Abstract
Background: Provision of human milk has important implications for the health and outcomes of extremely preterm (EP) infants. This study evaluated the effects of an exclusive human milk diet on the health of EP infants during their stay in the neonatal intensive care unit.
Subjects and Methods: EP infants <1,250 g birth weight received a diet consisting of either human milk fortified with a human milk protein-based fortifier (HM) (n=167) or a diet containing variable amounts of milk containing cow milk-based protein (CM) (n=93). Principal outcomes were mortality, necrotizing enterocolitis (NEC), growth, and duration of parenteral nutrition (PN).
Results: Mortality (2% versus 8%, p=0.004) and NEC (5% versus 17%, p=0.002) differed significantly between the HM and CM groups, respectively. For every 10% increase in the volume of milk containing CM, the risk of sepsis increased by 17.9% (p<0.001). Growth rates were similar between groups. The duration of PN was 8 days less in the subgroup of infants receiving a diet containing <10% CM versus ≥10% CM (p<0.02).
Conclusions: An exclusive human milk diet, devoid of CM-containing products, was associated with lower mortality and morbidity in EP infants without compromising growth and should be considered as an approach to nutritional care of these infants.
Introduction
Human milk provides important advantages to health outcomes of extremely preterm (EP) infants. The emergence of donor human milk to support policies recommending its use has increased rapidly in the United States and throughout the world.1,2
However, the use of human milk as a primary nutrition source in EP infants requires that the milk be fortified with nutrients, especially protein and micronutrients, to achieve adequate growth, body composition, and micronutrient status.3 Currently, most EP infants receive some cow milk-based protein (CM), either as their primary diet via infant formula or as a multinutrient fortifier directly added to a human milk-based diet. A human milk protein-based fortifier (HM) for human milk (Prolact+ H2MF; Prolacta Bioscience, City of Industry, CA) has been developed. The effect of an exclusive human milk diet on key outcomes in EP infants has been reported.4,5 The current report combines the original individual subject results from these studies to provide a comprehensive evaluation of the risk associated with the exposure of EP infants to cow milk protein.
Subjects and Methods
An exclusively human milk diet was evaluated in two clinical trials of EP infants, defined as those born at <1,250 g birth weight.4,5 Both trials used the same approach to feeding and evaluation of outcomes. The first of these trials,4 concluded in 2008, involved infants at 12 centers, all but one of these in the United States, whose mothers had committed to providing them with their milk during the neonatal intensive care unit stay. In brief, infants were randomly assigned to either an exclusive human milk diet (mother's own milk or donor human milk) that included a human milk-based human milk fortifier or to a routine approach in which mother's own milk was fortified with a cow milk-based fortifier containing cow milk protein. In that group, if mother's own milk was not available, a cow milk-based infant formula product was used to supplement the mother's own milk, but donor human milk was not provided.
The second trial5 was completed in 2010 and included infants whose mothers, for any reason, were unable or unwilling to provide their milk during the neonatal intensive care unit stay. In that trial, infants were assigned randomly to receive either an all human milk-based diet using donor human milk and human milk-based human milk fortifier (Prolact+) or cow milk-based formula products. The protocol, other than feeding assignments and blinding, and study end points were the same as for the first trial.
Taken together, these two trials provided a cohort of 260 EP infants who received a diet that ranged from 100% cow milk to 100% human milk. The preceding articles4,5 reported the results of each of the trials as analyzed by treatment group assignment. The results being reported here represent the post hoc analysis of these two randomized clinical trials and are, in a sense, a meta-analysis of those sets of data. Of course, the individual data points are available, making this a more detailed evaluation than in the typical meta-analysis. Here, the complete cohort data were combined and analyzed in two ways. First, the results of the two studies were combined to investigate differences between infants receiving any cow milk-based nutrition in their diet and infants receiving only a 100% human milk diet (no cow milk-based nutrition). Second, taking advantage of the larger number of patients and broader range of exposures to cow milk-based products, the combined results were analyzed to determine if any of the observed differences in outcome was attributed to the proportion of human milk or cow milk-based product received during the study period.
All analyses were based on the intent-to-treat designation, except as specifically noted. Infants in the two trials who were assigned to only human milk protein are designated as Group HM (human milk fortified with a human milk protein-based fortifier), and those who were assigned to receive any cow milk protein, either as human milk fortifier or infant formula, are designated as Group CM.
