Abstract
The incidences of skin cancers resulting from chronic ultraviolet radiation (UVR) exposure are on the incline in both Australia and globally. Hence, the cellular and molecular pathways that are associated with UVR-induced photocarcinogenesis need to be urgently elucidated, in order to develop more robust preventative and treatment strategies against skin cancers. In vitro investigations into the effects of UVR (in particular, the highly mutagenic UVB wavelength) have, to date, mainly involved the use of cell culture and animal models. However, these models possess biological disparities to native skin, which, to some extent, have limited their relevance to the in vivo situation. To address this, we characterized a three-dimensional, tissue-engineered human skin equivalent (HSE) model (consisting of primary human keratinocytes cultured on a dermal-derived scaffold) as a representation of a more physiologically relevant platform to study keratinocyte responses to UVB. Significantly, we demonstrate that this model retains several important epidermal properties of native skin. Moreover, UVB irradiation of the HSE constructs was shown to induce key markers of photodamage in the HSE keratinocytes, including the formation of cyclobutane pyrimidine dimers, the activation of apoptotic pathways, the accumulation of p53, and the secretion of inflammatory cytokines. Importantly, we also demonstrate that the UVB-exposed HSE constructs retain the capacity for epidermal repair and regeneration after photodamage. Together, our results demonstrate the potential of this skin equivalent model as a tool to study various aspects of the acute responses of human keratinocytes to UVB radiation damage.
Introduction
The skin's epidermis forms the frontline of protection against environmental hazards, including the ultraviolet radiation (UVR) wavelength subtypes UVA (320–400 nm), UVB (290–320 nm), and UVC (200–290 nm). Chronic, unprotected exposure to high levels of UVR and predisposing genetic risk factors have been shown to increase the likelihood of skin cancer development.1–3 Consequently, populations in countries that experience extreme levels of solar UVR experience the highest rates of skin cancers.4 In Australia, for example, nonmelanoma skin cancers (NMSC), resulting from the carcinogenic transformation of epidermal keratinocytes, represent the most frequently diagnosed cancer type.4,5 Furthermore, the treatment of NMSCs also represents the largest financial burden on the Australian healthcare system.6
Chronic UVA and UVB exposure gives rise to cutaneous immunosuppression and DNA damage, which together form the intrinsic pathways to NMSC photocarcinogenesis.7 The UVA wavelength penetrates into the dermis of the skin and is predominantly responsible for the photoaging effects of sun exposure.8 Considered the major causative factor in epidermal malignancies, UVB is known to be highly destructive to keratinocytes via direct DNA damage.9 UVB irradiation leads to the formation of DNA photolesions, predominantly cyclobutane pyrimidine dimers (CPDs) and (6–4) pyrimidine-pyrimidones, along with the generation of DNA-damaging reactive oxygen species.10 Defence mechanisms against cutaneous photocarcinogenesis include the activation of cell cycle arrest and the subsequent initiation of the nucleotide excision repair complex.11 Failure to correctly and completely repair UVR-induced DNA damage, however, can lead to the accumulation of signature mutations, such as C→T transitions, commonly found in UVR-induced skin tumors.12 Higher doses of UVB radiation trigger apoptosis (or the formation of sunburn cells), yet another cellular safety net to inhibit the replication of genetically damaged proliferative keratinocytes.13 The intricacies of keratinocyte responses to UVB, and in particular the processes surrounding NMSC formation, however, have yet to be fully mapped. Investigations into the biological reactions after UVB radiation exposure using human participants have several ethical constraints. These are attributed to the considerable risks involved with UVR exposure and the requirement of skin biopsy collection, which together have limited such studies. Hence, physiologically relevant in vitro experimental platforms that are capable of accurately modeling the human UVB epidermal response may represent a practical and accessible alternative to such in vivo studies.
A variety of animal, cell, and tissue culture-based models have been employed to investigate UVB-induced damage and repair in keratinocytes.14 Drawbacks associated with these techniques, however, include strict ethical regulations, significant costs, and inconsistencies related to structural and biological aspects of native skin.15–17 The establishment of three-dimensional (3D), tissue-engineered, organotypic human skin equivalents (HSEs) has addressed some of these limitations.15,18,19 Essentially, the construction of HSEs involves the culture of epidermal skin cells on natural or synthetic dermal substrates. These HSEs may then be cultured at the air-liquid interface in order to promote keratinocyte differentiation and epidermogenesis.20–22 This results in the formation of an epidermal layer that is histologically similar to native human skin.21 These tissue-engineered constructs serve as versatile and powerful research tools with numerous applications, such as the study of cancer, pigmentation, and toxicity.15
In this study, we characterize the novel application of a human keratinocyte-based HSE using a de-epidermized dermal (DED) culture substrate as a tool to investigate keratinocyte responses to UVB radiation. Importantly, compared with conventional two-dimensional (2D) keratinocyte cultures, the HSE-DED more closely recapitulates the in vivo tissue structure and microenvironment.23,24 The use of synthetic dermal substrates such as collagen,25 fibrin,26 and chitosan-gelatin-hyaluronic acid27 in HSE construction has shown mixed results in terms of keratinocyte attachment and differentiation.28,29 Moreover, countries such as Australia tightly regulate the import of commercially available HSE composites for research purposes.24 Hence, the aim of this study was to validate the HSE-DED model as an accessible and biologically compatible platform to study UVB radiation-induced damage and postirradiation repair in keratinocytes.
This HSE-DED model has been established in our laboratory as a tool to study wound healing and re-epithelialization processes.20,21 Here, we describe an additional application of the HSE-DED to characterize the epidermal UVB photoresponse in vitro. Specifically, the HSE-DED was validated in terms of (1) the expression of key epidermal differentiation markers compared with native skin; (2) the generation of characteristic markers of UVB photodamage; and (3) the capacity for postirradiation repair and regeneration at cellular and epidermal tissue levels.
Materials and Methods
Isolation and culture of human keratinocytes
Human keratinocytes were isolated from skin tissue that was obtained from surgical discard as previously described.30 Briefly, skin was washed in three serial washes with antibiotic-antimycotic solution (Invitrogen) that was supplemented with gentamicin sulfate. Skin tissue was cut into ∼3×3 mm pieces and incubated in 0.125% trypsin solution at 4°C overnight. After enzymatic digestion, the epidermis was detached from the dermis using forceps. Cells at the epidermal-dermal junction were scraped off gently and resuspended in Full Green's media (FGM) as previously described by Rheinwald and Green.24,30,31 Freshly isolated epidermal cells were then seeded into a T75 cell culture flask containing 2×106 irradiated 3T3 mouse fibroblasts at a ratio of 1:1. Keratinocyte cultures were maintained at 37°C with 5% CO2 until 80% confluency was reached. Cell culture media (FGM) was replaced every 2–3 days. Nonsun exposed skin was obtained from consenting donors undergoing elective cosmetic breast reduction or abdominoplasty procedures. Ethical approval for the collection of human tissue was obtained from ethics committees at participating hospitals and the Queensland University of Technology (St Andrew's Hospital; Approval #200 4/46, Brisbane Private Hospital and Princess Alexandra Hospital; Approval #QUT 3865H). All experimentation performed with donor tissue was in accordance with the Declaration of Helsinki, 2004.
