Abstract
Background
Marijuana is the most commonly used illicit substance in the United States. Use, particularly when it occurs early, has been associated with cognitive impairments in executive functioning, learning, and memory.
Methods
This study comprehensively measured cognitive ability as well as comorbid psychopathology and substance use history to determine the neurocognitive profile associated with young adult marijuana use. College-aged marijuana users who initiated use prior to age 17 (n=35) were compared to demographically-matched controls (n=35).
Results
Marijuana users were high functioning, demonstrating comparable IQs to controls and relatively better processing speed. Marijuana users demonstrated relative cognitive impairments in verbal memory, spatial working memory, spatial planning, and motivated decision-making. Comorbid use of alcohol, which was heavier in marijuana users, was unexpectedly found to be associated with better performance in some of these areas.
Conclusions
This study provides additional evidence of neurocognitive impairment in the context of adolescent and young adult marijuana use. Findings are discussed in relation to marijuana’s effects on intrinsic motivation and discrete aspects of cognition.
Keywords: marijuana, neurocognition, decision-making, memory, planning
Marijuana is the most commonly used illicit drug in the United States among adolescents and young adults, with 52.2% of 18–25 year olds reporting use during their lifetimes (Substance Abuse and Mental Health Services Administration, 2013). Currently, adolescents and young adults perceive the risks of marijuana use to be lower, and profess less disapproval of peer marijuana use, than in past years (Johnston, Bachman, & Schulenberg, 2012; Johnston, O’Malley, Bachman, & Schulenberg, 2013). Despite these popular perceptions that marijuana use is not a high-risk activity, a growing body of research indicates that use is associated with cognitive impairments. Given growing advocacy for marijuana’s legalization and its prevalence of use, it is crucial to better understand the nature of these impairments.
The primary psychoactive compound in marijuana, delta-9-tetrahydrocannabinol, acts directly on the central and peripheral nervous systems, binding to receptors for endogenous cannabinoids. Dense populations of endocannabinoid system (ECS) receptors are located in the prefrontal cortex, hippocampus, basal ganglia, thalamus, hypothalamus, and cerebellum. Within these regions, the ECS broadly modulates synaptic signaling (Freund, Katona, & Piomelli, 2003; Viveros, Llorente, Moreno, & Marco, 2005). Animal models indicate that the endocannabinoid system undergoes dramatic change during adolescence (Ellgren et al., 2008; Rodriguez de Fonseca, Ramos, Bonnin, & Fernandez-Ruiz, 1993). ECS receptor expression is lower in subcortical (Rodriguez de Fonseca et al., 1993) and frontal cortical regions (Ellgren et al., 2008; Heng, Beverley, Steiner, & Tseng, 2011) in adulthood than during earlier stages of development.
ECS receptors in the dorsolateral prefrontal cortex (DLPFC) are located in the presynaptic terminals of inhibitory GABAergic interneurons (Long, Lind, Webster, & Weickert, 2012). ECS receptor activation results in excitation through inhibition of the inhibitory GABAergic interneurons. Normative patterns of modulation of the ECS through adolescence may be a mechanism through which greater degrees of cognitive control are achieved. That is, as ECS receptors are pruned as part of the normative changes in brain structure that occur during adolescence (Gogtay & Thompson, 2010), greater neuronal inhibition develops in the DLPFC, increasing capacities for cognitive control, self-directed behavior, and other regulatory functions (Long et al., 2012).
Disruption of the ECS during adolescence through the introduction of outside cannabinoids can have long-term effects on synaptic transmission and associated behaviors, leading to persistent alterations in adulthood. In rodents, chronic cannabinoid administration during adolescence is linked to decreased adult serotonergic activity in the brain stem (Bambico, Nguyen, Katz, & Gobbi, 2010) and blunted dopamine activity in the midbrain (Pistis et al., 2004). Rodents exposed to exogenous cannabinoids during adolescence demonstrate decreased memory and learning ability (Jager & Ramsey, 2008; Rubino et al., 2009; Schneider & Koch, 2003, 2007) as well as decreased inhibitory control (Realini, Rubino, & Parolaro, 2009; Schneider & Koch, 2003) in adulthood.
Similarly, cognitive impairments are noted in human adolescents and young adults in the context of active marijuana use. As might be expected, impairments are evident during acute intoxication, including impaired attention, executive function, decision-making skills, and memory function (Crean, Crane, & Mason, 2011; Morrison et al., 2009; Ramaekers et al., 2006). Beyond acute intoxication, adolescent and young adult marijuana use is associated with numerous impairments, particularly in verbal memory and executive functioning. Marijuana users demonstrate poorer retrospective recall on list-learning tasks (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Gonzalez et al., 2012; Hanson et al., 2010; Harvey, Sellman, Porter, & Frampton, 2007; Solowij et al., 2011; Takagi et al., 2011) as well as poorer memory for stories (Fried, Watkinson, & Gray, 2005; Medina et al., 2007; Schwartz, Gruenewald, Klitzner, & Fedio, 1989). Similarly, marijuana users display diminished memory for future actions assessed through prospective memory tasks (Bartholomew, Holroyd, & Heffernan, 2010; McHale & Hunt, 2008; Montgomery, Seddon, Fisk, Murphy, & Jansari, 2012).
Executive functioning skills appear to be diminished in marijuana users as well. Marijuana users show decreased planning ability on a Tower of London task (Grant, Chamberlain, Schreiber, & Odlaug, 2012) and a task of logical organization (Montgomery et al., 2012). Marijuana users demonstrate less flexibility and abstract reasoning ability than non-users (Bolla et al., 2002; Pope & Yurgelun-Todd, 1996), and decision-making tends to be more risky (Clark, Roiser, Robbins, & Sahakian, 2009; Grant et al., 2012; Solowij et al., 2012).
Marijuana users demonstrate impairments inconsistently in other cognitive domains, including attention (Bolla et al., 2002; Dougherty et al., 2013; Lisdahl & Price, 2012), processing speed (Fried et al., 2005; Lisdahl & Price, 2012; Medina et al., 2007), and spatial reasoning (Harvey et al., 2007; Pope & Yurgelun-Todd, 1996). It is unclear if performance in these domains is directly related to marijuana use, or if other behaviors associated with marijuana use contribute to these findings.
Deficits remain during early (Cuttler, McLaughlin, & Graf, 2012; Dougherty et al., 2013; Fried et al., 2005; McHale & Hunt, 2008), as well as sustained (Bolla et al., 2002; Hanson et al., 2010; Lisdahl & Price, 2012; Medina et al., 2007) abstinence.
As lawmakers grapple with questions about the legalization of marijuana, studies of non-acutely intoxicated marijuana users allow us to understand how cognitive functioning may be affected in the context of regular marijuana use. In addressing that question, it is important to consider when individuals began to use the drug. Marijuana users who begin use early in life often demonstrate greater cognitive impairment than later-onset marijuana users on measures of memory and executive functioning (Ehrenreich et al., 1999; Fontes et al., 2011; Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Lisdahl, Gilbart, Wright, & Shollenbarger, 2013; Pope et al., 2003). Given the important role of the ECS during development, it is likely that disruption of the system at a younger age impacts later cognitive performance, particularly executive functions that emerge as frontostriatal brain networks reach their full maturational potential.
Two important difficulties emerge when trying to compare studies of young adult marijuana users. First, the age range tends to vary widely between studies. Studies exploring samples of college-aged subjects commonly use a broad age and developmental range, including people in their late twenties or thirties (Battisti et al., 2010; Bolla et al., 2002; Ehrenreich et al., 1999; Wagner, Becker, Gouzoulis-Mayfrank, & Daumann, 2010; Whitlow et al., 2004). While several studies have focused on adolescent marijuana users (Hanson et al., 2010; Harvey et al., 2007; Medina et al., 2007), it is not common to narrowly define age groups in young adult samples. Second, the majority of studies exploring neurocognitive profiles of adolescent marijuana users focus on a limited range of cognitive skills. This approach fails to provide a comprehensive cognitive profile, likely resulting in a limited understanding of any observed cognitive deficits. A broad assessment of cognitive ability can better reveal patterns of weaknesses but also potential strengths.
The current study provides a comprehensive cognitive profile of non-treatment seeking college student marijuana users in a narrow age-range (18–20). Participants were heavy marijuana users but had histories of typical development and were low-risk in relation to socioeconomic vulnerabilities, comorbid psychopathology, as well as general cognitive ability. Marijuana use in 18–20 year olds is common, and the assessment of cognition within this selective age band has the benefit of providing information regarding the functional skills and abilities of actively- and heavy-using individuals who are otherwise at low risk for impairment.
Users were compared to non-using controls in the context of an assessment battery that included measures of clinical symptoms, other externalizing behaviors, and neurocognition across multiple domains of function.
It was predicted that marijuana users would exhibit relative impairments in learning and memory, particularly when such skills recruit executive functions, as well as decision-making, working memory, and planning.
Methods
Participants
Seventy-three individuals, ages 18–20, were studied: healthy non-using controls (n = 37) and heavy marijuana users who began use before age 17 (n = 36). The average age of use onset was 15.2 (Table 1). Participants were recruited through university advertisements, and all were monetarily compensated.
Table 1.
