Abstract
Keramidas, Michail E, Roger Kölegård, Igor B. Mekjavic, and Ola Eiken. Acute effects of normobaric hypoxia on hand-temperature responses during and after local cold stress. High Alt Med Biol. 15:183–191, 2014.—The purpose was to investigate acute effects of normobaric hypoxia on hand-temperature responses during and after a cold-water hand immersion test. Fifteen males performed two right-hand immersion tests in 8°C water, during which they were inspiring either room air (Fio2: 0.21; AIR), or a hypoxic gas mixture (Fio2: 0.14; HYPO). The tests were conducted in a counterbalanced order and separated by a 1-hour interval. Throughout the 30-min cold-water immersion (CWI) and the 15-min spontaneous rewarming (RW) phases, finger-skin temperatures were measured continuously with thermocouple probes; infrared thermography was also employed during the RW phase to map all segments of the hand. During the CWI phase, the average skin temperature (Tavg) of the fingers did not differ between the conditions (AIR: 10.2±0.5°C, HYPO: 10.0±0.5°C; p=0.67). However, Tavg was lower in the HYPO than the AIR RW phase (AIR: 24.5±3.4°C; HYPO: 22.0±3.8°C; p=0.002); a response that was alike in all regions of the immersed hand. Accordingly, present findings suggest that acute exposure to normobaric hypoxia does not aggravate the cold-induced drop in hand temperature of normothermic males. Still, hypoxia markedly impairs the rewarming responses of the hand.
Key Words: : CIVD, cold injury, cold pain, cold tolerance, high altitude, rewarming
Introduction
Epidemiological studies have shown that exposure to high altitude (>2800 m) is associated with high incidence of local cold injury (Hashmi et al., 1998; Harirchi et al., 2005). In high altitude conditions, hypoxia coexists with a number of environmental (e.g., cold, wind) and behavioral (e.g., reduced physical activity and energy intake) components that, independently or synergistically, might constitute risk factors for local cold injury. To establish whether a causal relationship exists between hypoxia per se and cold injury, a number of studies (Daanen and van Ruiten, 2000; Mathew et al., 1977; 1979; Purkayastha et al., 1999; Takeoka et al., 1993) examining the vasomotor responses of the extremities during local cold stress in controlled laboratory conditions at high altitude have suggested that systemic arterial hypoxia exaggerates the cold-induced cutaneous vasoconstriction and impairs any cold-induced vasodilatation (CIVD) response.
However, in the above studies (Daanen and van Ruiten, 2000; Mathew et al., 1977; 1979; Takeoka et al., 1993; Purkayastha et al., 1999), the temperature of the digits prior to the local cold provocation was significantly lower in the hypoxic than in the normoxic cold test, despite the similar general thermal status of the subjects; this effect appears not to have been taken into account when concluding that hypoxia potentiates the cold-induced vasoconstrictor response. In view of the fact that the cold tests were performed a few hours (Daanen and van Ruiten, 2000; Mathew et al., 1977) or days (Daanen and van Ruiten, 2000; Mathew et al., 1977; 1979; Takeoka et al., 1993; Purkayastha et al., 1999) after the arrival of the subjects at high altitude, the enhanced peripheral vasoconstrictor tone was probably attributable either to the distinct effects of hypoxia (Heistad and Wheeler, 1970; Kollai, 1983), or to the synergistic actions of hypoxia and low ambient temperature (Cipriano and Goldman, 1975). Considering that the response magnitude of a physiological function may be significantly affected by its basal level of activity (cf. Hodges et al., 2007), it is not clear whether cold-induced effects on cutaneous circulation would be superimposed on, or mask those induced by hypoxia.
Aside from the temperature response during local cold stress, the skin temperature reaction, in a period of spontaneous rewarming following the cold provocation, has been used as a means to examine the cold sensitivity (cf. Davey et al., 2013) and to detect certain vascular dysfunctions in the extremities (cf. Ahle et al., 1990; Brandstrom et al., 2008; Ruijs et al., 2008). With regard to peripheral rewarming in hypoxia, a substantial reduction in skin temperature recovery of the middle fingertip (Fahim, 1992) and the center of the palm (Rai et al., 1978) has been observed following a short (1–2 min) local cold test. However, the magnitude of the rewarming response is dependent on the duration of the cooling phase (Wolff and Pochin, 1949), during which a drop in the temperature of deeper tissue layers is induced (Barcroft and Edholm, 1943). Furthermore, whether all exposed segments of the hand would react in a similar manner during a hypoxic rewarming period is uncertain, since the neurogenic control of the cutaneous arterioles differs between the glabrous and the nonglabrous areas of the hand (cf. Minson, 2003).
Accordingly, the purpose of the present study was to examine the acute effects of normobaric hypoxia on the hand-temperature responses of healthy males during and after a 30-min local cold-water immersion test. We hypothesized that hypoxia would diminish the finger CIVD response during the cold-water immersion phase, and also attenuate the rewarming response of the hand. To accomplish a detailed mapping of the spontaneous rewarming response of all segments of the hand and fingers, changes in skin temperature were monitored using both thermocouple probes and infrared thermography.
Materials and Methods
Subjects
Fifteen healthy males participated in the study (age: 24.9±3.0 years; body mass: 74.8±8.7; height: 181.0±5.7 cm; body fat: 22.2±5.3%; 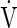 O2max: 3.2±0.5 L·min−1). They were nonsmokers, and had no history of any cardiovascular, circulatory, hematological, or pulmonary disease. All subjects were near-sea level residents, and had not been exposed to altitude >500 m during the month preceding the experiments. They were physically active on a recreational basis, and had no, or very limited previous experience with cold-exposure experiments. The subjects were informed in detail about the experimental procedures, and gave their written consent. They were instructed not to engage in any strenuous activity and to refrain from consuming alcohol or any caffeinated product a day before the tests. The experimental protocol was approved by the National Committee for Medical Ethics at the Ministry of Health of the Republic of Slovenia and conformed to the Declaration of Helsinki.