Preparation and culture of HSE-DED
The HSE-DED composites were prepared using a previously reported method.22 Briefly, the DED scaffold was prepared from 1.4 cm2 pieces of the dermis of ex vivo skin tissue according to previously described protocols22,23 by immersion in 1 M sodium chloride solution and incubated at 37°C overnight to decellularize the tissue. The epidermis was then separated from the dermal tissue and discarded. Next, ∼1.4 cm2 pieces of DED were prepared in 24-well cell culture plates (Thermo Fischer Scientific). The resulting DED was then washed with phosphate-buffered saline before being immersed in FGM overnight at 37°C before keratinocyte seeding. Cultured keratinocytes (passage 1, 2×104 cells) were then seeded (in sterile stainless-steel rings with silicone washer bases) (Aix Scientifics) onto the papillary surface of each prepared DED. The DED was then incubated with the rings in place at 37°C with 5% CO2 for 48 h before their removal. The HSE-DED composites were then cultured in FGM on stainless-steel grids at the air-liquid interface in six-well cell culture plates (Thermo Fischer Scientific) at 37°C with 5% CO2, for 9 days.21
UVB-irradiation of skin tissues
Skin tissues (HSE-DEDs and ex vivo skin explants) were irradiated with a single dose of UVB radiation using a calibrated medical UVB bulb (TL-40; Phillips) that was calibrated to deliver 9.27 mJ/cm2/min UVB radiation, with a stabilized power supply under sterile conditions in the dark at room temperature. Cellulose acetate film (Kodacel TA-407; Eastman-Kodak) was used to block out contaminating radiation (wavelengths below 290 nm) from the UVB source.32 The HSE-DED constructs were partially submerged in prewarmed PBS during irradiation. In the irradiated samples, the epidermal surface of HSE-DED constructs was placed 6 cm from the external surface of the UVB lamp and exposed to 50 mJ/cm2 UVB (two minimum erythemal dose) radiation. Irradiation was conducted inside a Class II Biological Safety Cabinet to maintain sterility. Mock irradiated samples were treated with similar conditions but in the absence of UVB radiation. The UVB irradiation dose was chosen based on dose-response studies in which 50 mJ/cm2 UVB generated classic markers of UVB radiation-induced damage without complete destruction of the HSE-DED epidermal layer (data not shown). The skin equivalent constructs were returned to the culture conditions as described in the previous section postirradiation. Ex vivo skin explant tissues that were used for a comparative analysis were UVB irradiated within 2 h of excision from the donor patient. The skin explant tissues were maintained in FGM on stainless-steel grids at the air-liquid interface in six-well cell culture plates (Thermo Fischer Scientific) at 37°C with 5% CO2 until analysis.
Histological analysis
At specific time points postirradiation, HSE-DED composite tissue was fixed in 10% neutral buffered formalin overnight at 4°C, before being dehydrated in a graded ethanol series and paraffin embedded. Sections of 5 μm thickness were cut (from the center of the HSE-DED samples) and mounted onto glass slides. The tissue sections were deparaffinized in xylene and rehydrated in a decreasing graded ethanol series before routine hematoxylin and eosin (H&E) staining.
Immunohistochemical analysis
The immunohistochemical analyses of the HSE-DED tissue sections were performed as per previously documented techniques.22 Briefly, 3 μm-thick tissue sections were deparaffinized and rehydrated before heat-induced epitope revival treatment in either tris-ethylendiaminetetraacetic acid (EDTA) buffer at pH 9.0 or sodium citrate buffer at pH 6.0 for 4 min at 97°C, followed by 10 min at 70°C in a decloaking chamber. Endogenous peroxidases were blocked with hydrogen peroxide solution (Biocare Medial), and nonspecific primary antibody binding was blocked using a blocking reagent (Biocare Medical). The primary antibodies used in immunohistochemical analysis are detailed in Table 1. Primary antibodies were diluted in sample diluent solution (Biocare Medical) and incubated in a humidified chamber overnight at 4°C. A horseradish peroxidase-linked secondary antibody was then applied to the tissue samples and detected with a 3,3′diaminobenzidine (DAB) chromogen system (Biocare Medical). The tissue sections were counterstained using hematoxylin Gill 1 (G1) nuclear stain (HD Scientific).
Table 1.
Primary Antibodies Used in Immunohistochemical Analysis
| Primary antigen | Host | Dilution | Antibody source |
|---|---|---|---|
| Keratin 1/10/11 | Mouse | 1:100 | USBioLogical, Texas |
| Cytokeratin 2e | Mouse | 1:100 | Abcam, Cambridge, United Kingdom |
| Keratin 14 | Mouse | 1:200 | Novus Biologicals, Colorado |
| Filaggrin | Mouse | 1:50 | Santa Cruz Biotechnology, California |
| p53 | Mouse | 1:50 | Clone DO-7, Dako, Hamburg, Germany |
| Collagen IV | Mouse | 1:50 | Clone CIV22, Dako |
| Ki-67 | Mouse | 1:50 | Clone MIB 5, Dako |
| Cyclobutane pyrimidine dimmers | Mouse | 1:1000 | Clone KTM53, Kamiya Biomedical Company, Japan |
Detection of apoptotic cells
Tissue sections of UVB-irradiated HSE-DED composites were analyzed for the presence of apoptotic cells using an in situ terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (Roche Applied Science) as per the manufacturer's protocol. Cell nuclei were counterstained with DAPI solution (Invitrogen) and imaged using fluorescence microscopy.
Quantification of secreted cytokines
To quantify levels of secreted cytokines in UVB-irradiated HSE-DED cultures, enzyme-linked immunoassay kits (Quantikine®; R&D Systems) for the detection of human IL-6 and IL-8 were utilized as per the manufacturer's instructions. Before irradiation, FGM was replaced with serum-free media (FGM without fetal calf serum) in the HSE-DED cultures. Cell culture supernatant samples were collected before and after UVB irradiation and were centrifuged to remove cellular debris. Samples were stored at −80°C until the assay was performed.
Image analysis
Digital image analyses of antibody-labeled tissue sections were performed using ImageJ software (public domain software downloaded from http://rsb.info.nih.gov/ij/).33
Statistical analysis
One-way ANOVA with Tukey's post hoc tests (all group comparisons) was applied to analyze the data. Statistical significance was accepted when p<0.05.
Results
Epidermal characterization of the HSE-DED model compared with native skin
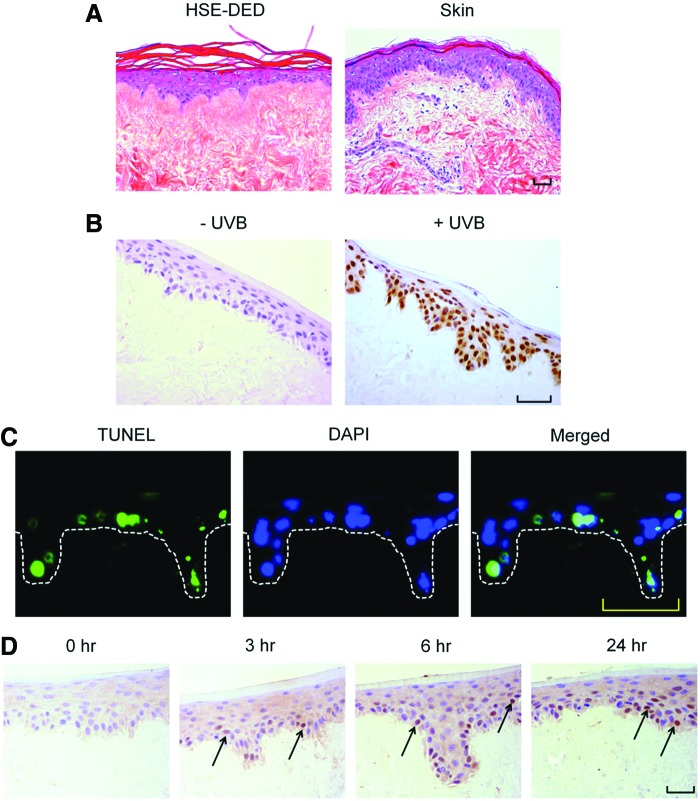
The human epidermis has a characteristic polarized pattern of keratinocyte growth, with actively proliferating cells situated at the dermal-epidermal junction. A comparative immunohistochemical study was conducted on unirradiated HSE-DED and native skin tissue to identify parallels in the expression of key epidermal markers (Fig. 1). Keratins 1, 10, and 11 were detected in terminally differentiated keratinocytes within the stratum spinosum, stratum granulosum, and stratum lucidum in both the HSE-DED and ex vivo skin sections. Upper spinous and granular keratinocytes, known to express cytokeratin 2e, were also detected in the HSE-DED composites.34,35 Similarly, the presence of keratin 14-expressing keratinocytes was detected in both native skin and HSE-DED sections, with higher immunoreactivity levels observed among the basal and suprabasal cell populations. Positive immunoreactivity of filaggrin was detected in the cornified envelope of keratinocytes within the stratum corneum in both the HSE-DED and skin tissue samples alike. The presence of type IV collagen, a major constituent of the BM zone in the skin, was detected at the dermal-epidermal junction in both the HSE-DED and skin tissue samples. Besides separating the epidermis and dermis, collagen IV is also known to line sweat glands, hair follicles, and blood vessels, which accounts for its positive immunoreactivity in these structures within the dermis and DED.36
FIG. 1.
