Abstract
In severe aplastic anemia, approximately one-third of responders to standard horse antithymocyte globulin (h-ATG) plus cyclosporine (CsA) will relapse. Anecdotal experience has suggested that a gradual CsA taper might avoid relapse, but this practice has not been rigorously assessed prospectively. In 2003, we adopted a strategy to taper CsA beyond 6 months, with the intention to reduce hematologic relapse compared to our extensive historical experience. In total, 102 patients received h-ATG/CsA for 6 months in two sequential clinical protocols: 67 patients (66%) responded and all had the CsA dose tapered per protocol over the subsequent 18 months (total of 2 years). The rate of relapse at 5 years was 33% (95% CI 27%-44%), which did not differ from our large historical relapse experience (patients treated before 2003) of 30-40%, in protocols in which CsA was simply discontinued at 6 months. However, time to relapse was prolonged by about 1 year with the longer CsA course. The rates of clonal evolution and overall survival did not differ between the two cohorts. We infer from this large prospective study that CsA taper as implemented delayed but did not prevent relapse. The kinetics of relapse with long course CsA does suggest that a lower long-term dose might be adequate to maintain patients in remission.
Introduction
Severe aplastic anemia (SAA) is successfully treated with horse ATG (h-ATG) and cyclosporine (CsA), with hematopoietic recovery achieved in 60-80% of cases [1]. However, this therapy is limited by relapse in 1/3 of responders, as well as clonal evolution to myelodysplasia and acute leukemias in about 15% of all treated patients. Most research protocols have specified a 6-month course of CsA, a duration also common in practice. Anecdotal experience has suggested that hematologic relapse among responders might be avoided by a more gradual discontinuation of CsA. In addition, the benefit of a taper has been inferred from large observational studies in the 1990s, in which some patients appeared to require CsA in order to maintain adequate blood counts [2-4]. Despite being logical, data to support this practice are limited, as no prospective study have formally addressed the possible benefits of long course CsA.
At our institution, CsA was discontinued at 6 months in all h-ATG protocols from 1989-2003. With this approach, hematologic relapse was observed in 30-40% of patients over the 5 years after ATG administration [2]. To lower this rate, we postulated that continuing CsA beyond 6 months might reduce relapse, as is observed in other autoimmune disorders with maintenance therapy [5, 6]. To test this hypothesis, we prospectively implemented a longer, 18-month taper of CsA in all responders to h-ATG. Here we report on the outcomes of the first prospective study of continued CsA use beyond 6 months in SAA.
Methods
Patients were enrolled into two sequential treatment-naive SAA protocols from June 2003 to July 2010 (registered at clinicaltrials.gov as NCT00061360 and NCT00260689). These studies investigated new regimens in SAA: the first specified randomization between standard h-ATG/CsA (n=42) and h-ATG/CsA/sirolimus (n=35) from 2003-2005 and in the second patients were randomized between standard h-ATG/CsA (n=60), rabbit ATG/CsA (n=60), and alemtuzumab (n=16) from 2005-2010 [7, 8]. For both randomized protocols, the control arm was standard h-ATG/CsA, which was administered in 102 patients in the context of these studies. For these protocols, SAA was defined as bone marrow cellularity of less than 30% and severe pancytopenia with at least two of the following peripheral blood count criteria: (i) absolute neutrophil count less than 0.5×109/L; (ii) absolute reticulocyte count less than 60×109/L; and (iii) platelet count less than 20×109/L. for Fanconi anemia conducted in patients under 40 years of age [1, 7, 8]. All patients (or their legal guardians) signed informed consent according to protocols approved by the Institutional Review Board of the National, Heart, Lung, and Blood Institute. All patients were treated at the Clinical Center of the National Institutes of Health (NIH) in Bethesda, MD. ATG administration and landmark visits for evaluation (at 3, 6, and 12 months, and then yearly thereafter) were conducted at the NIH. The 6-month hematologic response outcome was the primary endpoint in these protocols and defined as no longer meeting criteria for SAA [7, 8]. The combined long-term outcomes and comparative analysis (to historical control) in all patients who had a long course of CsA has not yet been reported. Hematologic relapse, clonal evolution and survival were specified secondary endpoints in both studies. A complete response (CR) was defined as ANC above 1.0×109/L, Hgb > 10 g/dL, and a platelet count > 100×109/L and a partial response (PR) defined as counts no longer meeting criteria for SAA but not sufficient for a CR. CsA taper was initiated at 6 months irrespective of the quality of response (PR or CR) with a 25% dose reduction every 3 months for 18 months. Cyclosporine levels were maintained at 200-400 ng/ml and monitored for the first 6 months only, after which no further blood level monitoring was conducted once the taper had been initiated. CsA was continued in all patients until relapse occurred or the taper was complete at 2 years from initiating immunosuppression. By protocol design, the rate of relapse with long-term CsA was to be compared to our extensive historical experience with standard h-ATG/CsA, where patients treated at the Clinical Center from 1989 to 2003 had CsA discontinued at 6 months.