O2max: 3.2±0.5 L·min−1). They were nonsmokers, and had no history of any cardiovascular, circulatory, hematological, or pulmonary disease. All subjects were near-sea level residents, and had not been exposed to altitude >500 m during the month preceding the experiments. They were physically active on a recreational basis, and had no, or very limited previous experience with cold-exposure experiments. The subjects were informed in detail about the experimental procedures, and gave their written consent. They were instructed not to engage in any strenuous activity and to refrain from consuming alcohol or any caffeinated product a day before the tests. The experimental protocol was approved by the National Committee for Medical Ethics at the Ministry of Health of the Republic of Slovenia and conformed to the Declaration of Helsinki.
Experimental protocol
The study was conducted at the Olympic Sports Center Planica (Rateče, Slovenia) that is situated at an altitude of 940 m. Subjects arrived at the Center 36 hours before the tests. On a first visit to the laboratory, subjects were thoroughly familiarized with the experimental procedure. Thereafter, they performed two cold-water hand immersion tests during which they were breathing either room air (AIR), or a hypoxic gas mixture (HYPO; Fio2: 0.14; simulated altitude of 4000 m). The tests were conducted in a counterbalanced order and separated by a 60–65 min interval. All tests were performed in May and at the same time of the day (morning hours). Subjects were dressed in T-shirts, short trousers, and socks; and throughout each test they remained in a sitting position on a chair. Prior to the start of each test, subjects were accustomed to the conditions of the laboratory for ∼20 min. The mean temperature, relative humidity, and barometric pressure during the tests were 20.9±0.8°C, 35.1±5.5%, and 682±4 mmHg, respectively.
Throughout the HYPO test, subjects breathed through a low resistance two-way respiratory valve (Model 2, 700 T-Shape, Hans Rudolph, Inc. Shawnee, USA). The inspiratory side of the valve was connected via a respiratory corrugated tubing to a 200 L Douglas bag filled with the pre-mixed humidified breathing gas.
Both tests commenced with a 5-min baseline phase, during which subjects rested with both hands on an emissivity neutral, customized hand-support at the level of their hips. After that, the right hand was covered with a thin plastic bag (thickness of 0.025 mm) sealed with air permeable tape to the skin (∼10 cm above the wrist), and the hand was then immersed up to the ulnar and radial styloids in warm water (35°C) for 5 min. Subsequently, the hand was removed from the warm-water tank, and placed without the plastic bag on the hand-support for ∼1 min, during which infrared-thermal images were obtained (see below for details). Thereafter, the hand was covered with a new plastic bag and was immersed in a tank containing cold water (8°C; see Mekjavic et al., 2013) for 30 min (CWI). The temperature of the water was maintained by means of a cooling system (Haake, Germany), and a small impeller continuously stirred the water inside the tank. After the completion of the CWI phase, the hand was removed from the water, dried with a towel, if necessary, and a 15-min spontaneous rewarming (RW) phase ensued, during which both hands were resting on the hand-support as in the baseline phase. Subjects were instructed to, throughout each test, keep the non-immersed hand immobile on the hand-support at the level of the hip.
Instrumentation
Temperature measurements
Thermocouple probes
Throughout the test, the finger skin temperatures of the immersed hand were measured with copper-constantan (T-type) thermocouple (each conductor was 0.2 mm in diameter) probes (Physitemp Instruments Inc, Clifton, NJ, USA), which were attached to the skin in the middle of the palmar side of the distal phalanx of each finger. The primary insulation of the thermocouples was polytetrafluoroethylene (PTFE); the non-insulated welded junctions of the thermocouple were attached directly to the skin with thin air-permeable tape (Tegaderm, 3M, Healthcare, St. Paul, MN, USA). All skin temperatures were sampled every second with a NI USB-6215 (National Instruments, Austin, Texas, USA) data acquisition system, processed with TestPoint software (TestPoint v7®, Norton, Massachusetts, USA), and stored in a PC (Dell, USA) for further analysis. Following a manual check of the raw data, a computer program written in TestPoint was used to calculate the average temperature (Tavg) of each finger during every phase, as well as the minimum (Tmin) and maximum (Tmax) temperatures reached during the CWI and RW phases. The same program was also used to detect any finger CIVD response, defined as a local skin-temperature wave (N) in terms of >1°C increase lasting for a minimum duration of 3 min. In case of a CIVD response, the following parameters were determined: (i) the temperature amplitude (ΔT), which was the difference between the lowest temperature recorded just before the CIVD and the highest temperature reached during the CIVD, and (ii) the duration of a local-skin temperature wave (Δt).
Two additional thermocouple probes measured the Tavg at two sites of the immersed hand, one being positioned at the center of the opisthenar region (Topisthenar) and the other on the radial side of the forearm (midway between the elbow and the wrist joints; Tforearm). Furthermore, Tavg of the distal phalanx of the thumb and of the center of the palm of the left non-immersed hand were also measured with thermocouple probes. All temperature variables were recorded every second using the aforementioned data acquisition system (National Instruments, USA).
Infrared thermography
At the end of the baseline phase, immediately after the warm-water phase, and during the RW phase (at minutes 1, 2, 3, 4, 5, 10, and 15), the skin temperature from the dorsal and ventral side of the immersed hand was recorded with an infrared camera (T365, FLIR Systems AB, Täby, Sweden), which was calibrated automatically. The field of the camera's view was 25°×19°, the spatial resolution was 1.36 mrad, the spectral range was 7.5–13 μm and the infrared detector resolution was 320×240 pixels. The distance between the camera and the hand was ∼60 cm. After the completion of the test, the thermal images were transferred to a PC (Dell, USA) via a USB cable, and subsequently analyzed using the ThermaCam Researcher PRO software (version 2.10, FLIR Systems, Sweden). Namely, Tavg was determined for the following anatomical areas of the right hand: (i) the palmar and the dorsal side of the distal phalanx of each finger, (ii) the palmar and the dorsal side of the proximal phalanx of each finger, (iii) the total palm and (iv) opisthenar areas.
Tympanic temperature
During the baseline and RW phases, the tympanic temperature (Ttympanic) was measured using a commercially available infrared thermometer (ThermoScan IRT 3020, Braun, Kronberg, Germany). Two consecutive measurements were obtained each time and the higher of the two values was used for subsequent analysis.
