This project was funded under contract HHSA 290 2007 10065 I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
FINANCIAL DISCLOSURE: Dr Dove has received training support from the National Institute of General Medical Sciences, the National Heart, Lung, and Blood Institute, the National Institute of Child Health and Human Development, the Maternal Child Health Bureau, the American Heart Association, and the Autism Speaks Autism Treatment Network; Dr Taylor has received research support from the National Institute of Mental Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Autism Speaks, and the Marino Autism Research Institute; Dr Warren has received research support from the National Institute of Mental Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Science Foundation, the Agency for Healthcare Research and Quality, Autism Speaks, the Marino Autism Research Institute, and the Simons Foundation; Dr Veenstra-VanderWeele has received research support from the National Institute of Mental Health, the National Institute of Child Health and Human Development, the Agency for Healthcare Research and Quality, Autism Speaks, the American Academy of Child and Adolescent Psychiatry, NARSAD, Seaside Therapeutics, Roche Pharmaceuticals, and Novartis. He has consulted for Novartis; and Dr McPheeters and Ms Sathe have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The full review project was supported by the Agency for Healthcare Research and Quality (contract HHSA 290 2007 10065 I). Dr. Lounds Taylor was also supported by the National Institute of Mental Health through a K01 award (K01 MH092598) during participation on the project and preparation of the manuscript.
References
- 1.Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):234–247 [DOI] [PubMed] [Google Scholar]
- 2.Warren Z, Veenstra-VanderWeele J, Stone W, et al. Therapies for children with autism spectrum disorders. Comparative Effectiveness Review No. 26. AHRQ Publication No. 11-EHC029-EF. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productID=656. Accessed July 6, 2012
- 3.Agency for Healthcare Research and Quality Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2008 [PubMed] [Google Scholar]
- 4.Hellings JA, Zarcone JR, Reese RM, et al. A crossover study of risperidone in children, adolescents and adults with mental retardation. J Autism Dev Disord. 2006;36(3):401–411 [DOI] [PubMed] [Google Scholar]
- 5.Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: a double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol. 2001;21(4):440–444 [DOI] [PubMed] [Google Scholar]
- 6.McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55(7):633–641 [DOI] [PubMed] [Google Scholar]
- 7.McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996;53(11):1001–1008 [DOI] [PubMed] [Google Scholar]
- 8.McDougle CJ, Brodkin ES, Naylor ST, Carlson DC, Cohen DJ, Price LH. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol. 1998;18(1):62–66 [DOI] [PubMed] [Google Scholar]
- 9.Brodkin ES, McDougle CJ, Naylor ST, Cohen DJ, Price LH. Clomipramine in adults with pervasive developmental disorders: a prospective open-label investigation. J Child Adolesc Psychopharmacol. 1997;7(2):109–121 [DOI] [PubMed] [Google Scholar]
- 10.Cook EH, Jr, Rowlett R, Jaselskis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry. 1992;31(4):739–745 [DOI] [PubMed] [Google Scholar]
- 11.Willemsen-Swinkels SH, Buitelaar JK, Nijhof GJ, van England H. Failure of naltrexone hydrochloride to reduce self-injurious and autistic behavior in mentally retarded adults. Double-blind placebo-controlled studies. Arch Gen Psychiatry. 1995;52(9):766–773 [DOI] [PubMed] [Google Scholar]
- 12.Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics. 2008;121(3). Available at: www.pediatrics.org/cgi/content/full/121/3/ e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPheeters ML, Warren Z, Sathe N, et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127(5). Available at: www.pediatrics.org/cgi/content/full/127/5/e1312. [DOI] [PubMed] [Google Scholar]
- 14.Hollander E, Soorya L, Chaplin W, et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am J Psychiatry. 2012;169(3):292–299 [DOI] [PubMed]
- 15.Rendell JM, Gijsman HJ, Bauer MS, Goodwin GM, Geddes GR. Risperidone alone or in combination for acute mania. Cochrane Database Syst Rev. 2006;(1):CD004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):CD006626. [DOI] [PMC free article] [PubMed] [Google Scholar]