Abstract
Young children who experience toxic stress are at high risk for a number of health outcomes in adulthood, including cardiovascular disease, cancers, asthma, and depression. The American Academy of Pediatrics has recently called on pediatricians, informed by research from molecular biology, genomics, immunology, and neuroscience, to become leaders in science-based strategies to build strong foundations for children’s life-long health. In this report, we provide an overview of the science of toxic stress. We summarize the development of the neuroendocrine-immune network, how its function is altered by early life adversity, and how these alterations then increase vulnerability to disease. The fact that early environments shape and calibrate the functioning of biological systems very early in life is both a cautionary tale about overlooking critical periods in development and reason for optimism about the promise of intervention. Even in the most extreme cases of adversity, well-timed changes to children’s environments can improve outcomes. Pediatricians are in a unique position to contribute to the public discourse on health and social welfare by explaining how factors that seem distal to child health may be the key to some of the most intractable public health problems of our generation. We consider the challenges and opportunities for preventing toxic stress in the context of contemporary pediatric practice.
KEY WORDS: toxic stress, health disparities, social determinants of health
In January 2012, the American Academy of Pediatrics (AAP) released a policy statement and accompanying technical report that detailed the role of early life “toxic stress” in shaping health across the life course.1 Toxic stress is the extreme, frequent, or extended activation of the stress response, without the buffering presence of a supportive adult.1,2 Risk factors for toxic stress in childhood include neglect and abuse, extreme poverty, family violence, substance abuse, and parental mental health problems.1,2 Young children who experience toxic stress are at high risk for a multitude of health outcomes in adulthood ranging from cardiovascular and obstructive pulmonary disease to cancers, asthma, autoimmune disease, and depression.3–8 Identifying the pathways by which early adverse experiences set in motion trajectories toward poor adult health is an area of intense scientific interest. To date, the evidence suggests that early adversity catalyzes a series of biological adaptations that change the way the brain, neuroendocrine stress response, and immune system function, both individually and cooperatively. Preventing toxic stress, however, entails an entirely different paradigm, with a focus not at the molecular level but at the level of family, society, and policy. The success of these prevention efforts depends, in part, on health professionals’ ability to successfully make the case in the popular discourse that improving child health requires interventions that seem quite distal to health.
With this in mind, the AAP has called on pediatricians to become leaders in new science-based strategies designed to build strong foundations for life-long health.9 To do this effectively, physicians must be familiar with a diverse body of evidence that draws on research from molecular and developmental biology, genomics, immunology, and neuroscience. In service of this goal, this report provides an overview of the biological pathways by which early life toxic stress shapes health. While many discussions of early adversity and health focus specifically on the impact on the developing brain, we take a broader view to consider how toxic stress shapes the development and calibration of the neuroendocrine-immune (NEI) network in the prenatal and early childhood periods. NEI functioning is at the heart of multiple goals of pediatric practice: addressing children’s acute medical needs, preventing communicable diseases, and, increasingly, identifying and intervening at the family and population level to limit the effects of social determinants of adverse health. In this report, we begin by outlining the core concepts of biological adaptation to stressful circumstances, including plasticity and critical and sensitive periods. We then provide an overview of the development and functioning of the NEI network, and how toxic stress during the prenatal and early childhood periods disrupts these processes. Given that toxic stress is defined by the absence of supportive caregiving, we pay particular attention to the role of caregiving in building a healthy, well-modulated NEI network. Finally, we consider the challenges and opportunities for preventing toxic stress in the context of pediatric practice.