Patient characteristics were described using summary statistics including means, proportions, standard errors and 95% confidence intervals. Survival analysis for years from the first course of IST to relapse, clonal evolution, and overall mortality was with the Kaplan-Meier method with patients who had hematopoietic stem cell transplantation (HSCT) or loss of follow-up treated as censored at their HSCT or loss to follow-up time. For the purpose of determining the impact of a long CsA taper, only responders from 1989-2003 and 2003-2010 who had CsA discontinued or tapered at 6 months, respectively, were compared for hematologic relapse, clonal evolution, and survival. Numerical results were computed using the S-PLUS 8.0 software package (TIBCO). A 2-sided P value was used throughout and considered statistically significant if < .05. Analysis was based on intention to treat.
Results
Of the 102 patient cohort treated with h-ATG/CsA, 67 (66%) responded at 6 months (PR+CR), and these patients had their dose of CsA gradually reduced per protocol (this hematologic response rate is in accordance with our large historical experience). Patient characteristics are shown in Table 1. Side effects such as hypertension, hirsutism, and gingival hyperplasia improved once the CsA dose was tapered at 6 months with the first 25% dose reduction. The tolerability remained good with subsequent tapers at which point patients no longer complained of drug-related side effects. The median follow-up in surviving patients was 4.9 (range, 1.3-8.3) years. At 5 years, the cumulative incidence of relapse was 33% (95% CI 11%-44%) in those treated from 2003-2010 who had a prolonged CsA course and 36% (95% CI 27%-44%; p=0.334) among historical controls, in whom CsA was simply discontinued at 6 months (Figure 1A). Of the 21 relapsed patients, 7 achieved a CR and 14 a PR by 6 months, suggesting that a robust response (CR) did not preclude a relapse. In those who relapsed (6 within the first 2 years), CsA dose was increased in 16 of whom 11 responded (leading to maintenance CsA dosing determined by blood counts). This response rate to CsA resumption in relapsed patients is in accordance with our historical experience suggesting that the taper did not affect responsiveness to CsA at relapse. The median time to relapse for the taper cohort was approximately 2 years, which contrasts to about 1 year when CsA was discontinued at 6 months in earlier protocols conducted from 1989-2003. Only 1 (1.5%) patient in the CsA taper group relapsed before 1-year post-ATG, while about 40-50% of the relapses occurred within the first year when CsA was discontinued at 6 months (from 1989-2003) [1, 2, 9, 10]. However, by 5 years the relapse rate with the CsA taper was almost identical to our reported relapsed rate of 30-40% when CsA was discontinued at 6 months (Figure 1A) [1, 2, 9, 10].
Table 1. Patient characteristics*.
| Variable | N = 67 |
|---|---|
| Mean age, yrs | 35.1 ± 21.5 |
| Male sex | 38 (56.7%) |
| Etiology | |
| Idiopathic | 66 (98.5%) |
| Post-hepatitis | 1 (1.5%) |
| Cell counts per ×109/L | |
| ANC | 0.432 ± 0.357 |
| ARC | 27.357 ± 20.955 |
| Platelets | 12.851 ± 30.443 |
| PNH clone (>1%) | 31 (46.3%) |
Plus-minus values are means ± SD
Figure 1. Rates of late events and survival after horse antithymocyte globulin (h-ATG)/cyclosporine (CsA) followed by CsA taper.
