Abstract
Bianba, Sveinung Bernsten, Lars Bo Andersen, Hein Stegum, Ouzhuluobu, Per Nafstad, Tianyi Wu, and Espen Bjertness. Exercise capacity and selected physiological factors by ancestry and residential altitude—Cross-sectional studies of 9–10-year-old children in Tibet. High Alt Med Biol. 15:162–169, 2014.—Aim: Several physiological compensatory mechanisms have enabled Tibetans to live and work at high altitude, including increased ventilation and pulmonary diffusion capacity, both of which serve to increase oxygen transport in the blood. The aim of the present study was to compare exercise capacity (maximal power output) and selected physiological factors (arterial oxygen saturation and heart rate at rest and during maximal exercise, resting hemoglobin concentration, and forced vital capacity) in groups of native Tibetan children living at different residential altitudes (3700 vs. 4300 m above sea level) and across ancestry (native Tibetan vs. Han Chinese children living at the same altitude of 3700 m).
Methods: A total of 430 9–10-year-old native Tibetan children from Tingri (4300 m) and 406 native Tibetan- and 406 Han Chinese immigrants (77% lowland-born and 33% highland-born) from Lhasa (3700 m) participated in two cross-sectional studies. The maximal power output (Wmax) was assessed using an ergometer cycle.
Results: Lhasa Tibetan children had a 20% higher maximal power output (watts/kg) than Tingri Tibetan and 4% higher than Lhasa Han Chinese. Maximal heart rate, arterial oxygen saturation at rest, lung volume, and arterial oxygen saturation were significantly associated with exercise capacity at a given altitude, but could not fully account for the differences in exercise capacity observed between ancestry groups or altitudes.
Conclusions: The superior exercise capacity in native Tibetans vs. Han Chinese may reflect a better adaptation to life at high altitude. Tibetans at the lower residential altitude of 3700 m demonstrated a better exercise capacity than residents at a higher altitude of 4300 m when measured at their respective residential altitudes. Such altitude- or ancestry-related difference could not be fully attributed to the physiological factors measured.
Key Words: : high altitude, adaptation, maximal exercise, arterial oxygen saturation, hemoglobin
Introduction
Approximately 17 million of the world's population lives at altitudes higher than 3500 m above sea level (Huddleston et al., 2003). In the Tibet Autonomous Region (TAR) of China, more than 2.8 million people (2009) live at similar altitudes, of whom 92% are native Tibetans and 8% are Han immigrants from lowland China (Han Chinese). Native Tibetans have the longest history of living at high altitude among world populations, approximately 22,000 years (approximately 1100 generations) (Niermeyer et al., 2001; Aldenderfer, 2003), as compared with Han Chinese immigrants, who have lived at high altitude in Tibet for 1–3 generations only.
Most previous studies have shown that native Tibetan adults (Sun et al., 1990; Groves et al., 1993; Zhuang et al., 1993; 1996) and adolescents (Chen et al., 1997) achieve a superior exercise capacity compared to Han Chinese immigrants. However, a few studies have shown that native Tibetan adults attained a higher exercise capacity, but a similar (Niu et al., 1995) or lower (Ge et al.,1994) peak oxygen uptake (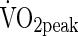 ).
). 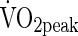 is considered the best single measure of cardiorespiratory fitness (Astrand and Rodahl, 1986), which provides a good index of the integrated function of the oxygen transport system in both children and adults.
is considered the best single measure of cardiorespiratory fitness (Astrand and Rodahl, 1986), which provides a good index of the integrated function of the oxygen transport system in both children and adults. 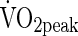 can be estimated from maximal power output (Wmax) (Woynarowska,1980; Hansen et al., 1989; Andersen et al., 1987; Andersen, 1995; Arngrimsson et al., 2008; Bianba et al., 2010), and has been used in the evaluation of the adaptation to live and work at high altitude (Grover et al.,1967; Ge et al.,1994; Wu and Kayser, 2006).
can be estimated from maximal power output (Wmax) (Woynarowska,1980; Hansen et al., 1989; Andersen et al., 1987; Andersen, 1995; Arngrimsson et al., 2008; Bianba et al., 2010), and has been used in the evaluation of the adaptation to live and work at high altitude (Grover et al.,1967; Ge et al.,1994; Wu and Kayser, 2006).
A person who moves from sea level to Lhasa (3700 m) will acclimatize after some days, but even after decades he or she will not reach a sea level performance of exercise or work. A person arriving in Lhasa from sea level can expect to reach an exercise performance or work that is approximately 70% of his or her performance at sea level, which will increase to 80% after a few weeks of acclimatization (Moore et al., 1994). Similar to the immigrant Han Chinese in Tibet, if a person stays at 3700 m for years, there may be a further small increase to nearly 85% of the initial sea level value (Moore et al., 1994). After generations of residence, Tibetans at 3700 m have an exercise performance or work that is 92% of sea level values for sedentary populations (Moore et al., 1994).
In relation to both a decrease in arterial oxygen content and a limitation in maximal cardiac output, moving lowlanders to higher altitudes is associated with a decreased aerobic exercise capacity (Fulco et al., 1998), while a higher pre-exposure residential altitude modulates the negative effect (Fulco et al., 1998). High altitude populations are continuously challenged in an environment of hypobaric hypoxia with a low ambient partial pressure of oxygen, thus influencing the capacity for work and exercise. Moreover, the capacity is reduced with an increasing residential altitude (Fulco et al., 1998). However, if high altitude natives move to a lower altitude, they will exhibit a better exercise capacity than natives living at the same, lower altitude, as demonstrated by Curran et al. (1998). The relatively better maximal exercise capacity in high altitude natives may primarily be due to their exposure to a high altitude environment during childhood (Frisancho et al., 1973). Independent of being a child or an adult, or of a low or high socioeconomic status, everyone is exposed to the same ambient hypoxia at a given altitude. As reviewed by Simonsen et al. (2012), native highland populations have genetic adaptations that enable them to live at high altitudes. Three out of several genes associated with natural selection among Tibetans, EPAS1, EGLN1, and PPARA, are associated with their relatively low hemoglobin concentration (Simonson et al., 2012).
