Abstract
Purpose: To determine the half-life of mycophenolic acid (MPA) in the vitreous of New Zealand albino rabbits after intravitreal injection and the retinal toxicity of different doses of MPA.
Methods: Ten micrograms of MPA (Roche Bioscience, Palo Alto, CA) was injected in the vitreous of 16 rabbits, animals were sacrificed at different time-points, and vitreous samples underwent high-performance liquid chromatography. For functional and morphological studies, 5 doses of MPA (0.05, 0.5, 2, 10, and 100 μg) were injected in the vitreous of 20 rabbits. As control, contralateral eyes were injected with aqueous vehicle. Electroretinograms (ERGs) were recorded before injection and at days 7, 15, and 30. Animals were sacrificed on day 30 and retinas were analyzed under light microscopy.
Results: MPA half-life in the vitreous was 5.0±0.3 days. ERG revealed photoreceptor functional impairment in eyes injected with 0.5 μg and higher on day 30, while eyes injected with 100 μg presented the same changes already from day 15. No morphological change was found.
Conclusions: MPA vitreous half-life is 5.0 days. Intravitreal injection of 0.5 μg MPA and higher causes dose- and time-related photoreceptor sensitivity decrease in rabbits. The MPA dose of 0.05 μg may be safe for intravitreal use in rabbits.
Introduction
Corticosteroids are the first-line drugs for the treatment of non-infectious uveitis. However, long-term use may be associated with severe systemic side effects and lead to treatment discontinuation.1,2 Immunosuppressant drugs downregulate chronic inflammation and prevent recurrences but also may present significant risk of severe side effects that can lead to dose tapering and even discontinuation.3
Mycophenolate mofetil (MMF) is an immunosuppressant drug extensively successfully used for the prevention of organ transplant rejection and for the treatment of many autoimmune diseases, such as vasculitis, systemic lupus erythematous, psoriasis, rheumatoid arthritis, and nephritic syndrome.4–12 MMF is a prodrug; after oral intake, it is converted in the liver to mycophenolic acid (MPA), the active substance. MPA is a selective and reversible inhibitor of inosine monophosphate dehydrogenase, an enzyme that blocks purine synthesis via a de novo pathway, which is preferentially used by T and B lymphocytes.13,14
In a preclinical study in rats, MMF inhibited the development of experimental autoimmune uveoretinitis induced by S-antigen after oral intake.15 In clinical studies that included patients with uveitis, orally administered MMF was an efficient adjuvant therapy, either in steroid-dependent or steroid-resistant uveitis.16–24 However, up to 21% of the patients treated with oral MMF discontinue treatment due to systemic side effects, mainly leukopenia and gastrointestinal disturbance.17,23
Intravitreal drug delivery has become a frequent and important approach for the treatment of posterior-segment diseases. It provides the eye with immediate exposure to therapeutic levels of the injected drug and avoids systemic side effects. Therefore, intravitreal injection of MPA may be a potential adjuvant therapy to autoimmune uveitis in patients who have to discontinue systemic steroid or immunosuppressive therapy due to systemic side effects. However, local side effects on the retina and other ocular structures must be considered.25–28 No information on the morphological and functional effects of intravitreal injection of MPA is available.
The aims of this experimental study are to determine the half-life of MPA in the rabbit vitreous, as well as the functional and morphological effects of MPA in the rabbit retina following a single intravitreal injection.
Methods
Animals
Thirty-six New Zealand albino rabbits (weight 2–3 kg) were included in this study. Animals were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments were approved by the Research Ethic Committee, Medical School, Universidade de São Paulo, and the Committee for Ethics in Animal Research, Psychology Institute, Universidade de São Paulo, São Paulo, Brazil.
Animals were housed in individual cages under 12/12 h light-dark cycle with free access to water and food. Before every procedure (intravitreal injection, electroretinogram [ERG], and sacrifice), animals were anesthetized with intramuscular injection of 50 mg/kg ketamine hydrochloride (Ketamina; Agener, São Paulo, Brazil) and 6.7 mg/kg xylazine hydrochloride (Calmiun; Agener). Pupils were dilated with topical 0.5% tropicamide (Mydriacyl; Alcon, São Paulo, Brazil) and the eyes were topically anesthetized with 0.5% proxymetacaine hydrochloride (Anestalcon; Alcon). All animals were sacrificed with intravenous injection of 70 mg/kg sodium pentobarbital (Euthanyle; Brouwer, Buenos Aires, Argentina) under deep anesthesia.
MPA preparation
MPA (C17H20O6, wt: 320.3) is a lipophilic compound slightly soluble in water (13.0 μg/mL). MPA (Roche Bioscience, Palo Alto, CA) intravitreal formulation was prepared as a sterile 10-μg suspension in single-use vials. The suspending medium (vehicle) was sterile saline solution containing the dispersion agent polysorbate 80 (0.4% w/v; Sigma-Aldrich Co., St. Louis, MO) and the suspending agent hydroxypropyl methylcellulose (0.5% w/v; Sigma-Aldrich Co.). Before injections, MPA was diluted with the vehicle to 0.05, 0.5, 2, 10, and 100 μg doses. The formulations were prepared immediately before injection.
There is no study in the literature that evaluates the levels of MPA in the vitreous after systemic use, either in humans or in rabbits that we can rely on. In addition, there are so many factors that influence drug absorption, bioavailability, and penetration into the eye (eg, age, nutritional status, time from last meal, drug solubility, and blood-retinal barrier) that theoretical calculations play a limited role and it is not possible to accurately predict the amount of drug that penetrates into the eye. Thus, we tested the highest possible concentration of MPA using the appropriate vehicle (1.0 mg/mL) and lower doses with differences from 1 to 2.3 log units (100, 10, 2, 0.5, and 0.05 μg of MPA).