Biological Embedding, Plasticity, and Critical and Sensitive Periods
More than 2 centuries ago, poet William Wordsworth observed that “the child is father of the man.”10 Not until recently, however, has the scientific evidence accumulated to identify the mechanisms of this “biological embedding.” Biological embedding is the process by which individuals’ previous experiences and environments systematically alter their health and functioning across the life span.11
One of the foundations of individuals’ ability to adapt to their environment is neural plasticity. Plasticity is the iterative process by which experience shapes the brain, allowing it to be exposed to new experiences, which, in turn, shape brain structure and function.12 While the brain is plastic across the life span, critical and sensitive periods are “windows of opportunity” during which experiences and environments have a disproportionately large impact on development.13 (The development of binocular vision in infancy is a common example of a critical period–dependent developmental process.12) Plasticity has been referred to as a “double-edged sword,” because the brain can adapt to either positive or negative environmental stimuli.12
The NEI Network
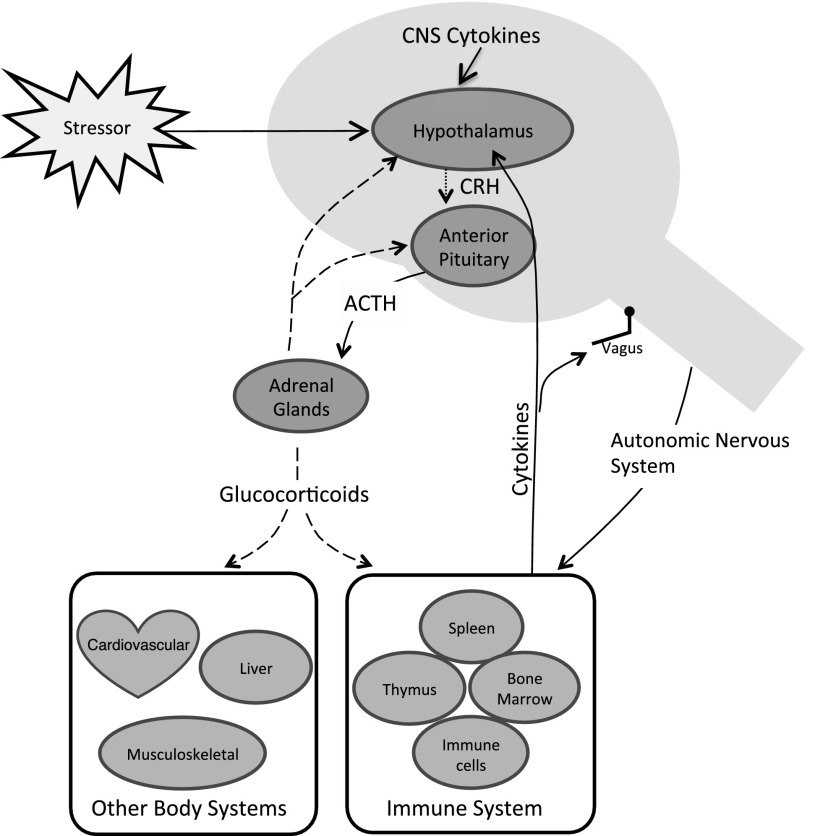
It is virtually impossible to parse the impact of experience on the developing brain from its simultaneous impact on the stress response and immune systems. Also calibrated by early experience, this NEI network plays a critical part in physical, cognitive, and socioemotional development by sensing, interpreting and orchestrating the body’s response to stress in the environment. The brain, endocrine, and immune systems share a common language of hormones, signaling molecules, receptors, and neurotransmitters, which facilitates communication across the network to maintain homeostatic balance14,15 (Fig 1). In addition, through interactions with the brain and neuroendocrine system, immune insults affect not only immune competence but also the building blocks of brain development, including neurogenesis and neural signaling.16
FIGURE 1.
Relationship between the HPA axis, immune systems, and other body systems. Glucocorticoids are indicated by dashed lines, corticotropin-releasing hormone (CRH) by dotted lines, and cytokines by solid lines. ACTH, adrenocorticotropic hormone (ie, corticotropin); CNS, central nervous system.
Toxic Stress and NEI Development
Figure 2 summarizes how early life adversities, including lack of nurturance and social support, poverty, and trauma, are translated into health and developmental outcomes via the NEI network. Importantly, outcomes vary considerably among children exposed to similar environments, underscoring the role of resilience factors. In Fig 2, this variability is captured by individual moderators, including variability in genetic endowment, coping skills, and stage of development.
FIGURE 2.
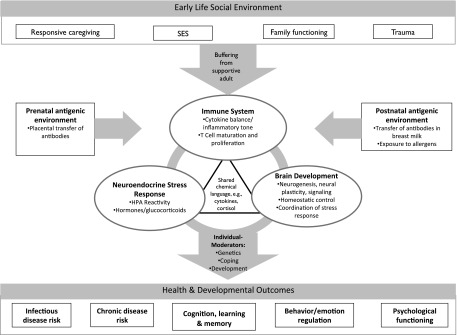
Mediating role of the NEI network in linking early life experiences to individual differences in health and functioning. SES, socioeconomic status.
Before reviewing the science illustrated in Fig 2, by way of background, we provide a very brief overview of human immune system development in early life. (We refer the reader to Janeway17 and Vedhara and Irwin18 for additional reading.)