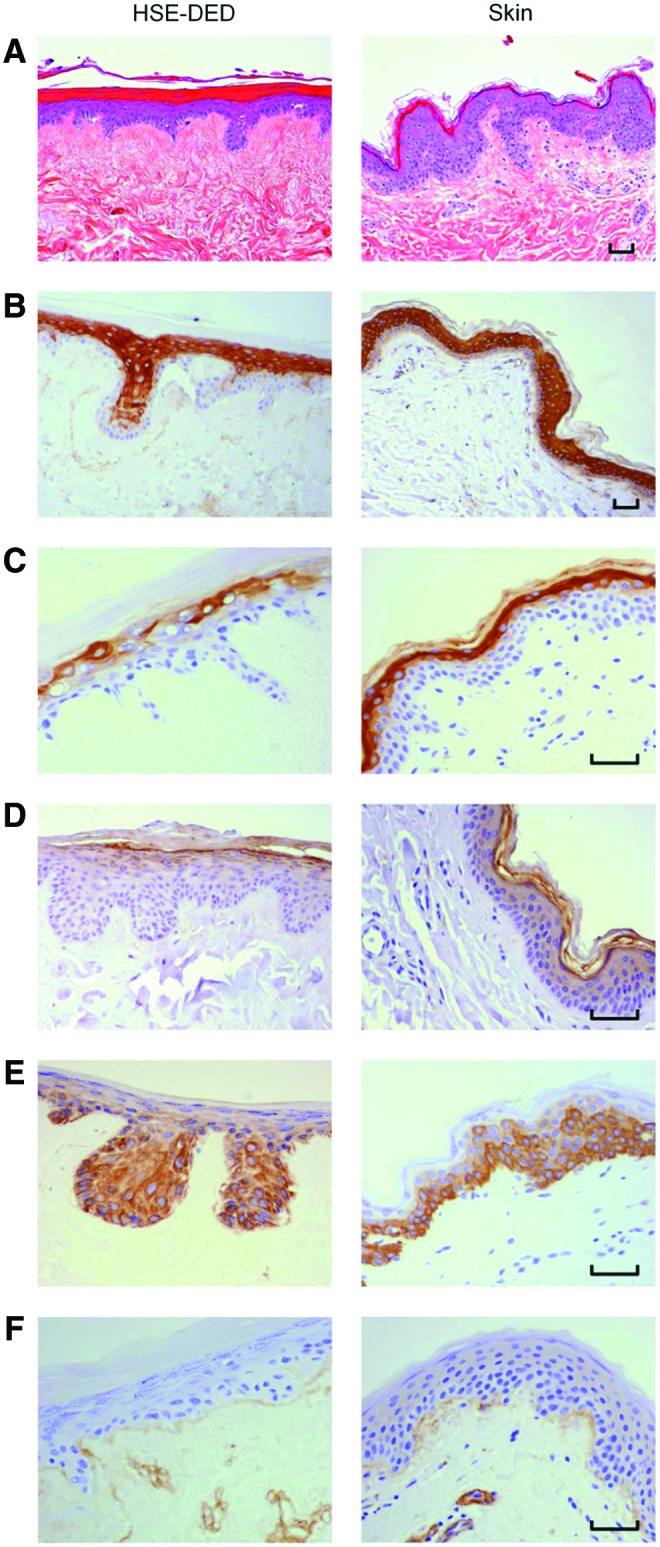
Characterization of unirradiated human skin equivalent-de-epidermized dermal (HSE-DED) constructs compared with native skin. Hematoxylin and eosin staining of HSE-DED and native skin sections (A) shows histological parallels between the two tissue types. The expression of epidermal markers in the HSE-DED and ex vivo skin was analyzed using immunohistochemistry (brown staining). Keratins 1/10/11 (B) were detected in the stratum spinosum, stratum lucidum, and stratum granulosum in both samples. The expression of cytokeratin 2e (C) was observed in upper suprabasal keratinocytes in both the HSE-DED and skin sections. Filaggrin (D) expression was detected in the stratum corneum layer in both ex vivo and skin equivalent tissues. Keratin 14 (E) appeared in basal and suprabasal keratinocytes in both sections of HSE-DED and native skin, with the latter showing a more intense immunoreactivity in the stratum basal. Collagen IV (F) was observed at the epidermal-dermal junction and lining dermal structures in both the skin equivalents and native skin. Images are representative of immunohistochemical analyses of native skin and HSE-DED constructs from three independent skin donor samples. Scale bars represent 50 μm in length. Color images available online at www.liebertpub.com/tec
UVB irradiation induces markers of photodamage in the HSE-DED
A histological analysis of UVB-irradiated HSE-DED and ex vivo skin explant tissue revealed the formation of apoptotic keratinocytes in the epidermal layer, characterized by the presence of pyknotic nuclei and cytoplasmic shrinkage (Fig. 2A). The formation of CPD molecular lesions in the nuclei of UVB-irradiated keratinocytes is a classic biomarker of DNA damage and an important factor in NMSC development.10 Immunohistochemical staining using an antibody that was specific for CPDs was performed on irradiated and nonirradiated HSE-DED tissue (Fig. 2B). CPD-positive keratinocytes were observed only in UVB-treated HSE-DED constructs, showing marked positive nuclear immunoreactivity in basal and suprabasal cells.
FIG. 2.
Analysis of UVB photodamage markers in irradiated HSE-DED constructs. Images represent the histology (A), formation of cyclobutane pyrimidine dimers (CPDs) (B), apoptotic keratinocytes (C), and p53 expression (D) in sections of HSE-DED exposed to two MED UVB radiation. At 24 h postirradiation, sections of the irradiated HSE-DED constructs appear histologically similar to those of irradiated ex vivo human skin. Immediately after UVB exposure, the presence of DNA dimers (B) within the nuclei of HSE-DED keratinocytes is shown by the immunohistochemical detection of CPDs. Sunburn cells or apoptotic keratinocytes were detected in irradiated HSE-DED using the TUNEL assay and immunofluorescence. The presence of sunburn cells (green fluorescence) was observed in the epidermal layer of irradiated HSE-DED (C) at 24 h after UVB exposure. The expression of p53, an important cellular mediator of UVB-induced pathways, was analyzed at 3, 6, and 24 h after irradiation in HSE-DED tissue. As indicated by the arrows, p53-positive keratinocyte nuclei appear in irradiated HSE-DED constructs at 3, 6, and 24 h postirradiation. Scale bars represent 50 μm in length. Color images available online at www.liebertpub.com/tec
Keratinocytes that have sustained irreparable DNA damage resulting from UVB damage undergo apoptosis to prevent the propagation of cells harboring photolesions. Using the TUNEL assay, DNA fragmentation (as a marker of apoptosis) was detected in UVB-exposed HSE-DED constructs (Fig. 2C). At 24 h after a single two MED UVB dose, cells undergoing apoptosis were found to be present along the stratum basale and among suprabasal keratinocyte populations. On average, 19.9%±4.1% of the total number of epidermal nuclei were found to be TUNEL positive at 24 h postirradiation.