In a review by Wu and Kayser (2006), they concluded that among adults, Tibetans were better adapted than other populations to live and work at high altitudes. In addition to a lower Hb concentration, as compared with lowlanders and Andeans living at the same altitude, several other physiological factors contributed to this adaptation, including: maintaining a higher arterial oxygen saturation at rest and during exercise, less loss of aerobic performance with an increasing altitude, a greater hypoxic and hypercapnic ventilatory responsiveness, larger lungs, a better lung function, and a greater lung diffusing capacity than lowlanders (Wu and Kayser, 2006). Lastly, Tibetans develop only minimal hypoxic pulmonary hypertension (Groves et al., 1993), and have higher levels of exhaled nitric oxide than lowlanders or Andeans (Wu and Kayser, 2006). Studies of physiological aspects of Tibetan children's capacity to work and live at high altitude are scarce, although differences similar to those seen in adults may be expected to occur. Children living at altitudes higher than 3000 m above sea level show several adaptive mechanisms in the uptake of oxygen in terms of increased ventilation and pulmonary diffusion capacity (de Meer et al., 1995). The latter depends on the diffusion rate per unit of the surface area and the total surface area in the lungs available for diffusion of oxygen, which is reflected in part by total lung capacity (de Meer et al., 1995). Oxygen transportation in the blood is highly dependent on blood flow, hemoglobin concentration ([Hb]), and arterial oxygen saturation (Sao2) (Astrand, 1986).
The objectives of the present study were to compare exercise capacity (maximal power output) and selected physiological factors (arterial oxygen saturation and heart rate at rest and during maximal exercise, resting hemoglobin concentration, and forced vital capacity) in groups of high altitude native Tibetan children across residential altitudes (3700 vs. 4300 m above sea level) and across ancestry (native Tibetan vs. Han Chinese children living at the same altitude of 3700 m). We also aimed to investigate the association of residential altitude and ancestry with exercise capacity, and the possible impact of selected physiological factors on this association.
Materials and Methods
Nine primary schools out of 20 were randomly selected from Lhasa City (3700 m), the capital of TAR, while all five primary schools were included from the rural Tingri district (4300 m) located under the North Face of Mt. Everest. An identical procedure for selecting participants was applied in both areas, and all 9–10-year-old children, 817 children from Lhasa and 460 children from Tingri, were invited. In Lhasa, one child refused to participate and four children on the list were excluded due to respiratory health problems, yielding 812 eligible children, which gave a participation rate of 99%. A total of 406 native Lhasa Tibetan children (207 boys and 199 girls) and 406 Lhasa Han Chinese children (235 boys and 171 girls) participated in the urban area. Among the Han Chinese, 313 (77%) were born at low altitude (lowland-born Han Chinese) and then migrated to Lhasa with their parents, while the remaining 93 (23%) were born in Lhasa (highland-born Han Chinese). In Tingri, 16 did not go to school on the days of the data collection and four children refused to participate, whereas 10 children participated but did not give permission to use their data for publication, thus yielding a response rate of 93%. A total of 215 girls and 215 boys participated from Tingri.
Ethical considerations
The Health and Education Office and the Tibet University Medical College in TAR approved the study, and information and consent forms were given to parents through the school leaders. Information about the study procedure was also given to the children, participation was voluntary, and the children could withdraw from the study for any reason at any time with no negative consequences.
Data collection and variables
Data were collected through a questionnaire, a clinical examination, and a maximal exercise test, and the same research team and methods were used in the studies in Lhasa and Tingri. The data collections were performed in a classroom at each school from August to November 2005 in Lhasa, and from September to October 2007 in Tingri. In addition, there was no vigorous physical activity the day before the test, no food was consumed less than 2 hours prior to the test, and no feast meals were eaten less than 4 hours before each test.
Study group
“Study group” is a variable that combines ancestry and residential altitude, which includes Tingri Tibetans, Lhasa Tibetans, and Lhasa Han Chinese. It is a measure of the effect of altitude when comparing Lhasa Tibetans (3700 m) and Tingri Tibetans (4300 m), and a measure of ancestry effect when comparing Lhasa Tibetans and Lhasa Han Chinese. Lhasa Han Chinese were further divided into lowland- and highland-born for selected analyses.
Maximal power output and heart rate
Maximal power output (Wmax) was assessed using an electronically braked cycle ergometer (Monark Ergomedic 839, Varberg, Sweden) and presented as exercise capacity according to a previously validated protocol (Hansen et al., 1989). We lack data on 350 children from the cycle ergometer test: 279 children could not fully reach the pedals, 49 children could not complete the test, and for 22 no reason was given.
Children were requested to have 5 minutes of practice on the ergometer cycle before the test to ensure that all inexperienced children had some cycling practice. A variable “owning a bicycle,” with answers of yes or no, was used to distinguish between experienced and inexperienced cyclists. Heart rate (Polar Electro OY, Kempele, Finland) was measured throughout the test, and recorded at the end of each step in the progressive cycle test. We applied methods similar to previous studies in children (Aandstad et al., 2006; Riddoch et al., 2005), and more details regarding the procedures and test criteria can be found elsewhere (Bianba et al., 2010). The children cycled at a pedaling rate of 70–80 revolutions per minute (rpm), and the power output was increased by 20 W (weight<30 kg) or 25 W (weight>30 kg) every third minute. The test was stopped when the child could no longer continue with a pedaling rate above 30 rpm.
