Abstract
Fully synthetic carbohydrate-based cancer vaccine is an attractive concept, while an important topic in the area is to develop proper vaccine carriers that can improve the immunogenicity and other immunological properties of tumor-associated carbohydrate antigens (TACAs). In this context, four monophosphoryl derivatives of Neisseria meningitidis lipid A were synthesized via a highly convergent and effective strategy and evaluated as vaccine carriers and adjuvants. The conjugates of these monophosphoryl lipid A (MPLA) derivatives with a modified form of the sTn antigen were found to elicit high titers of antigen-specific IgG antibodies, indicating T cell-dependent immune response, in the absence of an external adjuvant. It was concluded that MPLA’s could be utilized as potent vaccine carriers and built-in adjuvants to create fully synthetic self-adjuvanting carbohydrate-based cancer vaccines. The lipid composition and structure of MPLA were shown to have a significant influence on its immunological activity, and among the MPLA’s examined, natural N. meningitidis MPLA exhibited the most promising properties. Moreover, Titermax Gold, a conventional vaccine adjuvant, was revealed to inhibit, rather than promote, the immunological activity of MPLA conjugates, maybe via interacting with MPLA text goes here.
Introduction
Fully synthetic glycoconjugate cancer vaccines are currently a hot topic, since they have well-defined structures, reproducible physical, chemical and biological properties, and promising immunological activities.1–7 To develop functional conjugate cancer vaccines, a vital issue is the carrier molecule. An ideal vaccine carrier should be rather small to be synthetically manageable and highly immunoactive to be able to improve the immunogenicity and promote T cell-dependent immunity of tumor-associated carbohydrate antigens (TACAs) that are often poorly immunogenic and T cell-independent. While several vaccine carriers have been explored for this purpose,1–7 this paper presents a new type of vaccine carrier derived from lipid A for fully synthetic self-adjuvanting carbohydrate-based cancer vaccines.
Lipid A is the conserved hydrophobic core of lipopolysaccharides (LPSs) – the main component and virulent factor on the Gram negative bacterial cell surface.8 Lipid A is of great importance in that it not only serves as an anchor to attach bacterial O-polysaccharides to the bacterial cell membrane8 but also is a potent toxin that can stimulate strong immune responses in eukaryotic hosts.9 It binds specifically to the toll or interleukin-1 receptor domain-containing adaptor-inducing interferon-β (TRIF) of toll-like receptors (TLRs) in association with MD88 to induce a downstream signaling cascade, stimulate the release of cytokines and chemokines, such as tumor necrosis fctor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and interferon-β (IFN-β), and upgrade immune cell expression.10 Even though the immunostimulatory activity of lipid A can be extremely useful, its pro-inflammatory and septic property is a serious problem.11
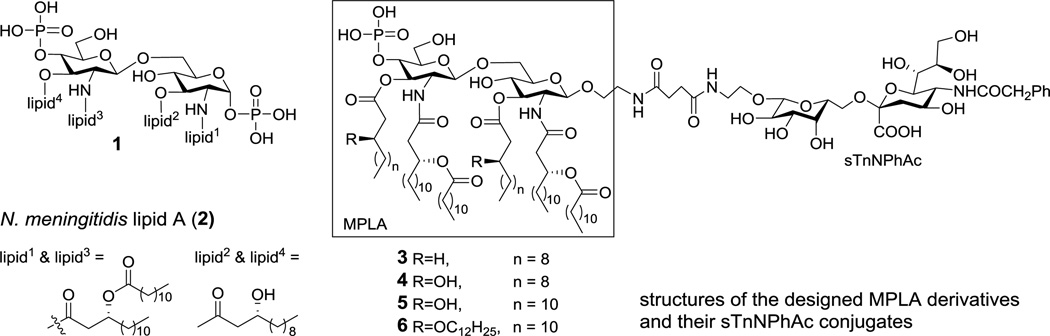
Regardless of source, all lipid A’s contain the highly conserved construct 1 (Figure 1) with several lipids, varying considerably in chain length, saturation, number and distribution, linked to the N-2-, N-2’-, O-3- and O-3’-positions and two phosphate groups linked to the O-1- and O-4’-positions of β-1,6-linked disaccharide of D-glucosamine (GlcNH2). Biological assays demonstrated that the number, structure and position of lipids and the degree of phosphorylation are important factors to determine the bioactivity of lipid A.12–14 A typical lipid A that exhibits a full spectrum of immunological and endotoxic activities consists of six lipids that can be distributed symmetrically (3+3), such as in the lipid A of Neisseria meningitidis (2, Figure 1),15 or asymmetrically (4+2), such as in the lipid A of Escherichia coli, on the two GlcNH2 units. The two phosphate groups are essential for the endotoxic activity of lipid A. However, modification of the O-1-position, for example, removal of the phosphate group at this position to generate monophosphoryl lipid A (MPLA), was shown to greatly reduce its endotoxicity with little impact on its immunostimulatory activity.16 Thus, MPLA has immerged as a potential adjuvant used in human vaccine formulation.17,18 However, the optimal lipid A structure required for binding to the cell surface receptors is different from the structure required for the activation of the host immune system, supported by the finding that most biosynthetic precursors of lipid A are nontoxic and that the toxicity of lipid A has multiple structural requirements.19 Consequently, systematic study on the structure-activity relationship of MPLA is necessary to discover the proper vaccine carrier and adjuvant.
Figure 1.
The conserved construct of lipid A (1) and the structures of N. meningitidis lipid A (2) and designed MPLA derivatives and their sTn conjugates (3–6).