Hemodynamic variables
Heart rate (HR) was recorded using a heart-rate monitor (S800CX; Polar, Kempele, Finland). Systolic (SAP) and diastolic (DAP) arterial pressure was measured at 5-min intervals using an automated oscillometric sphygmomanometer (Omron M6, Kyoto, Japan) with the cuff positioned around the distal portion of the left upper arm. The capillary oxyhemoglobin saturation (Spo2) was monitored with a pulse oxymeter (BCI 3301, Waukesha, WI, USA) on the left index finger.
Psychometric response scales
During the baseline, warm-water, CWI (at minutes 1, 2, 3, 4, 5, and every 5 min thereafter) and RW phases, the subjects were requested to provide ratings of their thermal sensation on a 7-point scale (TS; from 1-cold to 7-hot), thermal comfort on a 4-point scale (TC; from 1-comfortable to 4-very uncomfortable) and local pain on a 10-point scale (LP; from 0-no pain to 10-unbearable pain). All scales were explained to the subjects by the same investigator prior to each test.
Statistical analysis
Statistical analyses were performed using Statistica 5.0 (StatSoft, Inc., Tulsa, OK, USA). All data are presented as mean±SD, unless otherwise indicated. A two-way ANOVA for repeated measures was used for the temperature and hemodynamic measurements. The Tukey post hoc test was employed to identify specific differences between means when ANOVAs revealed significant F-ratio for main effects. Differences in N, TC, TS, and LP were evaluated with a Wilcoxon matched pairs nonparametric test. The alpha level of significance was set a priori at 0.05.
Results
Skin-temperature responses: Immersed hand
Baseline and warm-water immersion phases
During the baseline phase, there were no differences between the two conditions as regards Tavg of the fingers (AIR: 30.5±4.2°C, HYPO: 31.0±4.2°C; p=0.67), the Topisthenar (AIR: 30.8±1.8°C, HYPO: 30.4±2.2°C; p=0.99) or the Tforearm (Fig. 1). During the warm-water immersion phase, no differences were observed between the conditions for the Tavg of the fingers (AIR: 34.4±1.8°C, HYPO: 34.1±2.1°C; p=0.42), the Topisthenar (AIR: 34.1±1.0°C, HYPO: 34.1±1.4°C; P=0.92) or the Tforearm (Fig. 1).
FIG. 1.
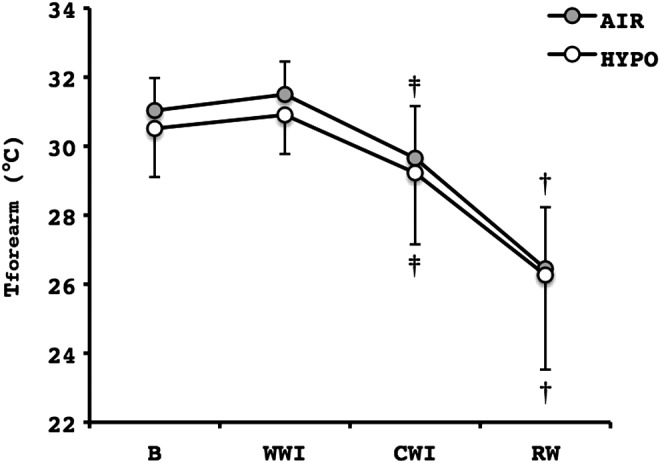
Average temperature of the radial side of the right forearm (Tforearm) during the control (AIR) and the normobaric hypoxic (HYPO) cold-water hand immersion test. Values are mean±SD. ‡Significant difference from B and WWI (p≤0.05); †Significant difference from B, WWI and CWI (p≤0.001). B, a 5-min baseline phase, a 5-min warm-water hand immersion phase, a 30-min cold-water hand immersion phase; RW, a 15-min rewarming phase; WWI, a 5-min warm-water hand immersion phase.
Cold-water immersion phase
Tavg of the fingers was not different between the first (10.1±0.6°C) and the second (10.1±0.5°C) CWI phase of the cold tests (p=0.81), thereby excluding any order effect. Tavg and Tmin of the fingers did not differ between the conditions (Table 1, Fig. 2). However, Tmax of the fingers was significantly lower in the HYPO than in the AIR CWI phase (Table 1), albeit the statistical analysis did not reveal any post hoc difference for any individual finger. Regarding the parameters of the finger CIVD response, N and Δt did not vary between the tests (Table 1); however, a statistical tendency for lower ΔT was identified during the HYPO CWI phase (p=0.06; Table 1). The drop in Topisthenar in the CWI phase was similar in the two conditions (AIR: 12.5±0.9°C, HYPO: 12.5±0.8°C; p=0.95). Tforearm was significantly reduced during CWI, and there were no differences between conditions (Fig. 1).
Table 1.
Average Temperature (Tavg), Minimum Temperature (Tmin), Maximum Temperature (Tmax), Number of Local Skin-Temperature Waves (N), Temperature Amplitude of the Wave (ΔT) and Duration of the Wave (Δt) in the Palmar Side of the Distal Phalanx of Each Finger of the Right Hand Obtained During the 30-Min Cold-Water Hand Immersion in the Control (AIR) and Normobaric Hypoxic (HYPO) Test
| AIR test | HYPO test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Finger | Finger | |||||||||||
| I | II | III | IV | V | Average | I | II | III | IV | V | Average | |
| Tavg (°C) | 10.3±0.8 | 10.4±0.7 | 10.3±0.8 | 10.1±0.6 | 9.7±0.5 | 10.2±0.5 | 10.0±0.6 | 10.4±0.7 | 10.1±0.6 | 10.0±0.6 | 9.6±0.6 | 10.0±0.5 |
| Tmin (°C) | 8.7±0.6 | 8.8±0.6 | 8.5±0.6 | 8.5±0.5 | 8.3±0.4 | 8.6±0.5 | 8.7±0.6 | 9.0±0.7 | 8.6±0.7 | 8.6±0.6 | 8.3±0.5 | 8.6±0.6 |
| Tmax (°C) | 12.3±1.2 | 12.1±1.4 | 12.3±2.2 | 11.7±1.0 | 11.0±1.1 | 11.9±1.1 | 11.6±0.9 | 11.8±0.8 | 11.5±0.5 | 11.3±1.1 | 10.8±1.2 | 11.4±0.6* |
| N | 0 (0–2) | 1 (0–2) | 1 (0–3) | 1 (0–3) | 1 (0–2) | 1 (0–2) | 0 (0–2) | 1 (0–3) | 1 (0–2) | 1 (0–3) | 1 (0–2) | 1 (0–2) |
| ΔT (°C) | 0.7±0.9 | 2.3±1.8 | 2.2±2.0 | 2.2±1.4 | 1.8±1.6 | 1.8±1.2 | 0.5±0.7 | 1.8±1.3 | 1.5±1.2 | 1.7±1.6 | 1.7±1.4 | 1.4±1.1 |
| Δt (min) | 3.2±3.7 | 8.5±5.0 | 7.3±5.6 | 7.1±3.9 | 7.3±4.9 | 6.7±2.8 | 2.2±3.4 | 7.3±4.4 | 5.7±4.5 | 6.1±4.1 | 6.1±3.6 | 5.5±3.3 |
Values are mean±SD. Values are median (range) for N. *Significant difference from the AIR test; (p=0.05).