Descriptive statistics for quantitative data were used to summarize the characteristics of the two groups on a univariate basis. These included mean±SD for nonskewed data and median±interquartile range for skewed data (e.g., milk intake). The groups were compared using the nonparametric Wilcoxon rank-sum test. The chi-squared test for homogeneity or Fisher's exact test (if the expected frequencies in any given cell were less than 5) was used to compare the proportions in the two groups from a univariate perspective. Ninety-five percent confidence intervals were calculated using large sample (normal distribution) methods for the difference in outcomes between the CM and HM groups, including necrotizing enterocolitis (NEC)/surgical NEC and sepsis risk, and growth velocities. For mortality, an exact calculation of the 95% confidence interval for the difference in risk was based on the Blythe–Still–Casella method.6 For parenteral nutrition (PN) days, because of the censoring in this variable, the method of Kaplan and Meier7 was used to estimate the distribution of these events, and the formula of Greenwood8 was used to help in the determination of the 95% confidence interval for the difference in medians.
Multivariate binary logistic regression was used to evaluate key end points, such as the occurrence of NEC, NEC surgery, and sepsis. These models were used to evaluate the effect of the amount of the diet from cow milk sources using odds ratios and 95% confidence intervals. The significance of the regression coefficients was assessed by the usual Wald's Z-statistic. In addition, adjustments for key covariates were incorporated into the models. For NEC and surgical NEC, we used Apgar scores, gestational age, use of antenatal steroids, African American race, and presence of bronchopulmonary dysplasia because each of these has been previously identified as potential risk factors for NEC.9–11 For sepsis, the adjustment covariates were birth weight, days of PN, days on a central line, days on a ventilator, and Apgar score. Again, these are known risk factors for the development of sepsis in this population.12–14 In all cases a p value of 5% or less was set as the definition of statistical significance.
Informed written consent was obtained from parents separately for each trial after review and approval of the Institutional Review Board for Human Subject Research at each site.
Results
Characteristics of the study subjects and study outcomes are given in Tables 1 and 2. The patient populations were not significantly different with respect to characteristics and various clinical outcomes. However, most importantly, significantly lower mortality (p=0.04), risk of NEC (p=0.002), and NEC requiring surgery within 2 weeks of diagnosis (p=0.0003) were seen in Group HM versus Group CM.
Table 1.
Patient Characteristics
| Parameter | Group CM (n=93) | Group HM (n=167) | p value |
|---|---|---|---|
| Gender (male) | 47 (51%) | 69 (41%) | 0.15a |
| Completed study | 70 (75%) | 134 (80%) | 0.35a |
| Transferred to another hospital | 16 (17%) | 21 (13%) | 0.10a |
| Withdrew consent | 0 | 2 (1%) | 0.54b |
| Race (black) | 21 (23%) | 50 (30%) | 0.22a |
| BPD | 28 (30%) | 50 (30%) | 0.98a |
| SGA | 10 (11%) | 15 (9%) | 0.36a |
| Prenatal steroids | 71 (82%) | 128 (81%) | 0.83a |
| Postnatal steroids | 23 (30%) | 39 (28%) | 0.71a |
| Apgar score <6 | 11 (12%) | 13 (8%) | 0.28a |
| Mechanical ventilation at study entry | 53 (76%) | 105 (76%) | 0.95a |
| Birth weight (mean±SD) | 938±200 | 939±192 | 0.97c |
| GA (mean±SD) | 27±2 | 27±2 | 0.85c |
| Volume (mL) [median (25th, 75th percentile)] | |||
| Mother's milk | 2,102 (1, 7,174) | 1943 (127, 6,960) | 0.96c |
| Donor human milk | 0 | 883 (0, 4,581) | |
| Formula | 2,109 (54, 5,764) | ||
| Total milk intake | 7,190 (4,402, 10,008) | 8,169 (5,331, 11,016) | 0.13c |
By chi-squared test.
By Fisher's exact test.
By Wilcoxon rank-sum test.
BPD, bronchopulmonary dysplasia; CM, milk containing cow milk-based protein; GA, gestational age; HM, human milk fortified with a human milk protein-based fortifier; SGA, small for gestational age.
Table 2.
