Abstract
OBJECTIVE:
To develop and validate heart and respiratory rate percentile curves for hospitalized children and compare their vital sign distributions to textbook reference ranges and pediatric early warning score (EWS) parameters.
METHODS:
For this cross-sectional study, we used 6 months of nurse-documented heart and respiratory rates from the electronic records of 14 014 children on general medical and surgical wards at 2 tertiary-care children’s hospitals. We developed percentile curves using generalized additive models for location, scale, and shape with 67% of the patients and validated the curves with the remaining 33%. We then determined the proportion of observations that deviated from textbook reference ranges and EWS parameters.
RESULTS:
We used 116 383 heart rate and 116 383 respiratory rate values to develop and validate the percentile curves. Up to 54% of heart rate observations and up to 40% of respiratory rate observations in our sample were outside textbook reference ranges. Up to 38% of heart rate observations and up to 30% of respiratory rate observations in our sample would have resulted in increased EWSs.
CONCLUSIONS:
A high proportion of vital signs among hospitalized children would be considered out of range according to existing reference ranges and pediatric EWSs. The percentiles we derived may serve as useful references for clinicians and could be used to inform the development of evidence-based vital sign parameters for physiologic monitor alarms, inpatient electronic health record vital sign alerts, medical emergency team calling criteria, and EWSs.
Keywords: percentile curves, early warning score, heart rate, hospital rapid response team, medical emergency team, physiologic monitoring, reference values, respiratory rate
What’s Known on This Subject:
Accurately identifying ill hospitalized children with vital signs concerning for clinical deterioration is fundamental to inpatient pediatrics. Normal vital sign ranges for healthy children are useful for outpatient practice but have limited application to detecting deterioration in the hospital setting.
What This Study Adds:
Percentile curves for heart and respiratory rate in hospitalized children were developed and validated. The distributions differed from existing reference ranges and early warning scores. They may be useful to identify vital signs deviating from ranges expected among hospitalized children.
The widespread implementation of rapid response systems (RRSs) over the past decade has called attention to the importance of recognizing and responding to early signs of clinical deterioration.1 RRSs aim to reduce mortality, cardiac arrest, and respiratory arrest outside of ICUs and include afferent and efferent limbs.2 The afferent limb of RRSs includes tools to aid clinicians in detecting patients exhibiting early signs of clinical deterioration.3 The efferent limb consists of medical emergency teams (METs)4–9 that can be urgently summoned to the bedside to assist in management.
Accurately interpreting vital signs is critical to the success of RRSs.3 To facilitate the recognition of vital signs consistent with clinical deterioration, clinicians may rely on several afferent tools, including monitor alarms, electronic health record (EHR) vital sign alerts, MET calling criteria, and early warning scores (EWSs) that assign point values to vital signs outside of age-based ranges.10–17
Limited data are available to inform the development of evidence-based parameters for monitor alarms, EHR alerts, MET calling criteria, and EWSs. Currently available sources to inform the configuration of these parameters include textbook reference ranges and existing EWSs with parameters developed using consensus opinion. No previous studies have described the actual distributions of heart rate (HR) or respiratory rate (RR), 2 vital signs critical to ongoing surveillance for deterioration, among hospitalized children. In this study, we sought to develop and validate HR and RR percentile curves for pediatric inpatients. We aimed to determine the proportion of vital signs in our cohort that fell outside textbook18–21 reference ranges and those that would be assigned points using existing EWSs.16,17 Finally, we aimed to directly compare our HR and RR percentile cut points to those from a recent meta-analysis of vital sign distributions in well children.22
Methods
Data Sources
We performed this cross-sectional study among children <18 years of age hospitalized on general medical and surgical wards in 2 tertiary-care children’s hospitals: Cincinnati Children’s Hospital Medical Center (CCHMC) and The Children’s Hospital of Philadelphia (CHOP). In both settings, nurses or nursing assistants entered vital signs into discrete fields on EHR flowsheets; CCHMC used the Integrating Clinical Information System (Siemens Medical Solutions, Malvern, PA) and CHOP used Epic Systems (Verona, WI) during the study periods. The minimum frequency of vital sign assessment was determined by physicians’ orders. The procedures used to measure HR and RR were neither standardized across patients nor documented in the record.
We extracted vital signs, demographics, and primary discharge diagnosis from CCHMC between July 1, 2008, and December 31, 2008, and CHOP between February 1, 2011, and July 30, 2011. The use of distinct time periods allowed us to create a merged data set including each of the 4 seasons, as well as both geographic and temporal variation among the included patients.
