Abstract
BACKGROUND AND OBJECTIVE:
Subcutaneous immunotherapy (SCIT) is approved in the United States for the treatment of pediatric asthma and rhinitis; sublingual immunotherapy (SLIT) does not have regulatory approval but is used in clinical practice. The objective of this study was to systematically review the evidence regarding the efficacy and safety of SCIT and SLIT for the treatment of pediatric asthma and allergic rhinoconjunctivitis.
METHODS:
Two independent reviewers selected articles for inclusion, extracted data, and graded the strength of evidence for each clinical outcome. All studies were randomized controlled trials of children with allergic asthma or rhinoconjunctivitis treated with SCIT or an aqueous formulation of SLIT. Data sources were Medline, Embase, LILACS, CENTRAL, and the Cochrane Central Register of Controlled Trials through May 2012.
RESULTS:
In 13 trials, 920 children received SCIT or usual care; in 18 studies, 1583 children received SLIT or usual care. Three studies compared SCIT with SLIT head-to-head in 135 children. The strength of evidence is moderate that SCIT improves asthma and rhinitis symptoms and low that SCIT improves conjunctivitis symptoms and asthma medication scores. Strength of evidence is high that SLIT improves asthma symptoms and moderate that SLIT improves rhinitis and conjunctivitis symptoms and decreases medication usage. The evidence is low to support SCIT over SLIT for improving asthma or rhinitis symptoms or medication usage. Local reactions were frequent with SCIT and SLIT. There was 1 report of anaphylaxis with SCIT.
CONCLUSIONS:
Evidence supports the efficacy of both SCIT and SLIT for the treatment of asthma and rhinitis in children.
Keywords: allergen-specific immunotherapy, asthma, pediatric, rhinitis, rhinoconjunctivitis, subcutaneous immunotherapy, sublingual immunotherapy, systematic review
Asthma is one of the most common chronic diseases of childhood, affecting >6 million children in the United States.1 Allergic rhinitis affects up to 40% of children in the United States.2 Allergen-specific immunotherapy (SIT) is frequently used to treat asthma and allergic rhinitis and may modify the course of the disease. SIT is typically recommended for children whose asthma and allergic rhinitis symptoms cannot be adequately controlled with medication or environmental changes.
The US Food and Drug Administration has approved the use of allergen extracts for subcutaneous immunotherapy (SCIT) to treat allergic rhinitis and allergic asthma. Considerable interest has developed in using sublingual immunotherapy (SLIT), which is currently prescribed off-label in the United States. SLIT involves placement of the allergen under the tongue for local absorption, to desensitize the allergic individual over a period of months to years; this method has gained favor in Europe,3 where sublingual tablets and aqueous immunotherapy have been approved.
The objective of the current systematic review was to summarize the evidence regarding the efficacy and safety of SCIT and SLIT for the treatment of pediatric asthma and allergic rhinoconjunctivitis. This review evaluated only the SCIT allergen formulations that are currently available in the United States or SLIT formulations with similar off-label substitutes. This report is derived from a comparative effectiveness review evaluating SIT in adult and pediatric populations commissioned by the US Agency for Healthcare Research and Quality (AHRQ).
Methods
Technical experts were recruited for input on the research questions and search strategy. We developed a protocol for this review and posted it online, following guidelines for systematic review (http://effectivehealthcare.ahrq.gov/ehc/products/270/665/SIT_Protocol_20110824.pdf). Additional details on the methods appear in the full AHRQ Evidence Report, Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma, Comparative Effectiveness Review (http://effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=665).
Data Sources and Selection
We searched Medline, Embase, LILACS, CENTRAL, and the Cochrane Central Register of Controlled Trials, from inception through May 21, 2012 (Supplemental Appendix Fig 1). Two investigators independently reviewed titles, abstracts, and full articles for possible inclusion. Disagreements were resolved by consensus. We included randomized controlled trials (RCTs), exclusively studying children with allergic asthma and/or rhinoconjunctivitis due to inhalant allergens, with diagnoses confirmed by using objective testing (positive result on skin allergy testing and/or in vitro specific immunoglobulin E allergy testing) (Supplemental Appendix Table 1). We included only studies evaluating SCIT formulations available in the United States or SLIT formuations with close off-label substitutes, alone or in combination with usual care, and compared them with placebo, pharmacotherapy, or other SIT regimens. Studies of sublingual tablets were not included because this formulation is currently not available for clinical use in the United States. We included only trials that clearly reported allergen dosages, evaluated clinical outcomes or safety, and were published in English.
Data Extraction and Quality Assessment
One investigator extracted data into standardized forms, and accuracy was confirmed by a second reviewer. We used DistillerSR for data management (Evidence Partners, Ottawa, Ontario, Canada). Data from the final time point were reported. For outcomes with multiple measurements during a single season, data collected at peak allergen season were used.
The quality of each study was assessed by using a modified Cochrane Collaboration Tool for Assessing Risk of Bias to record the adequacy of randomization, allocation concealment, blinding, completeness of data reporting, sponsor company involvement, and other sources of potential bias.4 Two independent reviewers assigned ratings of low, medium, or high risk of bias based on this assessment. Disagreements were resolved by consensus.
Data Synthesis and Analysis
Data were stratified according to outcome, intervention, and allergen, and synthesized qualitatively. We graded the quantity, quality, and consistency of the evidence by adapting an evidence-grading scheme recommended by the Guide for Conducting Comparative Effectiveness Reviews.5 The magnitude of effect was classified according to the percent difference in pre-to-post change comparing the SIT group and comparator group: <15% was defined as a weak difference; a 15% to 40% difference was defined as moderate; and >40% was defined as a strong effect (Supplemental Appendix Table 2). The body of evidence for each primary outcome was graded as high, moderate, low, or insufficient (Supplemental Appendix Table 3). The evidence grade reflects the likelihood that additional research will change the conclusions about the intervention. Adverse events were categorized as local or systemic. Only studies that observed adverse events were included in the safety evaluation.
