Abstract
Introduction
It is impossible to imagine a modern socially–active man who does not use mobile devices and/or computers with Wi–Fi function. The effect of mobile phone radiation on male fertility is the subject of recent interest and investigations. The aim of this study was to investigate the direct in vitro influence of mobile phone radiation on sperm DNA fragmentation and motility parameters in healthy subjects with normozoospermia.
Material and methods
32 healthy men with normal semen parameters were selected for the study. Each sperm sample was divided into two equal portions (A and B). Portions A of all involved men were placed for 5 hours in a thermostat, and portions B were placed into a second thermostat for the same period of time, where a mobile phone in standby/talk mode was placed. After 5 hours of incubation the sperm samples from both thermostats were re–evaluated regarding basic motility parameters. The presence of DNA fragmentation in both A and B portions of each sample was determined each hour using a standard sperm chromatin dispersion test.
Results
The number of spermatozoa with progressive movement in the group, influenced by electromagnetic radiation, is statistically lower than the number of spermatozoa with progressive movement in the group under no effect of the mobile phone. The number of non–progressive movement spermatozoa was significantly higher in the group, which was influenced by cell phone radiation. The DNA fragmentation was also significantly higher in this group.
Conclusions
A correlation exists between mobile phone radiation exposure, DNA–fragmentation level and decreased sperm motility.
Keywords: sperm motility, DNA fragmentation, mobile phones, electromagnetic radiation
INTRODUCTION
It is impossible to imagine a modern socially–active man who does not use mobile devices and/or computers with Wi–Fi function. During the last two decades, there has been a significant increase in cell phone usage throughout the world. Meanwhile, continuous electromagnetic radiation from mobile phones, through the development of oxidative stress and DNA fragmentation, can obviously lead to the development of different pathologies including tumors, and also can violate spermatogenesis [1, 2].
The effect of mobile device radiation on healthy male sperm parameters and fertility is the subject of recent interest and investigations.
Male infertility is a contemporary problem. It is well known that infertile men are distinguished by abnormal semen characteristics.
In general, the quality of sperm in recent years has worsened throughout the world.
The majority of infertile or subfertile men are subjects with sperm motility violations and/or DNA damage [3]. So, could radiation from mobile phones have an influence on these spermatozoa parameters?
The aim of this study was to investigate the direct in vitro influence of mobile phone radiation on sperm DNA fragmentation and motility in healthy subjects with normozoospermia.
MATERIAL AND METHODS
32 healthy middle–aged men (27.5 ±3.5 years old) with normal semen parameters (normozoospermia) were selected to study the direct influence of mobile phone radiation on sperm quality in vitro. The participants involved were chosen among normozoospermic men from childless couples with proved female infertility who were consulted at our clinics. All of them have given signed informed consent for permission to participate in the study. Wi–Fi laptop users were not included in the study a priori.
Men were instructed to avoid pocket bearing of mobile phones for a 2 month period before examination. After 3–4 days of sexual abstinence, the semen samples were obtained by masturbation. Each sperm sample was diluted with isotonic solution at a temperature of 37°C in a 1:1 ratio to prevent sperm–agglutination; the samples were then placed in an incubator for one hour. The calculation of key semen parameters was conducted after this period.
Immediately thereafter, each sperm sample was divided into two equal portions (A and B). Portions A of all involved men were placed for 5 hours in a thermostat, and portions B were placed in a second thermostat for the same period of time, where an enabled mobile phone in combined standby/talk mode was placed. The device was located 5 cm from the samples and was turned on in radiation frequency range 900/1800 MHz (GSM standard). For authenticity of the experiment a call was carried out on the phone every 10 minutes, to imitate the transmission mode ”talk“.
After 5 hours of incubation at a temperature of 27°C, the sperm samples from both thermostats were re– evaluated with respect to the basic semen parameters. Sperm vitality and motility were evaluated according to the specifications of the World Health Organization. which classifies sperm movement accordingly as progressively motile, nonprogressively motile and immotile [4].