Data Quality
We implemented several data integrity measures to ensure that the data were of the highest quality possible. We excluded admissions in which the primary discharge diagnosis was missing. We included only those observations in which the HR and RR were entered simultaneously. We excluded observations in which the RR exceeded the HR or the HR or RR included a decimal. In addition, we excluded observations in which either vital sign met criteria for physiologic implausibility, defined by consensus of the investigators as being more likely to represent keystroke errors than actual observations (HR >300 or <30, and RR >120 or <5).
Sampling Strategy
To minimize ascertainment bias from vital signs collected frequently over short periods of time, we first divided each admission into 6-hour intervals. We then randomly selected 1 HR and 1 RR from within each interval. To reduce ascertainment bias from single individuals with lengthy hospitalizations, we included a maximum of 10 intervals per admission. We then randomly divided the data into a curve development set made up of 67% of the patients and a curve validation set made up of 33%.
Data Analysis
Before developing the curves, we log-transformed the highly skewed RR data to more closely approximate a normal distribution. We also noted evidence of digit preference.23 For example, among those aged >15 years, 73% of RRs were accounted for by the values 16, 18, and 20. We therefore added uniformly distributed random noise to the RR data before developing the curves to facilitate identifying the underlying distribution of RR in older children. Adding random noise, or “jitter,” can be used to identify the underlying distribution in data that have been subject to rounding or digit preference.24
We then developed percentile curves with the vital sign on the y-axis and the patient age at the time the vital sign was measured on the x-axis using the Box-Cox power exponential (BCPE) distribution in the generalized additive models for location, scale, and shape (GAMLSS) package for R software.25–28 The use of GAMLSS models with the BCPE distribution in the development of percentile curves for growth has been demonstrated25,29–31; GAMLSS models can readily be applied to vital sign curves. BCPE is an appropriate distribution because it is useful for data exhibiting both skewness and kurtosis, and it allows for determination of z-scores at any point in the distribution. This second feature facilitates future development of EWSs that use z-scores to standardize the assignment of point values to vital signs across age groups.
Next, we determined the stability of the first and 99th percentiles by creating curves using 3 bootstrapped samples for both HR and RR and comparing the differences between the 3 bootstrapped samples.
To validate the curves, we divided the validation set into 5 age groups and determined the proportion of vital signs below the fifth and above the 95th percentiles for each age group. It would be expected that 5% of the values in the validation set would be below the fifth percentile and 5% would be above the 95th percentile derived using the development set.
To evaluate the impact of respiratory disease on the RR and HR curves, we performed a sensitivity analysis. We identified all admissions with a primary discharge diagnosis of a Disease of the Respiratory System as defined by the International Classification of Diseases, Ninth Revision, Clinical Modification, codes 460 through 519. We then excluded those admissions from the data set. We compared the curves excluding respiratory disease to the curves without disease exclusions.
To evaluate the clinical relevance of our findings to the care of hospitalized children, we determined the proportion of observations deviating from reference ranges that hospitals may use to identify vital signs consistent with deterioration, set monitor alarm parameters, or configure EHR vital sign alerts. We included 3 commonly used pediatric references: Nelson Textbook of Pediatrics,18 The Harriet Lane Handbook,19,20 and the American Heart Association Pediatric Advanced Life Support Provider Manual.21 We also determined the proportion of observations that would have resulted in increased pediatric EWSs by using 2 published scores that are sufficiently described to permit retrospective scoring: Parshuram’s Bedside Pediatric Early Warning System Score17 and Akre’s Pediatric Early Warning Score.16 Of note, Akre’s score was modified from a score previously described by Monaghan10 and Tucker15; however, neither Monaghan nor Tucker’s published description included vital sign ranges.
To facilitate the clinical use of our percentile curve data, we defined cut points at the first, fifth, 10th, 50th, 90th, 95th, and 99th percentiles for HR and RR by calculating the mean vital sign value within each of 13 age groups. We used the same age groups used in a recent meta-analysis of HR and RR normal ranges to enable direct comparison with that study.22
Because we used a deidentified data set for the analysis, this project was granted exemptions from the CHOP and CCHMC Institutional Review Boards.