Results
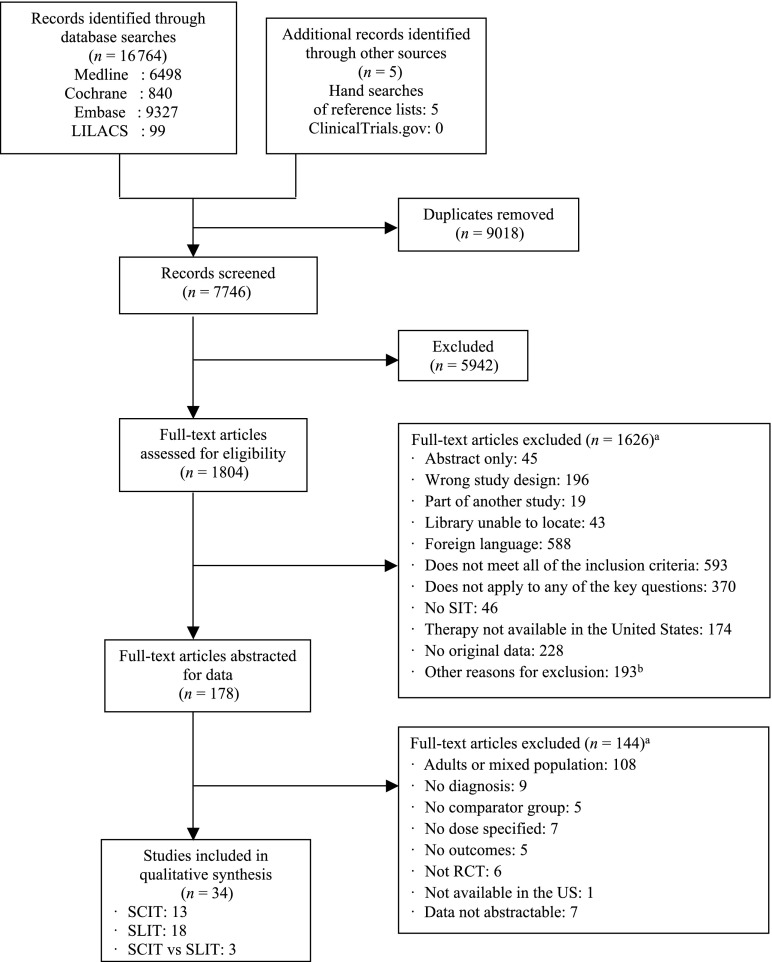
Our search generated 7746 citations (Fig 1). We included 34 articles relevant to children: 13 trials of SCIT, 18 trials of SLIT, and 3 trials comparing SCIT with SLIT. The findings are summarized according to intervention and outcomes (Table 1). We describe asthma findings only for studies that confirmed asthma diagnoses by using objective measures or previously established guidelines. Five of the included studies were not graded because all study arms received immunotherapy.6–10
FIGURE 1.
Flow diagram of evidence search and selection. aThe total number of articles excluded may be exceeded by the number of reasons for exclusion, because articles were excluded by 2 reviewers at this level. bOther reasons: control group is healthy population, routes of administration not included (eg, oral, nasal, lymph node), abandoned interventions, outcomes not reported, no comparator group, continued medical education reports, editorials or reviews, studies about mechanism of action, other allergies (food, aspirin), study in animals or in vitro, or ≤6 patients per arm.
TABLE 1.
Clinical Outcomes Summary for SCIT and SLIT in Children
| Outcome | No. of Studies | No. of Participants | Allergens (n Studies) | Comparators (n Studies) | Summary of Findings | Strength of Evidence |
|---|---|---|---|---|---|---|
| SCIT studies | ||||||
| Asthma symptoms | 6 | 550 | Dust mite (1) | SCIT (2) | The SCIT group showed greater improvement than the comparison group in 5 of 6 studies | Moderate that SCIT improves asthma symptoms more than comparators |
| Cladosporium (1) | vs placebo (4) | |||||
| Rye (1) | vs pharmacotherapy (2) | |||||
| Alternaria (1) | ||||||
| Multiple (2) | ||||||
| Asthma medication scores | 4 | 470 | Dust mite (1) | SCIT | 2 studies showed significant reduction in medication consumption with SCIT compared with usual care; 1 study found no difference between groups, 1 study did not report results from direct comparison between groups | Low that SCIT improves asthma medication scores more than comparators |
| Rye (1) | vs placebo (2) | |||||
| Multiple (2) | vs pharmacotherapy (2) | |||||
| Asthma plus rhinitis/rhinoconjunctivitis medication scores | 2 | 80 | Cladosporium (1) | SCIT | Both studies showed reduction in asthma and rhinoconjunctivitis medication consumption in the SCIT group | Low that SCIT improves asthma plus rhinitis/ rhinoconjunctivitis medication scores more than comparators |
| Alternaria (1) | vs placebo (2) | |||||
| Combined symptom-medication scores | 2 | 85 | Dust mite (1) | SCIT | Both studies showed significant improvement in the SCIT group compared with placebo | Low that SCIT improves asthma or asthma plus rhinoconjunctivitis combined symptom-medication scores more than comparators |
| Alternaria (1) | vs placebo (1) | |||||
| vs SCIT (1) (high- vs low-dose vs placebo) | ||||||
| Rhinitis/rhinoconjunctivitis symptoms | 3 | 285 | Alternaria (1) | SCIT | 2 studies showed statistically significant improvement in symptoms with SCIT; 1 study found no significant improvement with SCIT | Moderate that SCIT improves rhinitis/ rhinoconjunctivitis symptoms more than comparators |
| Cladosporium (1) | vs placebo (3) | |||||
| Birch (1) | ||||||
| Conjunctivitis symptoms | 3 | 285 | Alternaria (1) | SCIT | 2 studies reported significant improvement in symptoms with SCIT; 1 study found no significant improvement with SCIT | Low that SCIT improves conjunctivitis symptoms more than comparators |
| Cladosporium (1) | vs placebo (3) | |||||
| Birch (1) | ||||||
| Rhinoconjunctivitis QoL | 2 | 350 | Alternaria (1) | SCIT | Both studies reported significant improvement in disease-specific QoL in the SCIT arm | Low that SCIT improves rhinoconjunctivitis quality of life more than comparators |
| Multiple (1) | vs placebo (1) | |||||
| vs pharmacotherapy (1) | ||||||
| SLIT studies | ||||||
| Asthma symptoms | 9 | 471 | Dust mite (7) | SLIT | All 9 studies demonstrated improvement in the SLIT group. Higher dose of SLIT showed greater improvement compared with lower dose/placebo. There was a strong magnitude of effect in 6 studies. | High that SLIT improves asthma symptoms more than comparators |
| Tree mix (1) | vs placebo (8) | |||||
| Parietaria (1) | vs SLIT (1) (high versus low dose versus placebo) | |||||
| Rhinitis or rhinoconjunctivitis symptoms | 12 | 1065 | Grass mix (2) | SLIT | 11 studies showed greater improvement in symptoms with SLIT. One placebo controlled study found no improvement in symptoms. | Moderate that SLIT improves rhinitis or rhinoconjunctivitis symptoms more than comparators |
| Dust mite (6) | vs placebo (10) | |||||
| Parietaria (2) | vs control (1) | |||||
| Olive (1) | vs SLIT (1) (high- vs low-dose vs placebo) | |||||
| Tree mix (1) | ||||||
| Asthma plus rhinitis or rhinoconjunctivitis symptoms | 1 | 98 | Tree mix (1) | SLIT (high-dose) vs SLIT (low-dose) vs placebo | This study demonstrated improvement in the SLIT group, with higher dose showing greater improvement | Moderate that SLIT improves asthma plus rhinitis or rhinoconjunctivitis symptoms more than comparators |
| Conjunctivitis symptoms | 5 | 513 | Dust mite (2) | SLIT vs placebo | 4 studies showed greater improvement in symptoms in the SLIT group compared with placebo. Higher dose showed greater improvement. The direction of change could not be determined in one study. | Moderate that SLIT improves conjunctivitis symptoms more than comparators |
| Olive (1) | vs placebo (4) | |||||
| Tree mix (1) | vs SLIT (1) (high- vs low-dose vs placebo) | |||||
| Parietaria (1) | ||||||
| Medication use | 13 | 1078 | Dust mite (6) | SLIT | 11 studies showed a reduction in medication use in the SLIT group; 1 study showed no difference between SLIT and placebo. The direction of change could not be determined in one study. There was a moderate or strong magnitude of effect in 6 studies. | Moderate that SLIT improves medication use more than comparators |
| Grass mix (2) | vs placebo (11) | |||||
| Parietaria (2) | vs control (1) | |||||
| Olive (1)Tree mix (1)Multiple (1) | vs SLIT (1) (high- vs low-dose versus placebo) | |||||
| Combined medication plus symptoms | 2 | 329 | Grass mix (1) | SLIT | 1 study showed greater improvement in the SLIT group; 1 study showed no difference | Low that SLIT improves combined medication plus symptoms scores more than comparators |
| Dust mite mix (1) | vs control (2) | |||||
| Rhinoconjunctivitis QoL | 2 | 461 | Dust mite (1) | SLIT | 1 study showed no improvement in disease-specific QoL; 1 study showed no difference between SLIT and placebo | Insufficient that SLIT improves disease-specific QoL more than comparators |