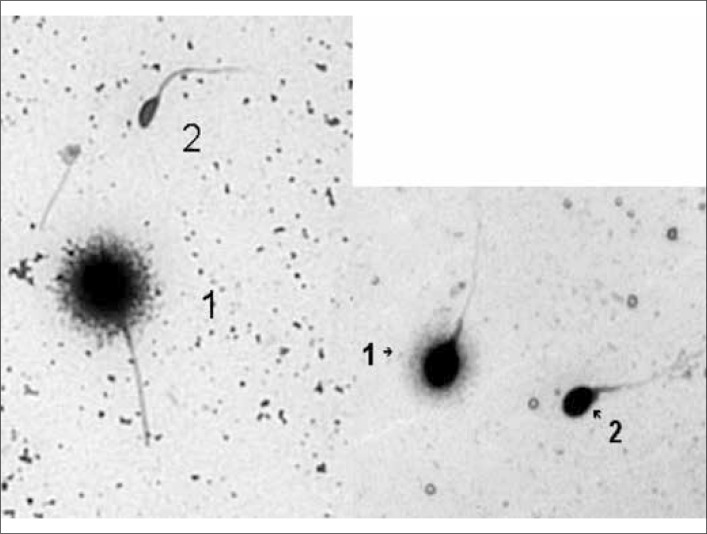
The sperm portions for evaluation of DNA damage were obtained from previously prepared and divided samples. A presence of DNA fragmentation in both A and B portions of each sample was determined every hour using the sperm chromatin dispersion (SCD) test with specific DNA breakage kit, which allows the detection of DNA breaks in lysed sperm nuclei. This test could be considered as simple, fast, versatile and reliable procedure to determine the frequency of sperm cells with fragmented DNA. Diagnostic procedure was performed as described before by Fernández JL et al. [5, 6]. Intact unfixed spermatozoa were immersed in an inert agarose microgel on a pretreated slide. An initial acid treatment denatures DNA in those sperm cells with fragmented DNA. Following this, the lysing solution removes most of the nuclear proteins, and in the absence of massive DNA breakage produces nucleotides with large halos of spreading DNA loops emerging from a central core. However, the nucleotides from spermatozoa with fragmented DNA either do not show a dispersion halo or the halo is minimal. We used “Halosperm®” in vitro diagnostic kit by Halotech Dna, SL that allows the measurement of sperm DNA fragmentation quickly, easily and without the need of complex laboratory equipment. After sample processing with Halosperm®, the slides were stained with DIFF– QUICK®/Panoptic stain. Spermatozoa without fragmentation were marked by dispersion halo, those with DNA strand breaks were without halo. After checking the sample with a bright field microscope, we classified sperm cells as follows (Figure 1).
Figure 1.
Spermatozoa with (2) and without (1) DNA fragmentation.
The results were expressed as percentage of sperm cells with fragmented DNA in 500 sperm cells. For interpreting of results we calculated Sperm DNA Fragmentation (SDF) index by formula:
Digital options of semen characteristics were expressed as “mean value ± statistical deviation”. The results were analyzed using the Mann–Whitney test to establish differences in the performance of both groups: the difference was considered statistically significant at p <0.05.
RESULTS
Basic semen parameters before incubation are presented in Table 1.
Table 1.
Basic semen parameters before incubation
| SEMEN PARAMETER | mean value ±SD |
|---|---|
| Volume (mL) | 3.1 ±1.2 |
| Concentration (x106/mL) | 95.0 ±25.3 |
| Vitality (%) | 91.4 ±3.5 |
| Progressive motility (%) | 86.3 ±7.6 |
| Non– progressive motility (%) | 10.2 ±3.4 |
| Immotility (%) | 6.1 ±2.8 |
| Normal morphology (%) | 9.5 ±3.1 |
| SDF index (%) | 3.3 ±1.2 |
| Dead sperm (%) | 8.6 ±3.5 |
Sperm count in groups A&B during 5 hours did not change in general and was not statistically different from each other: 92.3 ±22.7 x 106 / mL vs 90.8 ±24.2 x 106/mL respectively.