Results
We extracted data from 11 028 admissions among 8894 patients aged <18 years at CHOP and 6271 admissions among 5208 patients aged <18 years at CCHMC. Before implementing the sampling strategy described earlier, scatterplots of the data showed numerous “spikes” in the HR and RR distributions, many of which represented clusters of extreme values in individual patients with repeated measurement over short time periods, extreme values in individual patients with repeated measurement across lengthy admissions, or both. We minimized the presence of these spikes by applying the aforementioned sampling strategy, after which 116 383 HR values and 116 383 RR values from 14 014 patients across 17 153 admissions remained (3139, or 18% were repeat admissions). This sample represented 23% of the observations across 99% of the patients in the original data set. Demographics are shown in Tables 1 and 2.
TABLE 1.
Patient Characteristics
| Characteristic | Patients, n (%) |
|---|---|
| Race/ethnicitya | |
| White | 7558 (53.9) |
| African American | 4206 (30.0) |
| Hispanic | 604 (4.3) |
| Asian/Pacific Islander | 305 (2.2) |
| American Indian/Alaska Native | 15 (0.1) |
| Multiracial | 1 (<0.1) |
| Other | 1311 (9.4) |
| Unknown | 14 (0.1) |
| Total | 14 014 |
| Gender | |
| Male | 7539 (53.8) |
| Female | 6474 (46.2) |
| Unknown | 1 (<0.1) |
| Total | 14 014 |
Self-reported by patient or family and categorized according to the options available in the electronic health record.
TABLE 2.
Observations by Age Groups
| Age Groupa | Observations, n (%) |
|---|---|
| 0–<3 mo | 9872 (8.5) |
| 3–<6 mo | 5604 (4.8) |
| 6–<9 mo | 4248 (3.7) |
| 9–<12 mo | 3761 (3.2) |
| 12–<18 mo | 6582 (5.7) |
| 18–<24 mo | 4937 (4.2) |
| 2–<3 y | 8924 (7.7) |
| 3–<4 y | 6595 (5.7) |
| 4–<6 y | 10 896 (9.4) |
| 6–<8 y | 8815 (7.6) |
| 8–<12 y | 15 428 (13.3) |
| 12–<15 y | 14 477 (12.4) |
| 15–<18 y | 16 244 (14.0) |
| Total | 116 383 |
Because age at time of vital sign measurement changes during the course of the hospitalization, these data are presented in terms of the number of observations.
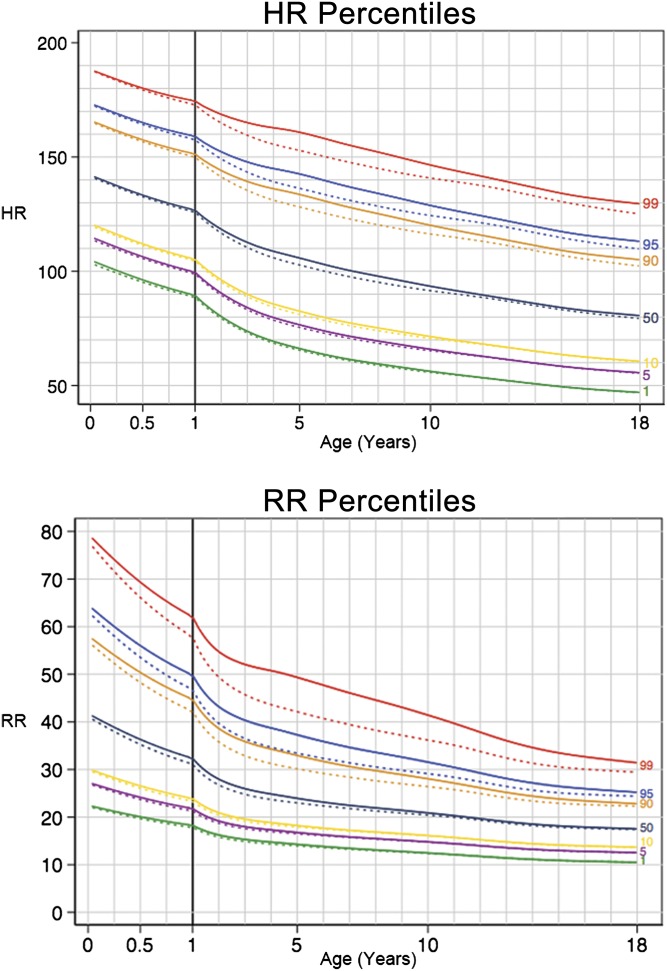
We then used the 77 825 HR and 77 825 RR values in the development set to generate HR and RR percentile curves, shown in Fig 1. Tables 3 and 4 show our suggested clinical cut points at the first, fifth, 10th, 50th, 90th, 95th, and 99th percentiles for HR and RR, respectively, among the 13 age groups. In evaluating the stability of the first and 99th percentiles, we found that the bootstrapped samples resulted in a maximum change in the first and 99th percentiles of 1 beat per minute for HR and 1 breath per minute for RR.