FIG. 2.
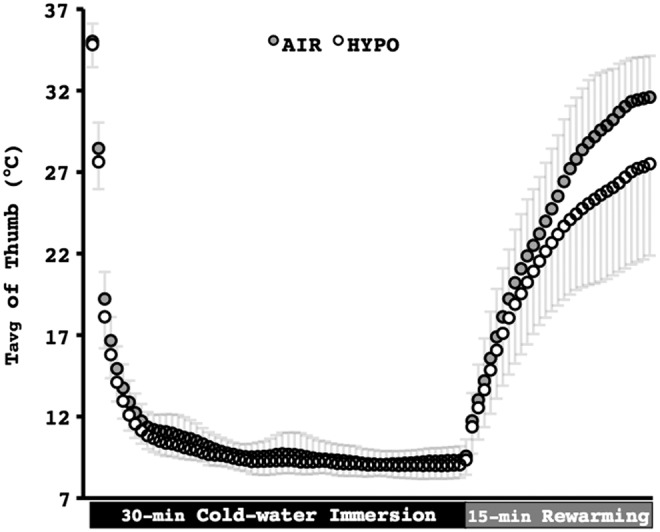
Average temperature (Tavg) of the palmar side of the distal phalanx of the right thumb during the control (AIR) and the normobaric hypoxic (HYPO) cold-water hand immersion test. Values are mean±SD. Data are averaged every 30 sec.
Rewarming phase
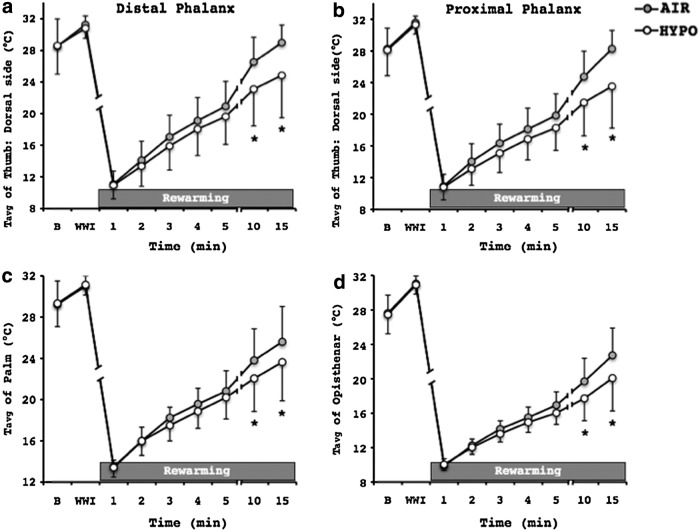
At the onset of the RW phase, there were no temperature differences between the conditions, as reflected in the similar Tmin of the fingers (AIR: 9.5±0.9°C, HYPO: 9.3±0.0°C; P=0.63). However, the hand rewarming response was substantially impaired in the HYPO test (Fig. 3). Namely, Tavg and Tmax of the fingers were significantly lower in the HYPO than the AIR RW phase (Fig. 4). At the 10th and the 15th min of the HYPO RW phase, Tavg of the fingers was markedly reduced on the palmar side of both distal (AIR: 27.3±4.0°C, HYPO: 25.3±5.3°C; p=0.02) and proximal (AIR: 26.7±3.8°C, HYPO: 25.1±5.1°C; p=0.03) phalanges, and on the dorsal side of both phalanges (Fig. 5a, b). No differences between the tests were observed in Topisthenar (AIR: 18.9±2.7°C, HYPO: 17.7±2.1°C; p=0.12). However, the Tavg of the total area of the palm and opisthenar region of the hand was significantly lower during the HYPO RW phase, in particular 10 and 15 min after the end of the CWI phase (Fig. 5c, d). Tforearm was further decreased in the RW phase, and it was significantly lower than in the CWI phase (p≤0.001); no differences between the conditions were detected (Fig. 1).
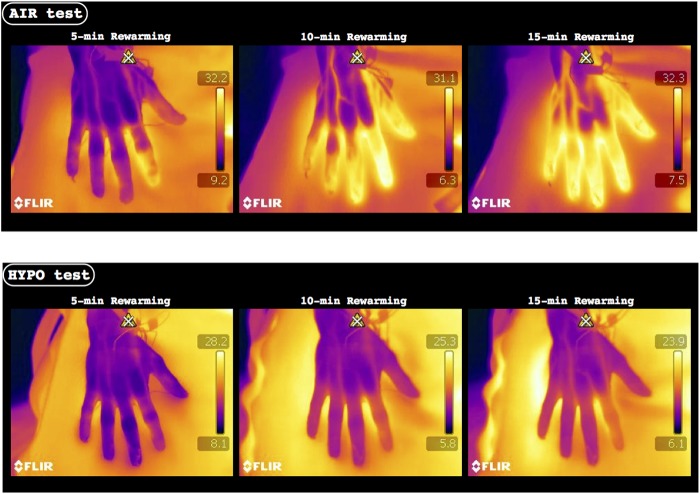
FIG. 3.