With this regard, many lipid A and MPLA derivatives have been prepared and evaluated in the literature.13,14,17,20–31 In association with our efforts to develop fully synthetic carbohydrate-based cancer vaccines, we synthesized a monophosphoryl analog32 of N. meningitidis lipid A and coupled it to N-phenylacetyl GM3, a modified form of TACA.33 The resultant conjugate alone induced promising immune responses in animals in the absence of any external adjuvant, suggesting that, as an immunostimulant, MPLA was functional not only as a vaccine carrier but also as a built-in adjuvant.34 Inspired by this discovery, here we have designed and synthesized three other monophosphoryl analogs of the lipid A of N. meningitidis, containing lipids of different chain length and linkage as well as different patterns of lipidation (Figure 1). To investigate the immunological properties of these MPLA derivatives as vaccine carriers and adjuvants, we conjugated them with N-phenylacetyl sTn (sTnNPhAc), a modified TACA that is currently studied for anticancer vaccine development,35 and examined in mouse the specific immune responses induced by the resultant MPLA-sTnNPhAc conjugates 3–6 (Figure 1). Through these studies, we anticipated to identify novel vaccine carriers and adjuvants with improved immunological properties useful for the development of fully synthetic glycoconjugate cancer vaccines.
Results and discussion
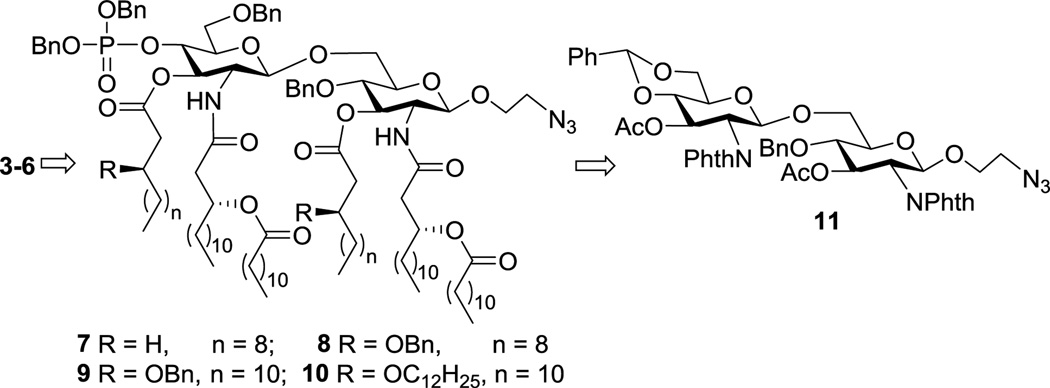
In our synthetic design for target molecules 3–6 (Scheme 1), the key intermediates were fully protected MPLA derivatives 7–10, which had a 2-azidoethyl group at the reducing end of the disaccharide backbone. Global deprotection of 7–10 would afford free MPLA derivatives that can be explored as independent vaccine adjuvants. On the other hand, the azido group in 7–10 could be selectively reduced to form a primary amine that would facilitate conjugation with sTnNPhAc or other TACAs to generate conjugate vaccines. In return, the MPLA derivatives would be constructed from a common disaccharide intermediate 11 having the 2,2’-N- and 3,3’-O-positions differently protected from other positions to enable regioselective deprotection and acylation later on. Moreover, the 4’,6’-O-positions in 11 were protected with a benzylidene group that can be regioselectively opened to expose the 4’-O-position for the introduction of the conserved phosphoryl group.
Scheme 1.
The retrosynthesis for 3–6
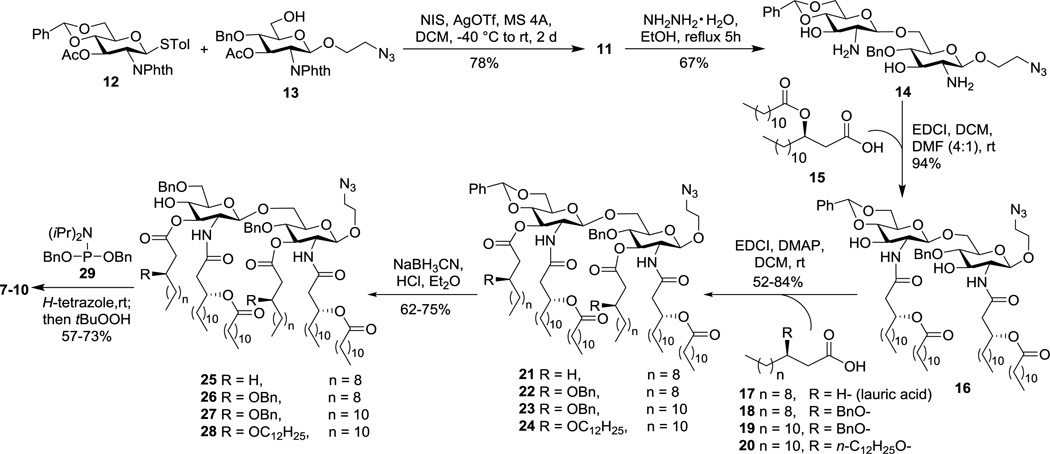
The synthesis started from the preparation of properly protected D-glucosamine derivatives 12 and 13 (Scheme 2), employed as a glycosyl donor and acceptor respectively, according to reported procedures.32 Glycosylation of 12 with 13 was realized with N-iodosuccinimide (NIS) and silver triflate (AgOTf) as promoters, which was unexpectedly slow and took two days to complete. The doublet signal of H-1’ at δ 5.88 in the 1H NMR spectrum of the resultant disaccharide 11 gave a coupling constant (J1’,2’) of 8.8 Hz, which confirmed the β-conformation of the newly generated glycosidic linkage. Treatment of 11 with hydrazine hydrate in refluxing ethanol removed both N-phthalyl and O-acetyl groups simultaneously to form 14, which was ready for the introduction of lipids. In the meanwhile, the chiral lipids 15 and 18–20 (Scheme 2) required for these syntheses were also prepared according to reported procedures32 with commercial (R)-epichlorohydrine as the stating material.
Scheme 2.