Immune System Overview
The immune system is sometimes called the “sixth sense” because of its ability to perceive and respond to the environment.14 Consequently, the immune system demonstrates its own sort of plasticity in response to environmental stimuli. The immune system is designed to be deployed in stages. The body’s first line of defense against disease, the innate immune response, it is activated very quickly, often within minutes. It relies on physical barriers, such as the skin, as well as on phagocytic cells and enzymes.18 A major component of innate immunity is the inflammatory response. After an immune threat has been eliminated, the immune system stops producing proinflammatory substances and inflammation subsides, protecting healthy cells and tissues. The innate immune response slows the progression of the immune insult until the second phase of the immune response, the acquired response, is deployed, if necessary.
Acquired immunity involves the activation of immune cells (ie, T and B lymphocytes) specific to the infecting agent; together, they result in the production of antibodies that bind to and neutralize or kill the antigen. Antigen-specific antibodies circulate in the bloodstream, making the immune response swift and efficient if the same antigen reinfects the body. To prevent the body from attacking itself, the healthy immune system can differentiate “self” from “nonself” antigens; only nonself antigens activate the immune response.
Linking the Brain and Immune System
Cytokines
Although the brain and immune system are physically segregated, cytokines are the chemical messengers that link them and they play a key role in regulating both innate and acquired immunity.19 As such, they are essential to development, growth, and maintenance of most body tissues and organ systems.20 In the face of an immune threat, the immune system produces proinflammatory cytokines to destroy it. Proinflammatory cytokines also act directly on the brain, leading to “sickness behavior” characterized by loss of appetite, fatigue, social withdrawal, depressed mood, irritability, and poor cognitive functioning.21 Accumulating evidence suggests that cytokines also play a role in the pathophysiology of depressive disorders, behavioral dysregulation, and posttraumatic stress symptoms in adults and children.22–27
Hypothalamic-Pituitary-Adrenal Axis
Central to the mammalian response to threats in the environment is the hypothalamic-pituitary adrenal (HPA) axis. The HPA axis is responsible for managing metabolic and cardiovascular responses to acute and chronic stress, among other functions.28 The HPA also plays an important role in the immune response.29 Specifically, proinflammatory cytokines activate the HPA axis; in turn, cortisol from the HPA creates a negative feedback loop and extinguishes the HPA and the inflammatory response.29,30 One of the primary consequences of early life toxic stress is HPA dysregulation, as the developing neuroendocrine system is chronically pressed into action.31,32 Because of the close links between the 2 systems, HPA dysregulation has broad effects on immune and inflammatory processes.29,30,33 Too much cortisol suppresses immunity and increases the chance of infection; too little cortisol and the inflammatory response persists after it is no longer needed.30
Prenatal/Perinatal Influences on NEI Network Development
The majority of immune system development occurs before birth and in the first year of life, and environmental input during this period refines the immune response and calibrates its life-long functioning.34 Coe and Lubach35 argue that maturational processes amplify the impact of early disruptions to immune development, akin to “changing the course of a rocket at the moment of take-off.”36
Early Environments and Immune Development
Maternal mental health and psychosocial factors are important for the development of the child’s immune system both before and after birth. Considerable evidence from animal models demonstrates that prenatal maternal distress undermines fetal immune development.34,35 Chronic maternal prenatal stress and anxiety have been linked in both humans and animals to an altered cellular immune response at birth37 and more illnesses and health complaints in newborns.38
Before birth, maternal, placental, and fetal cytokines interact to prevent rejection of the fetus by the mother’s immune system. After birth, however, a series of changes must occur to allow for the healthy development of the infant’s immune system. One of these essential changes is the polarization of the immune response to up-regulate T-helper 1 cellular immunity and down-regulate T-helper 2 cellular immunity.39 A dominant T-helper 2 cell response early in life creates life-long immune hyperreactivity, including allergies and asthma.39,40 Although animal models are abundant, studies of prenatal stress on cellular immune response in humans remain sparse. One study found that maternal prenatal poverty, life stress, and community violence were associated with alterations in their infants’ innate and adaptive immunity, as measured in cord blood.41 Other studies have linked cumulative trauma in the mother’s lifetime, even predating the pregnancy, to immunoglobulin E antibody levels in the neonatal period.42
After birth, maternal functioning continues to be a key risk factor for childhood toxic stress. In addition to adverse psychosocial environments, infants at risk for toxic stress are also more likely to encounter physical environments that increase the chance of immune hyperreactivity. For example, poor children are more likely to be exposed to secondhand smoke, mold, rodents, cockroaches, and dust mites.43,44 Sensitization to these allergens is highly correlated with the development of allergic and atopic disease.44 While some studies have suggested that sensitization to these allergens begins in utero,45 most conclude that the critical period for allergic sensitization is between birth and age 8.44,46,47
Early Caregiving and the Development of the NEI Network
Humans are distinguished by their altriciality; that is, they need a caregiver in early life to survive. During the fetal period, humans grow rapidly; gestation is shortened to allow the head to be delivered through the birth canal. We emerge from the womb more immature than virtually any other similar-sized species.48,49 The parent–child relationship is therefore “evolutionarily expected” as the context for a major part of postnatal maturation.36 In the absence of this expected parent–child bond, children must make adaptations that allow them to survive. These adaptations are essential in the short term, but they carry long-term costs by limiting an individual’s ability to cope with new demands are they mature.50,51
Evidence of the ways in which humans adapt to their caregiving environments is evident in virtually every biological process, including at the level of gene expression51 Research in the field of epigenetics has demonstrated that genes work together reciprocally, over time and across development, with cellular, familial, and even sociopolitical environments. Epigenetic changes control how genes are turned on or off and how proteins are transcribed, without altering the underlying DNA sequence. The genetic code can be thought of as the hardware of a computer and the epigenetic code as the software.52,53 The software, which can constantly be rewritten, determines how the computer works.52 The family environment, particularly early caregiving, has emerged as a particularly critical context for epigenetic regulation of the human stress response.