Representative infrared-thermal images from a single participant during the 5th, 10th, and 15th min of the rewarming phase of the control (AIR) and normobaric hypoxic (HYPO) test.
FIG. 4.
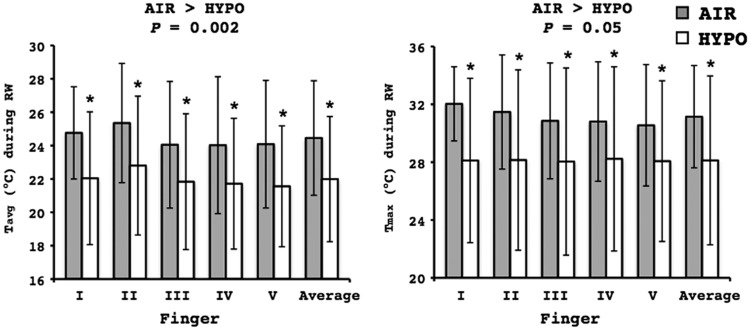
Average (Tavg; left graph) and maximum (Tmax; right graph) temperature of the palmar side of the distal phalanx of each finger of the right hand during the 15-min rewarming (RW) phase of the control (AIR) and normobaric hypoxic (HYPO) test. Values are mean±SD. *Significant difference between AIR and HYPO test. Finger I, thumb; Finger II, index; Finger III, middle; Finger IV, ring; Finger V, small.
FIG. 5.
Average temperature (Tavg) of the dorsal side of (a) the distal and (b) proximal phalanx of the thumb, (c) the total area of the palm, and (d) the total opisthenar area of the right hand during the 15-min rewarming phase of the control (AIR) and normobaric hypoxic (HYPO) test. Values are mean±SD. *Significant difference between AIR and HYPO test (p≤0.05). B, a 5-min baseline phase; WWI, a 5-min warm-water hand immersion phase.
Skin temperature responses: Non-immersed hand
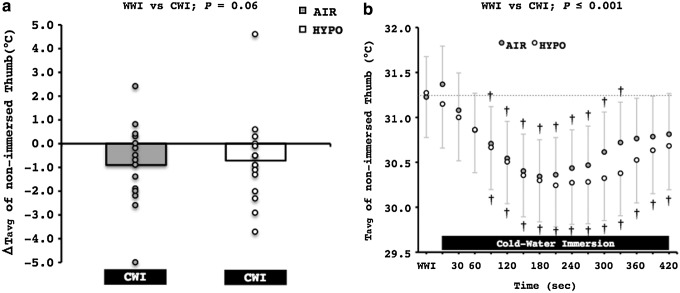
Mean and individual changes in the Tavg of the non-immersed thumb during the entire CWI phase are presented in Figure 6a. There was a statistical tendency for lower Tavg in the non-immersed thumb during the CWI phase (−0.8°C; p=0.06). Specifically, Tavg decreased in ten subjects during the AIR test, and in eleven subjects during the HYPO test. Tavg of the non-immersed thumb significantly decreased (−0.6°C) 90 sec after the immersion of the right hand in the cold-water, and it reached its nadir value (−1°C) ∼210 sec after the initiation of the CWI phase (Fig. 6b); no differences between the tests were observed.
FIG. 6.
(a) Mean and individual changes from the warm-water immersion (WWI) phase in the average temperature (Tavg) of the left non-immersed thumb during the entire control (AIR) and normobaric hypoxic (HYPO) cold-water immersion phase (CWI). (b) Average temperature (Tavg) in the left non-immersed thumb during the first 420 sec of the control (AIR) and normobaric hypoxic (HYPO) cold-water immersion phase (CWI). †Significant difference from WWI phase (p≤0.001).
Tavg of the center of the non-immersed palm was not altered throughout the tests (AIR: Baseline phase: 33.8±1.9°C, CWI phase: 34.1±2.3°C; HYPO: Baseline phase: 33.7±1.9°C, CWI phase: 34.1±2.1°C; p>0.05).
Ttympanic and hemodynamic responses
There were no differences in Ttympanic between the tests (AIR: Baseline=36.3±0.6°C, RW=36.3±0.6°C; HYPO: Baseline=36.4±0.6°C, RW=36.3±0.6°C; p=0.57).
The mean values of HR, SAP, DAP, and SpO2 obtained during each phase of the tests are summarized in Table 2. HR was higher (p=0.02) and Spo2 was lower (p≤0.001) throughout the HYPO than in the AIR test. No differences between the tests were observed for SAP and DAP (p>0.05).
Table 2.
Mean Heart Rate (HR), Systolic Arterial Pressure (SAP), Diastolic Arterial Pressure (DAP) and Capillary Oxyhemoglobin Saturation (SpO2) Obtained During the 5-Min Baseline (B), the 5-Min Warm-Water Immersion (WWI), the 30-Min Cold-Water Hand Immersion (CWI) and the 15-Min Rewarming (RW) Phases in the Control (AIR) and Normobaric Hypoxic (HYPO) Test
| AIR test | HYPO test | |||||||
|---|---|---|---|---|---|---|---|---|
| B | WWI | CWI | RW | B | WWI | CWI | RW | |
| HR (beats·min−1) | 79±12 | 80±13 | 76±14 | 72±14 | 83±13* | 87±12* | 83±14* | 78±14* |
| SAP (mmHg) | 121±9 | 120±12 | 128±10 | 123±10 | 120±10 | 124±13 | 129±9 | 123±13 |
| DAP (mmHg) | 73±14 | 74±11 | 86±8 | 82±7 | 75±14 | 77±13 | 84±8 | 80±11 |
| Spo2 (%) | 97±1 | 97±1 | 97±1 | 97±1 | 92±2* | 90±2* | 89±2* | 89±3* |
Values are mean±SD; SAP and DAP during CWI were significantly different from B, WWI and RW values (p≤0.001); *Significant difference from the AIR test; (p≤0.05).