Intravitreal injection
Under anesthesia and dilated pupils, 5% povidone iodine drops (Ophthalmos, São Paulo, Brazil) were instilled. Anterior chamber tap (0.1 mL of aqueous humor) was performed with a 30-gauge needle. Intravitreal injections were performed using a 30-gauge needle attached to a tuberculin syringe inserted ∼3 mm posterior to the limbus. The right eye (RE) was injected with 0.1 mL of the MPA solution and the left eye (LE) with 0.1 mL of the suspension vehicle as control.
Vitreous half-life determination
For MPA vitreous half-life determination, 16 animals received an intravitreal injection of 10 μg of MPA in the RE and 0.1 mL of vehicle in the LE. Groups of 4 animals were sacrificed at 1, 7, 14, and 28 days after intravitreal injection, eyes were enucleated, and anterior segment and lens were removed. Vitreous samples were frozen at −18°C for high-performance liquid chromatography (HPLC) assays, as well as peripheral blood samples that were drawn at the time of sacrifice to determine the serum concentration of MPA.
The ocular pharmacokinetic model was developed using previous studies as reference.29–32 All data were fit with a single exponential curve according to Equation (1) and the estimated half-time (t1/2) of MPA elimination was calculated with Equation (2).
 |
 |
Where C (μg/mL) and C0 (μg/mL) represent respectively, the MPA concentration at any time and at t0, t (day) is the time after injection, and k (day−1) represents the decay constant.
MPA determination
The chromatographic system consisted of a Merck-Hitachi LaChrom Elite apparatus equipped with an autosampler with sample loop of 100 μL (model L-2200; Merck-Hitachi, Darmstadt, Germany), a pump at a constant flow rate of 1.4 mL/min (model L-2130; Merck-Hitachi), and a diode array detector used at a wavelength of 215 nm (model L-2450; Merck-Hitachi). Separation chromatography was performed using an Ace 5 C18 column (250×4.6 mm id; Advanced Chromatography Technologies, Aberdeen, Scotland) maintained at 50°C (column oven model L-2300; Merck-Hitachi). A mixture of acetonitrile (Merck-Hitachi) and 40 mM phosphoric acid buffer at pH 3.0 (32:68 v/v) was used as mobile phase. Under these experimental conditions, the retention time of MPA was 14.0 min.
Frozen vitreous and blood samples were thawed out at ambient temperature. After brief mixing, 500 μL of mobile phase was added to aliquots of 500 μL of vitreous and blood samples. After mixing for 1 min, samples were filtered (Durapore, 0.2 μm; Millipore, Darmstadt, Germany) and 100 μL was injected into the column. Standard stock solutions of 0.5 mg/mL MPA were prepared in methanol. This solution was added to drug-free rabbit vitreous and blood to prepare 7 non-zero concentrations in the range of 1–200 μg/mL MPA.
Electroretinography
For functional study, 20 rabbits were divided in 5 groups of 4 animals each, according to the dose of MPA injected in the vitreous (0.05, 0.5, 2, 10, and 100 μg). RE was injected with MPA and LE with vehicle. Full-field ERG was performed in both eyes before intravitreal injection and 7, 15, and 30 days after injection. ERG was not performed on the first day after intravitreal injection because caged rabbits are very sensitive to deep and prolonged anesthesia33 (as needed for ERG recordings) and undergoing anesthesia in 2 consecutive days significantly increases the risk of death.
ERG responses were recorded using a corneal bipolar contact lens electrode (GoldLens; Doran Instruments, Inc., Littleton, MA) and a ground electrode (model E5; Grass Technologies, West Warwick, RI) placed on the ear. Light stimuli were generated by a Grass stimulator (PS33-PLUS Photic Flash Visual Stimulator; Grass Technologies) with a flash lamp bulb (PST-2100; Grass Technologies). Light stimuli were provided by a Ganzfeld Stimulator (2503B; LKC Technologies, Gaithersburg, MD) placed inside a Faraday cage (60×60 cm2) and were controlled by a computerized system (RETI-port; Roland Consult, Brandenburg, Germany). ERG signals were amplified and digitalized (PCI-1200; National Instruments Co., Austin, TX), and analyzed by a data acquisition software (LabVIEW; National Instruments Co.). The band pass for flash ERG was set to 0.3–1,000 Hz.
The ERG protocol was modified after the International Society for Clinical Electrophysiology of Vision (ISCEV) standards.34 Animals were dark-adapted for 30 min and anesthetized, pupils were dilated, and eyes were topically anesthetized. The ISCEV-modified ERG protocol included 5 flash luminances in dark-adapted state (0.003, 0.03, 0.3, 3, and 30 cd·s/m2), light-adapted state (after light adaptation with background luminance of 25 cd/m2 for 10 min, 3 cd·s/m2 flashes were presented), and light-adapted flicker (3 cd·s/m2 flashes presented at 12, 18, 24, and 30 Hz).
ERG data analysis
The a- and b-wave amplitudes and implicit times were quantified. The a-wave amplitude was measured from the baseline to the minimum amplitude after light stimulus onset. The a-wave time-to-peak, or implicit time, was measured from flash onset to the a-wave peak. The b-wave amplitude was measured from the a-wave through the b-wave peak amplitude. Similarly, the b-wave implicit time corresponded to the time of occurrence of its peak amplitude.