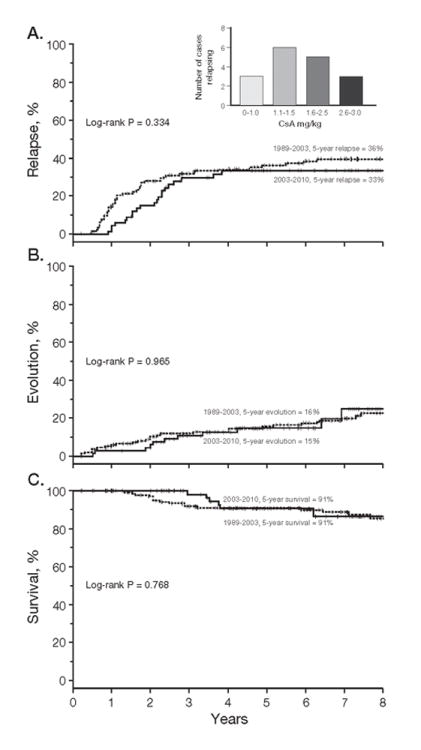
(A) The cumulative incidence of relapse at 5 years was 33%, which is comparable to our historical relapse rate of 30-40% in immunosuppression protocols at our institution where CsA was simply discontinued at 6 months [7, 8] Among responders, the CsA dose at the time of relapse during the taper phase was at a median of 1.32 (range, 0.52-4.18) mg/kg and a mean of 1.8 mg/kg (inset). This suggests that a CsA dose level above these levels might preclude relapse and improve tolerability of chronic maintenance. Not depicted in the inset are patients who did not experience relapse or that relapsed after conclusion of the 18-month taper Only responding patients who relapsed during the taper are included. (B) For clonal evolution the cumulative incidence at 5 years (15%) also did not differ from our historical experience of a rate of 10-20% [7, 8] (C) Survival rate at 5 years in the CsA taper group was 91% in this responding cohort which is in accordance to survival in this group of patients who responded to immunosuppression from 1989-2003 [18] Only patients who responded to immunosuppression by 6 months who had the CsA stopped at 6 months (from 1989-2003) or tapered (from 2003-2010) are shown in the figures.
Among the CsA taper group (n=67), the 5-year evolution rate was 15% (95% CI, 9%-23%), also not different from our historical figure of 10-20% in protocols in which CsA was simply discontinued at 6 months (p=0.965; Figure 1B) [2, 9]. There were 11 evolutions: monosomy 7 (5), acute leukemia (2), complex cytogenetics (1), del13q (1), del5q (1), del20q (1). This evolution pattern is comparable to our historical experience. Survival among responders between the 2 time periods (1989-2003 and 2003-2010) also did not differ for both group; at 5 years (p=0.768; Figure 1C).
Discussion
To our knowledge, ours is the first prospective study to test a simple strategy to avoid relapse in immune mediated marrow failure. Our results show that a prolonged CsA taper, over the course of two years post-ATG, delays hematologic relapse by approximately 1 year but do not ultimately prevent it. The mechanisms for hematologic relapse are not well understood but one plausible explanation is that expanded CD8+ T-cell clones that persist after effective immunosuppression are functionally suppressed but not eliminated by continued CsA but then become active effectors as the dose is reduced or the drug discontinued [11, 12]. We reasoned that testing long course CsA was rational, as continued immunosuppression might allow the ultimate elimination of residual auto-reactive lymphocytes, “burn-out”, as is observed long term in other immune-mediated diseases [13, 14]. That the CsA taper prolonged time to relapse and that the initial two dose reductions (3 months apart starting at 6 months) maintained remission blood count suggest that lower doses of CsA might be adequate to suppress immune-mediated progenitor suppression and destruction. Unfortunately, as the taper continued per protocol beyond the first year, relapses were observed at a higher rate. We cannot exclude the possibilities that maintaining some lower dose of CsA after 1 year for a longer period than two years or that a different (slower) taper regimen might be more effective in ultimately preventing relapses. The daily dose of CsA at the time of relapse during the taper was at a median of 1.3 (range, 0.52-4.18) mg/kg and mean of 1.8 mg/kg (Figure 1A, inset). This suggests that a dose level of 2-2.5-mg/kg daily—much lower than the initial (arbitrarily determined) dose of CsA utilized in this disease--might preclude hematologic relapse in most cases. This hypothesis could be validated in prospective trials.
Despite being frequently recommended [15] the presumed activity of CsA beyond 6 months derives only from anecdotal and retrospective analysis, both methods subject to reporting and post-hoc biases. The largest experience was based on a retrospective review of records from only 42 pediatric patients treated from 1991 and 1999 in Italy [16]. The CsA taper regimen varied between patients and three groups were retrospectively defined in this study. With a median follow-up of 118 months (range 49-185) in surviving patients, the cumulative incidence of relapse was 7.6% in the “slower” CsA taper group, compared to 60% in the “rapid” taper group. However, the retrospective nature of the study design, data collection, definitions, and analysis limit the interpretation of these results. It is possible that our results, which included patients of all ages, differed from the Italian pediatric experience due to the inclusion of adults in our study.
The current prospective study is limited by comparison to historical controls. However, our historical data set comprises a relatively large number of patients, uniformly treated in a single institution and with periodic and long-term evaluations. Protocol eligibility has remained consistent over the course of the two prospective trials. The CsA taper regimen was implemented prospectively since 2003 in consecutive patients enrolled on registered sequential clinical protocols with all responders participating in the taper and all patients accounted for and included in the final analysis. The definitions for clinical outcomes (including relapse) have remained consistent at our institution since the 1980s.