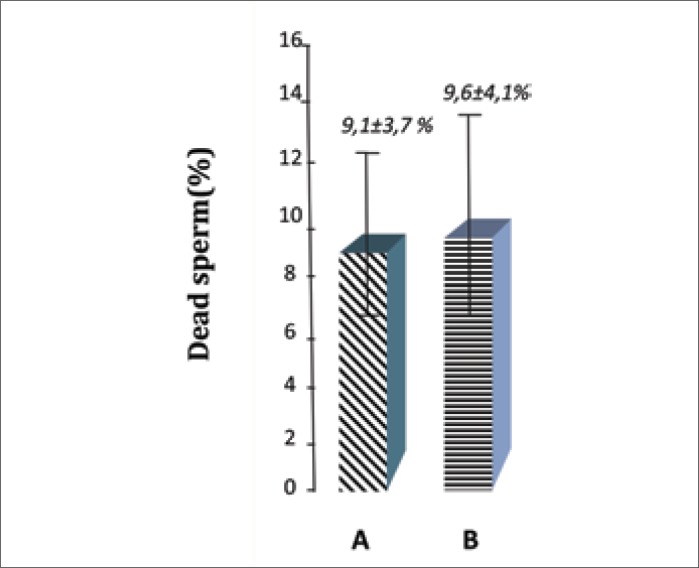
Furthermore, the number of dead sperm in both study groups after 5 hours of incubation was not statistically different (9.1% ±3.7% vs. 9.6% ±4.1%; p >0.05) (Figure 2).
Figure 2.
The dead sperm percentage in both groups A and B.
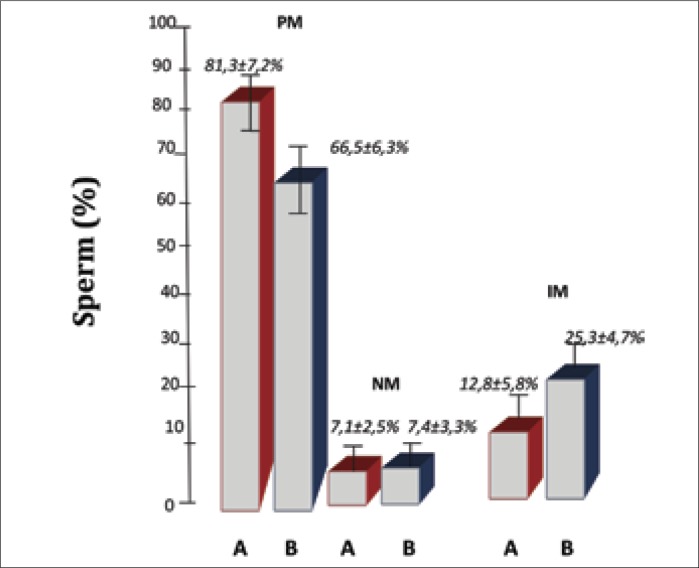
However, the number of spermatozoa with progressive movement in group B, under the influence of electromagnetic radiation, is statistically lower than the number of spermatozoa with progressive movement in group A with no effect of a mobile phone (66.5% ±6.3% vs 81.3% ±7.2%, p <0.05) (Figure 3).
Figure 3.
Medians of studied parameters in both groups A and B: the percentage of spermatozoa with progressive movement (PM); percentage of motionless sperm (NM); percentage of spermatozoa with non–progressive movement (IM).
No differences between the number of spermatozoa with motionless sperm (NM) between groups A and B were reported (7.1% ±2.5% vs. 7.4% ±3.3%; p >0.05), while the number of non–progressive movement spermatozoa (IM) was significantly higher in group B, that was under the influence of cell phone radiation (25.3% ±4.7% vs. 12.8% ±5.8%, p <0.05) (Figure 3).
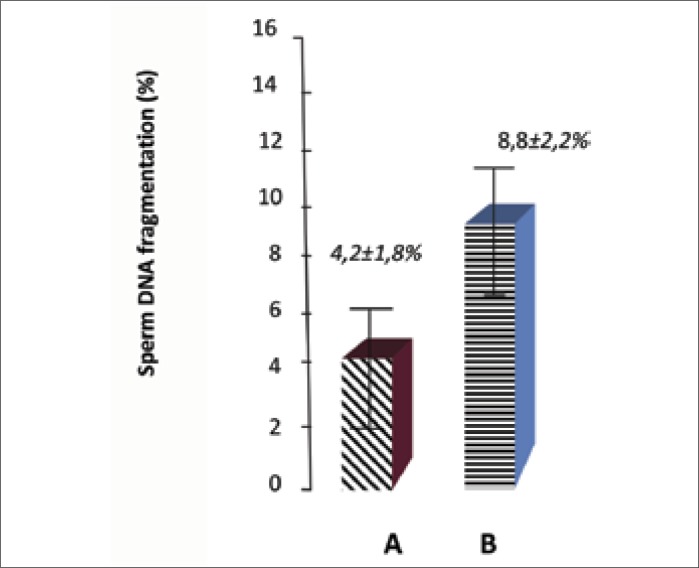
Figure 4 clearly shows a significant difference in the incidence of DNA fragmentation between the studied groups. The samples for DNA fragmentation were obtained from both thermostats each hour of incubation. Finally the sperm samples exposed to mobile phone radiation after 5 hours are characterized by sperm DNA fragmentation index of about 8.8% ±2.2%, while this index in the control group samples after 5 hours incubation without electromagnetic fields influence was 4.2% ±1.8% (p <0.05).