Dark-adapted b-wave mean amplitudes were plotted as log response versus light intensity curves and fitted with Naka–Rushton equation:
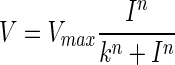 |
Where Vmax is the b-wave saturating amplitude, l is the light intensity, k is the intensity needed for 50% of Vmax, and n is the slope of the function, representing the dynamic range of the measured wave.
Correlations between b-wave and a-wave amplitudes in eyes injected with different doses of MPA and vehicle at different time-points for the dark-adapted state were plotted.
The 12, 18, 24, and 30 Hz flicker recordings were analyzed using the Fourier fast transform algorithm with 100 Hz low-pass filtering. The amplitudes and phases of the first, second, and third harmonics were calculated and plotted for all test conditions. The results of the eyes injected with MPA and vehicle were compared for each MPA dose, stimulus frequency, and time-point after treatment.
Morphological analysis
After the last ERG recording on day 30, animals were sacrificed and eyes were processed for light microscopy. Immediately after sacrifice, eyes were enucleated, the anterior segment and lens were removed, and the posterior segment was fixed in ALFAC fixative solution. Samples were included in paraffin and 7-μm-thick sections of the inferior midperipheral posterior segment (retina/choroid/sclera) were stained with hematoxylin and eosin (Sigma-Aldrich Co.) and were analyzed under light microscopy. Eyes injected with MPA were compared with vehicle-injected fellow eye of the same animal. Thickness and gross organization of each retinal layer were analyzed.
Statistical analysis
Amplitudes and implicit times are expressed as mean±standard deviation. Statistical analyses were carried out using repeated measures ANOVA and Fisher's least significant difference test as post hoc test. Parameters from Naka–Rushton equation of b-wave amplitude versus light intensity were analyzed with 1-way ANOVA and paired 2-tailed t-test with Bonferroni correction for the number of comparisons among treatment groups and intervals. P<0.05 was considered statistically significant.
Results
Determination of MPA half-life in the vitreous
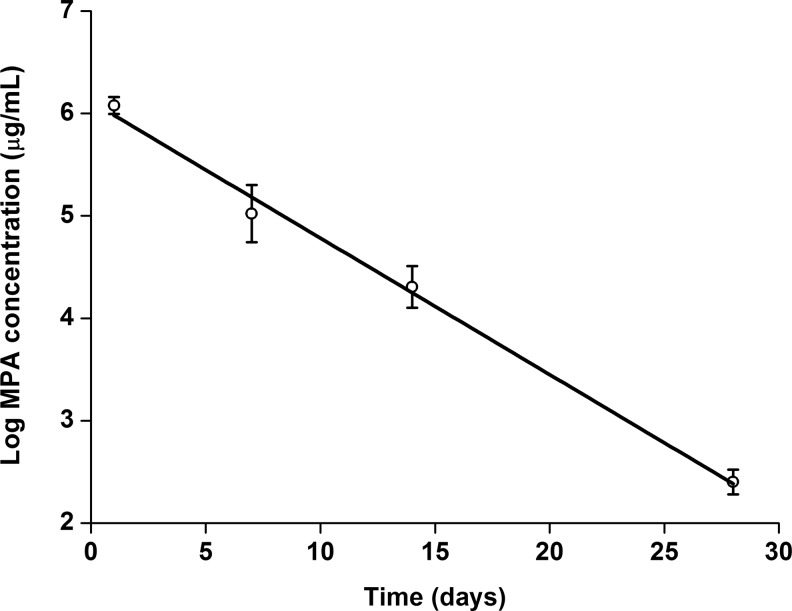
The HPLC method was validated and successfully used for the determination of MPA vitreous concentration. The chromatograms obtained during the analysis were very clean and no significant interference could be detected at the retention time of the blank samples (data not shown). The calibration curve was obtained by linear regression analysis plotting of peak-area ratio versus the MPA concentration (y=432700x−3472.9; R2=0.9996). These data suggest reproducible results and that the assay was accurate and reliable. MPA vitreous concentrations at different time-points are presented in Figure 1. Estimated half-time (t1/2) of MPA elimination from rabbit vitreous was 5.0±0.3 days (R2=0.993; P=0.004) and the drug was detectable for 29 days (sensitivity=1 μg/mL).
FIG. 1.
Linear regression of concentrations of mycophenolic acid (MPA) in the vitreous after intravitreal injection of 10 μg (4 eyes in each time-point).
MPA was not detectable in either eye injected with saline or peripheral blood samples.
Electrophysiological analysis
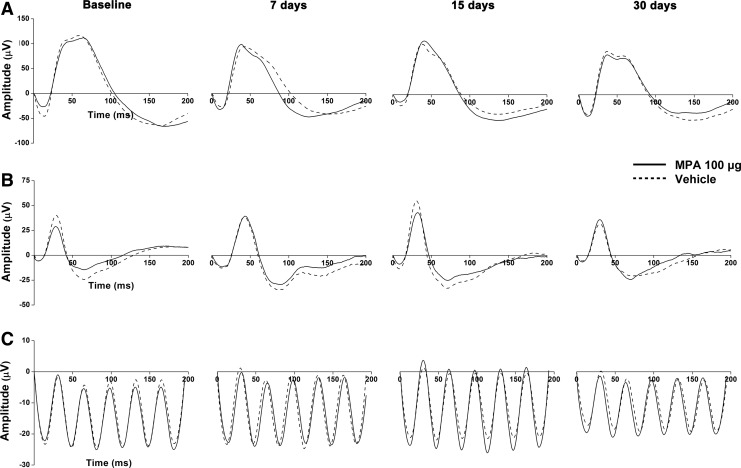
Dark- and light-adapted representative ERG records of 1 animal injected with the highest dose of MPA (100 μg) are shown in Figure 2.
FIG. 2.