| Grass mix (1) | vs placebo (2) | |||||
| SCIT vs SLIT studies | ||||||
| Asthma symptoms | 3 | 135 | Dust mite (3) | SCIT vs SLIT; 1 placebo-controlled, 2 pharmacotherapy- controlled | 1 study favored SLIT; 1 study favored SCIT; 1 study weakly favored SCIT, because SCIT, SLIT, and SCIT plus SLIT all showed significant reduction in symptoms | Low favoring SCIT over SLIT for improving asthma symptoms |
| Rhinitis or rhinoconjunctivitis symptoms | 3 | 135 | Dust mite (3) | SCIT vs SLIT; 1 placebo-controlled, 2 pharmacotherapy controlled | 3 studies favored SCIT | Low favoring SCIT over SLIT for improving rhinitis or rhinoconjunctivitis symptoms |
| Medication scores | 3 | 135 | Dust mite (3) | SCIT vs SLIT; 1 placebo-controlled, 2 pharmacotherapy- controlled | 2 studies favored SCIT for rhinitis medication use reduction, 1 study favored SLIT for total medication score reduction | Low favoring SCIT over SLIT for decreasing medication use |
Study and Population Characteristics for SCIT
Thirteen studies including 920 children aged 3 to 18 years evaluated SCIT for clinical outcomes. The primary diagnosis was asthma in 7 studies,6,7,11–15 rhinoconjunctivitis in 1 study,16 and asthma with rhinitis/rhinoconjunctivitis in 5 studies.8,9,17–19 The majority of studies used a single allergen for SCIT. Dust mites were evaluated in 8 of the 13 studies. All studies allowed either routine medications or rescue medications. The maintenance dosing interval varied from biweekly dosing to dosing every 6 weeks, and the duration of treatment ranged from 4 months to 3 years. There was great heterogeneity in the allergen dose delivered and the reporting of dosage units.
Clinical Outcomes of SCIT in Children
Asthma
Six RCTs with 550 subjects evaluated SCIT for control of asthma symptoms relative to placebo11,15,18,19 or pharmacotherapy13,17 (Table 1; Supplemental Appendix Table 4). Four studies evaluated a single allergen11,13,18,19 and 2 used multiple allergens.15,17 Single-allergen SCIT studies demonstrated improvement in asthma symptoms, compared with placebo or pharmacotherapy, with moderate to strong magnitudes of effect. In contrast, 1 study with a low risk of bias enrolled 121 children with moderate to severe asthma already receiving appropriate asthma medications and found no improvement, compared with placebo, with SCIT using multiple allergens.15 The overall strength of evidence is moderate that SCIT using a single allergen improves asthma symptoms relative to placebo or pharmacotherapy. However, there is low-grade evidence that SCIT using multiple allergens does not improve asthma symptoms in subjects with moderate to severe asthmatics.
Four studies with 470 participants evaluated SCIT for improvement of asthma medication usage (Supplemental Appendix Table 5),11,13,15,17 and 2 studies with 80 participants evaluated combined asthma and rhinitis/rhinoconjunctivitis medication usage (Supplemental Appendix Table 6).18,19 Four single-allergen studies demonstrated greater reduction in medication usage for asthma with or without rhinoconjunctivitis in the SCIT group than in the comparator.11,13,18,19 One study with 121 participants and low risk of bias showed similar reductions in asthma medication use in both the SCIT and placebo groups.15 The strength of evidence is low that SCIT improves medication use for asthma or combined asthma and rhinoconjunctivitis.
Two studies with 85 participants evaluated SCIT for improvement on a combined symptom and medication score (Table 1; Supplemental Appendix Table 7).12,19 Both studies showed greater improvement in the SCIT group compared with placebo. The strength of evidence is low that SCIT improves combined symptom and medication scores.
Rhinitis
Three placebo-controlled trials with 285 subjects evaluated SCIT for control of rhinitis and rhinoconjunctivitis symptoms (Table 1; Supplemental Appendix Table 8).16,18,19 These included single- and multiple-allergen regimens. Two studies allowed conventional therapy,16,18 and 1 study allowed only rescue therapy in the treatment arms.19 The largest study, with 205 participants and a medium risk of bias, strongly favored SCIT with grass/birch mix along with conventional therapy over placebo.16 The second study, with 50 participants and medium risk of bias, moderately favored SCIT over placebo.19 The smallest study, with 30 participants and a low risk of bias, weakly favored SCIT over placebo.18 Overall, we found moderate-strength evidence that SCIT controls rhinitis and rhinoconjunctivitis symptoms in children better than placebo.
Conjunctivitis
Three placebo-controlled trials with 285 participants evaluated SCIT, compared with placebo, for control of conjunctivitis symptoms (Table 1; Supplemental Appendix Table 9).16,18,19 Risk of bias was low to medium. One study with a medium risk of bias and 205 participants reported significant improvement in conjunctivitis symptom scores, although actual scores were not reported.16,19 Kuna et al19 also found significant improvement in symptoms, with strong magnitude of effect, after 3 years of SCIT. The third study, with 30 participants and low risk of bias, revealed no significant improvement in conjunctivitis symptoms.18 The strength of evidence is low that SCIT improves conjunctivitis symptoms.
Other Outcomes
Quality of life (QoL) was evaluated in 2 studies (Table 1; Supplemental Appendix Table 10).17,19 One study with 50 participants and medium risk of bias demonstrated a significant improvement in QoL scores after 3 years of SCIT in children and adolescents with asthma and rhinitis, as well as in the parents of children receiving SCIT.19 Another study with 300 participants and a high risk of bias reported no significant difference in QoL with SCIT compared with placebo.17
Prevention of asthma was evaluated in 1 study. Möller et al16 investigated asthma prevention as a primary outcome and observed, among 151 children with allergic rhinoconjunctivitis without asthma, a 2.5-fold greater odds of preventing new onset of asthma after 3 years of SCIT versus placebo. Benefit persisted after 5 years and after 10 years.20,21
Safety of SCIT in Children
Adverse events were observed in 10 of the 13 studies.6–8,12,14,17–19 Local reactions (injection site redness and swelling) were common in both the SCIT and placebo arms, occurring in up to 54% of SCIT and 53% of placebo injections in 1 study (Table 2).7 Systemic reactions included respiratory reactions such as mild to severe bronchospasm in 1% to 30% of patients or up to 4.6% of injections; unspecified or general systemic reactions in 3% to 34% of patients; and urticaria in 2% to 19% of patients. There were no reports of anaphylaxis or death.