Epigenetic Regulation by Caregiving
Animal models demonstrate that interactions with parents early in life program enduring aspects of HPA functioning. In rodents, naturally occurring variations in maternal care (demonstrated by levels of licking and grooming) are related to individual differences in HPA reactivity of their offspring. As adults, offspring born to high nurturing mothers demonstrate a well-regulated, modest HPA response, whereas those born to low nurturing mothers exhibit exaggerated HPA responses to stress.53–56 These group differences are due to differential expression of the glucocorticoid receptor in the brain, which is regulated epigenetically by caregiving.56,57 Encouragingly, however, when offspring born to low nurturing mothers are raised by high nurturing mothers, these animals develop the same well-regulated HPA response as the genetic offspring of high nurturing mothers54,58 In humans, several studies have documented similar HPA programming effects due to lack of caregiving among children raised in institutional care.32,59,60
Caregiving and Immune Function
Early caregiving also plays a pivotal role in the maturation of immunity.36,61 Reflecting the broader theme of altriciality discussed earlier, human immune development is premised on the expectation of consistent and positive mother–child interaction in the first weeks and months of life.35 For example, while some components of immunity (eg, immunoglobulin G antibodies) are transferred across the placenta, the organism expects some aspects of immunity to be provided by the mother after birth in breast milk (eg, secretory immunoglobulin A antibodies, lactoferrin).35,62 Young children cared for by individuals who are available and responsive to their emotional and material needs develop immune systems that are better equipped to deal with initial exposures to infections and to keep dormant infections in check over time.63,64
Animal Models
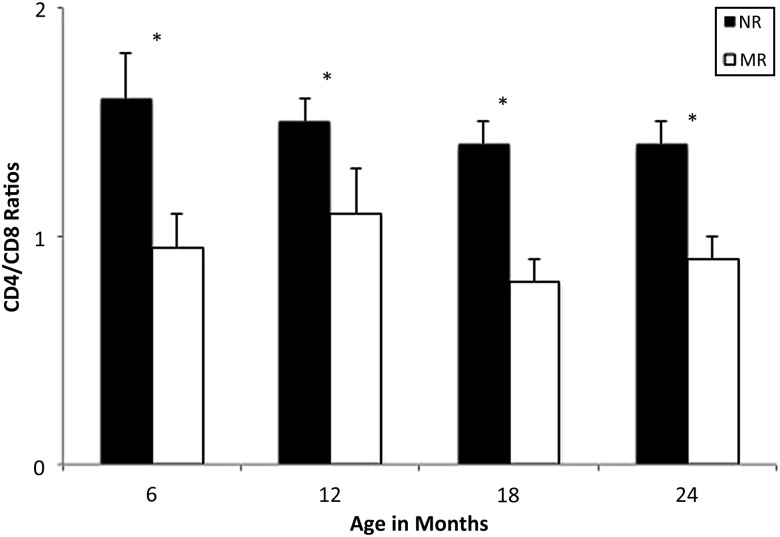
In primate models, infants who experience disruptions in caregiving show poorer immunity and resistance to disease over the long term.61,65 For example, primates raised by humans in nurseries rather than by their mothers have impaired thymic development, which negatively affects immune function.66 In seminal studies, Coe and colleague64 investigated why nursery-raised monkeys showed higher levels of blood lymphocytes than those raised by their mothers; they had expected that the stress of inadequate caregiving would dampen the monkeys’ immune response. They assessed the monkeys every 6 months from birth to age 2.67,68 Not only did lymphocytes proliferate in nursery-reared monkeys, but these monkeys also had different lymphocyte profiles. Mother-reared monkeys showed similar levels of CD4+ “helper” T cells, which facilitate immune reactions, and CD8+ “killer” T cells that destroy infected cells. In contrast, among nursery-reared monkeys, there were proportionately fewer CD8+ killer T cells, resulting in poorer immune competence (Fig 3). At 1 year of age, all of the monkeys were rehoused to identical living conditions consisting of small groups along with a supportive adult. Nonetheless, the immune differences between the nursery- and mother-reared groups persisted, highlighting the formative role of early caregiving in immune competence.67
FIGURE 3.