Figure 4.
Levels of DNA–fragmentation in sperm samples with (B) and without (A) electromagnetic exposure.
At first the DNA fragmentation analysis was performed at the start of investigation. After that this analysis was made every hour after placing semen samples into the irradiation zone. We aimed to establish the relationship between the duration of exposure of the semen samples and the level of DNA fragmentation (5B). The same analysis was made in the sperm samples without exposure (5A).
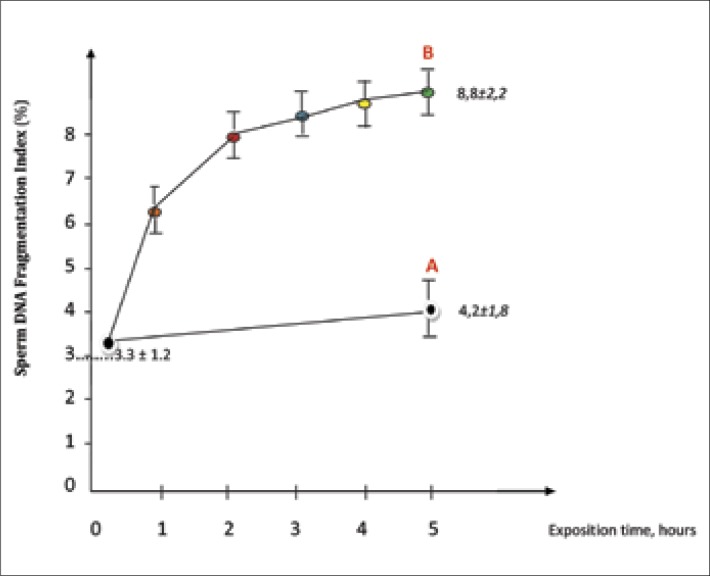
Obtained results are presented in Figure 5.
Figure 5.
The level of DNA fragmentation in semen samples exposed in the exposure area (B) and semen samples without electromagnetic field influence (A).
As shown in Figure 5, sperm DNA fragmentation index before and during 5 hours of electromagnetic field exposure constantly increases (3.3 ±1.2% vs. 8.8 ±2.2%, p <0.05). However, the SDF index in the semen samples without electromagnetic influence did not statistically change during 5 hours of examination (3.3 ±1.2% vs. 4.2 ±1.8%, p >0.05).
A positive correlation was noted between the duration of semen samples placement in the area of mobile phone electromagnetic radiation and the level of DNA fragmentation: the increase of exposure term increases the frequency of DNA damage. Moreover the majority of DNA damaging occurs during first 2 hours of exposure (Figure 5).
The individual influence of mobile phone electromagnetic waves in every semen sample was also determined. The percentage of spermatozoa with fragmented DNA after 5 hours exposure to the radiation zone was statistically higher, by 65.3 ±12.4% on average, than this parameter in the same semen sample before irradiation.
The results obtained are systematized in Table 2.
Table 2.