TABLE 2.
Adverse Event Outcomes Summary for SCIT and SLIT Studies in Children
| Type of Reaction | Allergen (n studies) | AEs | No. of AEs or Affected Patients/Total No. of Patients in Study Arms Reporting AEs | Range of AEs | Severity | |||
|---|---|---|---|---|---|---|---|---|
| Treatment Arm | Comparator Arm | Treatment Arm | Comparator Arm | Treatment Arm | Comparator Arm | |||
| SCIT | Placebo | SCIT | Placebo | SCIT | Placebo | |||
| SCIT studiesa | ||||||||
| Local reactions (patients), 4 studies | Dust mite (3) | Local swelling or edema | 13/82 patients | NR | 11%–17% | NR | Unspecified (23%) | NR |
| Alternaria (1) | Mild (44%) | |||||||
| Moderate (33%) | ||||||||
| Local reactions (events), 3 studies | Dust mite (1) | Redness or swelling | 578 events/61 patients | 251 events/12 patients (1 study reported AEs in control arm) | 53%–54% of injections (0.25–20 events/patient) | Percentage or range not quantifiable | Mild (100%) | Mild (100%) |
| Cladosporium (1) | 1 study: 265 events/492 injections (46 patients) | 1 study: (21 events/patient) | ||||||
| Dog (1) | ||||||||
| Cutaneous reactions (patients), 2 studies | Dust mite (1) | Urticaria | 6/167 patients | NR | 2%–19% | NR | Unspecified (100%) | NR |
| Cladosporium (1) | ||||||||
| Respiratory reactions (patients), 3 studies | Dust mite (2) | Bronchospasm | 10/203 patients | NR | 1%–30% | NR | Unspecified (14%) | NR |
| Multiple (1) | Wheezing | Severe (86%) | ||||||
| Respiratory reactions (events), 1 study | Dust mite (1) | Cough Dyspnea Bronchial asthma | 19 events/30 patients, 492 injections | NR | 3.5%–4.6% of injections | NR | Mild (96%) | NR |
| 0.7–0.8 events/patient | Moderate (4%) | |||||||
| Systemic reactions: General symptoms (patients), 2 studies | Multiple (1) | Systemic reactions | 22/91 patients | 6/80 patients | 3%–34% | 7%–10% | Unspecified Mild | Unspecified |
| Alternaria (1) | Headache, mild flushing, and redness | |||||||
| Unspecified reactions (patients), 1 study | Multiple (1) | Unspecified | 5/15 patients | NR | 33% | NR | Mild (100%) | NR |
| Unspecified reactions (events), 1 study | Cladosporium (1) | Unspecified systemic reactions | 45 events/16 patients in 1 study that did not report number of injections | NR | 2.8 events/ patient | NR | Unspecified (100%) | NR |
| SLIT | Placebo | SLIT | Placebo | SLIT | Placebo | |||
| SLIT studiesb | ||||||||
| Local reactions (patients), 12 studies | Grass mix (4) | Oral, labial, pharyngeal, itching (buccal, sublingual), swelling, irritation, reddening, tingling | 131/712 patients | 52/342 patients (7 studies reported AEs in control arm) | 0.2%–50% | 6%–25% | Unspecified (86%) | Unspecified (94%) |
| Dust mite (4) | Mild (14%) | Mild (6%) | ||||||
| Tree (2) | ||||||||
| Parietaria (2) | ||||||||
| Upper respiratory reactions (patients), 5 studies | Dust mite (2) | Nasal symptoms, rhinitis | 214/348 patients | 196/275 patients (4 studies reported AEs in control arm) | 3%–92% | 3%–94% | Unspecified (94%) | Unspecified (100%) |
| Trees (1) | Severe (4%) | |||||||
| Parietaria (1) | ||||||||
| Grass mix (1) | ||||||||
| Lower respiratory reactions (patients), 5 studies | Dust mite (2) | Asthma | 125/429 patients | 117/243 patients (3 studies reported AEs in control arm, 1 study had AE only in control arm) | 0%–67% | 10%–69% | Unspecified (92%) | Unspecified (98%) |
| Grass mix (2) | Shortness of breath | Mild (6%) | Mild (1%) | |||||
| Parietaria (1) | Cough | Severe (2%) | Severe (1%) | |||||
| Cutaneous reactions (patients), 5 studies | Dust mite (2) | Urticaria | 118/461 patients | 117/281 patients (3 studies reported AEs in control arm) | 0.7%–57% | 2%–65% | Unspecified (98%) | Unspecified (99%) |
| Grass mix (2) | Rash | |||||||
| Multiple (1) | Eczema | Mild (2%) | Mild (1%) | |||||
| Itch | ||||||||
| GI reactions (patients), 8 studies | Dust mite (3) | Abdominal pain | 186/548 patients | 153/243 patients (3 studies reported AEs in control arm) | 0.7%–74% | 33%–73% | Unspecified (83%) | Unspecified (100%) |
| Grass mix (2) | Abdominal swelling | |||||||
| Trees (1) | Diarrhea | Mild (0.5%) | ||||||
| Parietaria (1) | Gastrointestinal complaints | |||||||
| Multiple (1) | ||||||||
| Ocular reactions (patients), 7 studies | Dust mite (2) | Conjunctivitis, eye symptoms | 130/317 patients | 137/243 patients (3 studies reported AEs in control arm) | 3%–55% | 5%–65% | Unspecified (94%) | Unspecified (100%) |
| Grass mix (1) | Mild (1%) | |||||||
| Trees (1) | Severe (5%) | |||||||
| Parietaria (1) | ||||||||
| General symptoms (patients), 9 studies | Dust mite (6) | Tiredness | 120/393 patients | 103/282 patients (5 studies reported AEs in control arm) | 7%–63% | 8%–67% | Unspecified (81%) | Unspecified (91%) |
| Grass mix (1) | ||||||||
| Trees (1) | Flushing, allergic reaction, headaches | Mild (19%) | Mild (9%) | |||||
| Parietaria (1) | Allergy | |||||||
| SCIT/SLIT | Placebo | SCIT/SLIT | Placebo | SCIT/SLIT | Placebo | |||
| SCIT vs SLIT studiesc | ||||||||
| Local reactions (patients), 1 study SLIT | Dust mite (1) | Oral pharyngeal itching, mild edema | SLIT: 3/10 patients | Placebo: 2/10 patients | 30% | 20% | Unspecified (100%) | Unspecified (100%) |
| Local reactions (patients), 2 studies SCIT | Dust mite (2) | Injection site reaction | SCIT: 3/26 patients | Placebo: 2/10 patients | 12% (6%–20%) | 20% | Mild (100%) | Unspecified (100%) |
| Respiratory reactions (patients), 2 studies SCIT | Dust mite (2) | Asthma, dyspnea, wheezing | SCIT: 3/26 patients | NR | 12% (6%–18%) | NR | Moderate (67%) | NR |
| Severe (33%) | ||||||||
| Anaphylaxis (patients), 1 study SCIT | Dust mite (1) | Flushing, wheezing, dyspnea requiring adrenaline | SCIT: 1/16 patients | NR | 6% | NR | Severe | NR |
AEs, adverse events; NR, no AEs reported.
None of the SCIT studies reported gastrointestinal (GI), general symptoms, or anaphylactic reactions.