Ratios of CD4 and CD8 cells for mother-raised (MR) and nursery-raised (NR) monkeys over 2 years. Mean (+SE) of 2 or 3 samples per subject portrayed at 6-month intervals. NR monkeys had significantly higher ratios than MR monkeys at all ages (*P < .05). Reprinted from Lubach et al67 with permission from Elsevier.
Human Studies
In humans, there is similar empirical evidence that inadequate caregiving and nurturance very early in life have long-term and even permanent effects on immune and inflammatory responses.63,68–71 For example, 56% of children raised in Romanian orphanages were found to have antibody to herpes simplex virus and a bacterium associated with meningitis (Haemophilus influenzae), compared with only 5% of same-age noninstitutionalized children, which suggests that institutionalized children’s immune systems were less competent at keeping the illnesses dormant.36 Another study compared adolescents raised in orphanages as young children but subsequently adopted into stable homes with adolescents with recent histories of maltreatment and family disruption63 The 2 groups showed similar inability to keep the herpes simplex virus dormant, despite the fact that the adopted children had experienced significant periods of protective family environments.
Human studies also illustrate that disruptions to caregiver attachment early in life alter neuroimmune processes by sensitizing proinflammatory pathways.72,73 Children exposed to risk factors for toxic stress, including poverty, intimate partner violence, and community violence, are more likely to develop or report asthma; asthma has a known inflammatory/stress component.74–78 Similarly, HPA/immune links are increasingly implicated in metabolic syndrome.4 Chronic elevations in cortisol are linked to hypertension, insulin resistance, obesity, type 2 diabetes, and cardiovascular disease.4,15 As adults, children maltreated during childhood are more likely to have elevated inflammatory markers (eg, C-reactive protein) and greater inflammatory response to stress.70,79 Encouragingly, however, there is evidence that early maternal nurturance is sufficient to buffer children raised in poverty against the risk of metabolic syndrome in midlife.73 This suggests that ensuring that every child has a stable source of adult nurturance can foster resilience to a number of common disease outcomes in adulthood by transforming toxic stress into “tolerable” stress.
Conclusions
In this report, we have provided an overview of the development of the NEI network, how its function is altered by early life stress, and how these adaptations then increase vulnerability to a large number of immune and endocrine system–related mental and physical health conditions. The AAP statement “Early Childhood Adversity, Toxic Stress, and the Role of the Pediatrician: Translating Developmental Science Into Lifelong Health” calls for pediatricians to lead an “invigorated, science-based effort at transforming the way our society invests in the development of all children, particularly those who face significant adversity.”1 Becoming conversant in the science of toxic stress is the first step toward pediatricians assuming the mantle of scientist-advocate. However, the science of toxic stress draws heavily on research from genomics, neuroscience, molecular biology, and the basic health sciences, literature that falls outside the purview of the practicing physician. We suggest that just as there have been calls to translate basic research “from bench to bedside” in other domains of practice, research scientists outside of clinical practice should be mindful that the science of toxic stress must also be accessible to those who are providing care to vulnerable children and families. Pediatricians, in turn, can leverage their position of authority to educate the diverse community of stakeholders in child health (from families, to educators, policy makers, and insurers).