Wmax was determined as the number of watts in the last fully completed step (Wl), plus the increment in watts (Wd) of the last step, multiplied by the number of seconds completed at the last step (t) and then divided by 180 seconds (Hansen et al., 1989): Wmax=Wl + (Wd · t /180). The absolute Wmax and Wmax relative to body mass (Wmax/body mass) are presented.
Arterial oxygen saturation
Arterial oxygen saturation at rest (SaO2rest) was recorded after supine rest for 2–3 minutes using a hand-held pulse oximeter (Nellcor NPB-40, California, USA). An OXI-P/I OxiBand sensor was applied for children of less than 40 kg and a DURASENSOR DS-100A sensor for children above 40 kg, respectively. Furthermore, SaO2 was recorded at the last 30 seconds of each power output during the cycling and at maximal exercise (SaO2peak).
Hemoglobin concentration
A HemoCue Hb 201+ analyzer (Ängelholm, Sweden) was applied to measure [Hb] in capillary blood, and we calibrated the analyzer every testing day using a HemoCue Hemoglobin Calibrator (12.0±0.2 g•dL−1).
Lung volume
As a measure of total lung capacity, forced vital capacity (FVC) was measured using maximal expiratory flow volume maneuvers with Spiro USB (Micro Medical Limited, Rochester, Kent, UK), according to standardized international guidelines (Miller et al., 2005). The highest of the recorded FVC values was reported in the present study.
Anthropometrics
The body mass of the children was measured to the nearest 0.1 kg (without shoes and wearing light clothes) using an Electronic Scale (OMRON, HN-281, Shanghai, China), while height was measured to the nearest 0.5 cm using a stadiometer (TZG, Shanghai, China). Chest and waist circumferences were measured to the nearest 0.5 cm with children standing and breathing normally, and the average value both before and after expiration was recorded. The Body Mass Index (BMI) was calculated as the body mass (in kg) divided by the height squared (in meters).
Statistical methods
Data are reported as the mean plus or minus one standard deviation or as the mean with a 95% confidence interval. Differences in mean values between groups were tested using a one-way ANOVA. Three linear regression models were constructed for determining the relationship between the outcome variable (maximal power output) and the selected covariates. The association between the “study group” (i.e., residential altitude and ancestry) and Wmax was estimated in Model 1, with adjustment for sex, body mass, and having a bicycle. In Model 2, we estimated the association between physiological covariates ([Hb], SaO2, HR, and FVC) with Wmax, adjusting for sex, body mass, and having a bicycle. Model 3 was created to investigate whether the physiological covariates from Model 2 had any impact on the associations between “study group” and Wmax. Similar analyses (Models 1, 2, 3) were conducted with a change in the “study group” variable. The group Lhasa Han Chinese was further subdivided into two groups: those born at high altitude (highland-born Han Chinese) vs. those born at low altitude (lowland-born Han Chinese). The assumptions of the models were tested by plotting residuals vs. predicted values, no deviations from linearity were detected, and the residual variance was close to constant. Points with a high influence were looked for by plotting leverage vs. squared residuals, and no high influential points were found. No interactions between altitude and ancestry and physiological factors that distorted the main findings were identified. SPSS version 16.0 and Stata SE 9.0 were used. The level of statistical significance was set at p<0.05.
Results
The sex-specific descriptive data of Tingri Tibetan, Lhasa Tibetan, and Lhasa Han Chinese children are presented in Table 1. On average, the Lhasa Tibetans were 32% and 7% heavier than Tingri Tibetans and Lhasa Han Chinese, respectively. Furthermore, they were 10% and 1% taller, and had 8% and 4% larger chest circumferences than Tingri Tibetans and Lhasa Han Chinese, respectively.
Table 1.
Descriptive Data of 9- to 10-Year-Old Lhasa Tibetan, Lhasa Han Chinese, and Tingri Tibetan Children
| Boys | Girls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tingri Tibetan | Lhasa Tibetan | Lhasa Han Chinese | Tingri Tibetan | Lhasa Tibetan | Lhasa Han Chinese | |||||||
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Body mass (kg) | 203 | 22.5 (2.56)** | 207 | 29.7 (5.28)□□ | 235 | 27.8 (5.13)## | 195 | 22.4 (2.85)** | 198 | 29.4 (5.15)□□ | 169 | 27.6 (5.23)## |
| Stature (cm) | 203 | 122.2 (5.41)** | 207 | 134.8 (5.45)□ | 234 | 133.5 (6.43)## | 195 | 122.8 (6.31)** | 198 | 135.9 (6.68)□ | 169 | 133.7 (6.58)## |
| Chest circ. (cm) | 203 | 59.8 (2.61)** | 206 | 64.7 (4.74)□□ | 234 | 61.8 (4.26)## | 195 | 58.9 (2.88)** | 198 | 63.7 (4.61)□□ | 169 | 61.3 (4.47)## |
| Waist circ. cm) | 203 | 53.5 (2.44)** | 206 | 57.6 (6.06)□□ | 234 | 54.9 (5.38)# | 195 | 53.0 (2.51)** | 198 | 56.0 (5.09)□□ | 169 | 53.4 (5.24) |
| BMI (kg/m2) | 203 | 15.0 (1.02)** | 207 | 16.3 (2.12)□□ | 234 | 15.5 (1.79)# | 195 | 14.8 (1.07)** | 198 | 15.8 (1.81)□ | 169 | 15.4 (1.94)# |
| [Hb] (g/dl) | 201 | 13.9 (1.25)** | 206 | 14.6 (1.33)□□ | 233 | 15.3 (1.20)## | 192 | 14.0 (1.37)** | 199 | 14.6 (1.15)□□ | 169 | 15.4 (1.20)## |
| FVCpeak (L) | 190 | 1.94 (0.30)** | 204 | 2.22 (0.29)□□ | 233 | 2.04 (0.30)# | 197 | 1.82 (0.29)** | 193 | 2.06 (0.33) | 164 | 1.89 (0.26)## |
| HRpeak (beats/min) | 87 | 192 (13)** | 201 | 198 (10)□ | 216 | 194 (8) | 64 | 192 (10)** | 182 | 198 (9)□ | 141 | 195 (8) |
| Crude Wmax (W) | 87 | 57 (17)** | 201 | 85 (12)□□ | 216 | 76 (14)## | 64 | 51 (11)** | 182 | 72 (15)□ | 142 | 67 (12)## |
| Relative Wmax (W/kg) | 87 | 2.38 (0.62)** | 201 | 2.90 (0.44)□□ | 216 | 2.74 (0.47)## | 64 | 2.05 (0.40)** | 181 | 2.43 (0.41)□ | 142 | 2.38 (0.40)## |
p<0. 05 Tingri Tibetans vs. Lhasa Tibetans, **p<0. 001 Tingri Tibetans vs. Lhasa Tibetans.