None of the SLIT studies reported cardiovascular or anaphylactic reactions.
None of the SCIT versus SLIT studies reported upper respiratory, cutaneous, GI, cardiovascular, ocular, or general symptoms.
Study and Population Characteristics for SLIT
Eighteen studies that enrolled 1583 children aged 4 to 18 years evaluated SLIT for clinical outcomes.10,22–38 The primary diagnoses included asthma,22–24 rhinitis/rhinoconjunctivitis,25–30 and asthma with rhinitis/rhinoconjunctivitis.10,31–38 Immunotherapy targeted predominantly dust mite22–26,32–34,38 and grass.10,28–30 The majority of the studies (60%) used multiple allergens. Most of the comparator group(s) received placebo drops (15 studies), other SLIT regimens (3 studies), or conventional treatment or symptomatic therapy (2 studies). Studies allowed either conventional treatment (12 studies) or only rescue allergy medications (6 studies) in the SLIT arm. The maintenance dosing interval varied from daily to twice weekly, and treatment duration ranged from 6 months to 3 years.
Clinical Outcomes of SLIT in Children
Asthma
Nine studies including 471 participants evaluated SLIT for control of asthma symptoms (Table 1; Supplemental Appendix Tables 11 and 12).22–24,32–35,37,38 Seven studies evaluated dust mite allergen.22–24,32–34,38 The SLIT-treated children in the placebo-controlled studies demonstrated moderate to strong improvement in asthma symptoms. The risk of bias was low in 3 studies.22,32,34 Therefore, the strength of evidence is high that SLIT improves asthma symptoms, compared with placebo.
Rhinitis
Twelve trials including 1065 children evaluated SLIT for control of rhinitis or rhinoconjunctivitis symptoms (Table 1; Supplemental Appendix Table 13).25–29,32–38 One-half of the studies evaluated dust mite allergens.25,26,32–34,38 Risk of bias was low in 4 studies,27,29,32,34 medium in 6 studies,26,33,35–38 and high in 2 studies.25,28 Five studies demonstrated significant improvement in rhinitis/rhinoconjunctivitis scores with SLIT, compared with placebo, with moderate to strong magnitudes of effect.33,34,36–38 Four studies did not show significant improvement with SLIT,25,26,29,32 and 1 of these favored placebo.26 The strength of evidence is moderate that SLIT improves rhinitis symptoms.
Conjunctivitis
Five RCTs including 513 participants evaluated SLIT for control of conjunctivitis symptoms (Table 1; Supplemental Appendix Table 14).25,34–37 Two placebo-controlled trials of olive and tree mix allergens with medium risk of bias that enrolled 70 and 98 children, respectively, demonstrated moderate to strong magnitude of effect for SLIT.36,37 One study of dust mite immunotherapy in 58 children, with a low risk of bias and weak magnitude of effect, showed little improvement with SLIT compared with placebo.34 One study of dust mite allergen with 257 children and a high risk of bias and another study of Parietaria allergen with 30 children and a low risk of bias reported improvement with SLIT, although we could not determine the magnitude of effect.25,35 The strength of evidence is moderate that SLIT improves conjunctivitis symptoms.
Medication Scores
Medication scores were reported in 13 studies with 1078 participants (Table 1; Supplemental Appendix Table 15).22–29,32,33,35–37 Six studies evaluated dust mite allergen.22–26,32 The placebo-controlled studies demonstrated significant reductions in medication use in the SLIT group relative to the placebo group, with moderate to strong magnitudes of effect in patients with asthma and/or rhinitis. The magnitudes of effect could not be determined in 7 studies.25,27–29,32,35,36 The risk of bias across these studies was mixed. The strength of evidence is moderate that SLIT decreases medication use.
Other Outcomes
Combined symptom plus medication scores were reported in 2 SLIT trials with 329 participants (Table 1; Supplemental Appendix Table 16).28,31 Symptom scores included nasal, eye, and bronchial symptoms. One study of 216 participants with asthma and rhinitis and a medium risk of bias showed a strong effect, with lower scores on the symptom and medication use measure with SLIT than with conventional care.31 One study of grass mix allergen that included 113 children with rhinoconjunctivitis and had a high risk of bias reported no significant difference between SLIT and conventional therapy, although the magnitude of effect could not be determined.28 The strength of evidence is low that SLIT decreases the combination of symptoms and medication use for asthma and rhinitis.
QoL was reported in 2 studies involving 461 participants; QoL was measured by using the Pediatric and Adolescent Rhinoconjunctivitis Quality of Life questionnaires (Table 1; Supplemental Appendix Table 17).25,29 One study reported no improvement in QoL,29 and another reported no difference between the SLIT and placebo groups after 2 years.25 There is insufficient evidence to evaluate the impact of SLIT on disease-specific QoL.
Disease modification was addressed in 3 studies.24,27,31 Niu et al24 found significantly more patients with improved asthma classification from mild/moderate persistent asthma to mild intermittent asthma after 6 months of SLIT with dust mite allergen, compared with placebo. Marogna et al31 found no significant difference in the percentage of children with mild intermittent asthma after 3 years of SLIT, compared with placebo. La Rosa et al27 also found no difference in rhinitis symptoms during Parietaria pollen season after 8 years of follow-up in the SLIT and placebo groups.
Prevention of asthma was addressed in 3 studies.27,28,31 Novembre et al28 found that children receiving conventional therapy had a 3.8-fold increased risk of developing asthma compared with those receiving SLIT for 3 years. Marogna et al31 found a lower occurrence of new mild, persistent asthma in patients receiving SLIT compared with a conventional-therapy group after 3 years. La Rosa et al27 found no difference, after treatment for 2 years, in the number of patients with asthma in the SLIT versus placebo groups at 8 years of follow-up.
Two studies addressed the development of new sensitivities.27,31 Marogna et al31 found a 40% decreased odds of developing new sensitivities after 3 years of SLIT, compared with pharmacotherapy. La Rosa et al27 found no difference in the number of new sensitizations in monosensitized children treated with SLIT, compared with placebo, after 8 years of follow-up.
Safety of SLIT in Children
Local reactions, such as oral itching and swelling, were common but mild (Table 2). Twelve studies reported local reactions in 0.2% to 50% of patients receiving SLIT and 6% to 25% of patients receiving placebo.22,25,27–31,35–37
Systemic reactions were commonly reported in both the SLIT and placebo groups, but no life-threatening reactions, anaphylaxis, or deaths were reported in these trials. From most commonly to least commonly reported, the symptoms or reactions were characterized as general, gastrointestinal, ocular, respiratory, and cutaneous. Although severe systemic reactions were rare, 1 study reported severe rhinitis and severe asthma symptoms in children who exceeded their maximum dose.34 These adverse events resolved when the children returned to a lower dose.
Study and Population Characteristics: SCIT Versus SLIT
Three RCTs of dust mite immunotherapy reported on the efficacy and safety of SCIT, compared head-to-head with SLIT in children.39–41 These 3 studies included 135 children, 5 to 14 years of age, with a primary diagnosis of asthma with rhinitis. One study allowed the use of conventional medications,39 and 2 studies allowed only rescue medications.40,41 The maintenance dose for SCIT ranged from thrice weekly to monthly; for SLIT, it was thrice weekly in all studies. Treatment duration in each study was 1 year. Comparison groups in the studies included SCIT, SLIT, SCIT plus SLIT, and placebo or pharmacotherapy arms. All 3 studies had medium risks of bias.