Demographic and substance use characteristics of marijuana users and controls
| Control (n = 35) |
Marijuana user (n = 35) |
|||
|---|---|---|---|---|
| M (SD) or % | M (SD) or % | F or χ2 | U | |
| Age | 19.40 (0.93) | 19.52 (0.62) | 0.39 | |
| Sex Ratio (male:female) | 13:22 | 22:13 | 4.63* | |
| Race (% Caucasian) | 77.14% | 88.57% | 1.61 | |
| Years of education | 13.26 (1.24)a | 13.29 (0.94)a | 0.01 | |
| Estimated Full Scale IQa | 114.73 (9.40) | 115.27 (9.56) | 0.05 | |
| Vocabulary T-Scorea | 62.10 (6.85) | 61.27 (7.88) | 0.21 | |
| Matrix reasoning T-Scorea | 54.49 (5.98) | 56.09 (5.21) | 1.35 | |
| Alcohol Use Average | −0.59 (0.69) | 0.59 (0.76) | 46.54** | |
| ASR Substance Use | ||||
| Past 6 months: Tobacco use per day | 0.00 (0.00) | 0.91 (1.55) | 385.00** | |
| Past 6 months: Days drunk | 5.37 (9.24) | 25.07 (18.38) | 142.00** | |
| Past 6 months: Days using drugs | 0.14 (0.49) | 145.94 (40.58)a | 636.00** | |
| Marijuana Useb | ||||
| Age First Used | 15.24 (1.24) | |||
| Past year: Days MJ used | 333.43 (43.61) (range: 208–365) | |||
| Past 30 days: Days MJ used | 25.86 (3.24) (range: 20–28) | |||
| Past year: Avg. hits per day | 10.09 (8.82) (range: 2–50) | |||
| Past 30 days: Avg. hits per day | 10.20 (9.12) (range: 1.5–50) | |||
| Lifetime: Max hits in 24 hours | 38.72 (27.52) (range: 6–120) |
Mann–Whitney Us were computed when appropriate.
Marginal means presented, controlling for sex.
Variables only included for marijuana users.
p ≤ .05.
p ≤ .01.
Controls were selected from a larger study exploring adolescent brain development (described in more detail in Luciana, Collins, Olson, & Schissel, 2009; Olson et al., 2009). Controls were excluded if they met current or past Axis I DSM-IV-TR (American Psychiatric Association, 2000) criteria for any psychiatric disorder and/or if they reported marijuana use more than once monthly.
General inclusion criteria included being a native English speaker, right-handed, with normal/corrected-to-normal vision and hearing, and no reported history of neurological problems, mental retardation or current pregnancy.
Inclusion criteria for marijuana users consisted of self-reported marijuana use of at least 5 times per week for at least 1 year. Use onset was required to be before age 17 so that length of use across study participants would be relatively uniform. Marijuana use during this age span has been most strongly associated with cognitive impairment (Lisdahl et al., 2013), and use initiation is most common between the ages of 16 and 18 in the United States (Substance Abuse and Mental Health Services Administration, 2013). Marijuana users were excluded if they were daily cigarette smokers, if alcohol use exceeded 4 drinks for females and 5 drinks for males on more than 2 occasions per week, or if they met criteria for current or past substance dependence other than marijuana. One marijuana user met criteria for current and past alcohol dependence, despite meeting the project’s use frequency criteria, and was excluded from analyses. Marijuana users were asked to refrain from drug use for at least 12 hours before testing so as not to be acutely high during the assessment. Longer periods of abstinence were not required, because we did not wish to study individuals in the midst of drug withdrawal and because a goal of the study was to capture functional capacities in the context of active use. Formal drug testing was not implemented due to budgetary limitations and given that the study did not require long-term marijuana abstinence. This study was approved by the University of Minnesota’s Institutional Review Board. Participants provided informed consent prior to participation.
Procedure
Interested participants (those who responded to posted advertisements) completed a phone screening followed by an in-person structured interview, the Kiddie-Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K-SADS-PL: Kaufman et al., 1997) to assess for recent and past histories of psychological problems. The benefit of using the K-SADS to assess psychopathology in young adults is that it captures past histories of childhood disorders while also providing an in-depth assessment of DSM-IV-based adult psychopathology. Current (recent) ratings were based on the previous 2 months for non-substance use related disorders and the previous 6 months for SUDs. In addition, information was obtained about quantity and frequency of drug use across the past 30 days and past year. Intelligence was estimated by the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI: Wechsler, 1999). Participants completed detailed health and demographic questionnaires. Participants who met inclusion criteria returned for a second assessment, including behavioral questionnaires and a comprehensive neurocognitive battery. The battery was designed to capture a broad array of functions in the domains of motor behavior, processing speed, attention, spatial and verbal memory, and executive skills.
Neurocognitive Battery
Motor function
Finger Tapping Test
(Lezak, Howieson, & Loring, 2004). This test measures motor speed. Participants tapped a key as many times as possible within a 10-second period. Three trials were administered for each hand, and the number of taps per trial was recorded. The average of all three trials per hand is reported.
Grooved Pegboard
(Lafayette Instrument, 1989). This test measures psychomotor dexterity and speed. Participants were presented with a flat board containing rows of holes and small metal ‘pegs’ that fit into the holes on the board. The pegs were shaped so that one side is square. Each peg had to be correctly manipulated in order to fit the holes. Under timed conditions, participants used the pegs to fill the holes on the board using first the right hand, then the left hand. Accuracy and response time were recorded.
Processing speed
Digit Symbol
(WAIS-III Digit Symbol: Lezak et al., 2004; Wechsler, 1997). This test measures psychomotor performance, sustained attention, response speed, and visuomotor coordination. This test was administered according to WAIS-III standardized procedures. The score recorded is the number of squares filled in correctly out of a total possible 133 squares.
Letter cancellation task
(Lezak et al., 2004). This task measures immediate attention and vigilance but is also a speeded test. Participants viewed a piece of paper on which were printed rows of capitalized letters. They were instructed to work as quickly but as accurately as possible and to cross out all occurrences of the letters ‘E’ and ‘C’. Time-to-completion and numbers of errors were recorded.
Verbal fluency
Controlled oral word association test
(COWAT; Delis, Kramer, Kaplan, & Ober, 2000; Lezak et al., 2004). The COWAT assesses verbal production as well as rule maintenance and response monitoring. It was administered according to standardized procedures using the target letters F, A, and S. A total score for each participant was calculated, representing the total number of words generated across all three trials after deductions for rule violations, set-loss errors (i.e., words not beginning with target letters), and perseverations (i.e., saying the same word more than once).
Verbal attention and working memory
Digit Span
(WAIS–III Digit Span; Wechsler, 1997). This test measures immediate recall of auditory verbal information. Digit span forward and digit span backward conditions were administered according to WAIS-III standardized procedures.
Verbal learning and memory
Rey Auditory Verbal Learning Test
(RAVLT; Lezak et al., 2004; Rey, 1993). This test measures acquisition, storage, and retrieval of verbal information. During the learning stage, participants were read a list of 15 words 5 separate times and were asked to recall as many words as they were able after each presentation. Then, they were read a new (interference trial) list of 15 words once and asked to recall those words. Participants then were asked to freely recall as many words as they could from the first list (immediate recall). Following a 30-minute delay, participants were again asked to recall as many words as they could from the first list. The number of words recalled and errors during the learning trials, interference trial, immediate recall, and delayed recall trials were recorded. The learning trials assessed the participant’s immediate learning and temporary storage of verbal information. The interference trial assessed immediate learning of new information, presented only once. The immediate recall trial assessed learning recall when the items not actively rehearsed in working memory. The delayed recall trial performance represented learning that has been consolidated into memory. Intrusion and perseverative errors were also tabulated. Intrusion errors occurred when participants responded with non-list words. Perseverative errors occurred when participants repeated responses during a given trial.
Additional learning and memory variables were calculated to best characterize performance. Loss after consolidation was calculated as the percentage of words recalled during delay relative to words recalled during the final learning trial (Takagi et al., 2011). Retroactive interference (trial 5 vs. immediate recall) and proactive interference (trial 1 vs. interference) were examined. To explore learning efficiency and strategy, bidirectional serial ordering and response consistency were calculated (Delis, Kramer, Kaplan, & Ober, 2000). Bidirectional serial ordering refers to recall of stimulus words in the same order as they are presented, forward or backward. Response consistency measures how often the same words are recalled from trial to trial during free recall as a percentage of total words recalled during free recall trials:
Spatial memory
Spatial span
This test measures immediate recall of visually presented nonverbal information, and is a nonverbal analogue of the digit span test. The version used here was computerized using Eprime version 1.1 (Psychology Software Tools; www.psnet.com). Participants, seated at a computer terminal, viewed arrays of squares on the screen. One by one, some of the squares ‘lit up’ in a sequence. In the forward condition, participants repeated the sequence by touching the squares in the remembered sequence using a touch-pen device (FastPoint Technologies, Inc.). In the backward condition, participants repeated the sequence in reverse order. The forward and backward memory spans were recorded as the number of items recalled in correct sequence across trials in each condition.
Spatial Recognition
(Cambridge Neuropsychological Test Automated Battery, CANTAB; Fray, Robbins, & Sahakian, 1996). This test measures recognition memory for spatial locations. The participant viewed empty boxes at different locations on the screen. Five stimuli were presented in succession at different locations on the screen for 3 seconds each. After a five-second delay, the participant was shown two boxes, one of which was in a location previously displayed in the earlier sequence. The participant indicated which box position was shown previously. Accuracy and response time were recorded. The percentage of correct trials across all four blocks was used as the variable of interest.
Self-Ordered Search
(CANTAB; Owen, Downes, Sahakian, Polkey, & Robbins, 1990). This test measures spatial working memory, self-monitoring, and behavioral self-organization. Using a computerized touch-screen, participants searched for blue tokens hidden inside an array of boxes. The task was organized into 4, 6 and 8 box problems, with increased box number corresponding to increased task difficulty. Participants were instructed that at any one time there would be a single token hidden inside one of the boxes. Their task was to search until they found it, at which point the next token would be hidden. Once a given box yielded a token, that box would not be used to hide the token again during the trial. Every box was used once on every trial; thus, the total number of tokens to be found during each trial corresponded to the number of boxes on the screen. A “between-search” error was recorded when participants returned to open a box in which a token had already been found. Additionally, a strategy score was tabulated. The strategy score, which was based on responses to 6 and 8-item searches, reflects the participant’s tendency to search through available locations in an organized fashion. Between-search error scores for each level of search complexity, as well as strategy scores, were the variables of interest.