Basic semen parameters after 5 hours of incubation in groups A and B
| SEMEN PARAMETER | Group A, mean ± SD | Group B, mean ± SD | p |
|---|---|---|---|
| Concentration (x106 / mL) | 92.3 ±22.7 | 90.8 ±24.2 | >0.05 |
| Vitality (%) | 90.9 ±3.7 | 90.4 ±4.1 | >0.05 |
| Progressive motility (%) | 81.3 ±7.2 | 66.5 ±6.3 | <0.05 |
| Non– progressive motility (%) | 12.8 ±5.8 | 25.3 ±4.7 | <0.05 |
| Immotility (%) | 7.1 ±2.5 | 7.4 ±3.3 | >0.05 |
| Normal morphology (%) | 8.7 ±2.8 | 8.3 ±3.1 | >0.05 |
| SDF index (%) | 4.2 ±1.8 | 8.8 ±2.2 | <0.05 |
| Dead sperm (%) | 9.1 ±3.7 | 9.6 ±4.1 | >0.05 |
SD – statistical deviation
Thus, according to the presented data cell phone radiation decreases sperm motility, but only in previously motile sperm. The percentage of immotile sperm did not increase under electromagnetic waves’ influence. Cell phone radiation also leads to sperm DNA fragmentation.
DISCUSSION
Overall reduction of the basic normal semen analysis parameters that characterize the sperm count, motility and morphology have been noted worldwide in recent decades. Researchers are unanimous about the negative impact of certain environmental factors on sperm quality. The most well–known are the harmful effects of smoking, the abuse of alcohol, intake of spermicidal food products, etc. Likewise, the negative impact of increased local testicular temperature on spermatogenesis has been proven [7, 8, 9].
The reproductive toxicants like lead and cadmium should be considered the causes for the decline in semen quality. In idiopathic oligoasthenozoospermic males there were registered higher levels of lead and cadmium in their semen which correlated with impairment of sperm motility and vitality percentages and more importantly with higher sperm DNA fragmentation and semen reactive oxygen species level [10].
Different harmful environmental influences have led to changes in semen analysis standards by reducing the lower limits of normal ranges, which were declared by the World Health Organization [4].
The possible negative impact of mobile phone radiation on sperm quality has been established recently.
While no certain conclusions can be drawn from current evidence, a growing number of studies indicate a decrease in male fertility associated with cellular phone usage [11].
An excellent study, one of the first on this topic, was presented by Agarwal A et al. in 2008. The authors concluded that the use of cell phones by men is associated with a decrease in their semen quality. According to the researchers’ data the decrease in sperm count, motility, viability, and normal morphology was related to the duration of exposure to cell phones [12].
V. Boulos & H. Hassan, in 2013, concluded that cell phone use in men is associated with decreased semen quality in the form of decreased sperm count, motility, viability & normal morphology, which depend on the duration of cell phone exposure time. The authors found a significant positive correlation between the decrease in the different sperm parameters: the decrease in the value of one of these parameters is concomitant with other parameters changes [13].
Contrasting results were obtained by Yildirim M.E. et al. in 2013. Researchers compared sperm parameters in 4 groups from a total of 145 patients who completed the questionnaire: group 1, varying mobile phone usage duration (0–30 min, 30–60 min and over 60 min), group 2, different mobile phone carriage mode (in the pocket, on the belt and in the handbag), group 3, wireless internet users (duration: 0–30 min, 30–60 min and over 60 min), group 4, users of different types of internet connection (wireless, wired). The authors have concluded that there was no significant difference between period of mobile phone usage and sperm count and motility (p = 0.236 and p = 0.457, respectively). Similarly, they did not find a significant difference between the mobile phone carriage mode and sperm count and motility either (p = 0.837 and p = 0.157, respectively). However, according to their data, sperm motility decreased with the increased use of wireless internet (p = 0.03). Similarly, spermatozoa motility was worse in the group of wireless internet users than that of the wired internet usage group (p = 0.035) [14].
Furthermore, there are differences in the type of cellular device used, the transmission mode at which it operates (“talk” vs. “standby”) and also the distance between the sperm cells and phone. All of these variations contribute to the above–mentioned ambiguity of the results presented in the different cell phone studies [15].
Original research conducted in 2009, by subjecting in vitro samples of human spermatozoa to radio–frequency radiation at 1.8 GHz and specific absorption rates (SAR) of 0.4 to 27.5 W/kg, showed a correlation between increasing SAR and decreased motility and vitality of sperm, increased oxidative stress and 8–Oxo–2’–deoxyguanosine markers, stimulating DNA base adduct formation and increased DNA fragmentation [1].