Psychometric responses
There were no inter-condition differences (p>0.05) for either TS [CWI phase: AIR: 1 (1–4), HYPO: 1 (1–4); RW phase: AIR: 4 (3–6), HYPO: 4 (3–6)] or LP [CWI phase: AIR: 2 (1–5), HYPO: 3 (1–7); RW phase: AIR: 0 (0–1), HYPO: 0 (0–2)]. Subjects felt more uncomfortable at the 1st min of the hand immersion HYPO [2 (1–4)] than the AIR [1 (1–3)] (p=0.04); however, no other differences were observed between the tests at any other time-point [CWI phase: AIR: 2 (1–4), HYPO: 3 (1–4); RW phase: AIR: 1 (1–2), HYPO: 1 (1–2); p>0.05].
Discussion
Acute hypoxia during local cold stress
The main finding of the present study is that acute exposure to normobaric hypoxia did not aggravate the drop in hand temperature during local cold stress, despite the slightly lower Tmax (∼0.5°C). It may appear that current results are in conflict with previous studies that have shown an enhanced cold-induced vasoconstriction during CWI of a hand at high-altitude (Daanen and van Ruiten, 2000; Mathew et al., 1977; 1979; Purkayastha et al., 1999; Takeoka et al., 1993). However, in the aforementioned field studies, wherein the response to CWI was examined only in one finger, the pre-immersion temperature of the digit was considerably lower in the tests performed at high-altitude than in those at sea-level. The lower initial values in hypoxia, indicating a state of peripheral vasoconstriction already before the application of the local cold stimulus, were probably attributable either to distinct effects of hypoxia (Heistad and Wheeler, 1970; Kollai, 1983), or to the synergistic actions of hypoxia and low ambient temperature (Cipriano and Goldman, 1975) on peripheral circulation. In agreement with present results, Castellani et al., (2002) have observed no change in the finger CIVD response during acute mild hypoxia (Fio2: 0.16), whereas a marked decrease was obtained after 4 days in the hypoxic environment; notably, such a depression in the finger CIVD response was preceded by a reduction in the initial skin temperature values. Hence, it is postulated that, throughout the CWI phase, any hypoxia-induced vasoconstrictor effects in the extremities are masked by those induced by the cold stimulus, despite the increased overall sympathetic discharge caused by hypoxia.
Meeuwsen et al., (2009) had also detected no changes in almost all CIVD parameters, except that the ΔT was diminished during the hypoxic cold-water hand immersion. As recently discussed (Cheung and Daanen, 2012), there is a lack of consensus in the literature on the definition of CIVD, and hence on how to quantify this response. In the present study, the CIVD response was explored by determining a number of parameters, namely Tavg, N, Tmin, Tmax, ΔT, and Δt; among them, only the Tmax was slightly reduced during the HYPO CWI phase. Although the CIVD constitutes a complex physiological response and the impact of each parameter cannot be determined separately, the data of acclimatization/habituation studies (Cheung and Daanen, 2012) suggest that, in terms of its cryoprotective function (Daanen and van der Struijs, 2005; Mathew et al., 1974), it is the increased perfusion-induced elevation in Tavg that constitutes the most decisive parameter during local cooling (cf. Amon et al., 2012; Keramidas et al., 2010).
Acute hypoxia during spontaneous rewarming
Notwithstanding that acute hypoxia did not influence the hand temperature responses during the CWI phase, it significantly attenuated the rewarming process, in all regions alike, in the immersed hand. Likewise, previous studies have shown that hypoxia delayed cutaneous temperature recovery both for the middle finger (Fahim, 1992) and the palm (Rai et al., 1978) during hypoxic breathing following a short local cold provocation. Of interest in this regard is also the report by Cleophas et al., (1982), who have suggested that hypoxia induced by acute cigarette smoking hampers the finger skin temperature recovery after local cold stress.
The lower Tavg during the HYPO RW phase, indicating a protracted cutaneous vasoconstriction, was presumably mediated by sympathetic overactivity in the HYPO test (Heistad and Wheeler, 1970; Kollai, 1983). Thus, hypoxia is associated with hyperventilation and hence with hypocapnea, which lead to a reduced cutaneous perfusion in the distal portions of the extremities (Barker et al., 1991; Fahim, 1992; Heistad and Wheeler, 1970). Conceivably, a mild cerebral impairment induced by the hypoxic stimulus (cf. Virues-Ortega et al., 2004) could also have caused an inhibition in the efferent impulses to the periphery, and thus a slower after-reaction during the HYPO RW phase. A direct effect of hypoxia on the cutaneous vasculature might also have contributed to the diminished HYPO rewarming rate. That is to say, a recurring, or sustained sympathetic noradrenergic nerve activity in glabrous and nonglabrous skin (cf. Minson, 2003) resulted in a lower Tavg during the HYPO CWI phase. However, the mechanisms underlying the slow rewarming response in the HYPO test remain speculative, and need to be further investigated.
Temperature response of the non-immersed regions
Besides the direct effect of cold stress on the immersed hand segments, a vasoconstriction occurred in regions that were not in contact with the cold water, as the drop in Tforearm and Tavg of the contralateral thumb indicated during the initial portion of the CWI phase. It has previously been suggested that such a cold-induced “indirect” vasoconstriction is orchestrated either by the reflex response to a noxious stimulus and/or the action of a central mechanism excited by the cold blood returning from the skin (Folkow et al., 1963; Kregel et al., 1992; Pickering, 1932). The increased adrenal secretion triggered by the prolonged cold application constitutes an additional mechanistic explanation to the phenomenon (Freeman, 1935). In the present study, despite the increased general sympathetic discharge during the HYPO test, no differences in Tforearm and Tavg of the non-immersed thumb were detected between the tests. Indeed, this is in line with the findings of a similar degree of cold-induced vasoconstriction (judging from the Tavg in the immersed hand) and the similar size of noxious stimuli elicited by the cold stress (judging from the LP) in the AIR and HYPO tests.
In addition to the cooling during the CWI phase, a precipitous drop in Tforearm was observed initially during RW phases in both conditions. This “after-drop” was apparently evoked by the cold blood from the immersed hand rushing into the skin capillaries after the termination of the local cooling.