Spatial Delayed Response Task
(DRT; Luciana & Collins, 1997; Luciana, Collins, & Depue, 1998). This task measures working memory for the locations of spatial targets. During each of 48 trials, the participant first observed a central fixation point on a computer monitor. Next, a visual cue appeared in their peripheral vision for 200 ms. After the peripheral visual cue, the cue and fixation point disappeared, and the screen blackened for randomly interspersed delay intervals of 500 or 8,000 ms. After the delay interval, the participant indicated the remembered location of the cue with a touch-pen device (FastPoint Technologies, Inc.). A block of 16 “no delay” trials were also administered prior to the delay trials to measure basic perceptual and visuomotor abilities independent of memory. Average accuracy (in millimeters) and response times (in milliseconds) were recorded for each condition.
Planning
Tower of London
(CANTAB; Owen et al., 1990). This test measures future planning ability. A full task description can be found in Luciana et al. (2009). Using a computerized touch-screen, participants moved colored balls to match a target display (problem-solving block). Participants were told at the start of each problem-solving trial that the trial should be completed in X number of moves, where X was the minimum number of moves required to achieve a perfect solution. The total number of problems in which participants responded with the minimum number of moves was recorded and expressed as a proportion of total possible perfect solutions. Participants were instructed not to make the first move until they knew which balls to move and were encouraged to solve the problem correctly on the first try. Time from presentation of the problem to starting to solve the problem (planning time) was recorded. Planning time and percent of perfect solutions were examined.
Motivated decision-making
Iowa Gambling Task
(IGT: Bechara, Damasio, Damasio, & Anderson, 1994). This task measures motivated decision-making ability. Participants completed a computerized version of the IGT (Hooper et al., 2004) during which they selected from among four decks of cards varying in their amounts of monetary reward and punishment (Bechara et al., 1994). Participants worked to earn real money (maximum of $5). For each selection from Decks 1 or 2 (the “disadvantageous decks”), participants would win $0.25 but the losses were organized so that over 20 selections from these decks, participants would incur a net loss of $1.25. The difference between Decks 1 and 2 was in the frequency and magnitude of punishment: Deck 1 contained frequent (50% of cards) punishments, whereas Deck 2 contained less frequent (10% of cards) but much larger punishments. For each selection from Decks 3 or 4 (the “advantageous decks”), participants would win either $0.10 or $0.15 and the losses were organized so that over 20 selections from these decks, participants would accrue a net gain of $1.25. Similar to the disadvantageous decks, the two advantageous decks differed from each other in the frequency of punishment, such that small punishments occurred on 50% of the cards in Deck 3 and larger punishments occurred on 10% of the cards in Deck 4. Trials (n= 100) were split into 5 blocks with 20 trials per block. For each block, the number of choices from disadvantageous decks was subtracted from number of choices from advantageous decks. Thus, values above “0” correspond to relatively advantageous choices. In addition, the actual numbers of selections made from each deck were tabulated across the full task to analyze choice preferences.
Together, these measures took several hours to complete.
Self-Report Questionnaires
Participants completed Achenbach’s Adult Self-Report (ASR; Achenbach & Rescorla, 2003) questionnaire, which yields assessments of internalizing and externalizing tendencies as well as answers to specific substance use questions. Substance use scales consist of self-reported daily tobacco use, number of days drunk, and days using drugs (other than alcohol or tobacco) for the previous 6 months.
Marijuana users completed the Personal Experience Inventory (Henley & Winters, 1989) to assess other substance use. The PEI measures the frequency of substance use within the last 3 months on a 5-point scale (never, 1–5 times, 6–20 times, 21–49 times, 50–99 times, 100+ times). Non-marijuana drug use was calculated by summing the frequency ratings across illicit drug classes (psychedelics, cocaine, amphetamines, barbiturates, tranquilizers, heroin, narcotics, steroids, inhalants, and recreation use of prescription drugs). Controls had no history of use of these other substances.
Statistical Approach
Data were analyzed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA), Windows version 19. Distributions of all variables were examined. Error variables for the Letter Cancellation, RAVLT, and COWAT tasks were square root transformed to meet assumptions for parametric analysis. Chi-square tests were used to compare nominal variables (i.e., sex) between marijuana users and controls. Mann-Whitney U analyses assessed for group differences in substance use characteristics, in which variances were unequal between groups. Univariate and repeated measures analyses of variance (ANOVA) assessed for group differences in other characteristics. Sex, IQ, and alcohol use were covaried in all group comparisons. To best characterize a wide variety of alcohol use patterns, alcohol use was quantified as an average of two alcohol use variables that were standardized across the whole sample (controls and marijuana users). The first alcohol use variable was calculated by multiplying the participants’ self-reported average drinking occasions per week and the average number of alcoholic drinks per occasion for the previous 6 months, as assessed by direct interview. The second alcohol use variable was the number of days that the participant reported being drunk in the last 6 months, as assessed by the ASR questionnaire. Missing data from 2 controls and 1 marijuana user on an alcohol use variable reduced the sample size to 35 participants per group.
Due to recruitment procedures, controls had no history of tobacco use or non-alcohol-related drug use. Non-alcohol-related drug use was calculated from responses on the PEI. Tobacco use was quantified by responses to questions from the ASR and K-SADS regarding patterns of daily use.
Accordingly, levels of alcohol, tobacco, and other drug use differed between groups, but alcohol use was the only variable that could be used in between-group analyses. The contributions of tobacco and other drug use to cognition in the marijuana users were examined within marijuana users using partial correlations to explore the extent to which other substance use contributed to domains where group differences were observed. Alcohol use, tobacco use, and non-marijuana drug use was correlated with task performance in marijuana users, controlling for IQ, sex, and other substance use. For example, the examination of alcohol effects controlled for tobacco and other non-marijuana-related drug use.
Scatterplots of residuals were examined to assess for normal distributions, and data were screened for outliers and influential data points. To provide a conservative control for the number of statistical comparisons, alpha levels equal to or below 0.01 were considered significant, and alpha levels at or below 0.05 were considered trend effects.
Results
Demographics (Table 1)
Groups were comparable in age, years of education, race/ethnic distribution, and IQ. Consistent with similar studies of drug users with demographically matched college student controls (Croft, Mackay, Mills, & Gruzelier, 2001), IQs were generally above average, indicating that participants were generally high functioning from a cognitive standpoint. There were significantly more males among marijuana users, consistent with the gender distribution of marijuana users in this age range (Substance Abuse and Mental Health Services Administration, 2011).
Substance Use Characteristics
Marijuana users had significantly higher alcohol use as assessed by averaging the standardized scores of typical alcohol use patterns and days drunk in the past 6 months (p < 0.001; Table 1). Additionally, as would be expected from national norms (Substance Abuse and Mental Health Services Administration, 2013), marijuana users had greater tobacco use, days drunk, and days using other drugs in the last 6 months compared to controls. As a result of exclusion criteria, controls reported no marijuana use. Marijuana users reported a mean age of initiation of marijuana use during mid-adolescence (M = 15.24, SD = 1.24). Marijuana users reported nearly daily marijuana use during the past 30 days with a mean of 10.20 hits per day; however there was considerable variability in the number of reported hits per day, with a standard deviation of 9.12.
DSM-IV-TR diagnostic characteristics
Despite differences from the control sample, marijuana users reported relatively little substance use outside of marijuana and alcohol. The majority of marijuana users had tried other drugs less than 5 times, and no participant had used any other drug more than 15 times (Table 2). Almost all marijuana users met criteria for current and/or past marijuana substance use disorder (SUD) (Table 3) and many met criteria for current and past alcohol abuse.1 There was high concordance between current and past diagnosis of an SUD within subjects (Table 3). SUD symptom patterns were examined in detail to clarify symptom expression related to alcohol, marijuana, and other drug use. Marijuana users exhibited fewer symptoms related to current alcohol use (M = 0.89 symptoms per person, SD = 1.05) than related to marijuana use (M = 4.03 symptoms per person, SD = 1.90; U = 100.5, p < 0.001). Similarly, marijuana users reported fewer symptoms related to past alcohol use (M = 1.20 symptoms per person, SD = 1.32) than past marijuana use (M = 4.23 symptoms per person, SD = 1.77; U = 114.0, p < 0.001). No SUD symptoms related to other drug use were reported among marijuana users or controls. As a result of exclusion criteria, controls exhibited very few symptoms related to current alcohol use (M = 0.11 symptoms per person, SD = 0.32) or past alcohol use (M = 0.03 symptoms per person, SD = 0.17), and no symptoms related to other drug use.
Table 2.
Lifetime other drug usage in marijuana users. Number of participants who used each drug at different usage levels.
| Substance | 1–5 times | 6–10 times | 11–15 times | Mean Usage |
|---|---|---|---|---|
| Cannabis | ||||
| Hash | 1 | 0.14 (0.85) | ||
| Stimulants | ||||
| Adderall | 4 | 1 | 0.70 (2.06) | |
| Cocaine | 2 | 1 | 0.33 (1.34) | |
| Opioids | ||||
| Vicodin | 5 | 0.39 (1.35) | ||
| Codeine | 1 | 0.06 (0.34) | ||
| Opium | 4 | 0.26 (0.79) | ||
| Oxycodone | 2 | 0.05 (0.24) | ||
| Psychedelics | ||||
| Mushrooms/LSD | 16 | 3 | 2 | 1.24 (2.39) |
| Salvia | 4 | 0.31 (1.08) | ||
| Mescaline | 1 | 0.03 (0.17) | ||
| Benzodiazepines | ||||
| Xanax | 3 | 1 | 0.09 (0.28) | |
| Valium | 1 | 0.11 (0.69) | ||
| Sedative/Hypnotics | ||||
| Ambien | 1 | 0.29 (1.69) | ||
| Other | ||||
| Ecstasy | 12 | 0.81 (1.48) | ||
| Nitrous Oxide | 2 | 0.17 (0.86) |
Table 3.