The activation and downstream processes mediated by p53 are known to be critical for suppressing oncogenesis in UVB-irradiated keratinocytes. The presence of p53 in UVB-exposed HSE-DED keratinocytes was detected using immunohistochemical analysis (Fig. 2D). Sections from these HSE-DED constructs sacrificed at 0, 3, 6, and 24 h postirradiation reveal the intranuclear accumulation of p53.
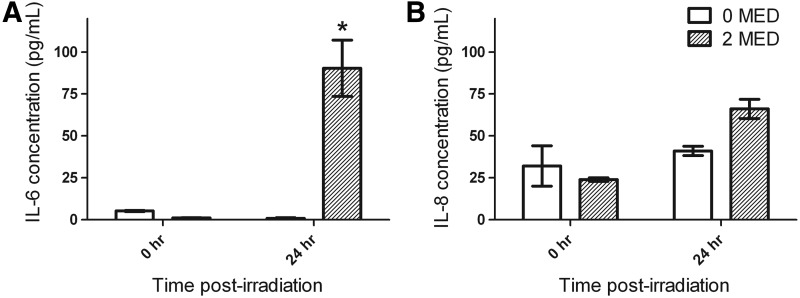
The up-regulation of IL-6 and IL-8 production by UVB-irradiated keratinocytes enable them to be considered central catalysts for the activation of local and systemic immune responses.37,38 The relative levels of secreted IL-6 and IL-8 were quantified in the media of HSE-DED cultures before and after UVB exposure with an ELISA (Fig. 3). Increases in the production of both cytokines were observed in irradiated HSE-DED at the 24 h postirradiation time point compared with samples obtained at t=0 h. This includes a significant increase from 1.00±0.3 to 90.3±16.8 pg/mL in detected IL-6. Basal levels of IL-8 that were detected were relatively higher (24.0±1.0 pg/mL) due to this cytokine being constitutively expressed by keratinocytes. UVB exposure resulted in a slight increase in IL-8 in HSE-DED media, with an average of 66.1±5.7 pg/mL.
FIG. 3.
Quantification of cytokine secretion in HSE-DED culture supernatants. Cell culture medium samples were obtained from HSE-DED cultures before and 24 h after irradiation with two MED UVB radiation. Supernatants were analyzed for the presence of interleukin-6 (A) and 8 (B) (IL-6/8) using an ELISA method. Data represent the average quantities of IL-6 and IL-8 detected at the two time points (mean±SEM), in experimental triplicates from two independent skin donors, expressed in pg/mL. All measurements were normalized to total protein levels in culture supernatants. Asterisks represent statistical significance (p<0.05) in the difference between matched samples at t=0 h.
HSE-DED composites retain the capacity for repair and regeneration after UVB irradiation
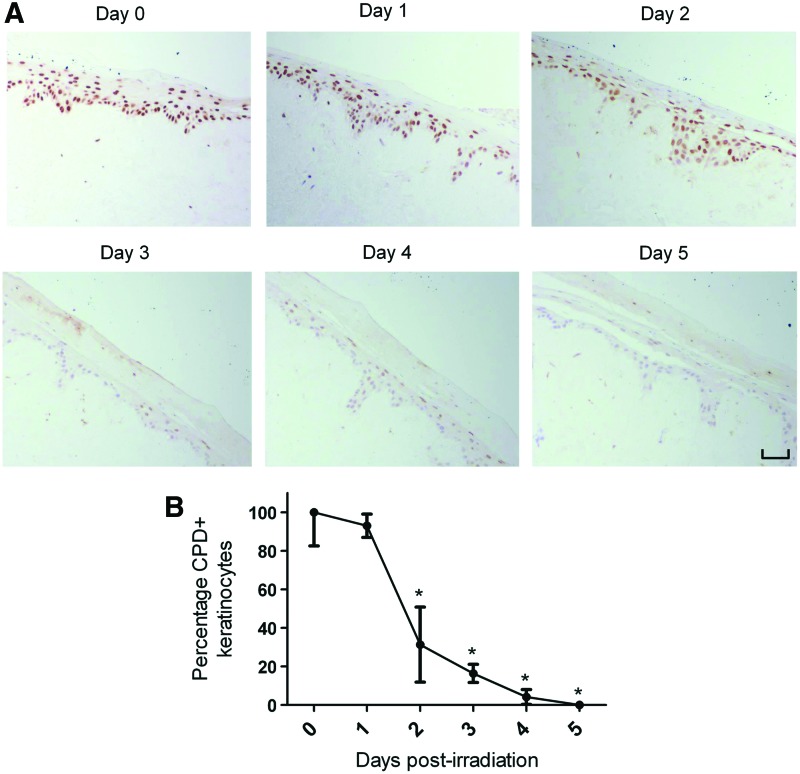
Due to the frequency of their exposure to damaging UVR, keratinocytes possess highly sophisticated repair mechanisms to cope with the formation of UVB-induced DNA mutations.39 The efficient removal of CPDs, for instance, has been strongly associated with the prevention of oncogenic transformation in irradiated keratinocytes.40 In view of this, an immunohistochemical analysis was performed to study the removal of UVB-induced CPDs in irradiated HSE-DED composites (Fig. 4A). After a single dose of UVB radiation, a gradual decrease in the intensity of CPD-positive immunoreactivity was observed in the epidermis of the HSE-DED over 5 days under culture conditions. At 2 to 5 days postirradiation, a significant decrease in the number of CPD-bearing keratinocytes was observed in these constructs as compared with levels immediately after UVB exposure (Fig. 4B).
FIG. 4.