Methodological considerations
Two different noninvasive techniques, viz. the thermal convection probes (thermocouples) and the infrared thermography, were employed to measure skin temperature during and after the local cold stress. Although both techniques are considered capable of detecting changes in the skin microcirculation (Kistler et al.,1998), it should be noted that they only provide an index of cutaneous vasomotor tone (Wright et al., 2006). Davey et al., (2013) have recently shown that the changes in skin temperature during a spontaneous rewarming period are not solely due to changes in skin blood flow, but also reflect the ambient temperature. Furthermore, skin temperature is but a slow indicator of temperature changes occurring in deeper tissues (Daanen, 2003).
A potential limitation of the present study is that the subjects were not naïve as regards the breathing gas mixture in each test; given especially, that the CIVD response is substantially influenced by the emotional status of the individual (Teichner, 1965). However, the reproducibility of the CIVD (O'Brien, 2005) and of the rewarming response (Cleophas et al., 1982), and the fact that no differences in temperature and psychometric responses were observed between the AIR and HYPO CWI phases suggest that such subject bias effect, if any, was minimal, and did not influence the interpretation of the data.
Lastly, based on the topical debate about potential differences between normobaric and hypobaric hypoxia (cf. Loeppky et al., 1997; Savourey et al., 2003), it could be argued that the present results might not be representative of those expected in a hypobaric hypoxic setting. However, a previous study has shown that the hand CIVD responses are similar in normobaric and hypobaric hypoxia (Meeuwsen et al., 2009).
Perspectives
A number of epidemiological studies have shown that the incidence of local cold injury is increased by exposure to high altitude (Harirchi et al., 2005; Hashmi et al., 1998). Present findings suggest that the previously reported aggravation of the cold-induced vasoconstrictor response to hypoxia during local cooling is mainly attributable to the increased baseline vasomotor tone. On the contrary, the baseline status and the level of vasoconstriction during the CWI phase seem to have negligible impact on the diminished peripheral rewarming response in hypoxia. Whether such a diminished rewarming response in hypoxia constitutes a mechanism underlying the increased risk for cold injury in high-altitude expeditions remains unknown; especially, in the light of the fact that in such expeditions, hypoxia is commonly accompanied by other potential risk factors, such as increased wind, reduced physical activity, and reduced fluid and energy intake.
Conclusions
In conclusion, present findings demonstrate that acute exposure to normobaric hypoxia neither aggravates the cold-induced drop in hand temperature, nor influences the finger CIVD responses of normothermic males during local cold stress. However, acute hypoxia markedly attenuates the spontaneous rewarming responses of both glabrous and nonglabrous regions of the hand. Hence, further investigation is needed to examine whether a prolonged period of hypoxic exposure would counter the adverse rewarming responses induced by acute hypoxic stimulus.
Acknowledgments
The current project was supported by grants from the Swedish Armed Forces (no 922: 0905) and the Slovene Research Agency. We would like to thank all the subjects for their participation. We are also grateful to Eddie Bergsten for the development of the analysis program.
Author Disclosure Statement
The authors state that there is no financial or personal conflict of interest in the present study.
References
- Ahle NW, Buroni JR, Sharp MW, and Hamlet MP. (1990). Infrared thermographic measurement of circulatory compromise in trenchfoot-injured Argentine soldiers. Aviat Space Environ Med 61:247–250 [PubMed] [Google Scholar]
- Amon M, Keramidas ME, Kounalakis SN, and Mekjavic IB. (2012). The effect of a sleep high-train low regimen on the finger cold-induced vasodilation response. High Alt Med Biol 13:32–39 [DOI] [PubMed] [Google Scholar]
- Barcroft H, and Edholm OG. (1943). The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol 102:5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SJ, Hyatt J, Clarke C, and Tremper KK. (1991). Hyperventilation reduces transcutaneous oxygen tension and skin blood flow. Anesthesiology 75:619–624 [DOI] [PubMed] [Google Scholar]
- Brandstrom H, Grip H, Hallberg P, Gronlund C, Angquist KA, and Giesbrecht GG. (2008). Hand cold recovery responses before and after 15 months of military training in a cold climate. Aviat Space Environ Med 79:904–908 [DOI] [PubMed] [Google Scholar]
- Castellani JW, O'Brien C, Stulz DA, Blanchard LA, DeGroot DW, Bovill ME, Francis TJ, and Young AJ. (2002). Physiological responses to cold exposure in men: A disabled submarine study. Undersea Hyperb Med 29:189–203 [PubMed] [Google Scholar]
- Cheung SS, and Daanen HA. (2012). Dynamic adaptation of the peripheral circulation to cold exposure. Microcirculation 19:65–77 [DOI] [PubMed] [Google Scholar]
- Cipriano LF, and Goldman RF. (1975). Thermal responses of unclothed men exposed to both cold temperatures and high altitudes. J Appl Physiol 39:796–800 [DOI] [PubMed] [Google Scholar]
- Cleophas TJ, Fennis JF, and van't Laar A. (1982). Finger temperature after a finger-cooling test: Influence of air temperature and smoking. J Appl Physiol 52:1167–1171 [DOI] [PubMed] [Google Scholar]
- Daanen HA. (2003). Finger cold-induced vasodilation: A review. Eur J Appl Physiol 89:411–426 [DOI] [PubMed] [Google Scholar]
- Daanen HA, and van der Struijs NR. (2005). Resistance Index of Frostbite as a predictor of cold injury in arctic operations. Aviat Space Environ Med 76:1119–1122 [PubMed] [Google Scholar]
- Daanen HA, and van Ruiten HJ. (2000). Cold-induced peripheral vasodilation at high altitudes—A field study. High Alt Med Biol 1:323–329 [DOI] [PubMed] [Google Scholar]
