Abstract
TiO2/graphene (TiO2-x/GR) composites, which are Ti3+ self-doped TiO2 nanorods decorated on boron doped graphene sheets, were synthesized via a simple one-step hydrothermal method using low-cost NaBH4 as both a reducing agent and a boron dopant on graphene. The resulting TiO2 nanorods were about 200 nm in length with exposed (100) and (010) facets. The samples were characterized by X-ray diffraction (XRD), UV-visible diffuse reflectance spectroscopy, X-band electron paramagnetic resonance (EPR), X-ray photoelectron spectra (XPS), transmission electron microscope (TEM), Raman, and Fourier-transform infrared spectroscopy (FTIR). The XRD results suggest that the prepared samples have an anatase crystalline structure. All of the composites tested exhibited improved photocatalytic activities as measured by the degradation of methylene blue and phenol under visible light irradiation. This improvement was attributed to the synergistic effect of Ti3+ self-doping on TiO2 nanorods and boron doping on graphene.
Since Fujishima and Honda discovered the phenomenon of photocatalytic splitting of water on TiO2 electrodes in 19721,2,3, titanium dioxide (TiO2) has emerged as one of the most promising oxide semiconductors and has been employed in diverse applications including air and waste water purifiers, solar energy cells and sensors4,5. However, the wide band gap and fast recombination of the photoexcited electron-holes of TiO2 restrict its use in many practical applications. Therefore, TiO2 modification is necessary for improving the optical sensitivity and activity of TiO2 in the presence of visible light. Such modifications might include impurity ion doping, noble metal loading, and others6,7. Among these, impurity doping is an efficient technology for improving the response of TiO2 to visible light. However, impurity doping could result in crystal or thermal instability and increased carrier recombination centers8.
Owing to its relatively high surface area and special photoelectrochemical properties compared to powder catalysts, many studies on TiO2 nanorods have been previously reported. Jun et al.9 varied the ratio of a nonselective and a surface selective surfactant (trioctylphosphine oxide and lauric acid, respectively) in dioctyl ether to induce the transformation of TiO2 nanoparticles into nanorods dissolved in dioctyl ether. Additionally, Li et al.10 synthesized tetragonal faceted-nanorods of single-crystalline anatase TiO2 with a large percentage of higher-energy (100) facets. Generally, the previous nanorods were prepared in organic solvent, increasing the tediousness of operational processes and subsequently reducing the working efficiency. In addition, modifications such as doping and controlling the morphology, reduced the amount of TiO2 containing Ti3+ or an oxygen vacancy and has also been confirmed to exhibit high photocatalytic activity11. Our group has previously reported studies on Ti3+. For example, Xing et al.8,12 successfully synthesized Ti3+ self-doped TiO2 with either NaBH4 as the reducing agent or using a vacuum-activated procedure. Both samples exhibited high photo-degradation of organic pollutants. In spite of the research progress achieved on Ti3+ and vacancy, there are still some controversies concerning especially the theoretical research on this topic. Rusu et al.13 concluded that the photocatalytic activity of rutile increased by vacuum pretreatment through the production of a large amount of anion on the (110) faces. Nevertheless, Hoffmann et al.4 ascribed this phenomenon to the reinforced crystallinity achieved via high temperature activation. Meanwhile, Sato et al.14 found that heating the material to 500°C induced desorption of surface oxygen and produced many oxygen defects resulting in improved photo-oxidation capacity. On the contrary, Yu et al.15 attributed the high photocatalytic activity of TiO2 to the existence of Ti3+ surface states. This was due to the ability of TiO2 to capture photogenerated electrons prior to transferring the electrons to the O2 adsorbed on the active sites of surface Ti3+, thus reducing the recombination of photogenerated electrons and holes.