DSM-IV-TR Diagnostic characteristics of marijuana users (n = 35)
| Current Diagnosis | Past Diagnosis | Current and Past | |
|---|---|---|---|
| Marijuana Dependence | 18 | 18 | 17 |
| Marijuana Abuse | 12 | 14 | 12 |
| Alcohol Dependence | 0 | 0 | 0 |
| Alcohol Abuse | 11 | 16 | 10 |
| Bipolar NOS | 1 | 1 | 0 |
| Oppositional Defiant Disorder | 0 | 2 | 0 |
| Specific Phobia | 0 | 1 | 0 |
| Comorbidity |
|||
| Only Marijuana Dependence | 14 | 9 | |
| Only Marijuana Abuse | 6 | 6 | |
| Only Alcohol Abuse | 2 | 0 | |
| Marijuana Dependence, Alcohol Abuse | 3 | 9 | |
| Marijuana Abuse, Alcohol Abuse | 6 | 7 |
Comorbid Psychopathology
Given selection procedures, controls were free of psychopathology. Outside of SUDs, marijuana users reported little psychopathology (Table 3). One participant met criteria for current Bipolar NOS; another met criteria for past Bipolar NOS. Both were due to episodic hypomania, consistent with the reported comorbidity between SUDs and bipolar disorder (Perlis et al., 2004; Wilens et al., 2008). Other psychological disorders included past Oppositional Defiant Disorder (n = 2) and past Specific Phobia (n = 1). As will be described, data were analyzed with and without inclusion of these individuals.
Overall, the sample of marijuana users is notable for its good overall psychological health independent of marijuana use, and for high levels of premorbid functioning (as indicated through estimated verbal IQ scores).
Neurocognitive Performance (Table 4)
Table 4.
Neuropsychological battery scores. Means reported are marginal means, controlling for sex, IQ, and alcohol use.
| Cognitive Measure | Control M (SD) |
Marijuana user M (SD) |
F | p | ηp2 |
|---|---|---|---|---|---|
| Finger Tapping Test | |||||
| Dominant hand (# taps) | 42.16 (10.19) | 46.93 (7.19) | 3.01 | 0.087 | 0.04 |
| Non-dominant hand (# taps) | 42.02 (8.49) | 44.76 (7.65) | 1.29 | 0.261 | 0.02 |
| Grooved Pegboard | |||||
| Dominant hand time (seconds) | 65.41 (8.14) | 64.12 (8.31) | 0.32 | 0.575 | 0.01 |
| Non-dominant hand time (seconds)a | 72.97 (9.98) | 71.13 (11.55) | 0.32 | 0.572 | 0.01 |
| Letter Cancellation | |||||
| Time (seconds) | 111.95 (16.60) | 96.79 (18.08) | 7.50* | 0.008 | 0.10 |
| Total omissionsc | 1.51 (0.71) | 1.49 (0.76) | 0.01 | 0.927 | 0.00 |
| Total commissionsc | 0.81 (0.27) | 0.72 (0.12) | 1.42 | 0.237 | 0.02 |
| Digit Symbol | |||||
| Total correct | 87.59 (15.12) | 89.35 (13.21) | 0.17 | 0.680 | 0.00 |
| COWAT – Verbal Fluency | |||||
| Total correct words generated | 43.24 (8.50) | 50.79 (10.98) | 6.78* | 0.011 | 0.09 |
| Total set-loss errorsc | 0.82 (0.29) | 1.11 (0.52) | 4.67^ | 0.034 | 0.07 |
| Total perseverative errorsc | 1.03 (0.39) | 0.95 (0.41) | 0.35 | 0.557 | 0.01 |
| Digit Span | |||||
| Digits forward (# recalled) | 7.52 (0.86) | 6.99 (1.03) | 3.28 | 0.075 | 0.05 |
| Digits backward (# recalled) | 5.76 (1.19) | 5.24 (1.17) | 1.95 | 0.167 | 0.03 |
| RAVLT – Verbal Learning and Memory | |||||
| Total words: Trial 1–5 | 55.05 (7.00) | 51.29 (8.34) | 2.70 | 0.105 | 0.04 |
| Total intrusions: Trial 1–5a | 1.02 (0.58) | 1.30 (0.63) | 2.26 | 0.121 | 0.04 |
| Total perseverative errors: Trial 1–5a | 1.96 (0.78) | 1.81 (1.01) | 0.26 | 0.609 | 0.00 |
| Total words: Interference trial list | 7.40 (1.95) | 5.83 (1.86) | 9.08* | 0.004 | 0.12 |
| Total words: Immediate recall | 12.24 (2.08) | 10.56 (2.44) | 6.65* | 0.012 | 0.09 |
| Total words: Delayed recall | 12.03 (2.13) | 9.68 (2.91) | 10.47* | 0.002 | 0.14 |
| Spatial Span | |||||
| Forward (# recalled) | 6.55 (0.98) | 6.99 (0.89) | 2.17 | 0.145 | 0.03 |
| Backward (# recalled) | 6.78 (1.22) | 6.50 (1.01) | 0.66 | 0.421 | 0.01 |
| Spatial Recognitiona | |||||
| % Correct recall | 86.50 (8.14) | 85.66 (7.20) | 0.12 | 0.731 | 0.00 |
| Self-Ordered Searcha | |||||
| Between search errors 4 | 0.12 (0.49) | 0.18 (0.44) | 0.15 | 0.700 | 0.00 |
| Between search errors 6 | 2.79 (3.25) | 2.60 (2.67) | 0.04 | 0.835 | 0.00 |
| Between search errors 8 | 10.65 (9.02) | 9.66 (8.32) | 0.13 | 0.716 | 0.00 |
| Total between search errors | 13.56 (10.21) | 12.43 (9.70) | 0.13 | 0.720 | 0.00 |
| Strategy Score: 6–8 | 30.28 (5.36) | 28.80 (5.52) | 0.76 | 0.386 | 0.01 |
| Spatial Delayed Response Task | |||||
| Error: No delay (millimeters) | 2.44 (0.76) | 2.55 (0.88) | 0.17 | 0.682 | 0.00 |
| Error: 500 ms delay (millimeters) | 6.44 (2.18) | 7.70 (2.23) | 3.15 | 0.080 | 0.05 |
| Error: 8,000 ms delay (millimeters) | 9.87 (3.36) | 11.98 (2.55) | 4.81^ | 0.032 | 0.07 |
| Mean reaction time: No delay | 1823.33 (575.62) | 1962.42 (419.96) | 0.75 | 0.390 | 0.01 |
| Mean reaction time: 500 ms delay | 1663.24 (352.62) | 2034.85 (374.35) | 10.93* | 0.002 | 0.14 |
| Mean reaction time: 8,000 ms delay | 1733.73 (345.42) | 2234.00 (475.77) | 15.60* | < 0.000 | 0.19 |
| Tower of Londona | |||||
| % Perfect Solutions | 83.71 (13.33) | 74.12 (14.47) | 5.24^ | 0.025 | 0.08 |
| Average moves 2 | 2.00 (0.00) | 2.00 (0.00) | |||
| Average moves 3 | 3.00 (0.16) | 3.28 (0.39) | 8.63* | 0.005 | 0.12 |
| Average moves 4 | 4.92 (0.89) | 5.16 (0.97) | 0.72 | 0.398 | 0.01 |
| Average moves 5 | 5.65 (1.00) | 6.24 (1.13) | 3.11 | 0.083 | 0.05 |
| First move initiation time 2 | 3190.52 (1161.58) | 3662.26 (735.35) | 2.27 | 0.137 | 0.05 |
| First move initiation time 3 | 5510.37 (2510.15) | 5442.88 (1485.40) | 0.01 | 0.920 | 0.00 |
| First move initiation time 4 | 8308.17 (5079.47) | 8420.53 (3091.36) | 0.01 | 0.935 | 0.00 |
| First move initiation time 5 | 12987.90 (6940.13) | 8805.07 (5108.86) | 4.75^ | 0.033 | 0.07 |
| Average first move initiation time | 7499.24 (3547.50) | 6582.68 (2138.04) | 0.94 | 0.337 | 0.01 |
| Iowa Gambling Taskb | |||||
| Good Choices-Bad Choices: Block 1 | −1.12 (9.58) | −3.56 (7.18) | 0.82 | 0.368 | 0.01 |
| Good Choices-Bad Choices: Block 2 | 3.25 (10.36) | −2.13 (7.54) | 3.85^ | 0.054 | 0.06 |
| Good Choices-Bad Choices: Block 3 | 3.73 (9.48) | −1.68 (8.67) | 3.47 | 0.067 | 0.05 |
| Good Choices-Bad Choices: Block 4 | 9.56 (9.32) | −1.29 (10.09) | 12.73* | 0.001 | 0.17 |
| Good Choices-Bad Choices: Block 5 | 9.80 (10.42) | −0.15 (10.66) | 9.89* | 0.003 | 0.13 |
| Good Choices: Total | 60.45 (18.17) | 49.77 (16.10) | 4.01^ | 0.049 | 0.06 |
Data unavailable for 1 marijuana user (n = 34).
Data unavailable for 1 control (n = 34).
Square root transformed.
p ≤ .05.
p ≤ .01.
Motor Function
Groups were equivalent in Finger Tapping and Grooved Pegboard performance with no evidence of laterality differences.
Processing Speed
Marijuana users demonstrated faster Letter Cancellation completion times. Omission and commission errors were equivalent between groups. Completion times were uncorrelated with overall errors in both groups. Groups were equivalent in Digit Symbol performance.
Verbal Fluency (COWAT)
Marijuana users displayed greater verbal fluency, producing more correct responses. Set-loss errors were only marginally greater among users. Perseverative errors were equivalent between groups.