Representative ERG records of 1 animal at different time-points after intravitreal injection of 100 μg MPA. (A) Dark-adapted state (stimulus of 30 cd·s/m2); (B) light-adapted state (stimulus of 3 cd·s/m2); (C) light-adapted flicker (stimulus of 3 cd·s/m2 at 30 Hz).
The light- and dark-adapted a- and b-wave mean amplitude and implicit time were analyzed. No statistically significant differences were found between baseline values and postinjection values on days 7, 15, and 30 at any MPA tested dose.
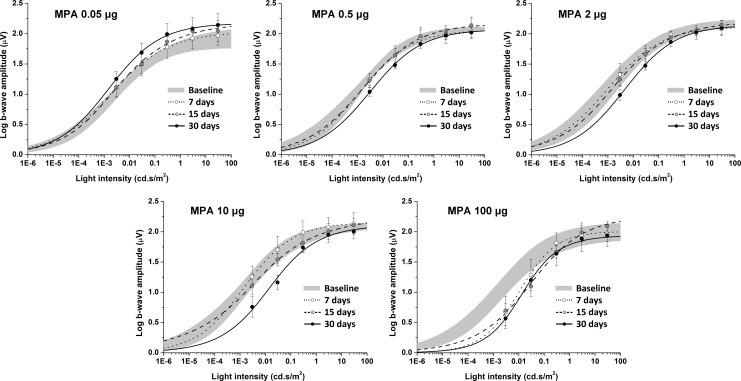
Dark-adapted log b-wave mean amplitudes versus flash intensity curves of eyes injected with MPA are presented in Figure 3. Curves of 7, 14, and 28 days postinjection were compared with preinjection curve. Parameters Vmax, k, and n are presented in Table 1. Vmax did not change over time after intravitreal injection of any dose of MPA. However, k significantly increased 30 days after intravitreal injection of 0.5 μg MPA and higher doses (0.5 μg, 305% increase, P<0.001; 2 μg, 423% increase, P=0.028; 10 μg, 882% increase, P=0.015; and 100 μg, 1,565% increase, P=0.005). As for 100 μg, k increased 931% already 15 days after intravitreal injection of MPA (P=0.006). The n parameter significantly increased 7 days (55% increase, P=0.013) and 30 days (51% increase, P=0.031) after 100 μg MPA injection. Eyes injected with aqueous vehicle did not present significant differences.
FIG. 3.
Log b-wave amplitude versus flash intensity curves of eyes injected with different doses of MPA. Gray areas represent the mean±standard deviation of 4 eyes before intravitreal injection. Dots and error bars represent mean±standard deviation of the 4 eyes after intravitreal injection. Contralateral eyes, injected with aqueous vehicle, did not present differences from baseline.
Table 1.
Parameters Obtained from b-Wave Mean Amplitude Versus Flash Intensity Curves Using the Naka–Rushton Equation (Dark-Adapted State)
| MPA (mg/mL) | Vmax (log, μV) | k (cd·s/m2) | n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 7 days | 15 days | 30 days | Baseline | 7 days | 15 days | 30 days | Baseline | 7 days | 15 days | 30 days | |
| 0.05 | 1.95 | 2.04 | 2.18 | 2.17 | 0.002 | 0.002 | 0.005 | 0.002 | 0.40 | 0.39 | 0.42 | 0.43 |
| 0.5 | 1.95 | 2.07 | 2.17 | 2.07 | 0.002 | 0.001 | 0.002 | 0.004a | 0.40 | 0.44 | 0.40 | 0.43 |
| 2 | 2.14 | 2.12 | 2.20 | 2.14 | 0.001 | 0.001 | 0.002 | 0.007b | 0.43 | 0.41 | 0.40 | 0.43 |
| 10 | 2.11 | 2.16 | 2.19 | 2.08 | 0.002 | 0.002 | 0.003 | 0.016c | 0.41 | 0.45 | 0.39 | 0.50 |
| 100 | 2.02 | 2.01 | 2.27 | 1.94 | 0.002 | 0.007 | 0.031d | 0.020e | 0.40 | 0.63f | 0.41 | 0.61g |
P=0.028.
P=0.015.
P=0.005.
P<0.001.
P=0.006.
P=0.013.
P=0.031.
Vmax, log b-wave saturating amplitude; k, light intensity at 50% of Vmax; n, curve slope; MPA, mycophenolic acid.
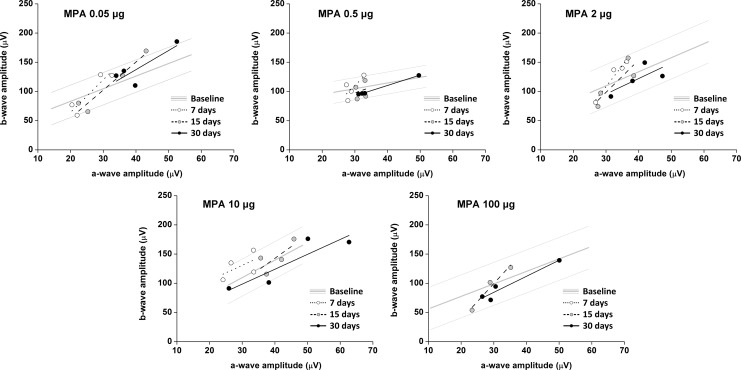
The b-wave to a-wave amplitude correlations for dark-adapted state before and after injection of the 5 tested MPA doses did not show significant differences at any time-point or flash intensity. Graphs of b-wave to a-wave amplitude correlations for 30 cd·s/m2 are shown in Figure 4.