On the other hand, after the discovery of an atomic sheet of sp2-bonded carbon atoms by Geim et al.16,17 in 2004, graphene has attracted great interest from both theoretical and experimental scientists. Graphene nanosheets, as two-dimensional (2D) conductors and monolayers of carbon atoms arranged into honeycomb network formations, have attracted attention as a consequence of their unique properties such as elasticity, low density, excellent electrical conductivity, chemical stability and their large surface area18,19. Additionally, graphene can also potentially act as a support material, allowing semiconductor particles (such as TiO2 nanoparticles) to anchor themselves to the surface20. Because of this feature, the surface properties of graphene can be widely adjusted by chemical modifications to form composites7,21. Combining TiO2 and graphene into composites is a promising approach to facilitate the effective photodegradation of pollutants under visible light irradiation.
Recently, the fabrication of hybrid materials, such as TiO2 loaded onto graphene, has been a popular topic of study. Zhang et al.17 synthesized a chemically bonded TiO2 (P25)/graphene nanocomposite using a facile, one-step hydrothermal method, affording impressive methylene blue degradation activity. Choi et al.22 reported the fabrication of TiO2/GR nanocomposites via a facile electrostatic attraction method. Lambert et al.23 obtained TiO2/GR hybrid materials by mixing graphene oxide (GO) and TiF4 followed by ultrasonication and heating before reduction by hydrazine hydrate (HHA) and hydrothermal processing for heightened stability. All of the reported composite hybrids have superior photocatalytic activities compared to other TiO2 materials used for the degradation of dyes. Yet, many open problem remain; for example, this process usually gives rise to TiO2 aggregation while loading P25 onto GO24. While HHA has been widely used in the reduction of GO, it is recognized, however, as an environmental pollutant. Additionally, solvothermal treatment is selective for the epoxy group of GO, leaving the hydroxyl and carboxyl groups unreduced. To mediate these problems, there is strong demand for environmentally friendly reducing agents and novel reduction processes.
Additionally, some doping modifications of graphene in order to improve its electronic properties have attracted a great deal of attention. Tran Van Khai et al.25 prepared boron-doped graphene oxides by means of annealing the films at 1100°C. The modified GOs were obtained from suspensions of GO and H3BO3 in a solution of N, N-dimethylformamide (DMF). Similarly, Niu et al.26 prepared boron-doped graphene through pyrolysis of graphene oxide with H3BO3 in an argon atmosphere at 900°C. Each of these experiments adopted high-temperature processes, increasing the economic cost of these methods. Theoretical studies on graphene nanoribbons doped with boron have demonstrated that edge-type as well as substitutional doping can induce half-metallic behavior and that the band gap can be tuned by doping27, thus highlighting the potential application of boron-doped graphene (B-GR) in photocatalysis.
Here, we report the preparation of TiO2 nanorods in deionized water via a simple one-step hydrothermal method. First, we exposed nanorods with (100) and (010) facets of about 200 nm in length. Next, the composite, consisting of Ti3+ self-doped TiO2 nanorods were loaded onto the boron-doped graphene sheets. This was successfully achieved using NaBH4 as the reducing agent as well as the boron source. The photocatalytic activity of Ti3+-TiO2/B-graphene composites will also be discussed.