Clinical Outcomes of SCIT Versus SLIT
For asthma outcomes, Yukselen et al39 favored SCIT and Eifan et al40 favored SLIT for improving asthma symptoms and medication use, with moderate magnitudes of effect (Table 1; Supplemental Appendix Table 18). Keles et al41 found that SCIT, SLIT, and SCIT plus SLIT all led to significant reductions in asthma symptoms, with little difference between them but weakly favoring SCIT over SLIT. Keles et al favored SCIT for decreasing asthma medication use, with a moderate magnitude of effect. For rhinitis outcomes, the studies demonstrated a moderate to strong magnitude of effect in favor of SCIT (Supplemental Appendix Table 19). Two studies favored SCIT for reducing rhinitis medication use, with moderate and strong magnitudes of effect,39,41 and 1 study favored SLIT for reducing total medication use, with a moderate magnitude of effect (Supplemental Appendix Table 20).40 Because of the inconsistent direction of change and the few studies available, the strength of evidence is low to support a greater decrease in asthma symptoms, rhinitis symptoms, and medication use with SCIT compared with SLIT.
Safety of SCIT Versus SLIT
Among these 3 studies, local injection site reactions were reported in 3 patients receiving SCIT, and local reactions (oral itching) were reported in 3 patients receiving SLIT (Table 2).39–41 No systemic reactions were reported in patients receiving SLIT. Among 37 patients receiving SCIT, 4 experienced systemic reactions, including 1 anaphylaxis event and 3 patients with moderate to severe respiratory symptoms. These studies suggest that SLIT may be safer than SCIT.
Discussion
In this comprehensive, systematic review of SIT for children with asthma and allergic rhinitis, we summarized data from 34 RCTS, including 13 testing SCIT, 18 testing SLIT, and 3 comparing SCIT with SLIT. We found moderate-strength evidence that SCIT improves asthma and rhinitis symptoms and low-strength evidence that SCIT improves conjunctivitis symptoms, lowers asthma medication scores, and improves rhinoconjunctivitis QoL. We found high-strength evidence that SLIT improves asthma symptoms and moderate-strength evidence that SLIT improves rhinitis and conjunctivitis symptoms and decreases medication usage. We found low-strength evidence to support SCIT over SLIT for improving asthma or rhinitis symptoms or medication usage. Local and systemic reactions were common with both regimens. Anaphylaxis was reported with SCIT in 1 study comparing SCIT with SLIT, and no deaths were reported.
Few previous systematic reviews have evaluated the efficacy of SCIT exclusively in children. Improvements in allergic rhinitis symptoms and medication use, and asthma symptoms and medication use, have been reported with SCIT in previous reviews of combined adult and pediatric populations, without separate pediatric results.42,43 Another systematic review reported conflicting results regarding the clinical efficacy of SCIT for allergic rhinoconjunctivitis in children.44
Our review of SLIT in children expands on the findings of previous pediatric systematic reviews. Significant reductions in asthma symptoms and medication use with SLIT were similarly reported in previous reviews.45,46 For allergic conjunctivitis symptoms, 1 review of 9 studies similarly showed significant reductions in children treated with SLIT,47 whereas another review of 7 studies found no significant reductions in conjunctivitis symptoms.46 In contrast, several reviews did not find significant reductions in rhinitis or rhinoconjunctivitis symptoms or medication use in children treated with SLIT,46,48,49 although decreasing trends were observed in 1 review.46
Our systematic review found more evidence to support the use of SLIT than SCIT. This finding may reflect the fact that there are fewer studies evaluating SCIT exclusively in children, and few head-to-head comparisons of SCIT and SLIT. Additional studies directly comparing these 2 modes of delivery or combination regimens may strengthen this evidence base.
Our safety results are consistent with previous studies evaluating SCIT and SLIT, both of which have been shown to be safe in children with allergic rhinitis and mild asthma. Adverse events associated with SCIT include local discomfort, pain, and serious reactions such as rare fatal and near-fatal reactions.50–52 Most adverse events reported with SLIT have been local reactions of the oral mucosa, with few serious systemic reactions. Only a few cases of anaphylaxis have been reported in children receiving SLIT, although none was found in our review.52–54
One important benefit of SIT specific to children may be the potential to modify the response to allergens at an early stage and thus prevent disease progression.16,20,21,24,28,31 SIT is currently the only treatment with this potential to modify and prevent progression of disease from allergic rhinitis to asthma.52 However, our study found few reports to support SCIT and SLIT for preventing the development of asthma and new sensitizations in children.
Challenges and Study Limitations
Our review involves several challenges and limitations. There was considerable heterogeneity in the study allergens, dosages, dose units, duration of treatment, and in reporting and scoring of outcomes and safety data. This heterogeneity precluded quantitative pooling of the data and made data synthesis challenging. The RCTs that were included in the review varied in their quality. Several studies had moderate or high risk of bias because they did not specify whether allocation schemes were concealed or if the intervention was concealed from the participants and outcome assessors, or did not clarify the role of industry support or sponsors. The majority of SCIT studies were single allergen, and thus the results cannot necessarily be generalized to the use of multiple-allergen regimens, which is common in the United States. In contrast, the SLIT studies mostly used multiple allergens, and the results are not necessarily generalizable to single-allergen regimens. Safety data were variably reported and only reflect observed reports from RCTs. A more complete evaluation of safety would require inclusion of data from observational studies. Publication bias may also be a concern because we only included studies published in English. Although we requested unpublished data from pharmaceutical companies, we did not receive any information.
Applicability
Our study findings are applicable to children and adolescents with allergic rhinoconjunctivitis or asthma. Our results are relevant to patients making decisions about therapy based on efficacy and safety of SIT, clinicians who provide care for children with asthma and allergic rhinitis, guideline developers making recommendations on SIT in children, and researchers evaluating SIT in children.
Conclusions
The evidence provides support for the efficacy of both SCIT and SLIT for treatment of allergic rhinitis and asthma in children. The evidence base is stronger for SLIT than for SCIT, which may reflect the fact that there are fewer studies evaluating SCIT exclusively in children and few head-to-head comparisons of SCIT and SLIT. SLIT has been thought to be a favorable alternative to SCIT, especially for children, based on convenience and ease of administration at home without multiple injections,55 whereas SCIT requires administration by an experienced provider. These benefits may influence the tolerability and adherence to treatment, especially in children, but this outcome remains to be seen. Additional studies directly comparing these 2 modes of delivery or combination regimens may strengthen this evidence.
Future pediatric studies should evaluate the real-world effectiveness of SCIT and SLIT, addressing issues of compliance, which are especially relevant in children. In addition, direct comparisons of SCIT versus SLIT should evaluate long-term outcomes such as prevention of asthma and potential for disease modification. Evaluating the differential effects of immunotherapy based on the developmental stage of children and adolescents can help to optimize treatment and identify the optimal dose, frequency, treatment duration, and age for initiating treatment in children.