p<0. 05 Tingri Tibetans vs. Lhasa Chinese,##p<0. 001 Tingri Tibetans vs. Lhasa Chinese.
p<0. 05 Lhasa Tibetans vs. Lhasa Chinese,□□p<0. 001 Lhasa Tibetans vs. Lhasa Chinese.
Physiological factors
The [Hb] was 5% higher in Lhasa Han Chinese compared with Lhasa Tibetans, and 4% higher in Lhasa Tibetans than in Tingri Tibetans. The lung volume (FVC) and peak heart rate (HRpeak) were higher in Lhasa Tibetans than both Lhasa Han Chinese and Tingri Tibetans. Lhasa Tibetan achieved 46% and 10% higher levels of absolute Wmax (W) than Tingri Tibetan and Lhasa Han Chinese, respectively, and 20% (21.8% in boys and 18.5% in girls) and 4% (5.8% in boys and 2.1% in girls) higher levels of relative Wmax (W/kg), respectively (Table 1).
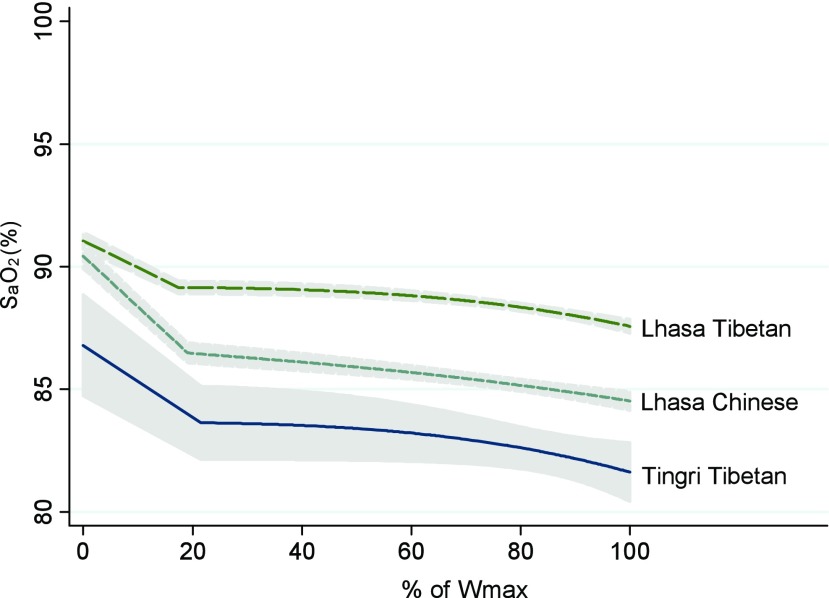
There was no difference in arterial oxygen saturation at rest (SaO2rest) between Lhasa Tibetans and Lhasa Han Chinese (i.e., no difference by ancestry), although a difference was found by residential altitude (Tingri vs. Lhasa) (Fig. 1). At rest, Lhasa Tibetan boys had a higher SaO2rest (91%±2.7%) than Tingri Tibetan boys (87%±3.5%, p<0.001), while Lhasa Tibetan girls had a higher SaO2rest (91%±2.6%) than both Tingri Tibetan girls (88%±3.2%, p<0.001) and Lhasa Han Chinese girls (90%±3.3%, p=0.013) (not shown in table). The oxygen saturation gradually decreased with an increasing power output for all three groups of children (Fig. 1), and at maximal power output, Lhasa Tibetan children had a significantly higher arterial oxygen saturation (SaO2peak) than both Tingri Tibetans (boys: 88%±3.7% vs. 82%±6.3%, p<0.001; girls: 88%±3.6% vs. 82%±6.7%, p<0.001), and Lhasa Han Chinese (boys: 88%±3.7% vs. 85%±4.8 %, p<0.001; girls: 88%±3.6% vs. 85%±4.8%, p<0.001).
FIG. 1.
Alterations in arterial oxygen saturation (SaO2) from rest to maximal exercise based on the cycle ergometer test in 9–10-year-old Tingri Tibetan, Lhasa Tibetan, and Lhasa Han Chinese children. Values are presented as means (95% CI) (y-axis: % SaO2; x-axis: % of maximal power output (Wmax)).