In one way, the science of toxic stress and the NEI network is a cautionary tale about the perils of failing to recognize critical periods in health risk. Perhaps more important, however, the science of toxic stress highlights extraordinary opportunities for improving lifelong health at the population level. In contrast to efforts such as immunization programs, which approach prevention one disease at a time, reducing toxic stress can target the common physiologic pathway implicated in an enormous array of health outcomes from asthma to cardiovascular disease. Within existing family-centered care models, pediatric providers are well positioned to identify distressed caregivers, to intervene on behalf of children without a source of stable responsive caregiving, and to advocate on behalf of systems, structures, and policies that support caregiving in young families. The AAP policy statement outlines a wide variety of specific steps that would help facilitate adopting the prevention of toxic stress as a core mission of pediatric practice.1 These include efforts to change reimbursement strategies to incentivize activities, including screening for risk factors for toxic stress; linking families with the clinical, community, and social resources they need; and working collaboratively with these stakeholders to ensure the best outcomes for vulnerable children.1
The concept of plasticity, whereby environments shape developmental biology, and the resulting biological adaptations shape subsequent experiences, is the scientific basis for renewed optimism about the promise of intervention. Even in the most extreme cases of adversity, improving the quality of children’s environments can change many, if not most, outcomes if carried out during critical and sensitive periods.50,80–82 Pediatricians are in a position of authority to explore and explain how distal factors, such as neighborhood violence, housing and zoning policies, the availability and affordability of quality childcare, and funding for mental health services for parents of young children, may be the key to some of the most intractable public health problems of our generation. In making the case for preventing risk factors for toxic stress, pediatricians can help build bridges in the public discourse between childhood experiences and lifelong health.
Acknowledgments
The authors thank the members of the Johns Hopkins Women’s and Children’s Health Policy Center for insightful comments on earlier drafts of this manuscript.
Glossary
- AAP
American Academy of Pediatrics
- HPA
hypothalamic-pituitary-adrenal
- NEI
neuroendocrine-immune
Footnotes
Dr Johnson conducted the literature review and wrote the manuscript. Dr Riley assisted with manuscript writing and literature review. Dr Granger assisted with conceptualizing and revising the manuscript. Ms Riis created Figure 2 and assisted with literature search as well as revising and editing of the manuscript.
FINANCIAL DISCLOSURE: Dr Granger is founder and chief strategy and scientific advisor at Salimetrics LLC (State College, PA). Dr Granger’s relationship with Salimetrics LLC is managed by the policies of the Conflict of Interest Committee at the Johns Hopkins University School of Medicine. Dr Riley worked through a contract with Pfizer Nutrition on the development and publication of an assessment of infant gastrointestinal distress. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Johnson is supported by a Career Development Award sponsored by the National Institute on Drug Abuse (K01DA027229).
References
- 1.Garner AS, Shonkoff JP, Committee on Psychosocial Aspects of Child and Family Health. Committee on Early Childhood, Adoption, and Dependent Care. Section on Developmental and Behavioral Pediatrics . Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/129/1/e224 [DOI] [PubMed] [Google Scholar]
- 2.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259 [DOI] [PubMed] [Google Scholar]
- 3.Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34(5):396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björntorp P, Rosmond R. The metabolic syndrome—a neuroendocrine disorder? Br J Nutr. 2000;83(suppl 1):S49–S57 [DOI] [PubMed] [Google Scholar]
- 5.Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun. 2011;25(7):1468–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55 [DOI] [PubMed] [Google Scholar]
- 7.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258 [DOI] [PubMed] [Google Scholar]
- 8.Maughan A, Cicchetti D, Toth SL, Rogosch FA. Early-occurring maternal depression and maternal negativity in predicting young children’s emotion regulation and socioemotional difficulties. J Abnorm Child Psychol. 2007;35(5):685–703 [DOI] [PubMed] [Google Scholar]
- 9.Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of Child and Family Health. Committee on Early Childhood, Adoption, and Dependent Care. Section on Developmental and Behavioral Pediatrics . The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/:e232 [DOI] [PubMed] [Google Scholar]
- 10.Wordsworth W. My Heart Leaps Up When I Behold. The Complete Poetical Works by William Wordsworth. London, UK: Macmillan and Co; 1888 [Google Scholar]