FIGURE 1.
Percentile curves for HR and RR in hospitalized children. Dotted lines represent sensitivity analysis excluding diseases of the respiratory system. The solid vertical line at 1 year of age represents a change in scale of the x-axis.
TABLE 3.
Suggested HR Cut Points Based on Average Predicted Value Within Each Age Group and Percentile
| Age group | 1st | 5th | 10th | 50th | 90th | 95th | 99th |
|---|---|---|---|---|---|---|---|
| 0–<3 mo | 103 | 113 | 119 | 140 | 164 | 171 | 186 |
| 3–<6 mo | 98 | 108 | 114 | 135 | 159 | 167 | 182 |
| 6–<9 mo | 94 | 104 | 110 | 131 | 156 | 163 | 178 |
| 9–<12 mo | 91 | 101 | 107 | 128 | 153 | 160 | 176 |
| 12–<18 mo | 87 | 97 | 103 | 124 | 149 | 157 | 173 |
| 18–<24 mo | 82 | 92 | 98 | 120 | 146 | 154 | 170 |
| 2–<3 y | 77 | 87 | 93 | 115 | 142 | 150 | 167 |
| 3–<4 y | 71 | 82 | 88 | 111 | 138 | 146 | 164 |
| 4–<6 y | 66 | 77 | 83 | 106 | 134 | 142 | 161 |
| 6–<8 y | 61 | 71 | 77 | 100 | 128 | 137 | 155 |
| 8–<12 y | 56 | 66 | 72 | 94 | 120 | 129 | 147 |
| 12–<15 y | 51 | 61 | 66 | 87 | 112 | 121 | 138 |
| 15–<18 y | 48 | 57 | 62 | 82 | 107 | 115 | 132 |
TABLE 4.
Suggested RR Cut Points Based on Average Predicted Value Within Each Age Group and Percentile
| Age group | 1st | 5th | 10th | 50th | 90th | 95th | 99th |
|---|---|---|---|---|---|---|---|
| 0–<3 mo | 22 | 27 | 30 | 41 | 56 | 62 | 76 |
| 3–<6 mo | 21 | 25 | 28 | 38 | 52 | 58 | 71 |
| 6–<9 mo | 20 | 23 | 26 | 35 | 49 | 54 | 67 |
| 9–<12 mo | 19 | 22 | 24 | 33 | 46 | 51 | 63 |
| 12–<18 mo | 18 | 21 | 23 | 31 | 43 | 48 | 60 |
| 18–<24 mo | 16 | 20 | 21 | 29 | 40 | 45 | 57 |
| 2–<3 y | 16 | 18 | 20 | 27 | 37 | 42 | 54 |
| 3–<4 y | 15 | 18 | 19 | 25 | 35 | 40 | 52 |
| 4–<6 y | 14 | 17 | 18 | 24 | 33 | 37 | 50 |
| 6–<8 y | 13 | 16 | 17 | 23 | 31 | 35 | 46 |
| 8–<12 y | 13 | 15 | 16 | 21 | 28 | 31 | 41 |
| 12–<15 y | 11 | 13 | 15 | 19 | 25 | 28 | 35 |
| 15–<18 y | 11 | 13 | 14 | 18 | 23 | 26 | 32 |
In validating the curves using the remaining 38 558 observations for each vital sign, we found that the proportion of vital signs below the fifth and above the 95th percentile lines for each age group in the validation set was within 0.9% of the expected 5% for the HR data at all ages. However, the proportion of RR observations in the validation set below the fifth and above the 95th percentile differed from the expected 5% by up to 4.7%; the proportion of RRs below the fifth percentile for RR was 9.7% among 12- to <18-year-olds. Because the majority of RRs in older children are accounted for by a small number of unique values, any distribution would likely result in some clustering at the most common values, precluding a truly normal distribution of RR.
We then evaluated the impact of our introduction of uniformly distributed random noise to the RR data as a means of overcoming digit preference. Using the validation data set, we analyzed the distribution with and without random noise added. We found that adding random noise caused minimal change in the distribution of the data (z-scores had a mean of 0.00 and SD of 0.93 for the data set without random noise, and a mean of 0.01 and SD of 0.98 for the data set with random noise) while improving the fit of the curves at the outer percentiles (the proportion of RRs below the fifth and above the 95th percentile lines for each age group in the validation set was within 1.4% of the expected proportion of 5%.).