Relationship between maximal power output (Wmax) and selected physiological factors
Coefficients from linear regression analyses are presented in Table 2, and in Model 1, Wmax for Lhasa Tibetans was 86 W (95% confidence interval: 83.2, 88.2). The effect of residential altitude on expected Wmax was estimated by contrasting the two study groups, Tingri vs. Lhasa Tibetans, yielding a 16 (−18.8, −14.0) W lower Wmax in Tingri Tibetans. The effect of ancestry was estimated by contrasting Lhasa Han Chinese vs. Lhasa Tibetans, yielding a 5 (−6.4, −2.9) W lower Wmax in Lhasa Han Chinese. These results indicate that Tibetans living at a higher altitude had a lower Wmax, and Han Chinese had a lower Wmax than the Tibetans at the same altitude. Model 2 shows that all the included physiological variables were associated with Wmax. For example, for every increase in maximal heart rate of one beat per minute, the exercise capacity (maximal power output, Wmax) increased by 0.4 W, and for a one liter increase in lung volume (FVC), the Wmax increased by 13.2 W, whereas a one kg body mass increase was associated with a 1 W increase in Wmax (Table 2, Model 2). Girls had an 8.8 W lower Wmax than boys (Table 2, Model 2), and the explained variance (R2) of Model 2 was 54%. The estimated Wmax was 79 W when the following were set as a reference: a boy with a body mass=27 kg; [Hb]=14.6 g/L; HRpeak=195 beats/min; SaO2rest=90%; SaO2peak=86% and FVC=2.00 (Table 2, Model 2). Model 3 shows that the “study group” (i.e., contrasting both Tingri vs. Lhasa Tibetans and Lhasa Han Chinese vs. Lhasa Tibetans) was significantly associated with Wmax after an adjustment for the explanatory physiological variables in Model 2, indicating that the effects of residential altitude and ancestry on Wmax were not due to the effects of these intermediate variables. Compared to Lhasa Tibetan, Tingri Tibetan and Lhasa Han Chinese had an 18.1 W and 4.1 W lower maximal power output, respectively. Maximal heart rate, arterial oxygen saturation at rest and at maximal exercise, and lung volume were associated with exercise capacity (maximal power output, Wmax). The explained variance in Model 3 was 63%, indicating that the combination of physiological variables, ancestry, and residential altitude increased the explained variance by 9% points compared with Model 2, which did not include ancestry and residential altitude. We conducted an additional analysis in which the “study group” included Tingri Tibetans, Lhasa Tibetans, and Lhasa Han Chinese Highland-born- and Lhasa Han Chinese Lowland-born children (not presented in table). Compared to Lhasa Tibetan, Tingri Tibetan had an 18.1 W lower maximal power output, while highland-born- and lowland-born Han Chinese had a 5.3 W and 3.7 W, respectively, lower maximal power output. Maximal heart rate, arterial oxygen saturation at rest and at maximal exercise, and lung volume did not affect the associations between “study group” (i.e., residential altitude and ancestry), with exercise capacity (maximal power output, Wmax) (not shown in table).
Table 2.
Coefficients of Maximal Power Output from Three Models, Based on Data from 9–10-Year-Old Native Tibetan and Han Chinese Children Living in Lhasa at 3700 m and Native Tibetan Children Living in Tingri at 4300 m Above Sea Level#
| Wmax | Coef. | 95% confidence interval |
|---|---|---|
| Model 1 | ||
| Constant | 85.7** | 83.2–88.2 |
| Lhasa Tibetan | 0 | |
| Tingri Tibetan | −16.4** | −18.8–−14.0 |
| Lhasa Han Chinese | −4.7** | −6.4–−2.9 |
| Sex | −10.6** | −12.2–−9.1 |
| Body mass | 1.5** | 1.3–1.6 |
| Owning bicycle▴ | −4.5** | −6.1–−2.9 |
Model 2
| ||
| Constant▵ | 78.9** | 76.4–81.4 |
| Sex | −8.7** | −10.4–−7.1 |
| Body mass | 1.3** | 1.1–1.5 |
| Owning bicycle▴ | −4.4** | −6.–−2.8 |
| [Hb] | 0.9* | 0.3–1.5 |
| HRpeak | 0.4** | 0.3–0.4 |
| SaO2rest | 0.8** | 0.5–1.0 |
| SaO2peak | −0.3* | −0.4–−0.1 |
| FVC | 13.2** | 9.8–16.5 |
Model 3  | ||
| Constant▵ | 82.5** | 80.1–84.9 |
| Lhasa Tibetan | 0 | |
| Tingri Tibetan | −18.1** | −20.6–−15.5 |
| Lhasa Han Chinese | −4.1** | −5.8–−2.4 |
| Sex | −8.4** | −9.9–−6.9 |
| Body mass | 0.9** | 0.7–1.0 |
| Owning bicycle▴ | −3.5** | −5.0–−2.0 |
| [Hb] | 0.3 | −0.3–0.9 |
| HRpeak | 0.2** | 0.2–0.3 |
| SaO2rest | 0.4* | 0.1–0.6 |
| SaO2peak | −0.6** | −0.7–−0.4 |
| FVC | 16.2** | 13.1–19.3 |
No bivariate association was found between HRrest and a maximal power output, thus not included in Models; ▴Own a bicycle (vs. no bicycle) was set as reference; ▵when boy is set as reference and body mass=27 kg, [Hb]=14.6g/L, HRpeak=195 beats/min, SaO2rest=90%, SaO2peak=86%, FVC=2.00; *0.001<p<0.01; **p<0.001;  The explained variance (R2) is 54%;
The explained variance (R2) is 54%; 
 The explained variance (R2) is 63%.
The explained variance (R2) is 63%.
Discussion
In the present study we have shown that native Lhasa Tibetan 9–10-year-old children presented a higher exercise capacity than both native Tingri Tibetans and Lhasa Han Chinese when measured at their respective residential altitudes. [Hb], SaO2rest, SaO2peak, HRpeak, and FVC were all associated with exercise capacity, but did not significantly impact on the associations between residential altitude and ancestry with exercise capacity (maximal power output, Wmax). Moreover, native Tibetans exhibited a lower [Hb] and higher SaO2rest than Han Chinese living at the same altitude, whereas native Tibetans at 4300 m had a lower [Hb] than native Tibetans at 3700 m.