- Davey M, Eglin C, House J, and Tipton M. (2013). The contribution of blood flow to the skin temperature responses during a cold sensitivity test. Eur J Appl Physiol 113:2411–2417 [DOI] [PubMed] [Google Scholar]
- Fahim M. (1992). Effect of hypoxic breathing on cutaneous temperature recovery in man. Int J Biometeorol 36:5–9 [DOI] [PubMed] [Google Scholar]
- Folkow B, Fox RH, Krog J, Odelram H, and Thoren O. (1963). Studies on the reactions of the cutaneous vessels to cold exposure. Acta Physiol Scand 58:342–354 [DOI] [PubMed] [Google Scholar]
- Freeman NE. (1935). The effect of temperature on the rate of blood flow in the normal and in the sympathectomized hand. Am J Physiol 113:384–398 [Google Scholar]
- Harirchi I, Arvin A, Vash JH, and Zafarmand V. (2005). Frostbite: Incidence and predisposing factors in mountaineers. Br J Sports Med 39:898–901; discussion 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi MA, Rashid M, Haleem A, Bokhari SA, and Hussain T. (1998). Frostbite: Epidemiology at high altitude in the Karakoram mountains. Ann R Coll Surg Engl 80:91–95 [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, and Wheeler RC. (1970). Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead. II. Responses to norepinephrine and angiotensin. 3. Effect of hypoxia and hypocapnia. J Clin Invest 49:1252–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges GJ, Kosiba WA, Zhao K, Alvarez GE, and Johnson JM. (2007). The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol 293:H3187–3192 [DOI] [PubMed] [Google Scholar]
- Keramidas ME, Musizza B, Kounalakis SN, and Mekjavic IB. (2010). Enhancement of the finger cold-induced vasodilation response with exercise training. Eur J Appl Physiol 109:133–140 [DOI] [PubMed] [Google Scholar]
- Kistler A, Mariauzouls C, and von Berlepsch K. (1998). Fingertip temperature as an indicator for sympathetic responses. Int J Psychophysiol 29:35–41 [DOI] [PubMed] [Google Scholar]
- Kollai M. (1983). Responses in cutaneous vascular tone to transient hypoxia in man. J Auton Nerv Syst 9:497–512 [DOI] [PubMed] [Google Scholar]
- Kregel KC, Seals DR, and Callister R. (1992). Sympathetic nervous system activity during skin cooling in humans: Relationship to stimulus intensity and pain sensation. J Physiol 454:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeppky JA, Icenogle M, Scotto P, Robergs R, Hinghofer-Szalkay H, and Roach RC. (1997). Ventilation during simulated altitude, normobaric hypoxia and normoxic hypobaria. Respir Physiol 107:231–239 [DOI] [PubMed] [Google Scholar]
- Mathew L, Purkayastha SS, and Nayar HS. (1979). Variation in the susceptibility to cold injury in Indians. Int J Biometeorol 23:263–270 [DOI] [PubMed] [Google Scholar]
- Mathew L, Purkayastha SS, Selvamurthy W, and Malhotra MS. (1977). Cold-induced vasodilatation and peripheral blood flow under local cold stress in man at altitude. Aviat Space Environ Med 48:497–500 [PubMed] [Google Scholar]
- Mathew L, Talwar JR, Purkayastha SS, and Mahotra MS. (1974). Prediction of susceptibility to cold injury in monkeys. In Selected Topics in Environmental Biology. Bhatia B, Chhina GS, Singh B, eds. Interprint Publications, New Delhi, India: pp: 191–195 [Google Scholar]
- Meeuwsen T, van Es EM, Smeets B, Layden JD, Simons R, and Daanen HAM. (2009). Reduced cold-induced vasodilation at altitude: Due to hypoxic or hypobaric circumstances? In Proceedings of the 13th International Conference on Environmental Ergonomics Boston (USA) pp: 540–543 [Google Scholar]
- Mekjavic IB, Dobnikar U, and Kounalakis SN. (2013). Cold-induced vasodilatation response in the fingers at 4 different water temperatures. Appl Physiol Nutr Metab 38:14–20 [DOI] [PubMed] [Google Scholar]
- Minson CT. (2003). Hypoxic regulation of blood flow in humans. Skin blood flow and temperature regulation. Adv Exp Med Biol 543:249–262 [DOI] [PubMed] [Google Scholar]
- O'Brien C. (2005). Reproducibility of the cold-induced vasodilation response in the human finger. J Appl Physiol 98:1334–1340 [DOI] [PubMed] [Google Scholar]
- Pickering CW. (1932). The vasomotor regulation of heat loss from human skin in reaction to external temperature. Heart 16:115–135 [Google Scholar]
- Purkayastha SS, Sharma RP, Ilavazhagan G, Sridharan K, Ranganathan S, and Selvamurthy W. (1999). Effect of vitamin C and E in modulating peripheral vascular response to local cold stimulus in man at high altitude. Jpn J Physiol 49:159–167 [DOI] [PubMed] [Google Scholar]
- Rai RM, Selvamurthy W, Purkayastha SS, and Malhotra MS. (1978). Effect of altitude acclimatization on thermoregulation efficiency of man. Aviat Space Environ Med 49:707–709 [PubMed] [Google Scholar]
- Ruijs AC, Jaquet JB, Brandsma M, Daanen HA, and Hovius SE. (2008). Application of infrared thermography for the analysis of rewarming in patients with cold intolerance. Scand J Plast Reconstr Surg Hand Surg 42:206–210 [DOI] [PubMed] [Google Scholar]
- Savourey G, Launay JC, Besnard Y, Guinet A, and Travers S. (2003). Normo- and hypobaric hypoxia: Are there any physiological differences? Eur J Appl Physiol 89:122–126 [DOI] [PubMed] [Google Scholar]
- Takeoka M, Yanagidaira Y, Sakai A, et al. (1993). Effects of high altitudes on finger cooling test in Japanese and Tibetans at Qinghai Plateau. Int J Biometeorol 37:27–31 [DOI] [PubMed] [Google Scholar]
- Teichner WH. (1965). Delayed cold-induced vasodilatation and behavior. J Exp Psychol 69:426–432 [DOI] [PubMed] [Google Scholar]
- Virues-Ortega J, Buela-Casal G, Garrido E, and Alcazar B. (2004). Neuropsychological functioning associated with high-altitude exposure. Neuropsychol Rev 14:197–224 [DOI] [PubMed] [Google Scholar]
- Wolff HH, and Pochin EE. (1949). Quantitative observations on vascular reactions in human digits in response to local cooling. Clin Sci (Lond) 8:145–154 [PubMed] [Google Scholar]
- Wright CI, Kroner CI, and Draijer R. (2006). Non-invasive methods and stimuli for evaluating the skin's microcirculation. J Pharmacol Toxicol Methods 54:1–25 [DOI] [PubMed] [Google Scholar]