In contrast to De Iuliis et al., our results did not demonstrate a correlation between mobile phone radiation exposure and sperm vitality, compared to the control group without exposure (Figure 2.). In our study we did not find differences between the percentages of dead and motionless sperm in samples with/without radiation exposure (9.1% ±3.7% vs. 9.6% ±4.1%; p >0.05; 7.1% ±2.5% vs. 7.4% ±3.3%; p >0.05, respectively). However, like the above–mentioned researcher's results, our study also has shown a correlation between radiation exposure time and decreased sperm motility, but only in previously motile sperm (Figure 3). Compared to the control group, our results demonstrate a significant decrease in the number of sperm with progressive movement (of 81.3% ±7.2% vs. 66.5% ±6.3%, p <0.05) and a concurrent increase in the number of sperm with non–progressive movement (12.8% ±5.8% vs. 25.3% ±4.7%, p <0.05) when under electromagnetic radiation exposure for a 5 hour period.
Our presented data also suggests that electromagnetic radiation causes DNA fragmentation of sperm. The SDF index before exposure in both groups was 3.3 ±1.2%, while after 5 hours of exposure the same index in the study group had increased to 8.8 ±2.2% (p <0.05). However, the DNA fragmentation levels in semen samples without electromagnetic influence did not statistically change during 5 hours of examination (3.3 ±1.2% vs. 4.2 ±1.8%, p >0.05) (Figure 5). The DNA fragmentation occurs predominantly during the first two hours of exposure. Its level correlates with semen exposure time in the area of electromagnetic radiation.
Avendano C.M.S et al. in 2012 evaluated the effects of laptop computers, connected to local area networks through Wi–Fi, on human spermatozoa. Their results demonstrate a significant decrease in sperm progressive motility and a significantly higher proportion of sperm with DNA fragmentation when samples were incubated for 4 hours under the laptop. These differences were seen in comparison with aliquots of the same semen samples incubated under similar conditions, but outside the proximity of any computer or electronic device. The investigators speculated that the use of a laptop computer wirelessly connected to the internet and positioned near the male reproductive organs may decrease human sperm quality [16]. Thus, exposing the semen to radio frequency electromagnetic waves from laptops connected to the internet through Wi–Fi has the same potential to damage spermatozoa as mobile phone electromagnetic radiation.
Besides affecting sperm motility, vitality and DNA fragmentation, mobile phone radiofrequency electromagnetic fields (RF–EMF) leads to changes in morphological spermatozoa parameters. According to Falzone N, et al, 2011, a significant reduction in sperm head area (9.2 ±0.7 μm2 vs. 18.8 ± 1.4 μm2) and acrosome percentage of the head area (21.5 ±4% vs. 35.5 ±11.4%) was reported among exposed sperm compared with unexposed controls. Also the mean number of zona–bound sperm of the test hemizona and controls was 22.8 ±12.4 and 31.8 ±12.8 (p <0.05), respectively. Authors concluded that although radiofrequency electromagnetic fields exposure did not adversely affect the acrosome reaction, it had a significant effect on sperm morphometry. Also, a significant decrease in sperm binding to the hemizona was observed. These results could indicate a significant effect of RF–EMF on sperm fertilization potential [17].
Atasoy H.I. et al. in 2012, after an investigation into the effects on rat testes of radiofrequency radiation emitted from indoor Wi–Fi Internet access devices, concluded that under RF–EMF impact significant increases occur in serum 8–hydroxy–2’–deoxyguanosine levels and 8–hydroxyguanosine staining in the testes of the experimental group. This fact is directly indicating DNA damage due to RF–EMF exposure. The authors also found decreased levels of catalase and glutathione peroxidase activity in the experimental group, which may have been due to radiofrequency effects on enzyme activity. The data obtained in this animal study raises questions about the safety of radiofrequency exposure from Wi–Fi Internet access devices for growing organisms of reproductive age, with a potential effect on both fertility and the integrity of their germ cells [18].
Neither we nor any of the above–mentioned authors studied prospectively the influence of mobile phones irradiation on paternity. Such investigations require long–term design and many previously healthy volunteers. Obviously, therefore, prolonged direct mobile phone radiation influence on sperm is able to progressively impair the basic semen motility parameters and bring about DNA fragmentation similar to other well–known causes of male infertility (varicocele, inflammatory gonadal diseases, etc.). Accordingly, it is likely that constant direct mobile phone radiation exposure on sperm (testis) could damage sperm and directly influence its motility. This is likely to affect the fertility of healthy men, possibly rendering them infertile in the future. It would be interesting to establish in the future if there is a decrease in fertility among heavy mobile phone users compared to men with short–term device use.