Postirradiation removal of UVB-induced CPDs in HSE-DED constructs. CPDs in the nuclei of HSE-DED keratinocytes irradiated with two MED UVB over 5 days were detected using immunohistochemical analysis (A). The HSE-DED constructs were then returned to culture conditions and sampled every 24 h to test for the presence of UVB-induced DNA damage. From days 0 to 5 after UVB exposure, there was an observable decrease in the number of CPD-positive keratinocytes in the epidermal layer of the HSE-DED. Keratinocytes harboring CPDs in the HSE-DED postirradiation were quantified using image analysis (B). The graph represents the mean ratio of immunopositive cells to total cell nuclei in five microscopy fields per sample±SEM. At days 2 to 5, there was a statistically significant (p<0.05) decrease in the proportion of CPD-positive cells (compared with day 0). The data presented are representative of results obtained using three independent tissue donors (n=3). HSE-DEDs were constructed in triplicate for each time point sampled. The scale bar represents 50 μm in length. Asterisks represents statistical significance of p<0.05. Color images available online at www.liebertpub.com/tec
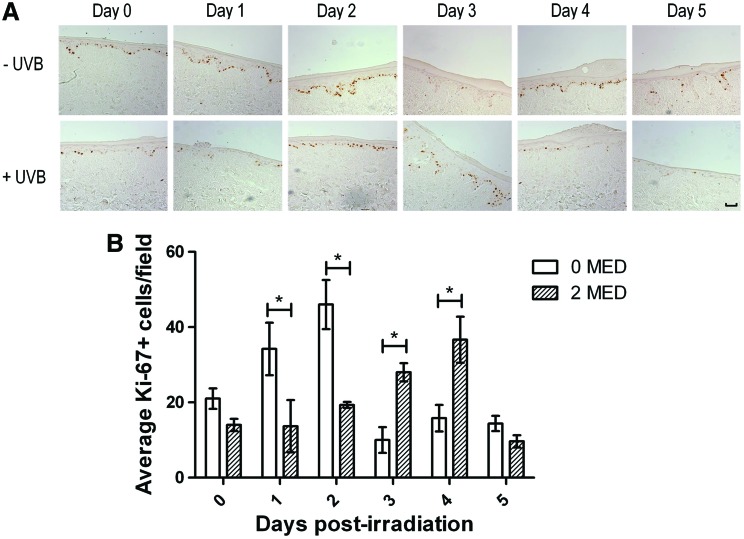
Epidermal regeneration after UVB-induced damage is facilitated by the stimulation of proliferation by various growth factors and cytokines.41 The nuclear expression of the Ki-67 antigen occurs during all active phases of cellular proliferation.42 Keratinocyte proliferation during the HSE-DED epidermal regenerative phase was investigated using immunohistochemical detection of Ki-67 in tissue sampled over 5 days postirradiation (Fig. 5). In UVB-exposed HSE-DED constructs, significantly lower numbers of Ki-67-expressing keratinocytes were observed at 1 and 2 days after irradiation (relative to unexposed HSE-DEDs). Subsequently, at 3 and 4 days post-treatment, there was a significant elevation in the number of proliferative keratinocytes in the UVB-treated constructs as compared with the untreated controls. Similar levels of Ki-67-positive keratinocytes were observed at Day 0 and 5 in both irradiated and nonirradiated samples. This indicates that UVB exposure triggers an initial inhibition of proliferation in the HSE-DED keratinocytes, potentially coinciding with the repair of DNA damage. This is followed by a proliferative burst that may function to replace UVB-damaged cells in which differentiation or apoptotic pathways have been activated.
FIG. 5.
Analysis of keratinocyte proliferation in HSE-DED constructs. The proliferation marker, Ki-67, was detected using immunohistochemistry in 2 MED UVB-irradiated and non-irradiated control HSE-DED constructs (A), and quantified in (B). Following UVB or sham exposure, the HSE-DEDs were returned to culture conditions and sampled every 24 hours for five days to investigate changes in epidermal proliferation during this period. Keratinocyte nuclei positive for Ki-67 are in the active phases of the cell cycle. The scale bar represents 50 μm. (B) The graph represents the mean counts of Ki-67-positive cells in five microscopy fields per sample ± SEM. At days 1 to 2, significantly higher (p < 0.05) numbers of proliferative cells were observed in non-irradiated HSE-DED constructs. However at days 3–4, a proliferative burst was observed in irradiated constructs, with significantly (p < 0.05) elevated numbers of Ki-67-positive keratinocyte observed relative to the non-irradiated controls. The images and data presented are representative of experimental triplicates from three independent skin donors (n = 3). HSE-DEDs were constructed in triplicate for each time-point sampled. Asterisks represent statistical significance of p < 0.05. Color images available online at www.liebertpub.com/tec
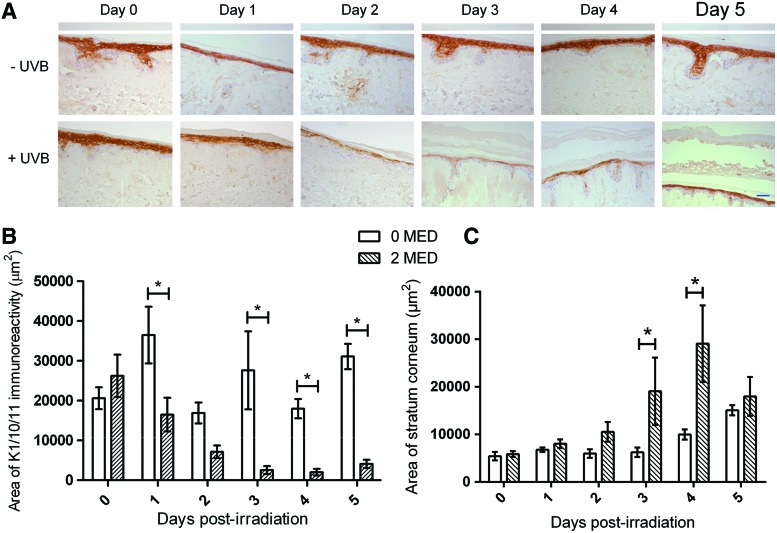
Another physiological epidermal response to UVR exposure is the thickening of the stratum corneum, which, along with the tanning, is considered a protective mechanism.43 This increase in thickness is associated with elevated levels of keratinocyte differentiation into corneocytes.44 The layer of differentiated keratinocytes (expressing keratins 1/10/11) in the HSE-DED epidermis was investigated subsequent to UVB exposure via immunohistochemistry to identify whether similar processes occur in these constructs (Fig. 6). These areas were then quantified using image analysis software. In the first 3 days after UVB irradiation, a decline in the average immunoreactive areas to keratins 1/10/11 was observed. This was localized to the spinous and granular layers of the epidermis. This was accompanied by a thickening of the stratum corneum layer in these constructs as observed in the tissue sections. Similar results of UVB-induced epidermal thickening have also previously been reported from in vivo studies involving irradiated test subjects.45,46
FIG. 6.
Epidermal blistering and regeneration after UVB irradiation in HSE-DED constructs. Immunohistochemical staining of keratin 1/10/11 in irradiated and mock-irradiated HSE-DED was conducted to identify changes to the thickness of the suprabasal layer containing differentiated keratinocytes (A). The images presented are representative of experimental triplicates from three independent skin donors. The scale bar represents 50 μm. The area of keratin 1/10/11 expression within the epidermis from immunohistochemical analysis was then quantified using image analysis software (ImageJ) (B). The mean area of keratin 1/10/11 positive immunoreactivity was expressed in μm2 from five microscopy fields in three independent experiments. The mean area of the stratum corneum layer was also quantified using image analysis (C). UVB irradiation causes a significant thickening of the HSE-DED stratum corneum. Error bars represent SEM values. Asterisks represent statistically significant differences (p<0.05) as compared with the nonirradiated controls at the same time point. Experiments were conducted on samples from three independent skin tissue donors. Color images available online at www.liebertpub.com/tec
Discussion
The gold standard of in vitro photobiology models should display biological parallels with native skin, as well as classical photodamage markers on UV irradiation. Conventional cell-based techniques, such as 2D cultures, do not adequately represent the complex morphology and organizational aspects of the human epidermis. Moreover, limitations associated with animal models include ethical restrictions, high costs, and differences to human skin anatomy and physiology. Accordingly, the HSE-DED reconstituted skin model constructed here was validated as an experimental model to investigate keratinocyte responses to UVB radiation. Composed of primary human keratinocytes cultured on a DED dermal substrate, the HSE-DED constructs maintain histological and physiological aspects of native skin, many of which are critical to the UVB photoresponse. Previously, we have reported that the 3D HSE-DED organotypic model can be applied to the investigation of a diverse range of epithelial processes, including woundbhealing.21, 22 In this current study, UVB irradiation of the HSE-DED was found to induce the generation of characteristic markers of photodamage in human keratinocytes. Significantly, our data indicate that the HSE-DED keratinocytes also retained their capacity to repair during epidermal regeneration postirradiation.