FIG. 4.
b-Wave to a-wave amplitude correlations in eyes injected with different doses of MPA at different time-points (dark-adapted state, stimulus of 30 cd·s/m2). Baseline data are represented as linear regression±standard deviation. Dots represent the a- and b-wave amplitudes of each animal, and lines represent the linear regression for each group. Contralateral eyes, injected with aqueous vehicle, did not present differences from baseline.
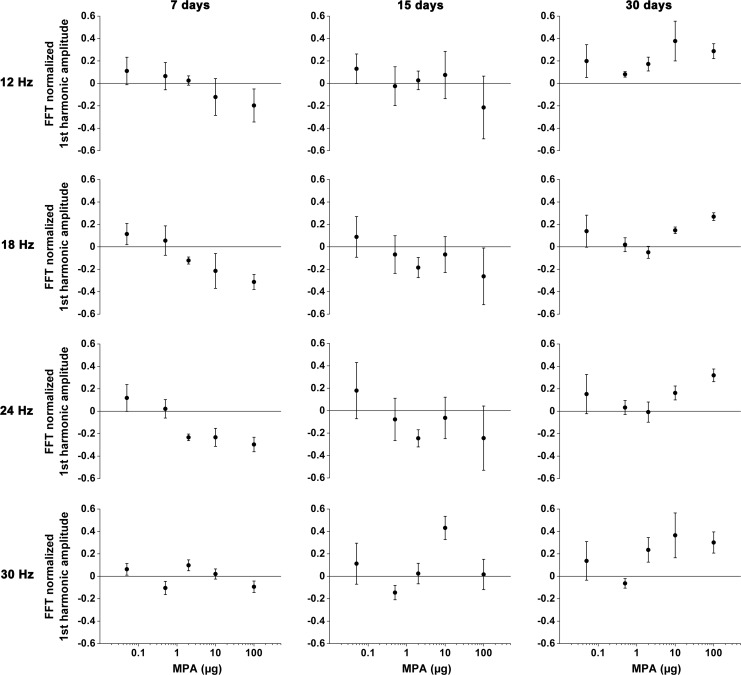
Flicker analysis showed that the amplitudes and phases of the different harmonics presented similar tendencies among eyes injected with MPA and vehicle. Therefore, we used the first-harmonic amplitudes for comparisons. Data were individually normalized to the mean amplitude of the eyes injected with vehicle, and the MPA and vehicle differences were calculated. Mean flicker amplitudes of eyes injected with MPA did not present statistical difference during follow-up (Fig. 5). However, graph analysis suggests dose- and time-related tendencies of flicker amplitude decrease with MPA dose increase at 12, 18, and 24 Hz on days 7 and 15 after intravitreal injection.
FIG. 5.
Flicker results of eyes injected with 5 doses of MPA for stimulus frequency and time-point after intravitreal injection. Differences between the MPA and vehicle normalized FFT first-harmonic amplitude values are plotted.
Morphological analysis
No major histological changes were observed in light microscopy of eyes injected with MPA and vehicle 30 days after intravitreal injection. Histological sections of representative retinas injected with the 5 tested MPA doses are shown in Figure 6.
FIG. 6.
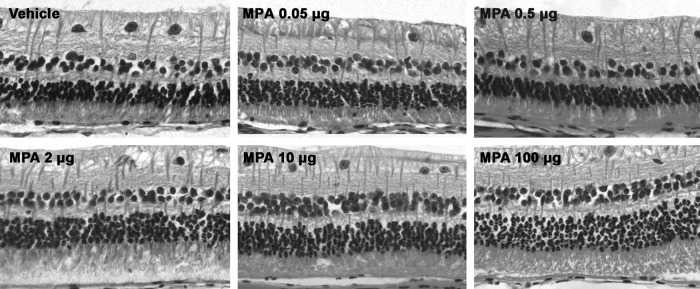
Light micrographs 30 days after intravitreal injection of different doses of MPA showing well-preserved retinal morphology.
Discussion
In this study, we investigated the pharmacokinetics of MPA in the rabbit vitreous and safety of different doses of MPA after intravitreal injection. MPA half-life in the vitreous of non-inflamed and phakic eyes is 5 days and the drug remains detectable for 29 days. MPA is not detectable in the vitreous of the fellow eye and in the serum, suggesting that systemic absorption, if any, may not be significant. With regard to safety, electrophysiological results suggest that MPA causes dose- and time-related photoreceptor functional impairment. MPA doses of 0.5 μg and higher cause photoreceptor sensitivity decrease on day 30, while eyes injected with 100 μg present the same changes already from day 15.
Although MPA has been successfully used to treat uveitis after oral administration of its prodrug MMF, systemic treatment has to be discontinued in up to 21% of the patients due to systemic side effects.17,23,24 Intravitreal injection may be an adjuvant treatment that may immediately deliver drug to the posterior segment of the eye. Our results show that MPA is detectable in the vitreous up to 29 days after a single intravitreal injection. The rate of elimination from the vitreous depends on drug characteristics and partition characteristics of the surrounding tissues. MPA is a lipophilic drug and its half-life is expected to be greater than that of hydrophilic drugs. Our pharmacokinetic data cannot be directly compared to other studies as this is the first report of MPA vitreous half-life determination. However, triamcinolone acetonide (TA) is also a lipophilic drug slightly soluble in water and its behavior in the vitreous can be considered a reference for this study. TA vitreous half-life in rabbit eyes varies from 1.6 to 8.2 days,35–37 confirming that MPA and TA, both lipophilic drugs, have vitreous half-lives in the range of few days.