Outcome of Study Infants
| Group CM (n=93) | Group HM (n=167) | p value | 95% CI (difference) | |
|---|---|---|---|---|
| Mortality | 7/93 (8%) | 3/167 (2%) | 0.04 | 0.3% to 13% |
| NEC | 16/93 (17%) | 9/167 (5%) | 0.002 | 2.4% to 21.3% |
| Surgical NEC | 11/93 (12%) | 2/167 (1%) | 0.0003 | 4.4% to 18.9% |
| Sepsis | 32/93 (34%) | 50/167 (30%) | 0.46 | −7.1% to 16.6% |
| TPN (median days)a | 25 | 23 | 0.26 | −2 to 5 |
| Weight (g/kg/day) | 13.6±4.1 | 14.9±7.2 | 0.11 | −0.1 to 2.7 |
| Length (cm/week) | 0.89±0.45 | 0.97±0.35 | 0.12 | −0.02 to 0.19 |
| FOC (cm/week) | 0.73±0.23 | 0.77±0.22 | 0.10 | −0.01 to 0.11 |
From the Kaplan and Meier7 estimate.
CI, confidence interval; CM, milk containing cow milk-based protein; FOC, fronto-occipital circumference; NEC, necrotizing enterocolitis; HM, human milk fortified with a human milk protein-based fortifier; TPN, total parenteral nutrition.
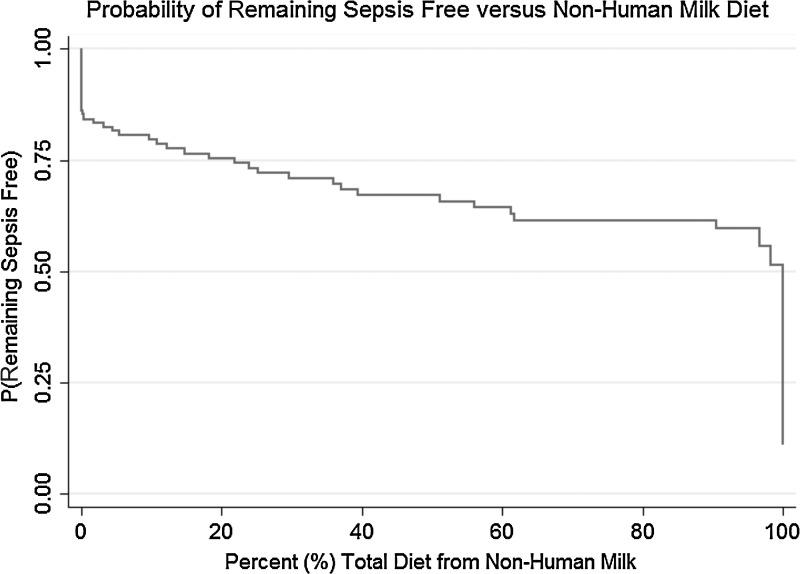
The incidence of NEC, NEC requiring surgery, and sepsis were evaluated using a multivariate logistic model to evaluate whether the percentage of the diet containing cow milk protein had an impact on those outcomes. Potentially mitigating covariates were included in the model. For the two NEC models these included Apgar score, gestational age, receipt of antenatal steroids, black race, and presence of bronchopulmonary dysplasia. The results of these models are given in Table 3. The percentage of diet containing cow milk was a significant predictor of NEC (p=0.047) and NEC surgery (p=0.01). The other covariates, although considered to be risk factors for NEC and NEC surgery, did not mitigate this effect. The odds ratios for cow milk percentage can be interpreted as follows: for each 10% (an incremental value selected to allow ease of interpretation of the odds ratios from the regression models) increase in the intake of other than an exclusive human milk diet, the risk of NEC increases by 11.8% (95% confidence interval, 0.2–24.8%), and the risk of surgical NEC increases by 21% (95% confidence interval, 4.2–39.6%). The multivariate model for sepsis risk as a function of the percentage of diet from cow milk is given in Table 4. The key potentially mitigating covariates in this model included Apgar score, birth weight, PN duration, central line days, and ventilation days. The percentage of diet from cow milk was significantly associated with the risk of sepsis (p=0.00006). For each 10% increase in the intake of other than an exclusive human milk diet, there was a 17.9% increase in risk in sepsis (95% confidence interval, 8.8–27.8%). The overall relationship between the risk of sepsis and amount of nonhuman milk is shown in Figure 1 as an inverse empirical cumulative distribution function.
Table 3.