Histological analysis of the HSE-DED revealed that several morphological features of the native epidermis, such as the presence of rete ridges and the epidermal structural organization, were retained. This was in accordance with previously reported studies on the histology of similar tissue-engineered skin constructed in vitro.47–49 The use of similar HSE models has also been reported in studies conducted on UVR-induced alterations to epidermal viability50 and protein expression.51 In these models, different dermal substrates such as collagen matrices, as well as various combinations of UVR wavelengths and doses were utilized. Importantly, to date, a fully characterized model using reconstituted human tissue is still lacking. By incorporating a single cell type into the HSE-DED model described here, we aimed at obtaining baseline data on the effects of UVB radiation in a physiologically relevant setting. This can then be used to compare, for example, the protective effects of other cutaneous cell populations, such as melanocytes and fibroblasts, on their incorporation into the HSE model.
The presence of a multilayered epithelium with a polarized differentiation gradient was further confirmed in the HSE-DED via the immunohistochemical detection of keratins 14, 1/10/11, 2e and filaggrin. Importantly, the HSE-DED enables the investigation into specific responses of basal keratinocytes to UVB radiation, in the context of a differentiated epidermis. Indeed, damage to DNA in basal keratinocytes as a result of UVB exposure is considered a major initiating factor in the development of squamous cell carcinoma and basal cell carcinomas.3 The protective function of the stratum corneum and filaggrin expression in the HSE-DED epidermis is also significant in the modeling of UVB-induced epithelial damage.52–54 Monolayer and tissue-engineered cultures lacking filaggrin, for example, have elevated levels of DNA damage and apoptosis observed in the stratum basale.54 In addition, the positive identification of the basement membrane component, collagen IV, at the epidermal-dermal junction is notable as a physiologically relevant photobiology model. Lacking in 2D cell cultures, such BM components have been found to profoundly influence keratinocyte responses to many external stimuli, including UVB radiation.55,56
The classical markers of acute cutaneous photodamage have been well documented, and they include the formation of DNA photolesions, apoptosis, inflammation, and the tanning response.57 Notably, in the studies reported here, key UVB-induced keratinocyte damage markers were shown to be inducible in the HSE-DED in an in vitro setting. The formation of CPDs and other photoproducts are a fundamental mutagenic event in the transformation of basal keratinocytes.3 In the HSE-DED, irradiation was found to result in the formation of these thymine dimers in keratinocyte nuclei, in line with investigations on UVB-exposed native skin biopsies.58,59 Interestingly, the rate of CPD removal in the HSE-DED was comparable not only to studies using other organotypic skin constructs, but also to the in vivo experiments mentioned earlier.49,60 This indicates that the cells in the HSE-DED retain the ability to initiate DNA damage response and repair pathways, which potentially enables the screening of novel mechanisms to enhance these processes.
The activation of both DNA damage-induced apoptosis and repair mechanisms are highly dependent on the function of p53.61–63 The UVB-induced accumulation of p53 in HSE-DED basal and suprabasal keratinocytes correlates with previously documented reports.63,64 Importantly, major differences in p53 activation have been identified between irradiated keratinocyte monolayers and in vivo skin.65 This has been attributed to factors such as keratinocyte differentiation status and cellular adhesion.66 The UVB-triggered secretion of inflammatory cytokines, including IL-1, -6, -8, -10, -12, and tumor necrosis factor-α, stimulates erythemal and immunomodulatory responses postirradiation.67–70 In line with earlier reports, our data demonstrate a significant augmentation of IL-6 and IL-8 levels in HSE-DED culture media after UVB irradiation.37,38 Potentially, the irradiated HSE-DED model could, thus, be applied to the analysis of pathways involved in inflammation as a result of UV exposure or cutaneous diseases such as psoriasis.71,72
Several previous studies on keratinocyte responses to irradiation involve the use of nonphysiological doses of UVB to induce photodamage.73,74 In part, this is because monolayer keratinocyte cultures are selective for cells with a more proliferative phenotype and an associated higher tolerance to UVB-induced damage.75 Moreover, the use of pigmented skin equivalents has been shown to withstand higher UVB doses; this is due to the shielding effect of melanin.76–78 Similarly, other in vivo studies in pigmented skin show relatively lower levels of apoptosis induction after irradiation compared with those observed in the HSE-DED.79 The studies reported here characterizing the HSE-DED model were conducted using physiological doses of UVB radiation. Indeed, the doses utilized have previously been reported to be experienced within the Australian population.80,81 The HSE-DED, therefore, may be applied to the testing of potential therapeutics or preventative strategies against keratinocyte damage in a biologically appropriate setting.
In summary, we demonstrate here that the HSE-DED model holds potential as a versatile and powerful photobiology tool due to the retention of several physiological cutaneous properties. Moreover, the HSE-DED represents a basic epidermal model into which additional cell types can be incorporated, resulting in the creation of an improved biologically relevant skin model. It is important to bear in mind that despite the HSE-DED model being flexible in terms of the origin and type of primary cells which may be incorporated, this may also contribute to inter-donor variability, a phenomenon that has been reported in a variety of skin studies.82–84 Indeed, studies have shown variability to exist, particularly in keratinocyte UVB responses between individuals.85 In the future, the development of increasingly elegant synthetic dermal substitutes may provide improved dermal scaffolds that can reduce variability while preserving the physiological properties of the dermis more faithfully.19,25–27
Disclosure Statement
The authors declare that no competing financial interests or conflicts of interest are associated with the work presented here.