Verbal Learning and Memory
The groups were equivalent on Digit Span forward and backward.
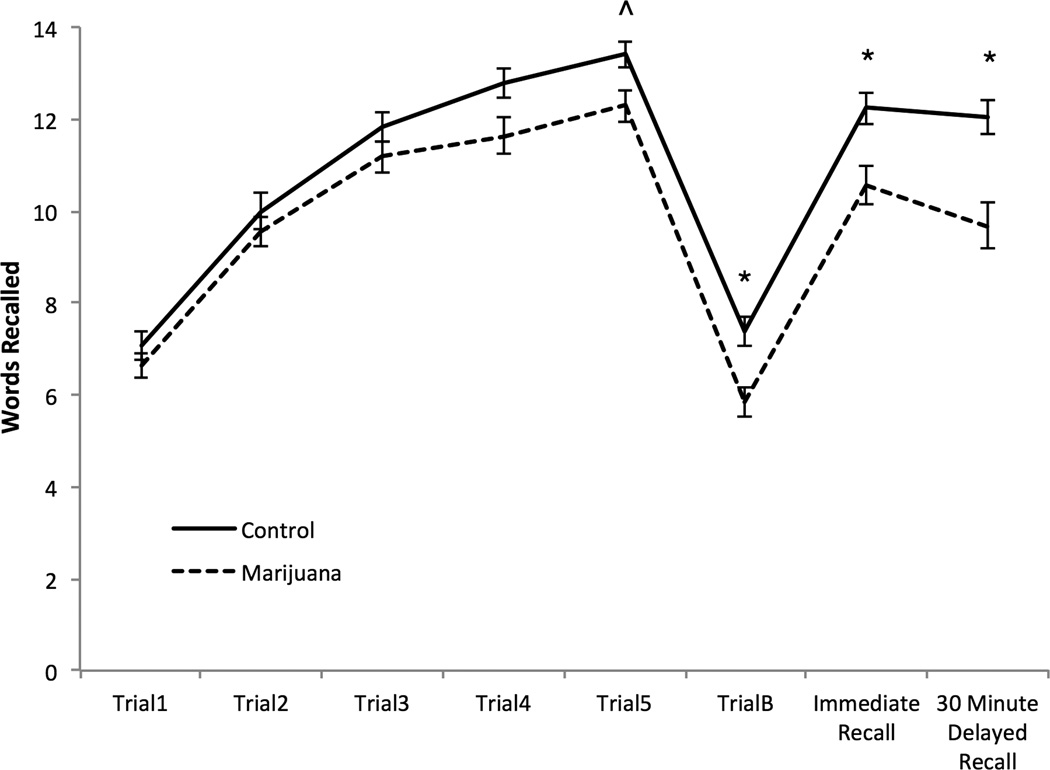
A repeated measures ANCOVA across RAVLT trials (T1-T5, interference trial, immediate recall, and delayed recall) revealed a group effect, F (1, 65) = 7.31, p = 0.009, ηp2 = 0.10, and a group by trial interaction, F (1, 65) = 8.74, p = 0.004, ηp2 = 0.12 (Figure 1). Follow-up analyses revealed that marijuana users had a trend toward poorer performance during list learning trial 5, F (1, 65) = 3.90, p = 0.05, ηp2 = 0.06. Marijuana users performed worse than controls on the interference trial list. Following interference, marijuana users demonstrated poorer immediate recall and poorer 30-minute delayed recall. Furthermore, there was a trend for marijuana users to have greater loss after consolidation, F (1, 65) = 6.30, p = 0.02, ηp2 = 0.09 (Marijuana user M = 78.04%, SD = 17.16, Control M = 90.90%, SD = 16.76)
Figure 1.
RAVLT Learning Curve. Average words recalled during learning trails 1–5, immediate recall, and 30 minute delayed recall.
^ p ≤ .05 * p ≤ .01
Examining retroactive interference (trial 5 vs. immediate recall) and proactive interference (trial 1 vs. interference) revealed no significant trial by group interactions. No significant group differences were noted during learning or delayed recall for bidirectional serial ordering or response consistency during learning. Marijuana users demonstrated less response consistency between short- and long-term recall, F (1, 65) = 15.78, p < 0.001, ηp2 = 0.20 (Marijuana user M = 80.64%, SD = 14.55, Controls M = 93.73%, SD = 6.58).
Errors during list learning and recall were equivalent between groups.
Spatial Working Memory
No significant group differences were evident on forward or backward Spatial Span, Spatial Recognition, or Spatial Self-Ordered Search. On the DRT, groups were equivalent in their accuracy and response latency for the no delay condition, indicating that basic sensorimotor functions recruited by the task were similar between groups. Additionally groups displayed equivalent accuracy during the 500 ms delay condition. Marijuana users demonstrated a trend toward decreased accuracy on the 8,000 ms delay condition. Marijuana users had significantly longer response latencies (slower performance) after both 500 ms and 8,000 ms delays. Accuracy and response latencies were uncorrelated in marijuana users and controls for the 500 ms and 8,000 ms delay conditions.
Planning
There was a trend for marijuana users to produce fewer perfect solutions on the Tower of London task, indicating that they made more moves than necessary to achieve accurate performance. When individual difficulty levels were examined, marijuana users made significantly more moves to complete 3-move problems; a similar pattern nominally characterized other levels of difficulty. Additionally, marijuana users had marginally faster initiation times during 5-move problems.
Motivated Decision-Making
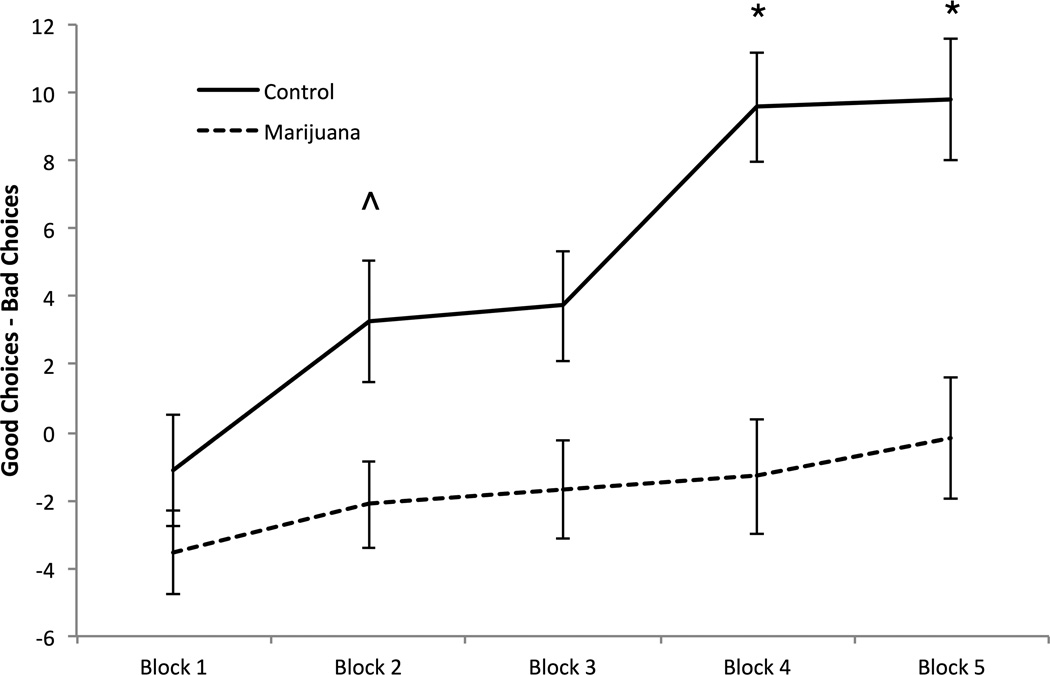
On the Iowa Gambling Task, total good minus bad choices over five blocks of the task were examined with block as the within subjects factor and group as the between subjects factor (Figure 2). A significant main effect of group, F (1,64) = 10.97, p = 0.002, ηp2 = 0.15, was observed with marijuana users displaying poorer performance.
Figure 2.
Iowa Gambling Task. Total good choices minus total bad choices over 5 blocks.
^ p ≤ .05. * p ≤ .01.
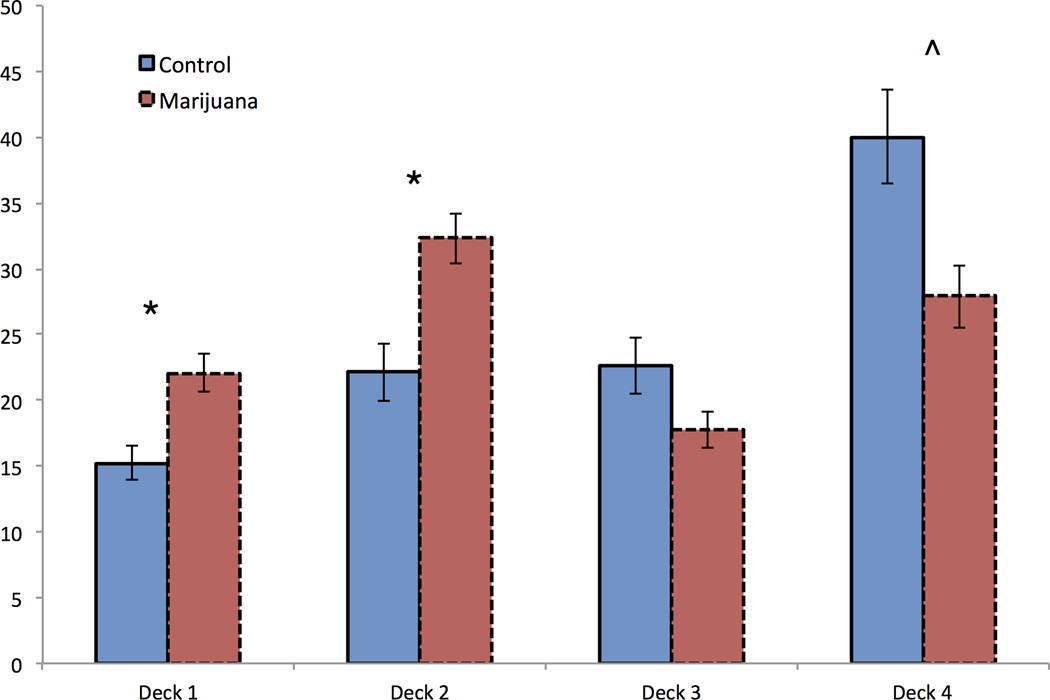
There was a significant group difference in choice of deck 1, F (1,64) = 7.63, p = 0.007, ηp2 = 0.11, deck 2, F (1,64) = 7.77, p = 0.007, ηp2 = 0.11, and a marginal group difference in choice of deck 4, F (1,64) = 5.26, p = 0.025, ηp2 = 0.08. Marijuana users made more choices from disadvantageous decks, decks 1 and 2, and fewer choices from advantageous deck 4 (Figure 3).