Compared to Lhasa Tibetan children living at 3700 m, the lower maximal power output in native Tingri Tibetans living at 4300 m may be due to the reduction in the inspired partial pressure of oxygen (PiO2). Furthermore, children's growth and development are closely correlated with their work and exercise performance, as well as altitude adaptation (Frisancho et al., 1975; 1985). The present study reveals that Tingri Tibetan children were shorter and lighter than Tibetan children from Lhasa of the same age, and such findings are consistent with a previous comparative study of Tibetan children and adolescents aged 7–18 years living at various altitudes from 2261 m to 4040 m in Qinghai-Tibet. The children from the highest altitude (4040 m) presented a slower growth (stature), lagging behind their counterparts at 2261 m by approximately 2 years. It was also indicated that hypoxia may be the main agent responsible for this delayed growth (Zhang et al., 1985), and Greksa et al. (1984) suggested that socioeconomic factors slowed the growth of high-altitude children (Greksa et al., 1984). In the present study, we have no valid information on socio-economic status (SES). It is a challenge to identify an indicator of SES that is valid for both Lhasa and Tingri. For example, in Tingri, parents generally have a low or no education and no income, but they may have many Yak or sheep, thus indicating a high SES. In contrast, in Lhasa, parents seldom own animals, and most males have an education, as well as income.
We speculate that the present finding of a lower [Hb] among Tingri Tibetan children compared with Lhasa Tibetan children, which stands in contrast to previous reports of an increase in [Hb] with an increasing altitude (Wu et al., 2005), may be due to poor nutrition, including less iron-rich foods at high altitude.
The present finding of a higher maximal power output in native Tibetan- compared to Han Chinese children at the same altitude may be due to a better oxygen transport system. The difference in oxygen concentration between arterial- and mixed-venous blood is a measure of tissue oxygen extraction, which can be inferred from [Hb] and the difference between the oxygen saturation in arterial- and mixed-venous blood (Sao2 - Svo2) (de Meer et al., 1995). In comparison with Han Chinese, a lower [Hb] in adult native Tibetans has previously been reported (Curran et al., 1997; Beall et al., 1998; Moore, 2000; Garruto et al., 2003; Wu et al., 2005). Additionally, the lower [Hb] in Tibetan subjects has been suggested to be a result of adaptation over many generations (Wu et al., 2005; Simonson et al., 2012), which has further been suggested to be more favorable than Andeans, who are characterized by a raised [Hb] (Stuber and Scherrer, 2010). Recent genetic studies identified genetic variants in native Tibetans living between 3200 and 4300 m compared with Han Chinese lowlanders (Beall et al., 2010; Bigham et al., 2010; Simonson et al., 2010; Yi et al., 2010; Peng et al., 2011; Xiang et al., 2013). Three genes, EPAS1, EGLN1, and PPARA, have been shown to be associated with hemoglobin concentration (Simonson et al., 2012), while no associations were found with EPAS1 and EGLN1 in Andeans (Bigham et al., 2013). Hence, genetic factors may explain the lower [Hb] in Lhasa Tibetans compared with Han Chinese in the present study.
Although the present Lhasa Tibetan children also had a lower [Hb] than Han Chinese, they sustained a higher SaO2 during maximal exercise than Lhasa Han Chinese children. This phenomenon could be explained by better maintained ventilation and/or a regulated production of [Hb] (Niermeyer et al., 2001). According to Moore et al. (1992), it is not likely that a leftward shift in the oxyhemoglobin dissociation curve in Lhasa Tibetans could help explain the present finding. As with other high altitude populations, they reported that the position of the Hb-O2 dissociation curve in Tibetans was the same as that seen in sea level populations (Moore et al., 1992). We suppose that the higher SaO2 across the entire range of exercise intensities in Lhasa Tibetan children may indirectly reflect their smaller alveolar-arterial O2 gradients ((A-a) DiffO2), thereby indicating a larger diffusion capacity. If this assumption is correct for children, they may better maintain oxygen saturation during exercise compared to Han Chinese children (Zhuang et al., 1996; Wu and Kayser, 2006). It has been suggested that diffusion capacity could be a decisive factor for oxygen transport during maximal exercise at high altitude (Chen et al., 1997), and De Meer et al. summarized that an increase in pulmonary diffusion capacity facilitates oxygen delivery to the tissues in both children and adults living at higher altitudes (de Meer et al., 1995). As shown in several studies, an increased lung volume is also accompanied by an increased pulmonary diffusion capacity (DeGraff et al., 1970; Guleria et al., 1971; Cerny et al., 1973; Johnson et al., 1985; Sun et al., 1990; Armstrong and Welsman, 1994). Moreover, large lung volumes and chest circumferences have been reported among schoolchildren and adolescents who are native to high altitude (Frisancho, 1969; Beall et al., 1977), though not in immigrants acclimatized to high altitude (Frisancho et al., 1973). In the present study, native Lhasa Tibetan children had a significantly larger FVC and chest circumference than Lhasa Han Chinese children, even after adjusting for body mass and stature (data not shown).
A large sample size and high participation rates are strengths of the present study, while a weakness is that the study could have underestimated the exercise capacity for children who were less accustomed to cycling. However, the adjustment by the variable “owning a bicycle” in Models 1–3 may have partly compensated for differential cycling practices across the three groups of children. A selection of healthier children for the schools in rural Tingri may have taken place because of the need to be sufficiently physically healthy to walk the long way to school, and to live at the school dormitory for long periods. This is in contrast to urban Lhasa children, who are staying with their families, and have short travel distances. As a result, this possible selection would most likely have distorted the results towards a smaller difference between Tingri- and Lhasa children.
Conclusion
The superior exercise capacity in native Tibetan children vs. Han Chinese immigrant children living at the same altitude of 3700 m may reflect a better adaptation to life at high altitude. The lower exercise capacity at a higher altitude in native Tibetans may partially be attributed to residential altitude differences. In addition to residential altitude and ancestry, selected physiological parameters explained some of the variance in exercise capacity, including higher arterial oxygen saturation and a higher forced vital capacity, but these physiological factors did not fully account for the associations between residential altitude and ancestry with a maximal power output. Future studies comparing Tibetan and Han Chinese children living at the same altitude should also take genetic factors into account, in addition to potentially unmeasured confounders such as SES and food habits. These confounders should also be included in future studies comparing native Tibetans living at different altitudes.