We found that relapse was delayed but not ultimately prevented with a taper over two years. The delay in relapse did not have an impact on survival, which was the same in patients who had the CsA stopped or tapered at 6 months. It is plausible that chronic CsA (even at low doses, which are better tolerated and less toxic) continue to suppress auto-reactive T-cells and decrease or eliminate relapse. However, as only 1/3 of responders experience relapse, such a strategy would commit 2/3 of responding patients who ultimately may not benefit from prolonged CsA. Relapse early in the taper in our study (between 6 and 12 months) were very infrequent, and during this period of dose reduction there were marked improvements in the usual CsA toxicities. Thus, while a strategy that employed lower dose and longer duration CsA can be tested in the clinic, ideally an accurate and applicable method to identify those at higher risk for relapse at baseline (for example, telomere length measurement) or during treatment by careful hematologic or immune monitoring would allow continued therapy to be targeted to those at greater risk, and allow early discontinuation of CsA in those who are not likely to experience this complication [11, 17].
In conclusion, CsA taper was effective in delaying relapse, but the optimal taper regimen is yet to be determined. These data have clinical implications, as CsA tolerance is limited by troubling symptoms and nephrotoxicity, hypertension, electrolyte imbalances, gingival hyperplasia and general risks associated to immunosuppression. Shorter or lower exposure to CsA abbreviates drug toxicity associated with more prolonged use.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute
Footnotes
Authorship: P Scheinberg was the Principal Investigator for the protocols, conceptualized, wrote and conducted the clinical trials, analyzed the data, interpreted the results and drafted the manuscript. O Rios and B Weinstein did the patient screening, data collection and attended to all patient's needs. Pr Scheinberg attended to all the regulatory protocol requirements including data collection. CO Wu was involved in the conceptualization, statistics, and writing of the protocols, and did the statistical analysis of the manuscript. NS Young was involved in the conceptualization, implementation and writing of the protocols, their conduct, interim discussions, data analysis, interpretation of results, and writing of the manuscript.
Disclosure of Conflicts: The authors have no conflicts to disclose.
References
- 1.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185–1196. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfeld S, Follmann D, Nunez O, et al. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 3.Bacigalupo A, Bruno B, Saracco P, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO) Blood. 2000;95:1931–1934. [PubMed] [Google Scholar]
- 4.Frickhofen N, Heimpel H, Kaltwasser JP, et al. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101:1236–1242. doi: 10.1182/blood-2002-04-1134. [DOI] [PubMed] [Google Scholar]
- 5.Langford CA, Talar-Williams C, Barron KS, et al. Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener's granulomatosis: extended follow-up and rate of relapse. Am J Med. 2003;114:463–469. doi: 10.1016/s0002-9343(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 6.Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350:971–980. doi: 10.1056/NEJMoa031855. [DOI] [PubMed] [Google Scholar]
- 7.Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheinberg P, Wu CO, Nunez O, et al. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009;94:348–354. doi: 10.3324/haematol.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheinberg P, Nunez O, Wu C, et al. Treatment of severe aplastic anaemia with combined immunosuppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. Br J Haematol. 2006;133:606–611. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- 10.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risitano AM, Maciejewski JP, Green S, et al. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004;364:355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- 12.Plasilova M, Risitano A, Maciejewski JP. Application of the molecular analysis of the T-cell receptor repertoire in the study of immune-mediated hematologic diseases. Hematology. 2003;8:173–181. doi: 10.1080/1024533031000107505. [DOI] [PubMed] [Google Scholar]
- 13.Boers M. The COBRA trial 20 years later. Clinical and experimental rheumatology. 2011;29:S46–51. [PubMed] [Google Scholar]
- 14.Klarenbeek NB, van der Kooij SM, Guler-Yuksel M, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis. 2011;70:315–319. doi: 10.1136/ard.2010.136556. [DOI] [PubMed] [Google Scholar]
- 15.Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 16.Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long-term observation follow-up. Br J Haematol. 2008;140:197–205. doi: 10.1111/j.1365-2141.2007.06903.x. [DOI] [PubMed] [Google Scholar]
- 17.Scheinberg P, Cooper JN, Sloand EM, et al. Association of Telomere Length of Peripheral Blood Leukocytes With Hematopoietic Relapse, Malignant Transformation, and Survival in Severe Aplastic Anemia. JAMA. 2010;304:1358–1364. doi: 10.1001/jama.2010.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2010;52:726–735. doi: 10.1093/cid/ciq245. [DOI] [PMC free article] [PubMed] [Google Scholar]