Figure 3.
Iowa Gambling Task. Number of choices from each deck across 5 blocks between groups.
^p ≤ .05. * p ≤ .01.
Deck choices were compared to choices expected by chance. Both groups showed aversion to frequent punishment decks (1 and 3), choosing from these less often than expected at a significant or trend level (Deck 1: Marijuana user t (34) = −2.12, p = 0.041, Control t (33) = −7.43, p < 0.001; Deck 3: Marijuana user t (34) = −3.70, p = 0.001, Control t (33) = −2.26, p = 0.031). Within infrequent punishment decks, controls demonstrated a preference for smaller wins with infrequent smaller punishment (Deck 4: t (33) = 4.24, p < 0.001), with choices from deck 4 correlating with overall good choices throughout the task (rsex, alcohol use, IQ = 0.80, p < 0.001). Conversely, marijuana users showed a preference for greater wins with infrequent but greater punishment (Deck 2: t (34) = 2.68, p = 0.011).
Associations with Other Substance Use
For all significant group effects described above, alcohol, tobacco, and non-marijuana drug use were each separately examined for associations with task performance within marijuana users (Table 5).
Table 5.
Follow-up partial correlations between substance use variable and cognitive measures in marijuana users controlling for sex and IQ.
| Alcohol Usea | Tobacco Useb | Non-Marijuana Drug Usec |
|
|---|---|---|---|
| Letter Cancellation | |||
| Time | 0.10 | −0.20 | 0.32 |
| COWAT – Verbal Fluency | |||
| Total words | −0.12 | −0.16 | 0.03 |
| Total set-loss errorsd | −0.21 | −0.04 | −0.32 |
| RAVLT – Verbal Learning & Memory | |||
| Total Words: Trial 5 | 0.47* | −0.15 | 0.01 |
| Total Words: Interference Trial List | 0.50* | 0.11 | 0.06 |
| Total Words: Immediate Recall | 0.56* | −0.23 | < −0.01 |
| Total Words: Delayed Recall | 0.48* | −0.18 | 0.33 |
| % of Learning Recalled During Delay | 0.26 | −0.18 | 0.43^ |
| Delayed Consistency | 0.48* | 0.07 | 0.01 |
| Spatial Delayed Response Task | |||
| Error: 8,000 ms delay | −0.40^ | 0.05 | 0.14 |
| Reaction Time: 500 ms delay | −0.28 | −0.29 | 0.01 |
| Reaction Time: 8,000 ms delay | −0.30 | −0.21 | < −0.01 |
| Tower of Londone | |||
| % Perfect Solutions | 0.35 | 0.04 | 0.05 |
| Average Moves 3 | −0.40^ | −0.24 | −0.20 |
| First move initiation time 5 | −0.20 | −0.17 | −0.36^ |
| Iowa Gambling Task | |||
| Good Choices-Bad Choices: Block 2 | < −0.01 | −0.13 | −0.15 |
| Good Choices-Bad Choices: Block 4 | 0.21 | 0.09 | −0.08 |
| Good Choices-Bad Choices: Block 5 | −0.04 | 0.15 | −0.18 |
| Good Choices: Total | −0.21 | −0.22 | 0.14 |
| Deck1 Choices | 0.05 | −0.08 | < 0.01 |
| Deck 2 Choices | −0.20 | −0.04 | 0.24 |
| Deck 4 Choices | −0.06 | 0.09 | −0.22 |
Controlling for tobacco and non-marijuana drug use.
Controlling for alcohol and non-marijuana drug use.
Controlling for alcohol and tobacco use.
Square root transformed.
Data unavailable for 1 participant (n = 34).
p ≤ .05.
p ≤ .01.
Alcohol Use. For learning and recall measures of the RAVLT, errors made within the 8,000 ms delay condition of the delayed response task, and average moves on 3-move problems of the TOL, greater alcohol use was unexpectedly associated with better task performance. Alcohol use was not significantly correlated, using our stringent alpha level, with other task performance variables.
Furthermore, within marijuana users, no group differences in cognitive function were noted between subjects with current alcohol abuse (n = 11) and subjects without current alcohol abuse (n = 24).
Tobacco Use. Cognitive performance was uncorrelated with tobacco use within marijuana users (Table 5).
Non-marijuana Drug Use. There was a trend for non-marijuana drug use to be positively correlated with RAVLT delayed recall as well as with faster initiation times (decreased planning) on 5-move Tower of London problems.
Impact of Comorbid Psychopathology
Significant group differences remained unchanged when marijuana users with psychopathology outside of SUDs (n = 5) were excluded.
Discussion
This study employed a comprehensive neurocognitive battery to assess a range of cognitive abilities in a low-risk sample of college-aged daily marijuana users who were studied in a non-intoxicated state but in the context of active use. The marijuana user sample was notable in terms of its relative psychological health, absence of externalizing psychopathology, and above-average levels of general intellect. Despite this general pattern of low risk, and in the context of several cognitive strengths, marijuana users demonstrated a number of cognitive deficits relative to demographically-matched controls.
Marijuana users performed particularly well on speeded measures, such as letter cancellation and verbal fluency. Both measures are short tasks (< 2 minutes), requiring short-term sustained attention. It is important to note that these tasks are externally motivated, with instructions to work quickly.
On the other hand, marijuana users demonstrated many relative deficits in the domains of memory, problem-solving, and motivated decision making. It appears marijuana users were generally less motivated and, as a consequence, less persistent in the absence of motivation-enhancing instruction, when tasks required sustained and internally motivated effort. For instance, marijuana users’ verbal learning performance was characterized by a pattern of sustained accurate performance during early learning trials and a slow and subtle divergence from controls’ performance as the task progressed (see Figure 1). Marijuana users demonstrated greater loss after consolidation when required to produce learned information after a time delay. Marijuana users’ relatively decreased response consistency indicates use of a less efficient recall strategy. The observation of relatively poor retention of learned material over time is consistent with other reports (Block et al., 2002; Fried et al., 2005; Medina et al., 2007; Pope & Yurgelun-Todd, 1996; Tait et al., 2011; Takagi et al., 2011; Wagner et. al., 2010) and could be explained by a combination of deficits in executive control as well as motivation. While the loss of information may appear to be relatively low in absolute magnitude (1 in 15 presented words), this same degree of information loss in the context of ongoing academic, occupational, and social interactions could have obvious impacts on social function and other areas of achievement. Similarly, the Tower of London and Iowa Gambling Tasks require sustained effort for optimal performance, and the longer trials of the delayed response task require intrinsically focused attention during the delay interval to support accurate responding (Luciana, Collins & Depue, 1998).
When these findings are considered in relation to the areas where marijuana users demonstrated relative strengths (short-term externally-motivated tasks that required quick performance), it could be that marijuana users are most impaired when tasks require intrinsic motivation and most successful when tasks are enhanced by the provision of motivation-enhancing instructions, such as the instruction to work quickly. External or extrinsic motivation reflects motivation that is cued through external means and is performance- or incentive-based. In contrast, internal/intrinsic motivation is reflected by a sense of purpose as well as voluntary engagement with a task in the absence of obvious external incentives (Lee, Reeve, Xue, & Xiong, 2012; Murayama, Matsumoto, Izuma, & Matsumoto, 2010). The speeded tasks within our battery can be construed as performance-based measures of external motivation. To some extent, all tasks in the battery require intrinsic motivation, but voluntary engagement is most strongly recruited when tasks are lengthy and require increasing amounts of self-organization and focus to assure accurate performance.
It has been proposed that a source of intrinsic motivation is the subjective value of achieving success on a task (Murayama et al., 2010). Subjective value is modulated within the brain through the dopaminergic reward network, including midbrain structures, ventral and dorsal striatum, and the prefrontal cortex (Kable & Glimcher, 2007; Levy & Glimcher, 2011). These regions are involved in both incentive (performance)-based and intrinsic motivational tasks. The lateral prefrontal cortex has been suggested as a substrate for the intrinsic preparatory cognitive control necessary to pursue distinct goals. In addition, conscious effort-based processing has been associated with the anterior cingulate and dorsolateral prefrontal cortices (Mulert, Menzinger, Leicht, Pogarell, & Hegerl, 2005). A recent series of studies has further linked personal agency and other aspects of intrinsic motivation to activation in the anterior insular region (Lee et al., 2012; Lee & Reeve, 2013). Although most neuroscientific studies of motivation involve manipulations of external incentive-based motivation and studies of intrinsic motivation are limited, we propose that overlapping subcortical systems are involved in both types of motivation given that incentive-based learning necessarily impacts subjective valuation but that higher-order frontal systems (involving multiple regions of the prefrontal cortex) are more strongly recruited in support of intrinsic motivation—the pursuit of goals in the absence of obvious external incentives.