In the sensitivity analysis (shown as dashed lines in Fig 1), we excluded 3713 (21.6%) admissions with primary diagnoses of “diseases of the respiratory system,” leaving 91 763 HR and 91 763 RR values. We found the impact of excluding respiratory disease to be most pronounced at the 99th percentile lines. For HR, excluding resulted in a reduction in the 99th percentile by up to 8 beats per minute, occurring between 5 and 6 years of age. For RR, excluding resulted in a reduction in the 99th percentile by up to 7 breaths per minute, occurring between 4 and 5 years of age.
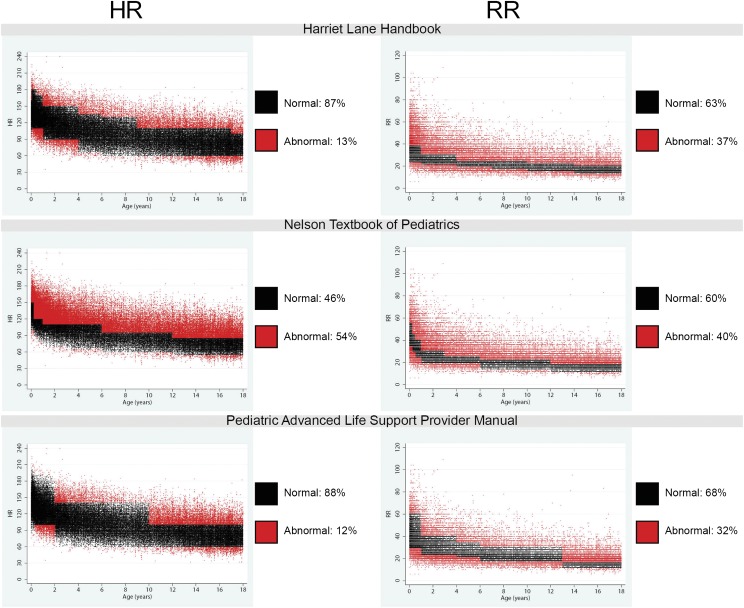
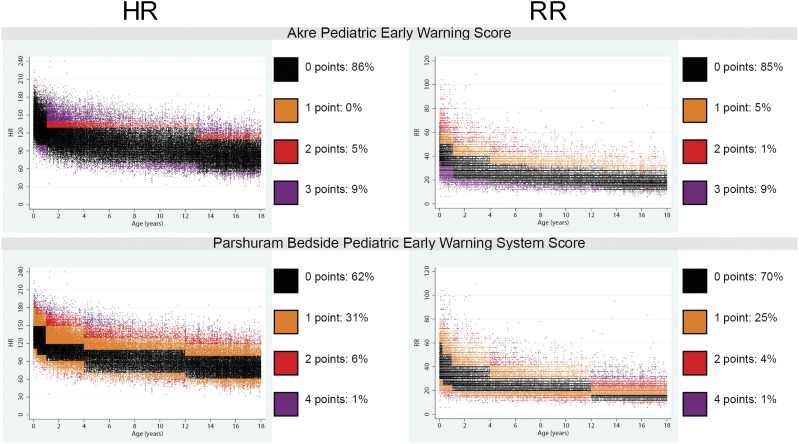
For each of the reference ranges and EWSs described, we determined the proportion of observations that either deviated from the reference range or earned points in the EWS. We found that 12% to 54% of HR observations and 32% to 40% of RR observations deviated from textbook ranges (Fig 2). In addition, 14% to 38% of HR observations and 15% to 30% of RR observations would have resulted in increased total EWSs (Fig 3). Compared with the data from a recent meta-analysis of HR and RR among well children,22 the curves we developed by using hospitalized children had a wider span from first to 99th percentile, primarily because of higher HR and RR at the 99th percentile in all age groups (Supplemental Fig 1).
FIGURE 2.
Scatterplot array showing the distribution of HR and RR in the study sample compared with textbook reference ranges. Each point on the scatterplot represents 1 vital sign observation. For each reference range, observations that would be considered normal are colored black, and observations that would be considered abnormal are colored red. We found that 12% to 54% of HR observations and 32% to 40% of RR observations in our study sample deviated from the ranges provided.
FIGURE 3.