Synthesis of the key intermediates 7–10
For lipid installation (Scheme 2), the free amino groups in 14 were acylated first by reacting with 15 under the influence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI). Despite the presence of two free hydroxyl groups in 14, the reaction was fast and selective for the amino groups, probably owing to their higher reactivity, to produce 16 in an excellent yield (94%). The two NH signals as doublets at δ 6.19 (J 4.8 Hz) and 5.99 (J 6.8 Hz) as well as the down-field shift of the H-2 and H-2’ signals in the 1H NMR spectrum of 16 confirmed the desired N-acylation. Compound 16 was employed as a common intermediate for the synthesis of all MPLA derivatives designed. Acylation of the 3-O- and 3’-O-positions in 16 with lauric acid (17) and EDCI required DMAP as a catalyst to afford 21. It was observed that the reaction to introduce the last lipid chain to either the 3-O- or the 3’-O-position was very slow and could complete only after prolonged reaction time, probably due to the further increased steric hindrance after introducing the third lipid to one of these two positions. Similarly, the coupling reactions between 16 and fatty acids 18–20 in the presence of EDCI and DMAP generated 22–24, respectively. Regioselective opening of the benzylidene ring in 21–24 was accomplished using NaBH3CN under acidic conditions to result in 25–28, which had a free hydroxyl group at the 4-O’-position. The regiochemistry of these intermediates was confirmed by the observation of down-field shift of 4’-H in the 1H acetylation products as well as by a similar down-field shift of the 4’-H observed in the O-phosphoryl products of the next step. Phosphorylation of 25–28 was achieved by the phosphoramidite approach in a two-step-one-pot manner to afford the key intermediates 7–10. As mentioned above, significant down-field shift of the H-4’ signals was observed with the 1H NMR spectra of 7–10 (δ 4.3–4.5), as compared to that of 25–28 (δ 3.5–3.6), which further substantiated the attachment of the phosphate group to the 4’-O-position. All of the intermediates involved in the syntheses were fully characterized by 1H, 13C and correlation NMR spectroscopy and by MALT-TOF MS.
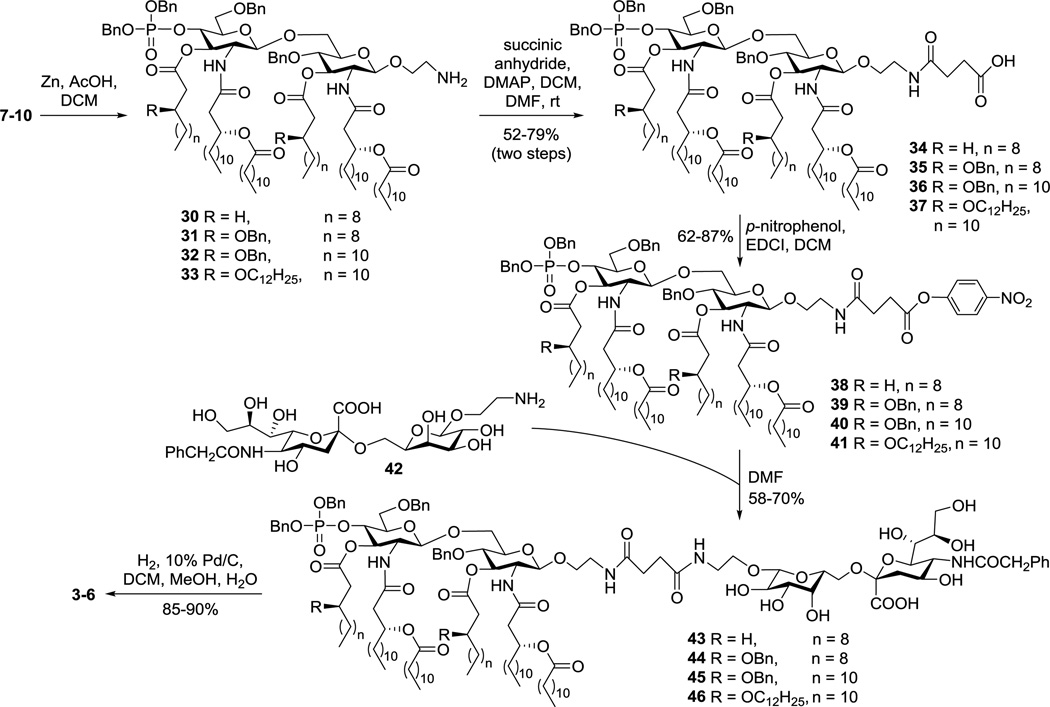
To be readied for conjugation with sTnNPhAc, MPLA derivatives 7–10 were treated with Zn and acetic acid to selectively reduce the azido group (Scheme 3). The resultant amines 30–33 were then acylated with succinic acid anhydride to produce 34–37, which had a free carboxylic acid group attached to the disaccharide reducing end. For coupling with sTnNPhAc derivative 42 that carried a free amino group, acids 34–37 were converted to active esters 38–41 by reacting with p-nitrophenol and EDCI. These reactions were fast, and the active esters formed were stable enough to enable silica gel column purification. Subsequently, 38–41 were coupled with 42 in DMF. These reactions were smooth and clean to afford 43–46 that were readily purified by silica gel column chromatography and by preparative TLC. Finally, catalytic hydrogenolysis of the benzyl ether linkages in 43–46 under a H2 atmosphere in the presence of 10% Pd/C afforded the desired glycoconjugates 3–6, which were confirmed by both NMR and MS. Thereafter, 3–6 were subjected to biological evaluation in mouse.
Scheme 3.