Acknowledgments
We are very thankful to the Network for University Cooperation Tibet-Norway for supporting this study. Thanks too to all the children who participated in this study and to our colleagues at the Tibet University Medical College, who gave great support in the data collection.
Author Disclosure Statement
Drs. Bianba, Bernstsen, Andersen, Stigum, Ouzhuluobu, Nafstad, Wu, and Bjertness have no conflicts of interest or financial ties to disclose.
References
- Aandstad A, Berntsen S, Hageberg R, Klasson-Heggebo L, and Anderssen SA. (2006). A comparison of estimated maximal oxygen uptake in 9 and 10 year old schoolchildren in Tanzania and Norway. Br J Sports Med 40:287-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenderfer MS. (2003). Moving up in the world; Archaeologists seek to understand how and when people came to occupy the Andean and Tibetan plateaus. Am Sci. 91:542–549 [Google Scholar]
- Andersen LB. (1995). A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports 5:143–146 [DOI] [PubMed] [Google Scholar]
- Andersen LB, Henckel P, and Saltin B. (1987). Maximal oxygen uptake in Danish adolescents 16–19 years of age. Eur J Appl Physiol Occup Physiol 56:74–82 [DOI] [PubMed] [Google Scholar]
- Armstrong N, and Welsman JR. (1994). Assessment and interpretation of aerobic fitness in children and adolescents. Exerc Sport Sci Rev 22:435–476 [PubMed] [Google Scholar]
- Arngrimsson SA, Sveinsson T, and Johannsson E. (2008). Peak oxygen uptake in children: Evaluation of an older prediction method and development of a new one. Pediatr Exerc Sci 20:62–73 [DOI] [PubMed] [Google Scholar]
- Astrand PO, and Rodahl K. (1986). Textbook of Work Physiology: Physiological Bases of Exercise. McGraw-Hill, Now York [Google Scholar]
- Beall CM, Baker PT, Baker TS, and Haas JD. (1977). The effects of high altitude on adolescent growth in southern Peruvian Amerindians. Hum Biol 49:109–124 [PubMed] [Google Scholar]
- Beall CM, Brittenham GM, Strohl KP, et al. (1998). Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol 106:385–400 [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, et al. (2010). Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107:11459–11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianba B, Berntsen S, Andersen LB, Stigum H, and Bjertness E. (2010). Estimation of peak oxygen uptake from maximal power output among 9–10-year-old children in Lhasa, Tibet. J Sports Med Phys Fitness 50:274–280 [PubMed] [Google Scholar]
- Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer Sw, Julian CG, Wilson MJ, Lopez Herraez D, Brutsaert T, Parra EJ, Moore LG, Shriver MD. (2010). Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet 6:e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Wilson MJ, Julian CG, et al. (2013). Andean and Tibetan patterns of adaptation to high altitude. Am J Hum Biol 25:190–197 [DOI] [PubMed] [Google Scholar]
- Cerny FC, Dempsey JA, and Reddan WG. (1973). Pulmonary gas exchange in nonnative residents of high altitude. J Clin Invest 52:2993–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, Kobayashi T, and Yoshimura K. (1997). Exercise performance of Tibetan and Han adolescents at altitudes of 3417 and 4300 m. J Appl Physiol 83:661–667 [DOI] [PubMed] [Google Scholar]
- Curran LS, Zhuang J, Droma T, and Moore LG. (1998). Superior exercise performance in lifelong Tibetan residents of 4,400 m compared with Tibetan residents of 3,658 m. Am J Phys Anthropol 105:21–31 [DOI] [PubMed] [Google Scholar]
- Curran LS, Zhuang J, Sun SF, and Moore LG. (1997). Ventilation and hypoxic ventilatory responsiveness in Chinese-Tibetan residents at 3,658 m. J Appl Physiol 83:2098–2104 [DOI] [PubMed] [Google Scholar]
- de Meer K, Heymans HS, and Zijlstra WG. (1995). Physical adaptation of children to life at high altitude. Eur J Pediatr 154:263–272 [PubMed] [Google Scholar]
- DeGraff AC, Jr., Grover RF, Johnson RL, Jr., Hammond JW, Jr., and Miller JM. (1970). Diffusing capacity of the lung in Caucasians native to 3,100 m. J Appl Physiol 29:71–76 [DOI] [PubMed] [Google Scholar]
- Frisancho AR. (1969). Human growth and pulmonary function of a high altitude Peruvian Quechua population. Hum Biol 41:365–379 [PubMed] [Google Scholar]
- Frisancho AR, Borkan GA, and Klayman JE. (1975). Pattern of growth of lowland and highland Peruvian Quechua of similar genetic composition. Hum Biol 47:233–243 [PubMed] [Google Scholar]
- Frisancho AR, Martinez C, Velasquez T, Sanchez J, and Montoye H. (1973a). Influence of developmental adaptation on aerobic capacity at high altitude. J Appl Physiol 34:176–180 [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Matos J, Leonard WR, and Yaroch LA. (1985). Developmental and nutritional determinants of pregnancy outcome among teenagers. Am J Phys Anthropol 66:247–261 [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Velasquez T, and Sanchez J. (1973b). Influence of developmental adaptation on lung function at high altitude. Hum Biol 45:583–594 [PubMed] [Google Scholar]
- Fulco CS, Rock PB, and Cymerman A. (1998). Maximal and submaximal exercise performance at altitude. Aviat Space Environ Med 69:793–801 [PubMed] [Google Scholar]