- 11.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. In: Adler NE, ed. Socioeconomic Status and Health in Industrialized Nations. New York, NY: New York Academy of Sciences; 1999:85–95 [DOI] [PubMed] [Google Scholar]
- 12.Nelson CA. Neural plasticity and human development. Curr Dir Psychol Sci. 1999;8(2):42–45 [Google Scholar]
- 13.Fox NA, Rutter M. Introduction to the special section on the effects of early experience on development. Child Dev. 2010;81(1):23–27 [DOI] [PubMed] [Google Scholar]
- 14.Blalock JE. The immune system as the sixth sense. J Intern Med. 2005;257(2):126–138 [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867 [DOI] [PubMed] [Google Scholar]
- 16.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–366 [DOI] [PubMed] [Google Scholar]
- 17.Janeway C. Immunobiology: The Immune System in Health and Disease. 6th ed. New York, NY: Garland Science; 2005 [Google Scholar]
- 18.Vedhara K, Irwin M. Human Psychoneuroimmunology. New York, NY: Oxford University Press; 2005 [Google Scholar]
- 19.Blalock JE, Smith EM. Conceptual development of the immune system as a sixth sense. Brain Behav Immun. 2007;21(1):23–33 [DOI] [PubMed] [Google Scholar]
- 20.Granger DA, Granger GA, Granger SW. Immunology and developmental psychopathology. In: Cicchetti D, Cohen DJ, eds. Developmental Psychopathology, Vol 2 Developmental Neuroscience. New York, NY: John Wiley and Sons; 2006:677–770 [Google Scholar]
- 21.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misener VL, Gomez L, Wigg KG, et al. International Consortium for Childhood-Onset Mood Disorders . Cytokine genes TNF, IL1A, IL1B, IL6, IL1RN and IL10, and childhood-onset mood disorders. Neuropsychobiology. 2008;58(2):71–80 [DOI] [PubMed] [Google Scholar]
- 23.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabbay V, Klein RG, Alonso CM, et al. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord. 2009;115(1–2):177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun. 2008;22(5):753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keller PS, El-Sheikh M, Vaughn B, Granger DA. Relations between mucosal immunity and children’s mental health: the role of child sex. Physiol Behav. 2010;101(5):705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pervanidou P, Kolaitis G, Charitaki S, et al. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32(8–10):991–999 [DOI] [PubMed] [Google Scholar]
- 28.Francis DD. Conceptualizing child health disparities: a role for developmental neurogenomics. Pediatrics. 2009;124(suppl 3):S196–S202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723 [DOI] [PubMed] [Google Scholar]
- 30.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163 [DOI] [PubMed] [Google Scholar]
- 31.Gunnar MR, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173 [DOI] [PubMed] [Google Scholar]
- 32.Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: a study of internationally adopted children. Dev Psychopathol. 2008;20(2):473–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonelli L, Webster JI, Rapp KL, Sternberg E. Neuroendocrine responses regulating susceptibility and resistance to autoimmune/inflammatory disease in inbred rat strains. Immunol Rev. 2001;184:203–211 [DOI] [PubMed] [Google Scholar]
- 34.Coe CL, Lubach GR. Prenatal origins of individual variation in behavior and immunity. Neurosci Biobehav Rev. 2005;29(1):39–49 [DOI] [PubMed] [Google Scholar]
- 35.Coe CL, Lubach GR. Mother-infant interactions and the devleopment of immunity from conception through weaning. In: Ader R, ed. Psychoneuroimmunology. Burlington, MA: Elsevier Academic Press; 2007 [Google Scholar]
- 36.Coe CL, Lubach GR. Critical periods of special health relevance for psychoneuroimmunology. Brain Behav Immun. 2003;17(1):3–12 [DOI] [PubMed] [Google Scholar]
- 37.Coe CL, Crispen HR. Social stress in pregnant squirrel monkeys (Saimiri boliviensis peruviensis) differentially affects placental transfer of maternal antibody to male and female infants. Health Psychol. 2000;19(6):554–559 [PubMed] [Google Scholar]
- 38.Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2). Available at: www.pediatrics.org/cgi/content/full/126/2/e401 [DOI] [PubMed] [Google Scholar]
- 39.Hertz-Picciotto I, Park H-Y, Dostal M, Kocan A, Trnovec T, Sram R. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin Pharmacol Toxicol. 2008;102(2):146–154 [DOI] [PubMed] [Google Scholar]
- 40.Hurtado A, Johnson RJ. Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int. 2005;68 (suppl 97):S62–S67 [DOI] [PubMed] [Google Scholar]
- 41.Wright RJ, Visness CM, Calatroni A, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sternthal MJ, Enlow MB, Cohen S, et al. Maternal interpersonal trauma and cord blood IgE levels in an inner-city cohort: a life-course perspective. J Allergy Clin Immunol. 2009;124(5):954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger JK, Takaro TK, Allen C, et al. The Seattle-King County Healthy Homes Project: implementation of a comprehensive approach to improving indoor environmental quality for low-income children with asthma. Environ Health Perspect. 2002;110(suppl 2):311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9(2):128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med. 2001;164(6):995–1001 [DOI] [PubMed] [Google Scholar]