Assembly of the target conjugates 3–6
Immunological investigation of the MPLA-sTnNPhAc conjugates 3–6 was carried out with female C57BL/6 mouse, a well-characterized inbred strain for vaccine evaluation and comparable to the human in immune reactions.33 To improve the solubility of these conjugates in buffer, they were incorporated in liposomes formed by using 1,2-distearoyl-sn-glycero-3-phosphocholine and cholesterol. Delivering glycoconjugate vaccines in liposomal forms can further improve their immunogenicity.36,37 For immunization, the liposomal preparations of 3–6 were each injected subcutaneously (s.c.) to a group of six mice. In the meantime, groups of mice were simultaneously inoculated with emulsions of 3–6 liposomes and Titermax Gold to evaluate the potential influence of an external adjuvant on the activities of these glycoconjugate vaccines. The adopted vaccination schedule was to inject to each mouse 0.1 mL of a vaccine preparation containing ca. 3 µg of sTnNPhAc on days 1, 14, 21 and 28, respectively. Each mouse was subjected to bleeding on day 0 before the first injection (used as blank controls) and on days 27 and 38 after immunization. The blood samples were treated according to standard protocols to prepare antisera for the analysis of sTnNPhAc-specific antibodies by enzyme-linked immunosorbent assay (ELISA) with the human serum albumin (HSA) conjugate of sTnNPhAc (sTnNPhAc-HSA)38 as a capture antigen. In addition to total antibodies, antibody isotypes such as IgG1, IgG2a, IgG3 and IgM were also individually assessed. Antibody titers were calculated from linear regression analysis of the curves of the optical density (OD) value against serum dilution number, and were defined as the dilution number yielding an OD value of 0.2.34
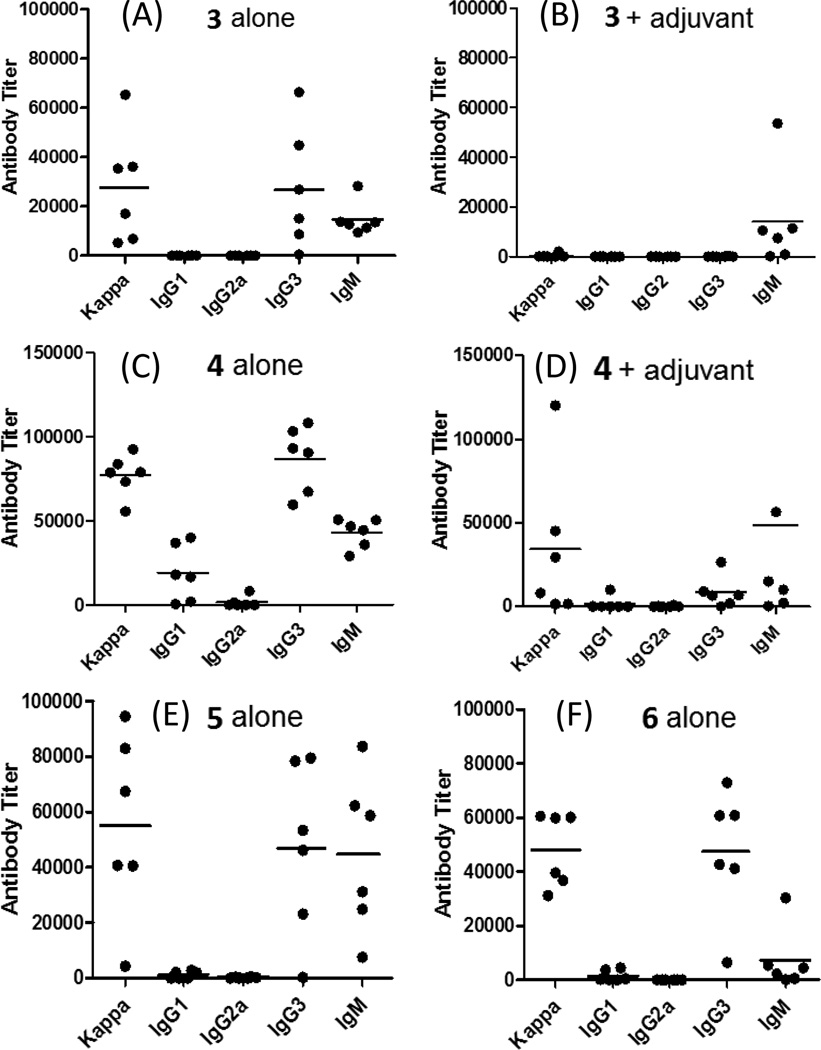
The ELISA results of antisera obtained with 3 without the use of an external adjuvant (Figure 2A) revealed that the conjugate itself provoked a strong sTnNPhAc-specific immune response in mice. More importantly, in addition to IgM antibody, a high titer of IgG3 and some IgG1 antibodies were also observed, suggesting a T cell-dependent immune response.39,40 Interestingly, 3 plus Titermax Gold failed to stimulate a significant antibody response (Figure 2B), although IgM antibodies were observed. These results suggest that the external adjuvant had an inhibitory effect on the T cell-mediated immune response against 3 but a relatively small influence on the B cell response. The results agreed well with our previous report about other MPLA-TACA conjugates.34
Figure 2.
ELISA results of the day 38 antisera of mice immunized with 3 alone (A), 3 plus Titermax Gold (B), 4 alone (C), 4 plus Titermax Gold (D), 5 alone (E), and 6 alone (F), respectively. The titers of various sTnNPhAc-specific antibodies are displayed. Each dot represents the antibody titer of an individual mouse, and the black bar shows the average antibody titer of a group of six mice.
The ELISA results of 4 (Figures 2C and 2D) were similar to that of 3 in terms of the type of immune responses provoked and the influence of Titermax Gold. However, the IgM and IgG antibody titers for 4 were much higher than that for 3. Moreover, 4 stimulated a strong immune response in all of the mice. Another interesting finding was that 4 also induced a significant level of IgG1 antibody which is usually observed with neoglycoprotein vaccines. The ELISA results of 5 (Figure 2E) and 6 (Figure 2F) were similar to that of 3 and 4, but their antibody titers were lower than that of 4 and higher than that of 3. In all of these cases, Titermax Gold showed inhibition on the immune responses against the conjugate vaccines.