These frontal systems are known to mediate tasks in our battery where marijuana users demonstrated deficits. For instance, success on delayed recall trials of the RAVLT has been linked to frontal mechanisms (Gershberg & Shimamura, 1995; Long, Oztekin, & Badre, 2010), and multiple regions of the dorsal, medial, and ventral prefrontal cortex, as well as the insular region, contribute to successful IGT performance (Bechara et al., 1994; Lawrence, Jollant, O’Daly, Zelaya, & Phillips, 2009). Accordingly, we hypothesize that neuroscience-based studies of intrinsic versus extrinsic motivation in marijuana users would reveal stronger evidence of disruption in the former. Although this suggestion is speculative, given that motivation was not directly measured in this study, motivation-enhancing instruction has been found to improve marijuana users’ performance, but not that of controls, on a verbal learning and memory task (Macher & Earleywine, 2012).
Several tasks in our battery, including the spatial delayed response task, are heavily influenced by dopamine neurotransmission in frontostriatal circuits, with increased dopamine activity facilitating better performance (Luciana et al., 1998). Similarly, acute but indirect reduction of dopaminergic activity has been found to produce impaired decision-making performance on the Iowa Gambling Task (Sevy et al., 2006), mirroring the disruptions noted in the current study. Throughout the Iowa Gambling Task, marijuana users failed to acquire an effective strategy. Marijuana users’ choice patterns suggest that reward feedback was more compelling to marijuana users than was punishment feedback.
The observed performance deficits on the IGT and spatial delayed response task may indicate blunted striatal or frontal dopamine activity in the marijuana users, a finding previously reported in adult marijuana users (Kowal, Colzato, & Hommel, 2011) and consistent with the animal literature (Pistis et al., 2004; Schneider & Koch, 2003). A recent study examined dopamine synthesis capacity in regular marijuana users who experienced psychotic-like symptoms while intoxicated with non-using sex- and age-matched control subjects. Findings revealed diminished dopamine synthesis capacity in marijuana users in multiple regions of the striatum (Bloomfield et al., 2013). Consistent with our proposed model, this diminished synthesis capacity was associated with a younger age onset of marijuana use as well as with higher current levels of use. A younger age of marijuana use onset is also associated with disrupted patterns of dopamine receptor binding (Urban et al., 2012). Bloomfield et al. (2013) have suggested that diminished striatal dopamine synthesis capacity accounts for amotivation in heavy marijuana users. Further research directly assessing dopaminergic activity in marijuana users and its relation to cognitive performance is needed to more comprehensively link dopamine activity to behavior in this population.
The performance deficits observed in the current study cohere with findings from the brain imaging literature indicating less efficient brain activation patterns in marijuana users. Marijuana users demonstrate increased activation across a wide range of brain regions and recruit alternative brain networks during task performance (Block et al., 2002; Chang et al., 2006; Harding et al., 2012; Jacobsen et al., 2004; Kanayama et al., 2004; Padula et al., 2007; Schweinsburg et al., 2010; Tapert et al., 2007). Increased activation and recruitment of alternative pathways may be compensatory and less efficient.
While marijuana users exhibited greater alcohol, tobacco, and non-marijuana drug use than controls, it does not appear that most observed cognitive differences were due to the impacts of these other substances. For the majority of tasks, alcohol, tobacco, and other substance use was unrelated to task performance. However, within the domain of verbal learning (RAVLT), relatively higher levels of self-reported alcohol use in marijuana users were associated with better cognitive functioning. This pattern is consistent with recent reports of more normative patterns of structural brain integrity in marijuana users who use alcohol versus those who do not (Jacobus et al., 2009; Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007). While counterintuitive, this finding may point to specific neural interactions between alcohol and marijuana such that alcohol use benefits some areas of memory consolidation function in the context of heavy marijuana use. Importantly, however, the interaction between alcohol and marijuana, if present, does not protect against diminished cognitive performance in other areas of function when marijuana users are compared to controls, nor does it ameliorate the overall performance deficit noted between marijuana users and controls on the RAVLT.
Moreover, when the limited number of users with histories of non-substance-related psychopathology were excluded, significant group differences remained. Marijuana users with and without current alcohol abuse did not differ in cognitive performance. Whether these findings would generalize to marijuana users who exhibit high levels of externalizing psychopathology or other forms of economic or psychological risk is unknown, but we speculate, based on the extant literature, that the presence of additional risk factors would result in an even more extensive pattern of group differences given that such factors are independently associated with cognitive difficulties.
In summary, marijuana users demonstrated an inconsistent pattern in terms of leveraging appropriate strategies to facilitate performance on complex memory, planning, and decision-making tasks. These tasks generally required high levels of self-organization, as well as potentially greater demands on intrinsic motivation, as opposed to areas where the marijuana users excelled (fast, short-term processing tasks), which were more externally motivated. These findings suggest that if individuals engage in use on a daily basis, they may become increasingly dependent upon external sources of reinforcement and motivation to structure their behavior as opposed to intrinsically driven self-reliance and self-organization. On the other hand, they may excel in settings where external sources of motivation are high. Marijuana users’ performance across domains of function suggests the possibility of diminished frontostriatal dopaminergic activity, affecting both decision-making and spatial working memory performance. This may be the mechanism driving the performance deficits noted and may be one avenue through which chronic marijuana use, particularly use that is initiated during adolescence, impacts longer-term function.
Limitations
One limitation of the current study is the overrepresentation of males in the marijuana user sample. However, this gender distribution is consistent with the gender distribution of marijuana users in the United States (Substance Abuse and Mental Health Services Administration, 2013). Sex was controlled in all statistical analyses; however, findings cannot be readily generalized to female marijuana users. A further limitation is that our design does not permit dose-response associations to be measured in marijuana users who tended to be relatively homogeneous in their use patterns. A related issue is that it is difficult to quantify the precise amount of drug ingested by marijuana users given that the potency of marijuana is not standard. While many studies have quantified dose by calculating hits per day (which we assessed), this measure does not address potency or the amount of drug ingested during a hit. Marijuana users were required to use marijuana at least 5 days per week, yielding a relatively homogeneous sample of marijuana users. There were no requirements for amount of hits during each episode of use.
Additionally, while marijuana users were not acutely high during testing, we cannot rule out the possibility that the cognitive differences observed in our sample are due to residual effects of marijuana use. However, the current assessment provides a comprehensive cognitive profile of otherwise high functioning individuals in the context of frequent current marijuana use. This profile allows us to make real-world inferences about how daily marijuana use might impact cognition. In order to minimize potential confounds, we recruited high functioning individuals, with comparable education and IQ to other college-aged controls, and a low level of psychopathology. While this feature of the study can be considered a strength since the sample of users represents college-aged individuals who heavily use the drug, it may limit generalizability to other marijuana-using samples who evidence more externalizing behavior, less education, and more psychopathology. Our expectation is that they would show greater levels of impairment.
Another possible concern is that marijuana users were in active states of withdrawal during testing, affecting the results. Marijuana users were asked to abstain for at least a twelve-hour period prior to the study. This possibility appears unlikely given the psychomotor performance exhibited by marijuana users, which is inconsistent with behaviors that individuals in the midst of marijuana withdrawal demonstrate (Haney et al., 2001). Finally, we did not employ marijuana drug testing, since the active compound in marijuana remains detectible long after prior use. The level of detail that participants conveyed regarding their use patterns was convincing in terms of the likelihood that they were, indeed, heavy marijuana users, an assumption validated by their reports of symptoms of marijuana dependence. However, because we did not employ drug testing, we cannot completely rule out the possibility that actual use in these participants is lower than what they self-reported. Finally, although our findings are suggestive of patterns of impairment that emerge as a consequence of use and replicate findings reported in the literature, cause-effect associations cannot be determined. It could be that premorbid levels of function were impaired in marijuana users prior to use onset.
We have suggested that marijuana use during active stages of brain development is more likely to result in cognitive impairment as opposed to use that begins later in life. Our sample of marijuana users had a relatively young age of marijuana use onset (15.2) relative to national norms (Substance Abuse and Mental Health Services Administration, 2013) with age of onset of use ranging from 13 to 18 years. This is an active period of brain and associated cognitive development (Giedd et al., 1999; Lebel & Beaulieu, 2011; Luciana, Conklin, Hooper, & Yarger, 2005; Sowell, Thompson, Tessner, & Toga, 2001). The animal literature supports our hypothesis that introducing marijuana during that critical time could lead to long-standing cognitive effects, which can be observed during assessment in adulthood, while use is ongoing. An obvious area of continued empirical study concerns the cognitive impacts of marijuana use at varying ages of onset, which we cannot comprehensively address within the current dataset given small sample size. Independent of length of drug exposure as a factor that might impact the current findings, this study is nonetheless informative regarding the cognitive profiles of young adults who are active marijuana users during the college years. We speculate that such use will become increasingly prevalent with marijuana legalization.
Conclusions
The current study provides a comprehensive cognitive profile of college-aged daily marijuana users. Marijuana users demonstrated strengths relative to controls in processing speed and verbal fluency. Marijuana users also demonstrated numerous cognitive deficits, most notably in verbal memory, engagement and use of efficient strategies with complex tasks, and motivated decision-making. Future dose-response studies in samples that are similarly free of comorbid pathology and in this well-defined age-range would be helpful in clarifying whether a single underlying deficit leads to these distinct behavioral patterns. Pharmacological challenge or positron emission tomography (PET) studies in marijuana users versus controls can further clarify the role dopamine activity plays in the observed behavioral patterns. Additionally, the relationship between concurrent marijuana and alcohol use and cognitive performance should continue to be explored in early-onset marijuana users to determine the unique and combined effects of the substances on performance.