Scatterplot array showing the distribution of HR and RR in the study sample in comparison with existing EWS point ranges. Each point on the scatterplot represents 1 vital sign observation. For each score, observations that would score 0 points are colored black, and observations that would score 1, 2, 3, or 4 points are colored according to each scatter plot’s legend. We found that 14% to 38% of HR observations and 15% to 30% of respiratory rate observations would have resulted in increased total EWSs.
Discussion
Our percentile curves, developed by using a large multicenter sample, provide the first evidence-based reference ranges for HR and RR among hospitalized children. These ranges differed from textbook reference ranges, EWS parameters created by using expert consensus, and distributions of vital signs from well children.
The ranges we developed represent the distributions of HR and RR among ill children hospitalized outside of ICU settings. By using these data, we found that up to 54% of vital sign values among patients in this study were abnormal according to textbook reference ranges, and up to 38% would be assigned points using EWSs with parameters developed based on the consensus of experts. Our results provide the most useful evidence to date for helping clinicians determine the degree to which vital signs deviate from the values expected of hospitalized children. These findings may serve as useful clinical references and can inform the development of evidence-based vital sign parameters for physiologic monitor alarms, EHR vital sign alerts, MET calling criteria, and EWSs.
A recent meta-analysis reviewed 69 studies with HR data for 143 346 children and RR data for 3881 well children in nonhospital settings and used the summary statistics from each study to derive percentile curves.22 While the meta-analysis provides the best available data to inform outpatient vital sign reference ranges, the uppermost percentiles for HR and RR in our study were higher than in the meta-analysis, raising questions about the application of vital sign distributions from well patients to inform deterioration surveillance in the hospital.
Developing valid vital sign-based tools to detect early signs of clinical deterioration with high sensitivity and low false-positive rates (high specificity) is challenging. Because children have vital sign ranges that vary by age, tool development in pediatrics is particularly complex. Several pediatric EWSs have been described10–17 with parameters developed using the consensus of experts. Parshuram’s Bedside Pediatric Early Warning System Score11,14,17 and Akre’s Pediatric Early Warning Score16 (adapted from Monaghan’s10 and similar to Tucker’s15) are the 2 most rigorously studied. Parshuram’s score had a sensitivity of 64% and a specificity of 94% in a validation study.17 Akre’s score had a sensitivity of 86%; its specificity was not reported.16 Neither has been evaluated in a clinical trial. The reference ranges for hospitalized children in our study provide an opportunity to take a data-driven approach to optimizing EWS parameters with the goal of increasing the sensitivity and specificity of these tools. The z-scores derived from the vital sign data could be used to assign EWS point values based on the degree to which the HR or RR deviates from the expected value for age.
In addition to EWSs, the percentiles derived in this study have potential application to configuring physiologic monitor alarm parameters and EHR vital sign alerts. Age-based monitor alarm thresholds based on clinical data from hospitalized children may help minimize alarm fatigue32 by safely reducing the frequency of false alarms that do not identify meaningful departures from expected vital signs in the hospital. For example, upon hospital admission, HR alarm thresholds could be set at the fifth and 95th percentiles for age. Similarly, inpatient EHR alerts, which may range from highlighting the text of an out-of-range vital sign entry in an electronic flowsheet to generating an alert if a vital sign exceeds an extreme threshold, could be set based on the percentiles.
Our study has several limitations. First, we derived these curves by using data from 2 tertiary-care children’s hospitals. The vital sign distributions from patients in community hospitals may differ, limiting the generalizability of our reference ranges to those settings. Second, developing percentile-based EWSs and alarm thresholds assumes that the patients at highest risk of deterioration are those with the most statistically abnormal vital signs, which is likely an oversimplification.33,34 Clinical trials are needed to determine whether percentile-based EWSs have a role in identifying critical but reversible disease states and improving patient outcomes. Third, although we noted that developing EWSs based on normal vital signs from healthy children may result in tools with low specificity and high false-positive rates, developing EWSs based on vital signs from ill children may result in tools with low sensitivity and high false-negative rates. Fourth, we used vital sign data that was manually entered in the course of clinical care. This introduced the issue of digit preference. This issue could likely be overcome either by using research staff to measure vital signs by counting HR and RR for at least 1 full minute or by using physiologic monitor data. We chose observations documented in the course of clinical care because our curves will be referenced in clinical settings, and we aimed for our distributions to be representative of that context. We chose manually entered data over data from monitors because only a fraction of inpatients at these hospitals are monitored, thus using monitor data would introduce selection bias. Fifth, just as within-subject changes in height, weight, and head circumference over time are critical to identifying abnormal growth, we suspect that within-subject changes in vital signs over time may be equally if not more important than exceeding absolute percentiles. To address this issue, the z-scores derived from these curves could be used to standardize the degree of within-subject change across age groups in an EWS.