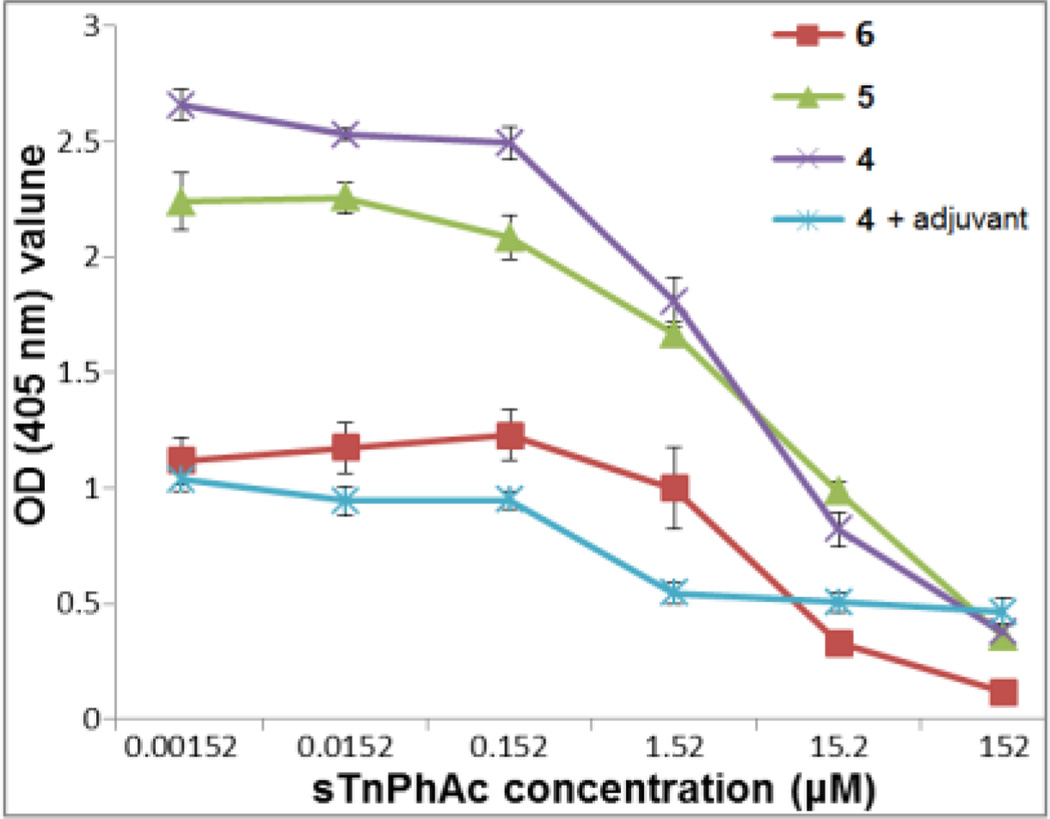
To further verify that the detected antibodies were indeed sTnNPhAc-specific, a competitive ELISA experiment for the pooled mouse antisera obtained with 4, 5, 6, and 4 plus adjuvant was performed. In this study, various concentrations (0, 0.00152, 0.0152, 0.152, 1.52, 15.2 and 152 µM) of free sTnNPhAc (42) were added to compete with sTnNPhA-HSA attached to the ELISA plate for antibody binding. Again, alkaline phosphatase-linked goat anti-mouse kappa antibody was employed as the secondary antibody. The OD values at 405 nm reflected the levels of total antibodies bond to the sTnNPhAc-HSA-coated plates. These studies (Figure 3) have revealed that 42 had concentration-dependent inhibition on antibody binding to the plates and that at concentrations higher than 152 µM 42 could essentially completely inhibit the binding, proving that the elicited antibodies were indeed specific to the sTnNPhAc antigen.
Figure 3.
Competitive ELISA results of the pooled mouse antisera obtained with conjugates 4, 5, 6, and 4 plus adjuvant. The OD values at 405 nm reflected the levels of total antibodies bound to the sTnNPhAc-HSA-coated ELISA plates in the presence of specified concentrations of 42.
The above ELISA results disclosed that the MPLA conjugates 3–6 alone, that is, in the absence of any external adjuvant, provoked a robust antigen-specific immune response in mouse. More importantly, they stimulated high titers of IgG3 antibodies, indicating a T cell-mediated immune response desirable for cancer immunotherapy.39,40 Therefore, all of the synthetic MPLA derivatives could act both as a vaccine carrier and as an adjuvant to effectively enhance the immunogenicity of sTnNPhAc and to efficiently promote T cell-dependent immune response toward TACAs.18,34,41 This property of MPLA should be particularly useful for the design and development of new fully synthetic carbohydrate-based conjugate vaccines. Titermax Gold is a commercial adjuvant that is commonly used with vaccines such as carbohydrate-protein conjugates42 to improve their immunogenicity. However, we showed in this work that it exhibited an inhibitory impact on the immunological activities of 3–6. As Titermax Gold mainly inhibited the elicitation of IgG antibodies, it seemed to mainly affect the T cell-mediated immune response. We proposed that Titermax Gold might interact with MPLA in the conjugate vaccine to prevent MPLA binding to cell surface TLRs and affect its engagement in the T cell-mediated immunological pathways. Another hypothesis is that Titermax Gold might interact with MPLA to affect vaccine delivery to the lymph system or antigen presenting cells required for effective T cell-mediated immunity.43,44 This discovery and further detailed studies may help understanding vaccine adjuvants, such as their binding sites, functional mechanisms and so on.