ERG analysis shows significant differences in b-wave amplitude versus flash intensity curves in the dark-adapted state, suggesting that MPA causes dose- and time-related retinal toxicity. The increase on the intensity needed to reach 50% of Vmax (parameter k) 30 days after intravitreal injection of 0.5 μg MPA and higher represents a decrease in retinal sensitivity, suggesting that injection of 0.5 μg MPA and higher causes time-related damage to either the photoreceptors or to the synaptic transmission between these and bipolar cells. Regarding the 100 μg dose, this effect is detectable after 15 days of MPA intravitreal injection and extends through day 30. However, postreceptoral changes seem less likely as the b- to a-wave amplitude ratios are not altered. If postreceptoral transmission pathways were affected by MPA, one might expect that b-wave would be reduced and a-wave would remain unchanged.
The increase in parameter n on days 7 and 30 after injection of 100 μg MPA represents a change in the gain of synaptic transmission between photoreceptors and bipolar cells, which ultimately drive the b-wave. Although the n value increase might corroborate the photoreceptor damage, the difference is not present on day 15. Therefore, it is not possible to assert that this finding represents experimental evidence of photoreceptor damage.
Flicker stimulation of the light-adapted retina isolates cone-driven ERG responses since the rod photoreceptors are saturated by the light adaptation and cannot respond even at low temporal frequencies. Some authors have shown that flicker responses may be more sensitive than light- and dark-adapted a- and b-wave parameters in detecting early toxic effects.38–40 Our graphs show that MPA tends to cause reduction in the first harmonic of the mean response amplitude at stimulus frequencies of 12, 18, and 24 Hz, at days 7 and 15. The light-adapted ERG response to flicker contains contributions from cones and from the inner retina at the temporal frequencies used here.41,42 The observed apparent reduction together with the other ERG findings may reflect a diffuse damage of the cones of eyes treated with the higher doses of MPA. However, rabbits have rod-rich retina and, therefore, toxicity studies of cone system should be viewed with caution.43
In contrast to the electrophysiological results, intravitreal injection of up to 100 μg of MPA did not cause morphological changes in the rabbit retina under light microscopy. Several studies using other drugs showed lack of association between functional and morphological findings.27,44–46 Although we have not found morphological evidence of retinal toxicity after injection of a wide range of MPA doses, it may not be discarded for some reasons. In this study we analyzed sections of the inferior midperipheral posterior segment. The whole retina was not examined by light microscopy and focal damage may not have been detected. It is also possible that the retinal changes may have occurred at the ultramicroscopic level, as shown by Inan et al. for bevacizumab.47 In this case, toxic findings could only be evidenced by electron transmission microscopy, which was not performed in the present study. The injection of higher doses of MPA might increase the odds of detecting morphological retinal changes. However, it is not possible to obtain a suitable suspension of MPA in concentrations higher than 1.0 mg/mL in the appropriate suspending vehicle used as the re-suspension of the drug particles is significantly compromised and the dose uniformity (accuracy and precision of unit dose) cannot be reached.
Different mechanisms may be involved in the retinal toxicity of MPA, such as oxidative stress. Our group has shown that 100 μg/mL MPA causes significant decrease in in vitro cell viability and increase in caspase 3/7 activity in retinal pigment epithelium (RPE) and Müller cells compared with untreated cells, suggesting increased apoptosis.48 The dispersion agent polysorbate 80 used in the vehicle of the intravitreal formulation of MPA may also play a role in the retina functional impairment. After systemic injection, polysorbate 80 has been shown to induce hypersensitivity reactions and plasma viscosity increase.49 However, polysorbate is part of the formulation of other drugs widely used in intravitreal injections, such as bevacizumab (Avastin®; Genentech, Inc., San Francisco, CA) and preservative-free TA (Triesence®; Alcon Laboratories, Inc., Fort Worth, TX), and no significant safety concerns have been raised in terms of retina functional damage.50–52 Differences in pH and osmolarity are also known as potential causes of retinal toxicity. However, the pH of the MPA preparation used in this study was 6.8±0.2, and its osmolarity was 300±8 mOsm, both in the physiological range. In addition, the lack of definite safe ranges for these parameters makes it difficult to predict whether a preparation is toxic or not.53
The design of this study does not allow drawing any conclusion on why MPA does not cause photoreceptor functional impairment on ERG early after intravitreal injection. It has been previously shown that MPA causes in vitro early RPE cell viability decrease and apoptosis increase.48 We can speculate that an ultramicroscopic RPE damage may be the primary event on the MPA retinal toxicity cascade and, due to the interdependency between RPE and photoreceptors, these cells may start to present functional changes weeks after intravitreal injection of MPA that are not detectable by ERG and light microscopy. Alternatively, MPA might cause focal early damage to photoreceptors that is not detected by ERG as it only detects the mass response of the retina to a light stimulus. However, the areas of focal damage may increase with time until they are so spread that ERG can detect functional changes.
This study has several limitations. First, the relatively small number of animals included in each group (4 animals) may impair the drawing of assertive conclusions. As we could not rely on previous studies, we tried to increase the odds of detecting toxic retinal changes by using different approaches and analyzing several variables. For this reason, the number of subjects per group was relatively small. Second, if MPA causes focal damage to the retina, it would not have been detected by our morphological analysis, as only the inferior midperipheral retina was used on the light microscopy analysis. Third, we used albino rabbits in our experiments, whose eyes do not contain melanin, a molecule highly expressed in the human retina. However, the use of albino rabbits may be more predictive of acute retinal toxicity than the use of pigmented animals, as some drugs partially bind to melanin and it partially protects the retina from drug toxicity.54 For this reason, a long-term administration of drugs with high melanin affinity may be necessary to induce ocular toxicity.55,56 Finally, this is an experimental study and limitations of the rabbit model include differences in the retina vascularization when compared with the human eye, as well as differences in eye volume. For these reasons, results may not fully represent the findings in inflamed human eyes.