References
- 1.Welsh M.M., Karagas M.R., Kuriger J.K., Houseman A., Spencer S.K., Perry A.E., et al. Genetic determinants of UV-susceptibility in non-melanoma skin cancer. PLoS One 6, e20019 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghiorzo P., Bonelli L., Pastorino L., Bruno W., Barile M., Andreotti V., et al. MC1R variation and melanoma risk in relation to host/clinical and environmental factors in CDKN2A positive and negative melanoma patients. Exp Dermatol 21,718, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y., and Ananthaswamy H.N.Molecular mechanisms of photocarcinogenesis. Front Biosci 7,d765, 2002 [DOI] [PubMed] [Google Scholar]
- 4.AIHW (Australian Institute of Health & Welfare) and AACR (Australasian Association of Cancer Registries) 2007. Cancer in Australia: an overview, 2006. Cancer series no. 37. Cat. no. CAN 32. Canberra: AIHW [Google Scholar]
- 5.Australian Institute of Health and Welfare A. Non-melanoma Skin Cancer: General Practice Consultations, Hospitalisation and Mortality. Canberra: Australian Institute of Health and Welfare A, 2008 [Google Scholar]
- 6.Australian Institute of Health and Welfare A. Health System Expenditures on Cancer and Other Neoplasms in Australia, 2000–2001. Canberra: Australian Institute of Health and Welfare A, 2005 [Google Scholar]
- 7.Battie C., and Verschoore M.Cutaneous solar ultraviolet exposure and clinical aspects of photodamage. Indian J Dermatol Venereol Leprol 78Suppl 1, S9.2012 [DOI] [PubMed] [Google Scholar]
- 8.Lavker R., and Kaidbey K.The spectral dependence for UVA-induced cumulative damage in human skin. J Invest Dermatol 108,17, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Kappes U.P., Luo D., Potter M., Schulmeister K., and Runger T.M.Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J Invest Dermatol 126,667, 2006 [DOI] [PubMed] [Google Scholar]
- 10.You Y.H., Szabo P.E., and Pfeifer G.P.Cyclobutane pyrimidine dimers form preferentially at the major p53 mutational hotspot in UVB-induced mouse skin tumors. Carcinogenesis 21,2113, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Svobodova A.R., Galandakova A., Sianska J., Dolezal D., Lichnovska R., Ulrichova J., et al. DNA damage after acute exposure of mice skin to physiological doses of UVB and UVA light. Arch Dermatol Res 304,407, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Wikonkal N.M., and Brash D.E.Ultraviolet radiation induced signature mutations in photocarcinogenesis. J Investig Dermatol Symp Proc 4,6, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Young A.R.The sunburn cell. Photodermatol 4,127, 1987 [PubMed] [Google Scholar]
- 14.Fernandez T.L., Dawson R.A., Van Lonkhuyzen D.R., Kimlin M.G., and Upton Z.A tan in a test tube–in vitro models for investigating ultraviolet radiation–induced damage in skin. Exp Dermatol 21,404, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Ponec M.Skin constructs for replacement of skin tissues for in vitro testing. Adv Drug Deliv Rev 54,S19, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Khavari P.A.Modelling cancer in human skin tissue. Nat Rev Cancer 6,270, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Adler S., Basketter D., Creton S., Pelkonen O., van Benthem J., Zuang V., et al. Alternative (non-animal) methods for cosmetics testing: current status and future prospects—2010. Arch Toxicol 85,367, 2011 [DOI] [PubMed] [Google Scholar]
- 18.MacNeil S.Progress and opportunities for tissue-engineered skin. Nature 445,874, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Supp D.M., and Boyce S.T.Engineered skin substitutes: practices and potentials. Clin Dermatol 23,403, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kairuz E., Upton Z., Dawson R.A., and Malda J.Hyperbaric oxygen stimulates epidermal reconstruction in human skin equivalents. Wound Repair Regen 15,266, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Xie Y., Rizzi S.C., Dawson R., Lynam E., Richards S., Leavesley D.I., and Upton Z.Development of a three-dimensional human skin equivalent wound model for investigating novel wound healing therapies. Tissue Eng Part C 16,1111, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Topping G., Malda J., Dawson R., and Upton Z.Development and characterisation of human skin equivalents and their potential application as a burn wound model. Prim Intention 14,14, 2006 [Google Scholar]
- 23.Chakrabarty K.H., Dawson R.A., Harris P., Layton C., Babu M., Gould L., Phillips J., Leigh I., Green C., Freedlander E., and MacNeil S.Development of autologous human dermal-epidermal composites based on sterilized human allodermis for clinical use. Br J Dermatol 141,811, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Poumay Y., Dupont F., Marcoux S., Leclercq-Smekens M., Herin M., and Coquette A.A simple reconstructed human epidermis: preparation of the culture model and utilization in in vitro studies. Arch Dermatol Res 296,203, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Valenta C., and Auner B.G.The use of polymers for dermal and transdermal delivery. Biopharmaceutics 58,279, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Mazlyzam A.L., Aminuddin B.S., Fuzina N.H., Norhayati M.M., Fauziah O., Isa M.R., Saim L., and Ruszymah B.H.I.Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns 33,355, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Liu H., Mao J., Yao K., Yang G., Cui L., and Cao Y.A study on a chitosan-gelatin-hyaluronic acid scaffold as artificial skin in vitro and its tissue engineering applications. J Biomater Sci Polym Educ 15,25, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Schafer I.A., Shapiro A., Kovach M., Lang C., and Fratianne R.B.The interaction of human papillary and reticular fibroblasts and human keratinocytes in the contraction of three-dimensional floating collagen lattices. Exp Cell Res 183,112, 1989 [DOI] [PubMed] [Google Scholar]
- 29.El Ghalbzouri A., Commandeur S., Rietveld M.H., Mulder A.A., and Willemze R.Replacement of animal-derived collagen matrix by human fibroblast-derived dermal matrix for human skin equivalent products. Biomaterials 30,71, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Dawson R.A., Upton Z., Malda J., and Harkin D.G.Preparation of cultured skin for transplantation using insulin-like growth factor I in conjunction with insulin-like growth factor binding protein 5, epidermal growth factor, and vitronectin. Transplantation 81,1668, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Rheinwald J.G., and Green H.Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6,331, 1975 [DOI] [PubMed] [Google Scholar]
- 32.Therrien J.P., Rouabhia M., Drobetsky E.A., and Drouin R.The multilayered organization of engineered human skin does not influence the formation of sunlight-induced cyclobutane pyrimidine dimers in cellular DNA. Cancer Res 59,285, 1999 [PubMed] [Google Scholar]
- 33.Abramoff M.D., Magalhaes P.J., and Ram S.J.Image processing with ImageJ. Biophotonics Int 11,36, 2004 [Google Scholar]
- 34.Smith L.T., Underwood R.A., and McLean W.H.Ontogeny and regional variability of keratin 2e (K2e) in developing human fetal skin: a unique spatial and temporal pattern of keratin expression in development. Br J Dermatol 140,582, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Collin C., Moll R., Kubicka S., Ouhayoun J.P., and Franke W.W.Characterization of human cytokeratin 2, an epidermal cytoskeletal protein synthesized late during differentiation. Exp Cell Res 202,132, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Konomi H., Hayashi T., Nakayasu K., and Arima M.Localization of type V collagen and type IV collagen in human cornea, lung, and skin. Immunohistochemical evidence by anti-collagen antibodies characterized by immunoelectroblotting. Am J Pathol 116,417, 1984 [PMC free article] [PubMed] [Google Scholar]
- 37.Shinoda S., Kameyoshi Y., Hide M., Morita E., and Yamamoto S.Histamine enhances UVB-induced IL-6 production by human keratinocytes. Arch Dermatol Res 290,429, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Kondo S., Kono T., Sauder D.N., and McKenzie R.C.IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol 101,690, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Mouret S., Charveron M., Favier A., Cadet J., and Douki T.Differential repair of UVB-induced cyclobutane pyrimidine dimers in cultured human skin cells and whole human skin. DNA Repair (Amst) 7,704, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Hanawalt P.C., Ford J.M., and Lloyd D.R.Functional characterization of global genomic DNA repair and its implications for cancer. Mutat Res 544,107, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Rosette C., and Karin M.Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274,1194, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Heenen M., Thiriar S., Noël J.C., and Galand P.Ki-67 immunostaining of normal human epidermis: comparison with 3H-thymidine labelling and PCNA immunostaining. Dermatology 197,123, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Elias P.M.Stratum corneum defensive functions: an integrated view. J Invest Dermatol 125,183, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Diffey B.L.Stratospheric ozone depletion and the risk of non-melanoma skin cancer in a British population. Phys Med Biol 37,2267, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Gambichler T., Kunzlberger B., Paech V., Kreuter A., Boms S., Bader A., et al. UVA1 and UVB irradiated skin investigated by optical coherence tomography in vivo: a preliminary study. Clin Exp Dermatol 30,79, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Lavker R.M., Gerberick G.F., Veres D., Irwin C.J., and Kaidbey K.H.Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol 32,53, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Prunieras M., Regnier M., and Woodley D.Methods for cultivation of keratinocytes with an air-liquid interface. J Invest Dermatol 81, 28s-33s 1983 [DOI] [PubMed] [Google Scholar]