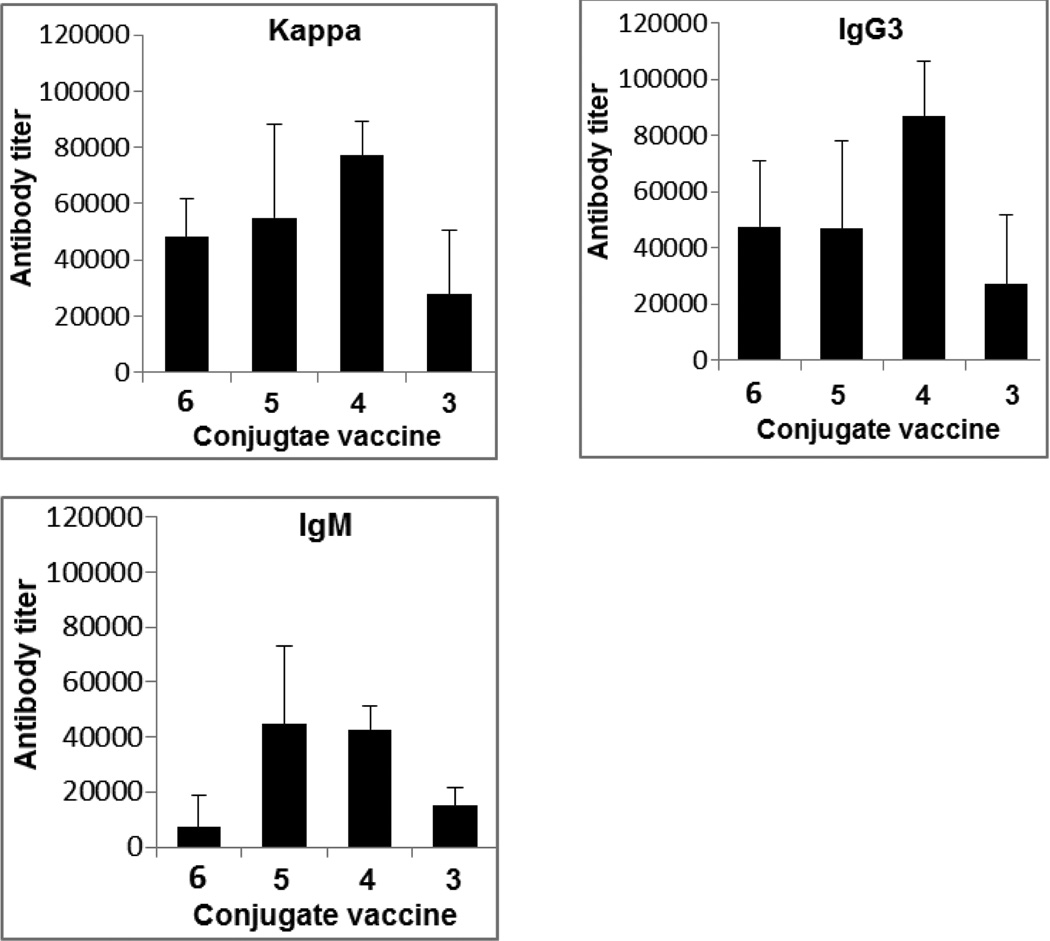
Despite the fact that 3–6 stimulated the similar pattern of immune responses in mouse, they did show difference in immunological activity. For example, conjugate 4 elicited much higher and more consistent titers of both total antibodies and IgG3 antibody than 3, 5 and 6 (Figure 4). Moreover, all of the mice showed strong immune responses to 4 but not to other conjugates. Clearly, 4 was the best vaccine among the MPLA conjugates investigated.
Figure 4.
Comparison of the average antibody titers of sTnNPhAc-specific kappa (total), IgG3, and IgM antibodies in the day 38 antisera of mice immunized with 3, 4, 5, and 6.
Structurally, 3–6 were different only in their MPLA moiety, and their antisera were obtained by means of the same immunization protocol and schedule. Consequently, any difference in their immunological activity should reflect the impact of MPLA structure. Conjugate 4 contained the monophosphoryl form of natural lipid A of N. meningitidis (H44/76 strain).15 In 3, the two free hydroxyl groups on the lipid chains of the MPLA moiety were removed; 5 and 6 were different in that their MPLA contained different lengths and different numbers of lipid chains from that of 4. As all these conjugates had essentially the same immunological profile, it seems that the free hydroxyl groups on the lipid chains and the length and number of lipid chains of MPLA had a quantitative, rather than qualitative, impact on its biological activities. With that said, it is clear that the hydroxyl groups on the lipid chains play an important role in the MPLA interaction with its receptors, as 3 was significantly less potent than both 4 and 5. Elongating the length of lipids at the 3-O-and 3’-O-positions and additional lipid chains linked to these lipids seemed to have a relatively small impact, although such changes did result in reduced immunological activity and some inconsistence of immune responses in individual mice.
Conclusion
In summary, a convergent and efficient strategy was developed for the synthesis of MPLA derivatives suitable for conjugation with carbohydrate antigens and was employed to synthesize four monophosphoryl derivatives of N. meningitidis lipid A. These MPLA derivatives were coupled with sTnNPhAc to form fully synthetic glycoconjugate cancer vaccines. The strategy should be generally applicable to preparing other MPLA derivatives and MPLA-carbohydrate conjugates. Studies on the resultant MPLA-sTnNPhAc conjugates revealed that they elicited strong and T cell-dependent immune responses without the use of any external adjuvant. Our previous work revealed that antisera derived from mice immunized with MPLA conjugates could effectively bind to and kill cancer cells metabolically engineered to express the corresponding antigen.34 MPLA has thus been demonstrated to be a useful platform for the development of new vaccine carriers and adjuvants and for the development of novel types of fully synthetic carbohydrate-based cancer vaccines with self-adjuvanting property. Our results have also revealed that MPLA derivatives containing six lipid chains exhibited more potent immunostimulatory activities than that with eight lipid chains (conjugate 6) and that the lipid structure and length had a significant impact on the immunology of MPLA. The monophosphoryl form of natural N. meningitidis lipid A was found to have the most promising immunological properties and its sTnNPhAc conjugate elicited the most potent and the most consistent T cell-dependent anti-sTnNPhAc immunity. As a result, the MPLA moiety in 4 is identified as the first generation of optimized vaccine carrier and adjuvant that is under further optimization and additional investigation.
On the other hand, Titermax Gold was found to inhibit the immunological activity of MPLA-sTnNPhAc conjugates, whereas it has the opposite influence on the activity of protein-sTnNPhAc conjugates.35,38 It is proposed that Titermax Gold may interact with MPLA to affect its binding to cell surface receptors and/or its delivery to the lymph system or antigen presenting cells. It is anticipated that these issues may be clarified by studies utilizing labeled MPLA derivatives and conjugates, the results of which should be useful for understanding the immunostimulatory and adjuvant activities and the functional mechanisms of MPLA and Titermax Gold, which is a very important topic in cancer vaccine immunology.