Conclusions
In summary, we developed and validated the first evidence-based percentile curves for HR and RR among children hospitalized on general wards at tertiary-care pediatric hospitals. Their vital sign distributions differed from existing reference ranges and EWS parameters. Inpatient clinicians may find value in using these curves and percentile cut points to identify patients with vital signs that deviate from the ranges observed in this sample of hospitalized children. These new findings also invite new research to validate the use of vital sign percentiles in the development of evidence-based parameters for physiologic monitor alarms, inpatient EHR vital sign alerts, MET calling criteria, and EWSs.
Supplementary Material
Acknowledgments
We thank William Nieczpiel, senior data architect at CHOP, and the research data warehouse team at CCHMC for their assistance obtaining the data.
Glossary
- BCPE
Box-Cox power exponential
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CHOP
The Children’s Hospital of Philadelphia
- EHR
electronic health record
- EWS
early warning score
- GAMLSS
generalized additive models for location, scale, and shape
- HR
heart rate
- MET
medical emergency team
- RR
respiratory rate
- RRSs
rapid response systems
Footnotes
Dr Bonafide conceptualized and designed the study, acquired the data from The Children’s Hospital of Philadelphia, participated in the analysis and interpretation of the data, and drafted the initial manuscript; Dr Brady conceptualized and designed the study, acquired the data from Cincinnati Children’s Hospital Medical Center, and participated in the analysis and interpretation of the data; Dr Keren conceptualized and designed the study and participated in the analysis and interpretation of the data; Dr Conway participated in the analysis and interpretation of the data; Dr Marsolo acquired the data from Cincinnati Children’s Hospital Medical Center; Dr Daymont conceptualized and designed the study, performed the statistical analysis, and participated in the analysis and interpretation of the data; and all authors critically revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Drs. Bonafide and Keren were funded in part by the Pennsylvania Health Research Formula Fund Award, a grant from the Pennsylvania Department of Health to perform research in pediatric hospital quality, safety, and costs. Staff at Cincinnati Children’s Hospital Medical Center involved with data extraction, including Dr. Marsolo, were funded in part by grant UL1RR026314 from the National Institutes of Health.
References
- 1.Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139–146 [DOI] [PubMed] [Google Scholar]
- 2.Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams [published correction appears in Crit Care Med. 2006:34(12):3070]. Crit Care Med. 2006;34(9):2463–2478 [DOI] [PubMed] [Google Scholar]
- 3.DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375–382 [DOI] [PubMed] [Google Scholar]
- 4.Brilli RJ, Gibson R, Luria JW, et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246, quiz 247 [DOI] [PubMed] [Google Scholar]
- 5.Hunt EA, Zimmer KP, Rinke ML, et al. Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children’s center. Arch Pediatr Adolesc Med. 2008;162(2):117–122 [DOI] [PubMed] [Google Scholar]
- 6.Sharek PJ, Parast LM, Leong K, et al. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a Children’s Hospital. JAMA. 2007;298(19):2267–2274 [DOI] [PubMed] [Google Scholar]
- 7.Tibballs J, Kinney S. Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306–312 [DOI] [PubMed] [Google Scholar]
- 8.Hillman K, Chen J, Cretikos M, et al. MERIT study investigators . Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091–2097 [DOI] [PubMed] [Google Scholar]
- 9.Priestley G, Watson W, Rashidian A, et al. Introducing Critical Care Outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398–1404 [DOI] [PubMed] [Google Scholar]
- 10.Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32–35 [DOI] [PubMed] [Google Scholar]
- 11.Duncan H, Hutchison J, Parshuram CS. The Pediatric Early Warning System score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271–278 [DOI] [PubMed] [Google Scholar]
- 12.Haines C, Perrott M, Weir P. Promoting care for acutely ill children-development and evaluation of a paediatric early warning tool. Intensive Crit Care Nurs. 2006;22(2):73–81 [DOI] [PubMed] [Google Scholar]