Common metallic objects like coins, rings or zips, in pockets or on trousers and the body, may intensify RF–EMF impact. Dr William Whittow and colleagues, in 2008, investigated specific absorption rates (SAR) in the human body with a realistic mobile phone source positioned in a ‘front trouser pocket’ of a truncated male heterogeneous anatomical body model. Realistic everyday metallic objects, including a coin, ring and zip were added to the model. The authors concluded that these objects increased the SAR in the body at different frequencies. The cumulative effect of these three objects generally increased the SAR in the waist section over the maximum levels of frequency range and exposure permitted in the United Kingdom [19].
At the present time nobody knows the real intercommunication between the constant direct mobile phones irradiation affects on testes and developmental delays in the offspring.
Another interesting implication of the findings of our study is that women wishing to conceive a child should be careful about laptop and mobile phones usage. The fertile life of human sperm in the female reproductive tract may be 80 hours or more. Spermatozoa can survive in the female cervix uteri and likely oviducts where they might be in danger from electromagnetic waves [20, 21].
A topic, also, for numerous studies regards the probably negative RF–EMF influence on female fertility and embryo/fetus. Some data in animals and humans declare an adverse impact caused by RF–EMR on granulosa cells, ovarian follicle numbers, endometrial tissue, quality of oocytes and embryos, and even alterations on fetal heart physiology during pregnancy [22]. For example, ninety women with uncomplicated pregnancies, and 30 full–term healthy newborn infants were included in the study by Rezk AY et al, 2008. The pregnant mothers were exposed to RF–EMR emitted by mobile phones while on dialing mode for 10 min during pregnancy and after birth. Researchers found that the exposure of pregnant women to mobile phones significantly increased fetal and neonatal heart rate, and significantly decreased fetal cardiac output [23].
That is why we consider that men readying themselves for fatherhood, as well as women wishing to conceive a child, especially when registered fertility problems are present, should be informed about the different risks and probably negative direct impact of long–term mobile phone radiation on semen quality and embryo/fetus development.
Maybe person who could be selected for assisted reproduction techniques or even sperm donors should avoid this influence during some time before semen extraction too [24]. Besides the semen parameters RF-EMR probably could negatively impact on sexual communication, fertility and quality of life by reducing the erectile function. Men with erectile dysfunction (ED) use their cell phones longer those without ED. Men who have ED carry their cell phones switched on much longer than men who do not have ED [25].
Currently, there is no consensus on mobile phone RF–EMR radiation effects on human fertility. Additional well–designed investigations are needed to evaluate the real consequences of long–term employment of these devices.
CONCLUSIONS
Long–term semen exposure in the area of mobile phone RF–EMR leads to a significant decrease in the number of sperm with progressive movement and an increase in those with non–progressive movement.
Prolonged direct mobile phone exposure may bring about sperm DNA fragmentation
For men readying themselves for fatherhood, especially when registered fertility problems exist, it would be better to avoid holding a mobile phone in a trouser pocket for long periods of time.