Experimental Section
Synthesis of the target glycoconjugates 3–6
Detailed experimental procedures for these syntheses and analytical data of compounds are described in the Electronic Supplementary Information document.
Preparation of liposomes of glycoconjugates 3–6
The protocol was similar to that reported in the literature.34 In brief, the mixture of a specific conjugate 3–6 (0.474 µmol), 1,2-distearoyl-sn-glycero-3-phosphocholine (2.44 mg, 3.08 µmol), and cholesterol (0.92 mg, 2.37 µmol) (in a 10:65:50 molar ratio) was dissolved in CH2Cl2 and MeOH (1:1, v/v, 2 ml) in a round-bottomed flask. The solvents were then removed in vacuum to form a thin lipid film on the flask wall, which was hydrated by adding 2.0 mL of HEPES buffer (20 mM, pH 7.5) containing NaCl (150 mM) and shaking the mixture under an argon atmosphere at 40 °C for 1 h. The suspension was finally sonicated for 1 min to obtain the desired liposomes.
Immunization of mouse
Each group of six female C57BL/6 mice were inoculated on day 1 by subcutaneous (s.c.) injection of 0.1 mL of the liposomal solution of a specific glycoconjugate containing 10 µg of the carbohydrate antigen or by injection of an emulsion of the liposomal solution (0.1 mL) of a specific glycoconjugate with Titermax Gold adjuvant (0.05 mL) prepared according to the manufacturer’s protocol. Following the initial immunization, mice were boosted 3 times on day 14, day 21 and day 28 by s.c. injection of the same conjugate and by means of the same immunization protocol. Mouse blood samples were collected prior to the initial immunization on day 0 and after immunization on day 27 and day 38, and were clotted to obtain antisera that were stored at −80 °C before use. The animal protocol (#A 03-10-11) for this investigation was approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University, and all animal experiments were performed in compliance with the relevant laws and institutional guidelines.
ELISA
ELISA plates were treated with 100 µL of a solution of sTnNPhAc-HSA conjugate (2 µg/mL) dissolved in coating buffer (0.1 M bicarbonate, pH 9.6) at 37 °C for 1 h, which was followed by treatment with a blocking buffer and washing 3 times with phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBST). Then, the pooled or an individual mouse antiserum with serial half-log dilutions from 1:300 to 1:656100 in PBS was added to the coated plates (100 µL/well) and incubated at 37 °C for 2 h. The plates were washed with PBS and incubated at rt for another hour with a 1:1000 diluted solution of alkaline phosphatase linked goat anti-mouse kappa, IgM or IgG2a antibody or a 1:2000 diluted solution of alkaline phosphatase linked goat anti-mouse IgG1 or IgG3 antibody (100 µL/well), respectively. Finally, the plates were washed with PBS and developed with 100 µL of a p-nitrophenylphosphate (PNPP) solution (1.67 mg/mL in buffer) for 30 min at rt for colorimetric readout using a microplate reader at 405 nm wavelength. For titer analysis, OD values were plotted against antiserum dilution values, and a best-fit line was obtained. The equation of this line was used to calculate the dilution value at which an OD of 0.2 was achieved, and the antibody titer was calculated at the inverse of the dilution value. For the competitive ELISA experiments, before the addition of antiserum (80 µL) to each well of the plate, a solution of free sTnNPhAc derivative 42 (20 µL) was added to reach the final concentration of 0, 0.00152, 0.0152, 0.152, 1.52, 15.2 and 152 µM, respectively. The remaining operations were the same as described above.
Supplementary Material
Acknowledgements
This research work was supported by an NIH/NCI grant (R01 CA95142).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental, 1H, 13C, and 2D NMR spectra and MS spectra of all new compounds. See DOI: 10.1039/b000000x/
Notes and references
- 1.Wang Q, Guo Z. Curr. Opin. Chem. Biol. 2009;13:608–617. doi: 10.1016/j.cbpa.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peri F. Chem. Soc. Rev. 2013;42:4543–4556. doi: 10.1039/c2cs35422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser A, Gaidzik N, Becker T, Menge C, Groh K, Cai H, Li Y, Gerlitzki B, Schmitt E, Kunz H. Angew. Chem. Int. Ed. 2010;49:3688–3692. doi: 10.1002/anie.201000462. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z, Shi L, Ma J, Sun Z, Cai H, Chen Y, Zhao Y, Li Y. J. Am. Chem. Soc. 2012;134:8730–8733. doi: 10.1021/ja211725s. [DOI] [PubMed] [Google Scholar]
- 5.Lakshminarayanana V, Thompsonb P, Wolfertb MA, Buskasb T, Bradleyc JM, Pathangeyc LB, Madsena CS, Cohenc PA, Gendlera SJ, Boons B-J. Proc. Nat. Acad. Sci. USA. 2012;109:261–266. doi: 10.1073/pnas.1115166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Aal AM, El-Naggar D, Zaman M, Batzloff M, Toth I. J. Med. Chem. 2012;55:6968–6974. doi: 10.1021/jm300822g. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Sun Z, Huang Z, Shi L, Zhao Y, Kunz H, Li Y. Chem. Eur. J. 2013;19:1962–1970. doi: 10.1002/chem.201203709. [DOI] [PubMed] [Google Scholar]
- 8.Erridge C, Bennett-Guerrero E, Poxton IR. Microbes Infect. 2002;4:837–851. doi: 10.1016/s1286-4579(02)01604-0. [DOI] [PubMed] [Google Scholar]