- 46.Rowe J, Kusel M, Holt BJ, et al. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J Allergy Clin Immunol. 2007;119(5):1164–1173 [DOI] [PubMed] [Google Scholar]
- 47.Lendor C, Johnson A, Perzanowski M, et al. Effects of winter birth season and prenatal cockroach and mouse allergen exposure on indoor allergen-specific cord blood mononuclear cell proliferation and cytokine production. Ann Allergy Immunol. 2008;101:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeveloff SI, Boyce MS. Why human neonates are so altricial. Am Nat. 1982;120:537–542 [Google Scholar]
- 49.Gould S. Ontogeny and Phylogeny. Cambridge, MA: Belknap; 1977 [Google Scholar]
- 50.Tottenham N. Risk and developmental heterogeneity in previously institutionalized children. J Adolescent Health. 2012;51(2 Suppl):S29-S33 [DOI] [PMC free article] [PubMed]
- 51.Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81(1):41–79 [DOI] [PubMed] [Google Scholar]
- 52.Brower V. Epigenetics: unravelling the cancer code. Nature. 2011;471(7339):S12–S13 [DOI] [PubMed] [Google Scholar]
- 53.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79(3):359–371 [DOI] [PubMed] [Google Scholar]
- 54.Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158 [DOI] [PubMed] [Google Scholar]
- 55.Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662 [DOI] [PubMed] [Google Scholar]
- 56.Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26(3–4):139–162 [DOI] [PubMed] [Google Scholar]
- 57.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103(9):3480–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84 [DOI] [PubMed] [Google Scholar]
- 59.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol. 2001;13(3):611–628 [DOI] [PubMed] [Google Scholar]
- 60.Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Dev Psychobiol. 2008;50(6):588–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solomon GF, Levine S, Kraft JK. Early experience and immunity. Nature. 1968;220(5169):821–822 [DOI] [PubMed] [Google Scholar]
- 62.Hanson L, Silfverdal SA, Strömbäck L, et al. The immunological role of breast feeding. Pediatr Allergy Immunol. 2001;12(Suppl 14):15–19 [DOI] [PubMed] [Google Scholar]
- 63.Shirtcliff E, Coe CL, Pollak S. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc Natl Acad Sci U S A. 2009;106(8):2863–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coe CL, Lubach GR, Schneider ML, Dierschke DJ, Ershler WB. Early rearing conditions alter immune responses in the developing infant primate. Pediatrics. 1992;90(3 pt 2):505–509 [PubMed] [Google Scholar]
- 65.Ader R. Developmental psychoneuroimmunology. Dev Psychobiol. 1983;16(4):251–267 [DOI] [PubMed] [Google Scholar]
- 66.Ruiz RJ, Avant KC. Effects of maternal prenatal stress on infant outcomes: a synthesis of the literature. ANS Adv Nurs Sci. 2005;28(4):345–355 [DOI] [PubMed] [Google Scholar]
- 67.Lubach GR, Coe CL, Ershler WB. Effects of early rearing environment on immune responses of infant rhesus monkeys. Brain Behav Immun. 1995;9(1):31–46 [DOI] [PubMed] [Google Scholar]
- 68.Boyce WT, Chesney M, Alkon A, et al. Psychobiologic reactivity to stress and childhood respiratory illnesses: results of two prospective studies. Psychosom Med. 1995;57(5):411–422 [DOI] [PubMed] [Google Scholar]
- 69.Chen E, Martin AD, Matthews KA. Socioeconomic status and health: do gradients differ within childhood and adolescence? Soc Sci Med. 2006;62(9):2161–2170 [DOI] [PubMed] [Google Scholar]
- 70.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104(4):1319–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179 [DOI] [PubMed] [Google Scholar]
- 72.Hennessy MB, Deak T, Schiml-Webb PA. Early attachment-figure separation and increased risk for later depression: potential mediation by proinflammatory processes. Neurosci Biobehav Rev. 2010;34(6):782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22(12):1591–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma. Am J Respir Crit Care Med. 2007;176(7):644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom Med. 2003;65(6):984–992 [DOI] [PubMed] [Google Scholar]
- 76.Chen E, Schreier HM. Does the social environment contribute to asthma? Immunol Allergy Clin North Am. 2008;28(3):649–664, x [DOI] [PubMed] [Google Scholar]
- 77.Suglia SF, Duarte CS, Sandel MT, Wright RJ. Social and environmental stressors in the home and childhood asthma. J Epidemiol Community Health. 2010;64(7):636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suglia SF, Enlow MB, Kullowatz A, Wright RJ. Maternal intimate partner violence and increased asthma incidence in children: buffering effects of supportive caregiving. Arch Pediatr Adolesc Med. 2009;163(3):244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65(4):409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vanderwert RE, Marshall PJ, Nelson CA, III, Zeanah CH, Fox NA. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS ONE. 2010;5(7):e11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis-Morrarty E, Dozier M, Bernard K, Terracciano SM, Moore SV. Cognitive flexibility and theory of mind outcomes among foster children: preschool follow-up results of a randomized clinical trial. J Adolesc Health 2012;51:S17–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]