- 13.Edwards ED, Powell CVE, Mason BW, Oliver A. Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system. Arch Dis Child. 2009;94(8):602–606 [DOI] [PubMed] [Google Scholar]
- 14.Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13(4):R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker KM, Brewer TL, Baker RB, Demeritt B, Vossmeyer MT. Prospective evaluation of a pediatric inpatient early warning scoring system. J Spec Pediatr Nurs. 2009;14(2):79–85 [DOI] [PubMed] [Google Scholar]
- 16.Akre M, Finkelstein M, Erickson M, Liu M, Vanderbilt L, Billman G. Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/125/4/e763. [DOI] [PubMed] [Google Scholar]
- 17.Parshuram CS, Duncan HP, Joffe AR, et al. Multicentre validation of the bedside paediatric early warning system score: a severity of illness score to detect evolving critical illness in hospitalised children. Crit Care. 2011;15(4):R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman ME, Cheifetz IM. Pediatric emergencies and resuscitation. In: Kliegman RM, Stanton BF, St Geme JW, III, Schor NF, Behrman RE, eds. Nelson Textbook of Pediatrics, 19th ed. Philadelphia, PA: Elsevier Saunders; 2011:280 [Google Scholar]
- 19.Kirk A. Pulmonology. In: Tschudy MM, Arcara KM, eds. The Harriet Lane Handbook, 19th ed. Philadelphia, PA: Elsevier Mosby; 2011:585 [Google Scholar]
- 20.Lennox EG. Cardiology. In: Tschudy MM, Arcara KM, eds. The Harriet Lane Handbook, 19th ed. Philadelphia, PA: Elsevier Mosby; 2011:170 [Google Scholar]
- 21.Ralston M, Hazinski MF, Zaritsky AL, Schexnayder SM, Kleinman ME, eds. Pediatric assessment. In: Pediatric Advanced Life Support Provider Manual. Dallas, TX: American Heart Association; 2006:9–16 [Google Scholar]
- 22.Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graves JW, Bailey KR, Grossardt BR, et al. The impact of observer and patient factors on the occurrence of digit preference for zero in blood pressure measurement in a hypertension specialty clinic: evidence for the need of continued observation. Am J Hypertens. 2006;19(6):567–572 [DOI] [PubMed] [Google Scholar]
- 24.Chambers JM, Cleveland WS, Kleiner B, Tukey PA. Graphical Methods for Data Analysis. Wadsworth & Brooks/Cole Statistics/Probability Series. Boston, MA: Duxbury Press; 1983:20 [Google Scholar]
- 25.Rigby RA, Stasinopoulos DM. Smooth percentile curves for skew and kurtotic data modelled using the Box-Cox power exponential distribution. Stat Med. 2004;23(19):3053–3076 [DOI] [PubMed] [Google Scholar]
- 26.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. J Roy Stat Soc C Appl Stat. 2005;54(3):507–544 [Google Scholar]
- 27.Rigby RA, Stasinopoulos DM. Using the Box-Cox t distribution in GAMLSS to model skewness and kurtosis. Stat Model. 2006;6(3):209–229 [Google Scholar]
- 28.Stasinopoulos DM, Rigby RA. Generalized additive models for location scale and shape (GAMLSS) in R. J Stat Softw. 2007;23(7). Available at: http://www.jstatsoft.org/v23/i07. Accessed January 9, 2013 [Google Scholar]
- 29.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva, Switzerland: World Health Organization; 2006: www.who.int/childgrowth/standards/Technical_report.pdf. Accessed April 1, 2012
- 30.van Buuren S, Hayes DJ, Stasinopoulos DM, Rigby RA, ter Kuile FO, Terlouw DJ. Estimating regional percentile curves from mixed data sources and countries. Stat Med. 2009;28(23):2891–2911 [DOI] [PubMed] [Google Scholar]
- 31.Daymont C, Hwang WT, Feudtner C, Rubin D. Head-circumference distribution in a large primary care network differs from CDC and WHO curves. Pediatrics. 2010;126(4). Available at: www.pediatrics.org/cgi/content/full/126/4/e836. [DOI] [PubMed] [Google Scholar]
- 32.Graham KC, Cvach M. Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19(1):28–34, quiz 35 [DOI] [PubMed] [Google Scholar]
- 33.Tarassenko L, Clifton DA, Pinsky MR, Hravnak MT, Woods JR, Watkinson PJ. Percentile-based early warning scores derived from statistical distributions of vital signs. Resuscitation. 2011;82(8):1013–1018 [DOI] [PubMed] [Google Scholar]
- 34.Subbe CP. Percentile-based Early Warning Scores derived from statistical distributions of vital signs [editorial]. Resuscitation. 2011;82(8):969–970 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.