Multivariate Logistic Models for Necrotizing Enterocolitis (NEC) and Surgical NEC as a Function of the Percentage of the Diet Containing Cow Milk Protein
| Parameter | Odds ratio (95% confidence interval) | p value |
|---|---|---|
| All NECa | ||
| % dietary volume from cow milk | 1.01 (1.00, 1.02) | 0.047 |
| Apgar score | 1.12 (0.82, 1.52) | 0.48 |
| GA | 0.86 (0.68, 1.10) | 0.22 |
| Antenatal steroids | 1.10 (0.34, 3.55) | 0.87 |
| Black race | 0.76 (0.29, 2.03) | 0.59 |
| BPD | 0.84 (0.30, 2.33) | 0.74 |
| Surgical NECb | ||
| % dietary volume from cow milk | 1.02 (1.004, 1.03) | 0.01 |
| Apgar score | 1.29 (0.80, 2.09) | 0.30 |
| GA | 0.79 (0.56, 1.12) | 0.19 |
| Antenatal steroids | 0.82 (0.16, 4.28) | 0.81 |
| Black race | 1.20 (0.27, 5.31) | 0.81 |
| BPD | 0.98 (0.24, 3.92) | 0.97 |
Fit of the regression model: pseudo-R2=0.039, area under the receiver operator characteristic curve=0.65.
Fit of the regression model: pseudo-R2=0.087, area under the receiver operator characteristic curve=0.70.
BPD, bronchopulmonary dysplasia; GA, gestational age.
Table 4.
Multivariate Logistic Models for Sepsis as a Function of the Percentage of the Diet Containing Cow Milk Protein
| Parameter | Odds ratio (95% confidence interval) | p value |
|---|---|---|
| % dietary volume from cow's milk | 1.02 (1.01, 1.025) | 0.00006 |
| Birth weight | 0.999 (0.997, 1.001) | 0.21 |
| TPN days | 1.04 (1.01, 1.06) | 0.009 |
| Central line days | 1.02 (0.996, 1.04) | 0.10 |
| Ventilation days | 0.99 (0.98, 1.005) | 0.19 |
| Apgar score | 1.03 (0.85, 1.24) | 0.80 |
Fit of the regression model: pseudo-R2=0.15, area under the receiver operator characteristic curve=0.77.
TPN, total parenteral nutrition.
FIG. 1.
Inverse empirical cumulative distribution of sepsis as a function of the amount of nonhuman milk in the diet.
We did not find any overall effect on length of time for PN. However, in a subgroup analysis of infants receiving <10% (a reasonably small number) of their diet as formula (n=182), the number of PN days was lower than in the subgroup receiving ≥10% of the diet as formula (n=78): 21 versus 29 days (p=0.02). The incidence of sepsis also was significantly lower in the <10% formula group (24% versus 49% [p<0.0001]), as was the percentage of NEC (6% versus 18% [p<0.001]) and NEC surgery (3% versus 10% [p=0.01]).
Discussion
We have shown that provision of an exclusively human milk diet during the early postnatal period, a diet devoid of cow milk protein, is associated with lower risks of death, NEC, NEC requiring surgery, and sepsis in EP infants. This information is necessary to calculate a cost:benefit ratio so that healthcare teams can support the optimization of nutritional outcomes in EP infants. The cost of major complications of extreme prematurity, such as sepsis and NEC, is high, typically more than $250,000 for each case of NEC that requires surgery.15 Lifetime costs are likely much higher because of the increased risk of long-term neurodevelopmental problems in infants who have had NEC requiring surgery.16 These outcomes are the major drivers of costs and long-term morbidity in this population, and their avoidance is a principal goal of contemporary neonatal care. A recent analysis confirmed that human milk costs are minimal compared with the costs of the major morbidities in very low birth weight infants.17
The results of this analysis indicate a significant effect on overall mortality as well as sepsis that was not identified in the individual studies previously reported.4,5 The combined studies demonstrate a dose-related effect of nonhuman milk intake in increasing negative patient outcomes. Furthermore, by including infants with a range of cow milk protein intake, we have shown a significant benefit to using very minimal cow milk protein on the duration of PN, a measure of overall morbidity in EP infants.
In interpreting these findings, clinicians should recognize that there is firm support to the American Academy of Pediatrics and other statements regarding the importance of human milk for preterm infants. Efforts should be developed to provide adequate donor milk to supplement mother's own milk so that these goals can be achieved.
Conclusions
The implications of these data are that efforts should be extended to provide as much human milk as possible to high-risk EP infants. The Surgeon General has advocated for expanding the use of donor milk that is pasteurized and safely provided to such infants.1 Efforts to enhance the availability and affordability of pasteurized donor human milk should be increased. Our data suggest that the use of an exclusively human milk-based diet, using a nutritionally appropriate human milk-based fortifier, should be considered for all EP infants.