- Garruto RM, Chin CT, Weitz CA, Liu JC, Liu RL, and He X. (2003). Hematological differences during growth among Tibetans and Han Chinese born and raised at high altitude in Qinghai, China. Am J Phys Anthropol 122:171–183 [DOI] [PubMed] [Google Scholar]
- Ge RL, Chen QH, Wang LH, et al. (1994). Higher exercise performance and lower VO2max in Tibetan than Han residents at 4,700 m altitude. J Appl Physiol 77:684–691 [DOI] [PubMed] [Google Scholar]
- Greksa LP, Spielvogel H, Paredes-Fernandez L, Paz-Zamora M, and Caceres E. (1984). The physical growth of urban children at high altitude. Am J Phys Anthropol 65:315–322 [DOI] [PubMed] [Google Scholar]
- Grover RF, Reeves JT, Grover EB, and Leathers JE. (1967). Muscular exercise in young men native to 3,100 m altitude. J Appl Physiol 22:555–564 [DOI] [PubMed] [Google Scholar]
- Groves BM, Droma T, Sutton JR, et al. (1993). Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J Appl Physiol 74:312–318 [DOI] [PubMed] [Google Scholar]
- Guleria JS, Pande JN, Sethi PK, and Roy SB. (1971). Pulmonary diffusing capacity at high altitude. J Appl Physiol 31:536–543 [DOI] [PubMed] [Google Scholar]
- Hansen HS, Froberg K, Nielsen JR, and Hyldebrandt N. (1989). A new approach to assessing maximal aerobic power in children: The Odense School Child Study. Eur J Appl Physiol Occup Physiol 58:618–624 [DOI] [PubMed] [Google Scholar]
- Huddleston B, Ataman E, and Fe d'Ostiani L. (2003). Towards a GIS-Based Analysis of Mountain Environments and Populations. FAO, Rome [Google Scholar]
- Johnson RL, Jr., Cassidy SS, Grover RF, Schutte JE, and Epstein RH. (1985). Functional capacities of lungs and thorax in beagles after prolonged residence at 3,100 m. J Appl Physiol 59:1773–1782 [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, et al. (2005). Standardisation of spirometry. Eur Respir J 26:319–338 [DOI] [PubMed] [Google Scholar]
- Moore LG. (2000). Comparative human ventilatory adaptation to high altitude. Respir Physiol 121:257–276 [DOI] [PubMed] [Google Scholar]
- Moore LG, Zamudio S, Curran-Everett L, et al. (1994). Genetic adaptation to high altitude. In: Advances in Exercise and Sports Medicine. Wood S. and Roach RC, eds.: New York: Marcel Dekker Inc., pp. 225–262 [Google Scholar]
- Niermeyer S, Zamudio S, Moore LG. (2001). The People. In High Altitude: An Exploration of Human Adaptation. Hornbein TF, Schoene R.B., ed. Marcel Dekker, New York: Pp. 43–99 [Google Scholar]
- Niu W, Wu Y, Li B, Chen N, and Song S. (1995). Effects of long-term acclimatization in lowlanders migrating to high altitude: Comparison with high altitude residents. Eur J Appl Physiol Occup Physiol 71:543–548 [DOI] [PubMed] [Google Scholar]
- Peng Y, Yang Z, Zhang H, et al. (2011). Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol 28:1075–1081 [DOI] [PubMed] [Google Scholar]
- Riddoch C, Edwards D, Page A, Froberg K, Anderssen SA, and Wedderkopp Nea. (2005). The European Youth Heart Study-Cardiovascular Disease Risk Factors in Children: Rationale, aims, study design, and validation of methods. J Phys Activity Health 2:115–129 [Google Scholar]
- Simonson TS, McClain DA, Jorde LB, and Prchal JT. (2012). Genetic determinants of Tibetan high-altitude adaptation. Hum Genet 131:527–533 [DOI] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, et al. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329:72–75 [DOI] [PubMed] [Google Scholar]
- Stuber T, and Scherrer U. (2010). Circulatory adaptation to long-term high altitude exposure in Aymaras and Caucasians. Prog Cardiovasc Dis 52:534–539 [DOI] [PubMed] [Google Scholar]
- Sun SF, Droma TS, Zhang JG, et al. (1990). Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Respir Physiol 79:151–161 [DOI] [PubMed] [Google Scholar]
- Tibet Statistical Yearbook (2009). China statistics press; National Bureau of Statistics of China, Beijing 100826, P. R. China [Google Scholar]
- Woynarowska B. (1980). The validity of indirect estimations of maximal oxygen uptake in children 11–12 years of age. Eur J Appl Physiol Occup Physiol 43:19–23 [DOI] [PubMed] [Google Scholar]
- Wu T, and Kayser B. (2006). High altitude adaptation in Tibetans. High Alt Med Biol 7:193–208 [DOI] [PubMed] [Google Scholar]
- Wu T, Wang X, Wei C, et al. (2005). Hemoglobin levels in Qinghai-Tibet: Different effects of gender for Tibetans vs. Han. J Appl Physiol 98:598–604 [DOI] [PubMed] [Google Scholar]
- Xiang K, Ouzhuluobu , Peng Y, et al. (2013). Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation. Mol Biol Evol 30:1889–1898 [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, et al. (2010). Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YB, Wang Y, Wu TY, et al. (1985). High Altitude Diseases. Qinghai People's Publishing House, Xining [Google Scholar]
- Zhuang J, Droma T, Sutton JR, et al. (1996). Smaller alveolar-arterial O2 gradients in Tibetan than Han residents of Lhasa (3658 m). Respir Physiol 103:75–82 [DOI] [PubMed] [Google Scholar]
- Zhuang J, Droma T, Sutton JR, et al. (1993). Autonomic regulation of heart rate response to exercise in Tibetan and Han residents of Lhasa (3,658 m). J Appl Physiol 75:196819–73 [DOI] [PubMed] [Google Scholar]