In conclusion, our data suggest that 0.05 μg MPA is safe for intravitreal injection. However, 0.5 μg MPA and higher may cause functional retinal changes that are detected 30 days after the intravitreal injection. The highest dose tested (100 μg) causes early functional retinal changes that may be irreversible, as the retina does not seem to functionally recover by day 30. This study contains evidences that may have both clinical and experimental significances of the use of intravitreal MPA as an adjuvant approach for the treatment of specific cases of non-infectious uveitis. It is unknown whether 0.05 μg MPA has therapeutic effects in an animal model of autoimmune uveitis or in humans, but our findings may be used as a reference for future in vivo studies.
Acknowledgments
R.G.A. (FAPESP Fellowship: 07/56624-1), G.L.I. (FAPESP Fellowship: 07/56844-1), B.V.N. (FAPESP Fellowship: 09/54292-7), N.N.O. (CNPq Fellowship: 151011/2008-7), D.F.V. (FAPESP Research Grant: 07/02696-1, CNPq 1A Fellowship), and F.M.D. (CNPq Fellowship: 150614/2009-8).
Author Disclosure Statement
All authors do not have any competing financial interests.
References
- 1.Durrani K., Zakka F.R., Ahmed M., et al. Systemic therapy with conventional and novel immunomodulatory agents for ocular inflammatory disease. Surv. Ophthalmol. 56:474–510, 2011 [DOI] [PubMed] [Google Scholar]
- 2.LeHoang P.The gold standard of noninfectious uveitis: corticosteroids. Dev. Ophthalmol. 51:7–28, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Kruh J., and Foster C.S.Corticosteroid-sparing agents: conventional systemic immunosuppressants. Dev. Ophthalmol. 51:29–46, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Wiesner R.H., Steffen B.J., David K.M., et al. Mycophenolate mofetil use is associated with decreased risk of late acute rejection in adult liver transplant recipients. Am. J. Transplant. 6:1609–1616, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Lake J.R., Shorr J.S., Steffen B.J., et al. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am. J. Transplant. 5:549–557, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y., Rosenthal D., Dutz J., and Ho V.Mycophenolate mofetil (CellCept) for psoriasis: a two-center, prospective, open-label clinical trial. J. Cutan. Med. Surg. 7:193–197, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Schwarz A.New aspects of the treatment of nephrotic syndrome. J. Am. Soc. Nephrol. 12Suppl 17:S44–S47, 2001 [PubMed] [Google Scholar]
- 8.Wiesner R., Rabkin J., Klintmalm G., et al. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transp. 7:442–450, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Boumpas D.T., Kritikos H.D., and Daskalakis N.G.Perspective on future therapy of vasculitis. Curr. Rheumatol. Rep. 2:423–429, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chan T.M., Li F.K., Tang C.S., et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N. Engl. J. Med. 343:1156–1162, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Warrens A.N.The evolving role of mycophenolate mofetil in renal transplantation. QJM. 93:15–20, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Goldblum R.Therapy of rheumatoid arthritis with mycophenolate mofetil. Clin. Exp. Rheumatol. Suppl 8:S117–S119, 1993 [PubMed] [Google Scholar]
- 13.Allison A.C., and Eugui E.M.Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 47:85–118, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Jiang A., Wang J., Joshi M., and Christoforidis J.B.Systemic treatments for noninfectious vitreous inflammation. Mediators Inflamm. 2013:515312, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chanaud N.P., 3rd, Vistica B.P., Eugui E., et al. Inhibition of experimental autoimmune uveoretinitis by mycophenolate mofetil, an inhibitor of purine metabolism. Exp. Eye Res. 61:429–434, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Thorne J.E., Jabs D.A., Qazi F.A., et al. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 112:1472–1477, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Baltatzis S., Tufail F., Yu E.N., et al. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology. 110:1061–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Lau C.H., Comer M., and Lightman S.Long-term efficacy of mycophenolate mofetil in the control of severe intraocular inflammation. Clin. Exp. Ophthalmol. 31:487–491, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Larkin G., and Lightman S.Mycophenolate mofetil. A useful immunosuppressive in inflammatory eye disease. Ophthalmology. 106:370–374, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Kilmartin D.J., Forrester J.V., and Dick A.D.Rescue therapy with mycophenolate mofetil in refractory uveitis. Lancet. 352:35–36, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Choudhary A., Harding S.P., Bucknall R.C., and Pearce I.A.Mycophenolate mofetil as an immunosuppressive agent in refractory inflammatory eye disease. J. Ocul. Pharmacol. Ther. 22:168–175, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Siepmann K., Huber M., Stubiger N., et al. Mycophenolate mofetil is a highly effective and safe immunosuppressive agent for the treatment of uveitis: a retrospective analysis of 106 patients. Graefes Arch. Clin. Exp. Ophthalmol. 244:788–794, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Sobrin L., Christen W., and Foster C.S.Mycophenolate mofetil after methotrexate failure or intolerance in the treatment of scleritis and uveitis. Ophthalmology. 115:1416–1421, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Doycheva D., Zierhut M., Blumenstock G., et al. Long-term results of therapy with mycophenolate mofetil in chronic non-infectious uveitis. Graefes Arch. Clin. Exp. Ophthalmol. 249:1235–1243, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Kwak H.W., and D'Amico D.J.Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch. Ophthalmol. 110:259–266, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Yu S.Y., Damico F.M., Viola F., et al. Retinal toxicity of intravitreal triamcinolone acetonide: a morphological study. Retina. 26:531–536, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Manzano R.P., Peyman G.A., Khan P., et al. Testing intravitreal toxicity of rapamycin in rabbit eyes. Arq. Bras. Oftalmol. 