Appendix
The H2MF Study Group is composed of the following investigtors (listed alphabetically): Cynthia L. Blanco, MD, Pediatrics, University of Texas Health Science Center, San Antonio, TX; Gary M. Chan, MD, Pediatrics, University of Utah Medical Center, Salt Lake City, UT; C. Michael Cotten, MD, MHS, Pediatrics, Duke University Medical Center, Durham, NC; Elizabeth A. Cristofalo, MD, MPH, Pediatrics, Johns Hopkins School of Medicine, Baltimore, MD; Golde Dudell, MD, Pediatrics, Children's Hospital and Research Center, Oakland, CA; Richard A. Ehrenkranz, MD, Pediatrics, Yale University School of Medicine, New Haven, CT; Ursula Kiechl Kohlendofer, MD, Pediatrics, Innsbruck Medical University, Innsbruck, Austria; Jae H. Kim, MD, PhD, Pediatrics, University of California, San Diego Medical Center, San Diego, CA; Nirupama Laroia, MD, Pediatrics, University of Rochester, Rochester General Hospital, Rochester, NY; Alan Lucas, MD, MRC Child Nutrition Research Center, Institute of Child Health, London, United Kingdom; Aloka L. Patel, MD, Pediatrics, Rush University Medical Center, Chicago, IL; Sandra Sullivan, MD, Pediatrics, University of Florida, Gainesville, FL; and Rudolph Trawoeger, MD, Pediatrics, Innsbruck Medical University.
Contributor Information
Collaborators: the Prolacta Study Group
Acknowledgments
Prolacta Bioscience paid for the clinical studies discussed in this article.
Disclosure Statement
D.L.R. and M.J.L. are employees of Prolacta Bioscience. S.A.A. and R.J.S. declare no competing financial interests exist.
References
- 1.U.S. Department of Health and Human Services. The Surgeon General's Call to Action to Support Breastfeeding. Washington, DC: Office of the Surgeon General, U.S. Department of Health and Human Services, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841 [DOI] [PubMed] [Google Scholar]
- 3.Schanler RJ. Evaluation of the evidence to support current recommendations to meet the needs of premature infants: The role of human milk. Am J Clin Nutr 2007;85:625S–628S [DOI] [PubMed] [Google Scholar]
- 4.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156:562–567 [DOI] [PubMed] [Google Scholar]
- 5.Cristofalo EA, Schanler RJ, Blanco CL. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr 2013;163:1592–1595 [DOI] [PubMed] [Google Scholar]
- 6.Blyth CR, Still HA. Binomial confidence intervals. J Am Stat Assoc 1983;78:108–116 [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481 [Google Scholar]
- 8.Greenwood M. The Natural Duration of Cancer. Reports on Public Health and Medical Subjects. Vol. 33 London: Her Majesty's Stationery Office, 1926:1–26 [Google Scholar]
- 9.Wójkowska-Mach J, Różańska A, Borszewska-Kornacka M, et al. Necrotising enterocolitis in preterm infants: Epidemiology and antibiotic consumption in the Polish neonatology network neonatal intensive care units in 2009. PLoS One 2014;9:e92865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BM, Holditch-Davis D. Risk factors for necrotizing enterocolitis in preterm infants: How race, gender, and health status contribute. Adv Neonatal Care 2008;8:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guthrie SO, Gordon PV, Thomas V, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003;23:278–285 [DOI] [PubMed] [Google Scholar]
- 12.Ozkan H, Cetinkaya M, Koksal N, et al. Culture-proven neonatal sepsis in preterm infants in a neonatal intensive care unit over a 7 year period: Coagulase-negative Staphylococcus as the predominant pathogen. Pediatr Int 2014;56:60–66 [DOI] [PubMed] [Google Scholar]
- 13.Leal YA, Alvarez-Nemegyei J, Velázquez JR, et al. Risk factors and prognosis for neonatal sepsis in southeastern Mexico: Analysis of a four-year historic cohort follow-up. BMC Pregnancy Childbirth 2012;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troger B, Gopel W, Faust K, et al. Risk for late-onset blood-culture proven sepsis in very-low-birth weight infants born small for gestational age: A large multicenter study from the German Neonatal Network. Pediatr Infect Dis J 2014;33:238–243 [DOI] [PubMed] [Google Scholar]
- 15.Ganapathy V, Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med 2012;7:29–37 [DOI] [PubMed] [Google Scholar]
- 16.Ganapathy V, Hay JW, Kim JH, et al. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: A retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr 2013;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson TJ, Patel AL, Bigger HR, et al. Economic benefits and costs of human milk feedingss: A strategy to reduce the risk of prematurity-related morbidities in very-low-birth-weight infants. Adv Nutr 2014;5:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]