Supplementary Material
Acknowledgments
Peter S. Creticos, MD, Franklin Adkinson, MD, Daniela Vollenweider, MD, and Darcy Ward, MS, assisted in the initial review of the abstracts and publications that were screened and subsequently selected for inclusion. Drs Creticos and Adkinson also helped develop the initial protocol for application of the EPC Methods guide on grading of the included papers, which constituted the work product for this evidence-based review. Dr Creticos was the initial principal investigator. Per the funder’s (AHRQ) request, to ensure compliance with established AHRQ conflict of interest policy including perceived conflicts of interest, Drs Creticos and Adkinson were recused from certain review activities (including data extraction, assessment of study quality, and report writing) due to their previous consulting arrangements.
Glossary
- AHRQ
Agency for Healthcare Research and Quality
- QoL
quality of life
- RCTs
randomized controlled trials
- SCIT
subcutaneous immunotherapy
- SIT
allergen-specific immunotherapy
- SLIT
sublingual immunotherapy
Footnotes
Dr Kim selected articles for inclusion, extracted data, graded the strength of the evidence, and drafted and revised the manuscript; Dr Lin selected articles for inclusion, extracted data, graded the strength of the evidence, and reviewed and revised the manuscript; Dr Suarez-Cuervo designed the data abstraction forms, coordinated data abstraction and data management, selected articles for inclusion, extracted data, and reviewed the manuscript; Drs Chelladurai and Ramananthan selected articles for inclusion, extracted data, graded the strength of the evidence, and reviewed the manuscript; Dr Segal supervised all steps of the systematic review process (including conceptualization and design), and critically reviewed and revised the manuscript; Dr Erekosima selected articles for inclusion, extracted data, graded the strength of the evidence, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Agency for Healthcare Research and Quality (AHRQ) under contract 290-2007-10061-1. The AHRQ program officers and technical experts participated in developing the research questions and reviewed draft manuscripts. The authors are solely responsible for the content and decision to submit for publication. Approval was required from AHRQ for copyright assertion before the manuscript could be submitted for publication. Dr Kim was supported by an AHRQ Comparative Effectiveness Development Training grant (T32HS019488).
References
- 1.National Asthma Education and Prevention Program . Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120suppl 5):S94–S138 [DOI] [PubMed] [Google Scholar]
- 2.Wallace DV, Dykewicz MS, Bernstein DI, et al. Joint Task Force on Practice. American Academy of Allergy Asthma & Immunology. American College of Allergy, Asthma and Immunology. Joint Council of Allergy, Asthma and Immunology . The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(suppl 2):S1–S84 [DOI] [PubMed] [Google Scholar]
- 3.Cox L, Jacobsen L. Comparison of allergen immunotherapy practice patients in the United States and Europe. Ann Allergy Asthma Immunol. 2009;103(6):451–459 [DOI] [PubMed] [Google Scholar]
- 4.Altman D, Antes G, Gøtzsche P, et al. Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. London, England: The Cochrane Collaboration; 2009 [Google Scholar]
- 5.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the effective health-care program. J Clin Epidemiol. 2010;63(5):513–523 [DOI] [PubMed] [Google Scholar]
- 6.Van Bever HP, Stevens WJ. Evolution of the late asthmatic reaction during immunotherapy and after stopping immunotherapy. J Allergy Clin Immunol. 1990;86(2):141–146 [DOI] [PubMed] [Google Scholar]
- 7.Schubert R, Eickmeier O, Garn H, et al. Safety and immunogenicity of a cluster specific immunotherapy in children with bronchial asthma and mite allergy. Int Arch Allergy Immunol. 2009;148(3):251–260 [DOI] [PubMed] [Google Scholar]
- 8.Akmanlar N, Altintaş DU, Güneşer KS, Yilmaz M, Bingöl G. Comparison of conventional and rush immunotherapy with der PI in childhood respiratory allergy. Allergol Immunopathol (Madr). 2000;28(4):213–218 [PubMed] [Google Scholar]
- 9.Hedlin G, Wille S, Browaldh L, et al. Immunotherapy in children with allergic asthma: effect on bronchial hyperreactivity and pharmacotherapy. J Allergy Clin Immunol. 1999;103(4):609–614 [DOI] [PubMed] [Google Scholar]
- 10.Pajno GB, Caminiti L, Crisafulli G, et al. Direct comparison between continuous and coseasonal regimen for sublingual immunotherapy in children with grass allergy: a randomized controlled study. Pediatr Allergy Immunol. 2011;22(8):803–807 [DOI] [PubMed] [Google Scholar]
- 11.Hill DJ, Hosking CS, Shelton MJ, Turner MW. Failure of hyposensitisation in treatment of children with grass-pollen asthma. Br Med J (Clin Res Ed). 1982;284(6312):306–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altintaş D, Akmanlar N, Güneşer S, et al. Comparison between the use of adsorbed and aqueous immunotherapy material in Dermatophagoides pteronyssinus sensitive asthmatic children. Allergol Immunopathol (Madr). 1999;27(6):309–317 [PubMed] [Google Scholar]
- 13.Pifferi M, Baldini G, Marrazzini G, et al. Benefits of immunotherapy with a standardized Dermatophagoides pteronyssinus extract in asthmatic children: a three-year prospective study. Allergy. 2002;57(9):785–790 [DOI] [PubMed] [Google Scholar]
- 14.Valovirta E, Viander M, Koivikko A, Vanto T, Ingeman L. Immunotherapy in allergy to dog. Immunologic and clinical findings of a double-blind study. Ann Allergy. 1986;57(3):173–179 [PubMed] [Google Scholar]
- 15.Adkinson NF, Jr, Eggleston PA, Eney D, et al. A controlled trial of immunotherapy for asthma in allergic children. N Engl J Med. 1997;336(5):324–331 [DOI] [PubMed] [Google Scholar]
- 16.Möller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J Allergy Clin Immunol. 2002;109(2):251–256 [DOI] [PubMed] [Google Scholar]
- 17.Cantani A, Arcese G, Lucenti P, Gagliesi D, Bartolucci M. A three-year prospective study of specific immunotherapy to inhalant allergens: evidence of safety and efficacy in 300 children with allergic asthma. J Investig Allergol Clin Immunol. 1997;7(2):90–97 [PubMed] [Google Scholar]
- 18.Dreborg S, Agrell B, Foucard T, Kjellman NI, Koivikko A, Nilsson S. A double-blind, multicenter immunotherapy trial in children, using a purified and standardized Cladosporium herbarum preparation. I. Clinical results. Allergy. 1986;41(2):131–140 [DOI] [PubMed] [Google Scholar]
- 19.Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol. 2011;127(2):502–508.e1–6 [DOI] [PubMed]
- 20.Niggemann B, Jacobsen L, Dreborg S, et al. PAT Investigator Group . Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. 2006;61(7):855–859 [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen L, Niggemann B, Dreborg S, et al. the PAT Investigator Group . Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62(8):943–948 [DOI] [PubMed] [Google Scholar]