- 9.Alving CA, Rao M. Vaccine. 2008;26:3036–3045. doi: 10.1016/j.vaccine.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Dobrovolskaia MA, Vogel SN. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 11.Van Amersfoort ES, Van Berkel TJC, Kuiper J. Clin. Microbiol. Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Di Padova SUF, Schreier M, Brade H. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 13.Stover AG, Correia JDS, Evans JT, Cluff CW, Elliott MW, Jeffery EW, Johnson DA, Lacy MJ, Baldridge JR, Probst P, Ulevitch RJ, Persing DH, Hershberg RM. J. Biol. Chem. 2004;279:4440–4449. doi: 10.1074/jbc.M310760200. [DOI] [PubMed] [Google Scholar]
- 14.Kanegasaki S, Tanamoto K, Yasuda T, Homma JY, Matsuura M, Nakatsujka M, Kumazawa Y, Yamamoto A, Shiba T, Kusumoto S, Imoto M, Yoshimura H, Shimamoto T. J. Biochem. 1986;99:1203–1210. doi: 10.1093/oxfordjournals.jbchem.a135583. [DOI] [PubMed] [Google Scholar]
- 15.Kulshin VA, Zahringer U, Lindner B, Frasch CE, Tsai CM, Dmitriev BA, Rietschel ET. J. Bacteriol. 1992;174:1793–1800. doi: 10.1128/jb.174.6.1793-1800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribi E, Cantrell J, Feldner T, Myers K, Peterson J. Microbiol. 1986:9–13. [Google Scholar]
- 17.Baldridge J, Myers K, Johnson D, Persing D, Cluff C, Hershberg R. In: Vaccine Adjuvants. Hackett CJ, Harn DAJ, editors. Totowa, N.J.: Humana Press Inc.; 2006. pp. 235–255. [Google Scholar]
- 18.Casella CR, Mitchell TC. Cell. Mol. Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama K, Qureshi N, Ribi E, Cantrell J. Rev. Infect. Dis. 1984;6:439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- 20.Fukase Y, Fujimoto Y, Adachi Y, Suda Y, Kusumoto S, Fukase K. Bull. Chem. Soc. Jpn. 2008;81:796–819. [Google Scholar]
- 21.Jiang Z, Budzynski WA, Qiu D, Yalamati D, Koganty RR. Carbohydr. Res. 2007;342:784–796. doi: 10.1016/j.carres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Zamyatina A, Sekljic H, Bradeb H, Kosmaa P. Tetrahedron. 2004;60:12113–12137. [Google Scholar]
- 23.Santhanam B, Wolfert MA, Moore JN, Boons G-J. Chem. Eur. J. 2004;10:4798–4807. doi: 10.1002/chem.200400376. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe Y, Miura K, Shiozaki M, Kanai S, Kurakata S, Nishijimac M. Carbohydr. Res. 2003;338:47–54. doi: 10.1016/s0008-6215(02)00357-9. [DOI] [PubMed] [Google Scholar]
- 25.Demchenko AV, Wolfert MA, Santhanam B, Moore JN, Boons G-J. J. Am. Chem. Soc. 2003;125:6103–6112. doi: 10.1021/ja029316s. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki T, Iwano Y, Shiozaki M, Kurakata S, Kanaic S, Nishijima M. Tetrahedron. 2000;56:7691–7703. doi: 10.1016/s0008-6215(99)00326-2. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DA, Keegan DS, Sowell CG, Livesay MT, Johnson CJ, Taubner LM, Harris A, Myers KR, Thompson JD, Gustafson GL, Rhodes MJ, Ulrich JT, Ward JR, Yorgensen YM, Cantrell JL, Brookshire VG. J. Med. Chem. 1999;42:4640–4649. doi: 10.1021/jm990222b. [DOI] [PubMed] [Google Scholar]
- 28.Fukase K, Fukase Y, Oikawa M, Liu W, Suda Y, Kusumoto S. Tetrahedron. 1998:4033–4050. (and references therein). [Google Scholar]
- 29.Christ WJ, McGuinness PD, Asano O, Wang Y, Mullarkey MA, Perez M, Hawkins LD, Blythe TA, Dubuc GR, Robidoux AL. J. Am. Chem. Soc. 1994;116:3637–3638. [Google Scholar]
- 30.Ulmer AJ, Heine H, Feist W, Kusumoto S, Kusama T, Brade H, Schade FU, Rietschel ET, Flad HD. Infect. Immun. 1992;60:3309–3314. doi: 10.1128/iai.60.8.3309-3314.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galanos C, Luderitz O, Rietschel ET, Westphal O, Brade H, Brade L, Freudenberg M, Schade U, Imoto M, Yoshimura H. FEBS. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Guo Z. Chem. Commun. 2009:5536–5537. doi: 10.1039/b907351e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Y, Chefalo P, Nagy N, Harding CV, Guo Z. J. Med. Chem. 2005;48:875–883. doi: 10.1021/jm0494422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Zhou Z, Tang S, Guo Z. ACS Chem. Biol. 2012;7:235–240. doi: 10.1021/cb200358r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Guo Z. Bioconjugate Chem. 2006;17:1537–1544. doi: 10.1021/bc060103s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buskas T, Ingale S, Boons GJ. Angew. Chem. Int. Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 37.Estevez F, Carr A, Solorzano L, Valiente O, Mesa C, Barroso O, Sierra GV, Fernandez LE. Vaccine. 1999;18:190–197. doi: 10.1016/s0264-410x(99)00219-4. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Ekanayaka SA, Wu J, Zhang J, Guo Z. Bioconjugate Chem. 2008;19:2060–2068. doi: 10.1021/bc800243f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markham RB, Pier GB, Schreiber JR. J. Immunol. 1991;146:316–320. [PubMed] [Google Scholar]
- 40.Gavin AL, Barnes N, Dijstelbloem HM, Hogarth PM. J. Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 41.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Trends Microbiol. 2002;10(Suppl.):S32–S37. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Guo Z. ACS Med. Chem. Lett. 2011;2:373–378. doi: 10.1021/ml100313d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H. Immunol. Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 44.O'Hagan DT, MacKichan ML, Singh M. Biomol. Eng. 2011;18:69–85. doi: 10.1016/s1389-0344(01)00101-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.