References
- 1.De Iuliis GN, Newey RJ, King BV, Aitken RG. Mobile Phone Radiation Induces Reactive Oxygen Species Production and DNA Damage in Human Spermatozoa In Vitro. In Zhang, Baohong. 2009;4:e6446. doi: 10.1371/journal.pone.0006446. PLoS ONE (Callaghan, New South Wales, Australia) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardell L, Carlberg M, Hansson Mild K. Epidemiological evidence for an association between use of wireless phones and tumor diseases. Pathophysiology. 2009;16:113–122. doi: 10.1016/j.pathophys.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010;27:3–12. doi: 10.1007/s10815-009-9359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 5th edn. Cambridge: Cambridge University Press; 2010. WHO laboratory manual for the examination of and procesing of human semen and sperm–cervical mucus interaction. [Google Scholar]
- 5.Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 6.Fernández JL, Cajigal D, López–Fernández C, Gosálvez J. Assessing sperm DNA fragmentation with the sperm chromatin dispersion test. Methods Mol Biol. 2011;682:291–301. doi: 10.1007/978-1-60327-409-8_21. [DOI] [PubMed] [Google Scholar]
- 7.Burton A. Study Suggests Long–Term Decline in French Sperm Quality Environ Health Perspect. 2013;121:a46. doi: 10.1289/ehp.121-a46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geoffroy–Siraudin C, Loundou AD, Romain F, Achard V, Courbière B, Perrard MH, et al. Decline of semen quality among 10 932 males consulting for couple infertility over a 20–year period in Marseille, France. Asian J Androl. 2012;14:584–590. doi: 10.1038/aja.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jørgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl. 2011;34:e37–e48. doi: 10.1111/j.1365-2605.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taha EA, Sayed SK, Ghandour NM, Mahran AM, Saleh MA, Amin MM. Correlation between seminal lead and cadmium and seminal parameters in idiopathic oligoasthenozoospermic males. Centr European J Urol. 2013;66:84–92. doi: 10.5173/ceju.2013.01.art28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Singh A, Hamada A, Kesari K. Cell Phones and Male Infertility: A Review of Recent Innovations in Technology and Consequences. International Braz J Urol. 2011;37:432–454. doi: 10.1590/s1677-55382011000400002. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Deepinder F, Sharma R, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89:124–128. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 13.Boulos V, Hassan H. Do cell phones affect semen quality? Eur Urol Suppl. 2013;12:e843–e844. [Google Scholar]
- 14.Yildirim ME, Kaynar M, Badem H, Cavis M, Karatas OF, Cimentepe E. The effect of radiofrequency–electromagnetic radiation on semen parameters. Eur Urol Suppl. 2013;12:e1113, S5. [Google Scholar]
- 15.Hamada A, Singh A, Agarwal A. Cell Phones and their Impact on Male Fertility: Fact or Fiction. The Open Reproductive Science Journal. 2011;5:125–137. [Google Scholar]
- 16.Avendano C, Mata A, Sanchez Sarmiento CA, Doncel GF. Use of laptop computers connected to internet through Wi–Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertility and Sterility. 2012;97:39–45. doi: 10.1016/j.fertnstert.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Falzone N, Huyser C, Becker P, Leszczynski D, Franken DR. The effect of pulsed 900–MHz GSM mobile phone radiation on the acrosome reaction, head morphometry and zona binding of human spermatozoa. Int J Androl. 2011;34:20–26. doi: 10.1111/j.1365-2605.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 18.Atasoy HI, Gunal MY, Atasoy P, Elgun S, Bugdayci G. Immunohistopathologic demonstration of deleterious effects on growing rat testes of radiofrequency waves emitted from conventional Wi–Fi devices. J Pediatr Urol. 2013;9:223–229. doi: 10.1016/j.jpurol.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Whittow WG, Panagamuwa CJ, Edwards MA, Ma L. Indicative SAR levels due to an active mobile phone in a front trouser pocket in proximity to common metallic objects. 2008 Loughborough Antennas and Propagation Conference; March 17–18; Loughborough, UK. pp. 149–152. [Google Scholar]
- 20.Gould JE, Overstreet JW, Hanson FW. Assessment of human sperm function after recovery from the female reproductive tract. Biol Reprod. 1984;31:888–894. doi: 10.1095/biolreprod31.5.888. [DOI] [PubMed] [Google Scholar]
- 21.Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 22.Merhi ZO. Challenging cell phone impact on reproduction: A Review. J Assist Reprod Genet. 2012;29:293–297. doi: 10.1007/s10815-012-9722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezk AY, Abdulqawi K, Mustafa RM, Abo El–Azm TM, Al–Inany H. Fetal and neonatal responses following maternal exposure to mobile phones. Saudi Med J. 2008;29:218–223. [PubMed] [Google Scholar]
- 24.Campagne DM. Can male fertility be improved prior to assisted reproduction through the control of uncommonly considered factors? Int J Fertil Steril. 2013;6:214–223. [PMC free article] [PubMed] [Google Scholar]
- 25.Al–Ali BM, Patzak J, Fischereder K, Pummer K, Shamloul R. Cell phone usage and erectile function. Centr European J Urol. 2013;66:75–77. doi: 10.5173/ceju.2013.01.art23. [DOI] [PMC free article] [PubMed] [Google Scholar]