- 22.Pajno GB, Morabito L, Barberio G, Parmiani S. Clinical and immunologic effects of long-term sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, placebo-controlled study. Allergy. 2000;55(9):842–849 [DOI] [PubMed] [Google Scholar]
- 23.Lue KH, Lin YH, Sun HL, Lu KH, Hsieh JC, Chou MC. Clinical and immunologic effects of sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, randomized, placebo-controlled study. Pediatr Allergy Immunol. 2006;17(6):408–415 [DOI] [PubMed] [Google Scholar]
- 24.Niu CK, Chen WY, Huang JL, Lue KH, Wang JY. Efficacy of sublingual immunotherapy with high-dose mite extracts in asthma: a multi-center, double-blind, randomized, and placebo-controlled study in Taiwan. Respir Med. 2006;100(8):1374–1383 [DOI] [PubMed] [Google Scholar]
- 25.de Bot CM, Moed H, Berger MY, et al. Sublingual immunotherapy not effective in house dust mite-allergic children in primary care. Pediatr Allergy Immunol. 2012;23(2):150–158 [DOI] [PubMed] [Google Scholar]
- 26.Tseng SH, Fu LS, Nong BR, Weng JD, Shyur SD. Changes in serum specific IgG4 and IgG4/IgE ratio in mite-sensitized Taiwanese children with allergic rhinitis receiving short-term sublingual-swallow immunotherapy: a multicenter, randomized, placebo-controlled trial. Asian Pac J Allergy Immunol. 2008;26(2–3):105–112 [PubMed] [Google Scholar]
- 27.La Rosa M, Ranno C, André C, Carat F, Tosca MA, Canonica GW. Double-blind placebo-controlled evaluation of sublingual-swallow immunotherapy with standardized Parietaria judaica extract in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 1999;104(2 pt 1):425–432 [DOI] [PubMed] [Google Scholar]
- 28.Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114(4):851–857 [DOI] [PubMed] [Google Scholar]
- 29.Röder E, Berger MY, Hop WC, Bernsen RM, de Groot H, Gerth van Wijk R. Sublingual immunotherapy with grass pollen is not effective in symptomatic youngsters in primary care. J Allergy Clin Immunol. 2007;119(4):892–898 [DOI] [PubMed] [Google Scholar]
- 30.Stelmach I, Kaluzińska-Parzyszek I, Jerzynska J, Stelmach P, Stelmach W, Majak P. Comparative effect of pre-coseasonal and continuous grass sublingual immunotherapy in children. Allergy. 2012;67(3):312–320 [DOI] [PubMed]
- 31.Marogna M, Tomassetti D, Bernasconi A, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008;101(2):206–211 [DOI] [PubMed] [Google Scholar]
- 32.Hirsch T, Sähn M, Leupold W. Double-blind placebo-controlled study of sublingual immunotherapy with house dust mite extract (D.pt.) in children. Pediatr Allergy Immunol. 1997;8(1):21–27 [DOI] [PubMed] [Google Scholar]
- 33.Bahçeciler NN, Işik U, Barlan IB, Başaran MM. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double-blind, placebo-controlled study. Pediatr Pulmonol. 2001;32(1):49–55 [DOI] [PubMed] [Google Scholar]
- 34.Tari MG, Mancino M, Monti G. Efficacy of sublingual immunotherapy in patients with rhinitis and asthma due to house dust mite. A double-blind study. Allergol Immunopathol (Madr). 1990;18(5):277–284 [PubMed] [Google Scholar]
- 35.Pajno GB, Vita D, Parmiani S, Caminiti L, La Grutta S, Barberio G. Impact of sublingual immunotherapy on seasonal asthma and skin reactivity in children allergic to Parietaria pollen treated with inhaled fluticasone propionate. Clin Exp Allergy. 2003;33(12):1641–1647 [DOI] [PubMed] [Google Scholar]
- 36.Vourdas D, Syrigou E, Potamianou P, et al. Double-blind, placebo-controlled evaluation of sublingual immunotherapy with standardized olive pollen extract in pediatric patients with allergic rhinoconjunctivitis and mild asthma due to olive pollen sensitization. Allergy. 1998;53(7):662–672 [DOI] [PubMed] [Google Scholar]
- 37.Valovirta E, Jacobsen L, Ljørring C, Koivikko A, Savolainen J. Clinical efficacy and safety of sublingual immunotherapy with tree pollen extract in children. Allergy. 2006;61(10):1177–1183 [DOI] [PubMed] [Google Scholar]
- 38.Ippoliti F, De Santis W, Volterrani A, et al. Immunomodulation during sublingual therapy in allergic children. Pediatr Allergy Immunol. 2003;14(3):216–221 [DOI] [PubMed] [Google Scholar]
- 39.Yukselen A, Kendirli SG, Yilmaz M, Altintas DU, Karakoc GB. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: a randomized, placebo-controlled, double-blind, double-dummy study. Int Arch Allergy Immunol. 2012;157(3):288–298 [DOI] [PubMed] [Google Scholar]
- 40.Eifan AO, Akkoc T, Yildiz A, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled study. Clin Exp Allergy. 2010;40(6):922–932. [DOI] [PubMed] [Google Scholar]
- 41.Keles S, Karakoc-Aydiner E, Ozen A, et al. A novel approach in allergen-specific immunotherapy: combination of sublingual and subcutaneous routes. J Allergy Clin Immunol. 2011;128(4):808–815.e7 [DOI] [PubMed]
- 42.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186. [DOI] [PubMed] [Google Scholar]
- 44.Röder E, Berger MY, de Groot H, van Wijk RG. Immunotherapy in children and adolescents with allergic rhinoconjunctivitis: a systematic review. Pediatr Allergy Immunol. 2008;19(3):197–207 [DOI] [PubMed] [Google Scholar]
- 45.Penagos M, Passalacqua G, Compalati E, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest. 2008;133(3):599–609 [DOI] [PubMed] [Google Scholar]
- 46.Olaguíbel JM, Alvarez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol. 2005;15(1):9–16 [PubMed] [Google Scholar]
- 47.Calderon MA, Penagos M, Sheikh A, Canonica GW, Durham SR. Sublingual immunotherapy for allergic conjunctivitis: Cochrane systematic review and meta-analysis. Clin Exp Allergy. 2011;41(9):1263–1272 [DOI] [PubMed] [Google Scholar]
- 48.Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893. [DOI] [PubMed] [Google Scholar]
- 49.Sopo SM, Macchiaiolo M, Zorzi G, Tripodi S. Sublingual immunotherapy in asthma and rhinoconjunctivitis; systematic review of paediatric literature. Arch Dis Child. 2004;89(7):620–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117(1):169–175 [DOI] [PubMed] [Google Scholar]
- 51.Bernstein DI, Wanner M, Borish L, Liss GM, Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology . Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004;113(6):1129–1136 [DOI] [PubMed] [Google Scholar]
- 52.Calderón MA, Larenas D, Kleine-Tebbe J, et al. European Academy of Allergy and Clinical Immunology task force report on ‘dose-response relationship in allergen-specific immunotherapy.’ Allergy. 2011;66(10):1345–1359 [DOI] [PubMed] [Google Scholar]
- 53.La Rosa M, Lionetti E, Leonardi S, et al. Specific immunotherapy in children: the evidence. Int J Immunopathol Pharmacol. 2011;24(suppl 4):69–78 [DOI] [PubMed] [Google Scholar]
- 54.Calderón MA, Simons FE, Malling HJ, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67(3):302–311 [DOI] [PubMed] [Google Scholar]
- 55.Cox L, Wallace D. Specific allergy immunotherapy for allergic rhinitis: subcutaneous and sublingual. Immunol Allergy Clin North Am. 2011;31(3):561–599 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.