- 48.Ponec M., Weerheim A., Kempenaar J., Mommaas A.M., and Nugteren D.H.Lipid composition of cultured human keratinocytes in relation to their differentiation. J Lipid Res 29,949, 1988 [PubMed] [Google Scholar]
- 49.Bernerd F., Marionnet C., and Duval C.Solar ultraviolet radiation induces biological alterations in human skin in vitro: relevance of a well-balanced UVA/UVB protection. Indian J Dermatol Venereol Leprol 78Suppl 1, S15, 2012 [DOI] [PubMed] [Google Scholar]
- 50.El-Abaseri T.B., Putta S., and Hansen L.A.Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 27,225, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Bernerd F., and Asselineau D.Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev Biol 183,123, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Sandilands A., Sutherland C., Irvine A.D., and McLean W.H.Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci 122,1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanitakis J., Ramirez-Bosca A., Reano A., Viac J., Roche P., and Thivolet J.Filaggrin expression in normal and pathological skin. A marker of keratinocyte differentiation. Virchows Arch A Pathol Anat Histopathol 412,375, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Mildner M., Jin J., Eckhart L., Kezic S., Gruber F., Barresi C., et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol 130,2286, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Stenback F., and Wasenius V.M.Basement membranes in ultraviolet light-induced skin lesions and tumors. Photo Dermatol 2,347, 1985 [PubMed] [Google Scholar]
- 56.Amano S.Possible involvement of basement membrane damage in skin photoaging. J Invest Dermatol Symp Proc 14,2, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Black H.S., deGruijl F.R., Forbes P.D., Cleaver J.E., Ananthaswamy H.N., deFabo E.C., et al. Photocarcinogenesis: an overview. J Photochem Photobiol B 40,29, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Katiyar S.K., Matsui M.S., and Mukhtar H.Kinetics of UV light-induced cyclobutane pyrimidine dimers in human skin in vivo: an immunohistochemical analysis of both epidermis and dermis. Photochem Photobiol 72,788, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Chadwick C.A., Potten C.S., Nikaido O., Matsunaga T., Proby C., and Young A.R.The detection of cyclobutane thymine dimers, (6–4) photolesions and the Dewar photoisomers in sections of UV-irradiated human skin using specific antibodies, and the demonstration of depth penetration effects. J Photochem Photobiol B 28,163, 1995 [DOI] [PubMed] [Google Scholar]
- 60.Marionnet C., Pierrard C., Lejeune F., Sok J., Thomas M., and Bernerd F.Different oxidative stress response in keratinocytes and fibroblasts of reconstructed skin exposed to non extreme daily-ultraviolet radiation. PLoS One 5, e12059 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Will K., Neben M., Schmidt-Rose T., Deppert W., Wittern K-P., and Bergemann J.p53-dependent UVB responsiveness of human keratinocytes can be altered by cultivation on cell cycle-arrested dermal fibroblasts. Photochem Photobiol 71,321, 2000 [DOI] [PubMed] [Google Scholar]
- 62.van Laethem A., Garmyn M., and Agostinis P.Starting and propagating apoptotic signals in UVB irradiated keratinocytes. Photochem Photobiol Sci 8,299, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Cotton J., and Spandau D.F.Ultraviolet B-radiation dose influences the induction of apoptosis and p53 in human keratinocytes. Radiat Res 147,148, 1997 [PubMed] [Google Scholar]
- 64.Lu Y.P., Lou Y.R., Yen P., Mitchell D., Huang M.T., and Conney A.H.Time course for early adaptive responses to ultraviolet B light in the epidermis of SKH-1 mice. Cancer Res 59,4591, 1999 [PubMed] [Google Scholar]
- 65.Enk C.D., Jacob-Hirsch J., Gal H., Verbovetski I., Amariglio N., Mevorach D., et al. The UVB-induced gene expression profile of human epidermis in vivo is different from that of cultured keratinocytes. Oncogene 25,2601, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Kumar M.G., Hurwitz S.A., Cotton J., and Spandau D.F.Subphysiological concentrations of extracellular calcium sensitize normal human keratinocytes to UVB-induced apoptosis. Arch Dermatol Res 291,37, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Petit-Frere C., Clingen P.H., Grewe M., Krutmann J., Roza L., Arlett C.F., et al. Induction of interleukin-6 production by ultraviolet radiation in normal human epidermal keratinocytes and in a human keratinocyte cell line is mediated by DNA damage. J Invest Dermatol 111,354, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Enk C.D., Mahanty S., Blauvelt A., and Katz S.I.UVB induces IL-12 transcription in human keratinocytes in vivo and in vitro. Photochem Photobiol 63,854, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Kock A., Schwarz T., Kirnbauer R., Urbanski A., Perry P., Ansel J.C., et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med 172,1609, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grewe M., Gyufko K., and Krutmann J.Interleukin-10 production by cultured human keratinocytes: regulation by ultraviolet B and ultraviolet A1 radiation. J Invest Dermatol 104,3, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Grossman R.M., Krueger J., Yourish D., Granelli-Piperno A., Murphy D.P., May L.T., et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A 86,6367, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nickoloff B.J., Karabin G.D., Barker J.N., Griffiths C.E., Sarma V., Mitra R.S., et al. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol 138,129, 1991 [PMC free article] [PubMed] [Google Scholar]
- 73.Leverkus M., Yaar M., and Gilchrest B.A.Fas/Fas ligand interaction contributes to UV-induced apoptosis in human keratinocytes. Exp Cell Res 232,255, 1997 [DOI] [PubMed] [Google Scholar]
- 74.Bender K., Blattner C., Knebel A., Iordanov M., Herrlich P., and Rahmsdorf H.J.UV-induced signal transduction. J Photochem Photobiol B 37,1, 1997 [DOI] [PubMed] [Google Scholar]
- 75.Liu S.C., Parsons S., and Hanawalt P.C.DNA repair in cultured keratinocytes. J Invest Dermatol 81,179s.1983 [DOI] [PubMed] [Google Scholar]
- 76.Nakazawa K., Nakazawa H., Sahuc F., Lepavec A., Collombel C., and Damour O.Pigmented human skin equivalent: new method of reconstitution by grafting an epithelial sheet onto a non-contractile dermal equivalent. Pigment Cell Res 10,382, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Todd C., Hewitt S.D., Kempenaar J., Noz K., Thody A.J., and Ponec M.Co-culture of human melanocytes and keratinocytes in a skin equivalent model: effect of ultraviolet radiation. Arch Dermatol Res 285,455, 1993 [DOI] [PubMed] [Google Scholar]
- 78.Brenner M., and Hearing V.J.The protective role of melanin against UV damage in human skin. Photochem Photobiol 84,539, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamaguchi Y., Takahashi K., Zmudzka B.Z., Kornhauser A., Miller S.A., Tadokoro T., et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. Faseb J 20,1486, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Kimlin M.G., Parisi A.V., and Wong J.C.The facial distribution of erythemal ultraviolet exposure in south-east Queensland. Phys Med Biol 43,231, 1998 [DOI] [PubMed] [Google Scholar]
- 81.Parisi A.V., Kimlin M.G., Wong J.C., and Wilson M.Personal exposure distribution of solar erythemal ultraviolet radiation in tree shade over summer. Phys Med Biol 45,349, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Judge M.R., Griffiths H.A., Basketter D.A., White I.R., Rycroft R.J., and McFadden J.P.Variation in response of human skin to irritant challenge. Contact Dermat 34,115, 1996 [DOI] [PubMed] [Google Scholar]
- 83.Basketter D.A., Griffiths H.A., Wang X.M., Wilhelm K.P., and McFadden J.Individual, ethnic and seasonal variability in irritant susceptibility of skin: the implications for a predictive human patch test. Contact Dermat 35,208, 1996 [DOI] [PubMed] [Google Scholar]
- 84.Hiroshima Y., Bando M., Kataoka M., Inagaki Y., Herzberg M.C., Ross K.F., et al. Regulation of antimicrobial peptide expression in human gingival keratinocytes by interleukin-1α. Arch Oral Biol 56,761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sesto A., Navarro M., Burslem F., and Jorcano J.L.Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci U S A 99,2965, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]