72:18–22, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Modorati G., and Miserocchi E.Intravitreal injection therapy in the treatment of noninfectious uveitis. Dev. Ophthalmol. 51:110–121, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Bahrami G., and Mohammadi B.An isocratic high performance liquid chromatographic method for quantification of mycophenolic acid and its glucuronide metabolite in human serum using liquid-liquid extraction: application to human pharmacokinetic studies. Clin. Chim. Acta. 370:185–190, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Khoschsorur G., and Erwa W.Liquid chromatographic method for simultaneous determination of mycophenolic acid and its phenol- and acylglucuronide metabolites in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 799:355–360, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Watson D.G., Araya F.G., Galloway P.J., and Beattie T.J.Development of a high pressure liquid chromatography method for the determination of mycophenolic acid and its glucuronide metabolite in small volumes of plasma from paediatric patients. J. Pharm. Biomed. Anal. 35:87–92, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Pastore A., Lo Russo A., Piemonte F., et al. Rapid determination of mycophenolic acid in plasma by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 776:251–254, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Lichtenberger M., and Ko J.Anesthesia and analgesia for small mammals and birds. Vet. Clin. North Am. Exot. Anim. Pract. 10:293–315, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Marmor M.F., Fulton A.B., Holder G.E., et al. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc. Ophthalmol. 118:69–77, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Scholes G.N., O'Brien W.J., Abrams G.W., and Kubicek M.F.Clearance of triamcinolone from vitreous. Arch. Ophthalmol. 103:1567–1569, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Chin H.S., Park T.S., Moon Y.S., and Oh J.H.Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 25:556–560, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Oliveira R.C., Messias A., Siqueira R.C., et al. Vitreous pharmacokinetics and retinal safety of intravitreal preserved versus non-preserved triamcinolone acetonide in rabbit eyes. Curr. Eye Res. 37:55–61, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Yoshizumi M.O., Bhavsar A.R., Dessouki A., et al. Safety of repeated intravitreous injections of antibiotics and dexamethasone. Retina. 19:437–441, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Theodossiadis P.G., Liarakos V.S., Sfikakis P.P., et al. Intravitreal administration of the anti-TNF monoclonal antibody infliximab in the rabbit. Graefes Arch. Clin. Exp. Ophthalmol. 247:273–281, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Tsilimbaris M., Diakonis V.F., Naoumidi I., et al. Evaluation of potential retinal toxicity of adalimumab (Humira). Graefes Arch. Clin. Exp. Ophthalmol. 247:1119–1125, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Bush R.A., and Sieving P.A.Inner retinal contributions to the primate photopic fast flicker electroretinogram. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 13:557–565, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Viswanathan S., Frishman L.J., and Robson J.G.Inner-retinal contributions to the photopic sinusoidal flicker electroretinogram of macaques. Macaque photopic sinusoidal flicker ERG. Doc. Ophthalmol. 105:223–242, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Perlman I.Testing retinal toxicity of drugs in animal models using electrophysiological and morphological techniques. Doc. Ophthalmol. 118:3–28, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Xu W., Wang H., Wang F., et al. Testing toxicity of multiple intravitreal injections of bevacizumab in rabbit eyes. Can. J. Ophthalmol. 45:386–392, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Komarowska I., Heilweil G., Rosenfeld P.J., et al. Retinal toxicity of commercially available intravitreal ketorolac in albino rabbits. Retina. 29:98–105, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Kazi A.A., Jermak C.M., Peyman G.A., et al. Intravitreal toxicity of levofloxacin and gatifloxacin. Ophthalmic Surg. Lasers Imaging. 37:224–229, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Inan U.U., Avci B., Kusbeci T., et al. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Invest. Ophthalmol. Vis. Sci. 48:1773–1781, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Zacharias L.C., Damico F.M., Kenney M.C., et al. In vitro evidence for mycophenolic acid dose-related cytotoxicity in human retinal cells. Retina. 33:2155–2161, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Engels F.K., Mathot R.A., and Verweij J.Alternative drug formulations of docetaxel: a review. Anticancer Drugs. 18:95–103, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Zacharias L.C., Lin T., Migon R., et al. Assessment of the differences in pharmacokinetics and pharmacodynamics between four distinct formulations of triamcinolone acetonide. Retina. 33:522–531, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Chen H., Sun S., Li J., et al. Different intravitreal properties of three triamcinolone formulations and their possible impact on retina practice. Invest. Ophthalmol. Vis. Sci. 54:2178–2185, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Martin D.F., Maguire M.G., Fine S.L., et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 119:1388–1398, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penha F.M., Rodrigues E.B., Maia M., et al. Retinal and ocular toxicity in ocular application of drugs and chemicals-part II: retinal toxicity of current and new drugs. Ophthalmic Res. 44:205–224, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Zemel E., Loewenstein A., Lei B., et al. Ocular pigmentation protects the rabbit retina from gentamicin-induced toxicity. Invest. Ophthalmol. Vis. Sci. 36:1875–1884, 1995 [PubMed] [Google Scholar]
- 55.Boke W., Baumer A., Muller-Limmroth W., and Mludek M.On the problem of chloroquine damage to the eye. Klin. Monatsbl. Augenheilkd. 151:617–633, 1967 [PubMed] [Google Scholar]
- 56.Marmor M.F., Carr R.E., Easterbrook M., et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 109:1377–1382, 2002 [DOI] [PubMed] [Google Scholar]