Acknowledgements
This study was supported by grant R01DA017843 awarded to M. Luciana by the National Institute on Drug Abuse, by grant R01AA020033 awarded to M. Luciana by the National Institute on Alcohol Abuse and Alcoholism, and by the University of Minnesota's Center for Neurobehavioral Development. M. P. Becker was supported by the Pearson Assessment Fellowship in Clinical Psychology awarded by the Pearson Clinical Assessment Division. We thank Dr. Snežana Uroševicć for helpful comments on prior versions of the manuscript. We also thank Brittany Schmaling for her contribution to data collection.
Role of Funding Source: Nothing declared. Funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Note that the inclusion criterion of limiting alcohol use frequency to 4–5 drinks per occasion less than twice weekly still allows for the possibility of alcohol abuse symptoms
Disclosure
Conflict of Interest: No conflict declared by all authors.
Contributor Information
Mary P. Becker, Department of Psychology, Center for Neurobehavioral Development, University of Minnesota, 75 East River Road, Minneapolis, MN 55455
Paul F. Collins, Department of Psychology, Center for Neurobehavioral Development, University of Minnesota, 75 East River Road, Minneapolis, MN 55455
Monica Luciana, Department of Psychology, Center for Neurobehavioral Development, University of Minnesota, 75 East River Road, Minneapolis, MN 55455.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiology of Disease. 2010;37(3):641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? Journal of Psychopharmacology. 2010;24(2):241–246. doi: 10.1177/0269881109106909. [DOI] [PubMed] [Google Scholar]
- Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology (Berl) 2010;212(4):613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology, Biochemistry, and Behavior. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bloomfield MaP, Morgan CJA, Egerton A, Kapur S, Curran HV, Howes OD. Biological Psychiatry. Advance online publication; 2013. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Clark L, Roiser JP, Robbins TW, Sahakian BJ. Disrupted “reflection” impulsivity in cannabis users but not current or former ecstasy users. Journal of Psychopharmacology. 2009;23:14–22. doi: 10.1177/0269881108089587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence-based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine. 2011;5(1):1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft RJ, Mackay AJ, Mills ATD, Gruzelier JGH. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology (Berl) 2001;153(3):373–379. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- Cuttler C, McLaughlin RJ, Graf P. Mechanisms underlying the link between cannabis use and prospective memory. PloS one. 2012;7(5):e36820. doi: 10.1371/journal.pone.0036820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition. San Antonio: Texas; 2000. Psychological Corporation, Ed. [Google Scholar]
- Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, Acheson A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology (Berl) 2013;226:307–319. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142(3):295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. European Neuropsychopharmacology. 2008;18(11):826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Lacerda ALT. Cannabis use before age 15 and subsequent executive functioning. The British Journal of Psychiatry. 2011;198(6):442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW, Sahakian BJ. Neuorpsychiatyric applications of CANTAB. International Journal of Geriatric Psychiatry. 1996;11:329–336. [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiological Review. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana - A comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27(2):231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia. 1995;13(10):1305–1333. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. Journal of Clinical and Experimental Neuropsychology. 2012;34(9):962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug and Alcohol Dependence. 2012;121:159–162. doi: 10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012;26(3):496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155(2):171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35(11):970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, Yücel M. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. 2012;37(8):1923–1933. doi: 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26(3):309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Heng L, Beverley Ja, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65(4):278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley GA, Winters KC. Development of psychosocial scales for the assessment of adolescents involved with alcohol and drugs. International Journal of Addictions. 1989;24:973–1001. doi: 10.3109/10826088909047324. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Annals of the New York Academy of Sciences. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: A cross-sectional multicenter functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(6):561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Ramsey NF. Long-term consequences of adolescent cannabis exposure on the development of cognition, brain structure and function: An overview of animal and human research. Current Drug Abuse Reviews. 2008;1(2):114–123. doi: 10.2174/1874473710801020114. [DOI] [PubMed] [Google Scholar]
- Johnston LD, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2011: Volume II, College students and adults ages 19–50. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 2012 Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: A functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children - Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kowal MA, Colzato LS, Hommel B. Decreased spontaneous eye blink rates in chronic cannabis users: Evidence for striatal cannabinoid-dopamine interactions. PloS one. 2011;6(11):e26662. doi: 10.1371/journal.pone.0026662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafayette Instrument. Instruction manual for the 32025 Grooved Pegboard Test. Lafayette, IN: Author; 1989. [Google Scholar]
- Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cerebral Cortex. 2009;19(5):1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of Neuroscience. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Reeve J. Self-determined, but not non-self-determined, motivation predicts activations in the anterior insular cortex: An fMRI study of personal agency. Social Cognitive and Affective Neuroscience. 2013;8(5):538–545. doi: 10.1093/scan/nss029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Reeve J, Xue Y, Xiong J. Neural differences between intrinsic reasons for doing versus extrinsic reasons for doing: An fMRI study. Neuroscience Research. 2012;73(1):68–72. doi: 10.1016/j.neures.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. Comparing apples and oranges: Using reward-specific and reward-general subjective value representation in the brain. The Journal of Neuroscience. 2011;31(41):14693–14707. doi: 10.1523/JNEUROSCI.2218-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society. 2012;18(4):678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Lind J, Webster M, Weickert CS. Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neuroscience. 2012;13:87. doi: 10.1186/1471-2202-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, Oztekin I, Badre D. Separable prefrontal cortex contributions to free recall. The Journal of Neuroscience. 2010;30(33):10967–10976. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Collins PF. Dopaminergic modulation of working memory for spatial but not object cues in normal humans. Journal of Cognitive Neuroscience. 1997;9(3):330–347. doi: 10.1162/jocn.1997.9.3.330. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Depue RA. Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cerebral Cortex. 1998;8(3):218–226. doi: 10.1093/cercor/8.3.218. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Olson EA, Schissel AM. Tower of London performance in healthy adolescents: the development of planning skills and associations with self-reported inattention and impulsivity. Developmental Neuropsychology. 2009;34(4):461–475. doi: 10.1080/87565640902964540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76(3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Macher RB, Earleywine M. Enhancing neuropsychological performance in chronic cannabis users: The role of motivation. Journal of Clinical and Experimental Neuropsychology. 2012;34(4):405–415. doi: 10.1080/13803395.2011.646957. [DOI] [PubMed] [Google Scholar]
- McHale S, Hunt N. Executive function deficits in short-term abstinent cannabis users. Human Psychopharmacology. 2008;23(5):409–415. doi: 10.1002/hup.941. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13(5):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery C, Seddon AL, Fisk JE, Murphy PN, Jansari A. Cannabis-related deficits in real-world memory. Human Psychopharmacology. 2012;27:217–225. doi: 10.1002/hup.1273. [DOI] [PubMed] [Google Scholar]
- Morrison PD, Zois V, McKeown Da, Lee TD, Holt DW, Powell JF, Murray RM. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychological Medicine. 2009;39(10):1607–1616. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, Hegerl U. Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. International Journal of Psychophysiology. 2005;56(1):65–80. doi: 10.1016/j.ijpsycho.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Murayama K, Matsumoto M, Izuma K, Matsumoto K. Neural basis of the undermining effect of monetary reward on intrinsic motivation. Proceedings of the National Academy of Sciences. 2010;107(49):20911–20916. doi: 10.1073/pnas.1013305107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson Ea, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: A diffusion tensor imaging study. Journal of Cognitive Neuroscience. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21(4):478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Nierenberg AA. Long-term implications of early onset in bipolar disorder: Data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Biological Psychiatry. 2004;55(9):875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biological Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA: The Journal of the American Medical Association. 1996;275(7):521–527. [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31(10):2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacological Research. 2009;60(2):132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4(2):135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19(8):763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28(10):1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. European Neuropsychopharmacology. 2007;17(3):180–186. doi: 10.1016/j.euroneuro.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases of Children. 1989;143(10):1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010;42(3):401–412. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, Malhotra A. Emotion-based decision-making in healthy subjects: Short-term effects of reducing dopamine levels. Psychopharmacology (Berl) 2006;188(2):228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Jones Ka, Rozman ME, Davis SM, Ciarrochi J, Heaven PCL, Yücel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011;216(1):131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PCL, Yücel M. Reflection impulsivity in adolescent cannabis users: A comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology (Berl) 2012;219(2):575–586. doi: 10.1007/s00213-011-2486-y. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of Neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13–4795. Rockville, MD: 2013. pp. 1–84. [Google Scholar]
- Takagi M, Yücel M, Cotton SM, Baliz Y, Tucker A, Elkins K, Lubman DI. Verbal memory, learning, and executive functioning among adolescent inhalant and cannabis users. Journal of Studies on Alcohol and Drugs. 2011;72(1):96–105. doi: 10.15288/jsad.2011.72.96. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NBL, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Abi-Dargham A. Dopamine release in chronic cannabis users: A [11c]raclopride positron emission tomography study. Biological Psychiatry. 2012;71(8):677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros M-P, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behavioural Pharmacology. 2005;16:353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Wagner D, Becker B, Gouzoulis-Mayfrank E, Daumann J. Interactions between specific parameters of cannabis use and verbal memory. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(6):871–876. doi: 10.1016/j.pnpbp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Third Revision. San Antonio, Texas: The Psychological Corporation; 1997. [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76(1):107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Adamson JJ, Henin A, Sgambati S, Gignac M, Monuteaux MC. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: A controlled study. Drug and Alcohol Dependence. 2008;95(3):188–198. doi: 10.1016/j.drugalcdep.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Zalesky A, Takagi MJ, Bora E, Fornito A, Ditchfield M, Lubman DI. White-matter abnormalities in adolescents with long-term inhalant and cannabis use: A diffusion magnetic resonance imaging study. Journal of Psychiatry Neuroscience. 2010;35(6):409–412. doi: 10.1503/jpn.090177. [DOI] [PMC free article] [PubMed] [Google Scholar]