Results
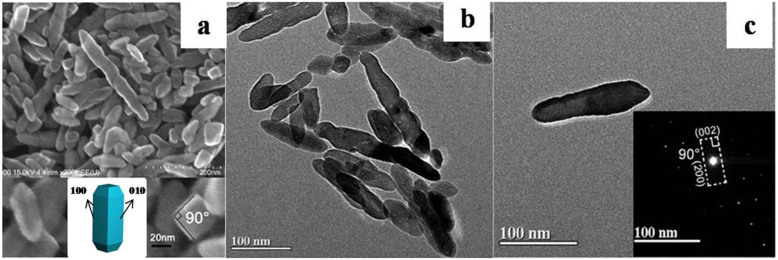
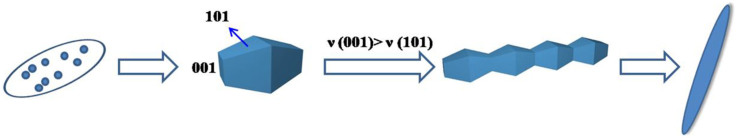
The FESEM and TEM images of TiO2 nanorods are presented in Figure 1. The prepared TiO2 nanoparticles are shaped like nanorods with lengths in the range of 50–200 nm. It is obvious from the cross-section of the FESEM image (Figure 1a) that the angle between two adjacent sides is 90°. For increased clarification, we set up a structural modeling image. From this image, it is obvious that the nanorod exists with the (100) and (010) facets exposed and at an angle of 90°, which is in agreement with the above result. To further characterize the exposure of the (100) facet, TEM (Figure 1b) and fast-Fourier transform (FFT) (Figure 1c) were performed. The axis direction of the nanorod is parallel to the (002) facet, as determined by FFT, confirming that the nanorod is extended along the (001) direction. Considering the observation of the (200) facet perpendicular to the (002) facet in the FFT image, it can be concluded that the prepared TiO2 nanorod exposes the (100) facet. Theoretical studies demonstrated that anatase (100) facets are more active and accordingly exhibit higher catalytic activity than (001) or (101) facets10. The mechanism of formation of TiO2 nanorods can be explained in the kinetic growth region9, shown in Figure 2. The structure of anatase TiO2 is tetragonal with the (101) and (001) facets exposed. The added ammonia results in the growth of the (001) facet, resulting in a change in growth velocity, namely, ν (001)> ν (101), ultimately resulting in the formation of nanorods.
Figure 1. (a) FESEM and (b) TEM images of TiO2 nanorods, and (c) the corresponding fast-Fourier transform (FFT).
The inset of (a) is the amplified image of TiO2 nanorods and a structural moduling image.
Figure 2. The formation mechanism of TiO2 nanorods.
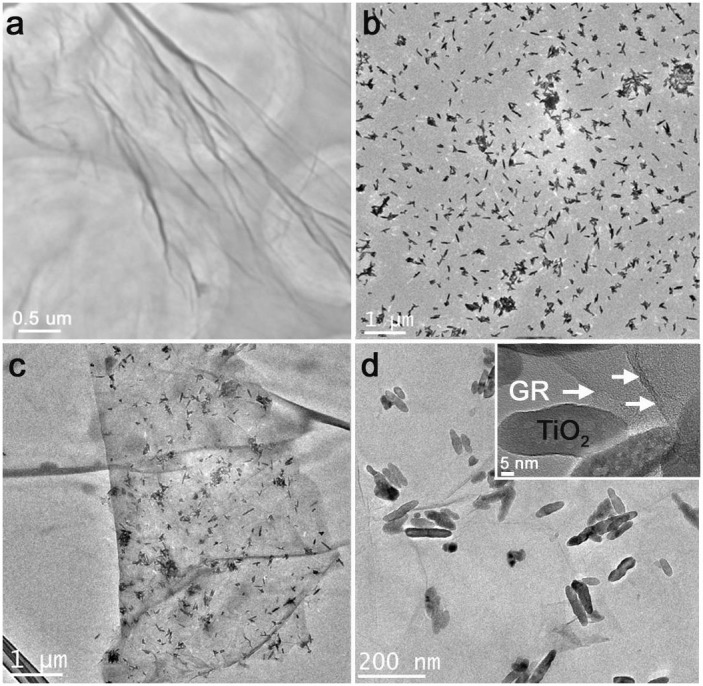
The loading of TiO2 nanorods on graphene sheets was characterized by TEM. Images of pure graphene and TiO2-x/GR composites are shown in Figure 3. Figure 3a demonstrates that the prepared sheet-like graphene oxide was a transparent, smooth, and 2D-layered material well suited for the addition of TiO2. We intended to load the TiO2 nanorods on the wrinkled or edged areas of the GO where carboxyl functional group are likely to be abundant14 (Figure 3b–d). Accordingly, the TiO2 nanoparticles were covalent bonded to GO, forming a composite favoring the separation of electron-hole pairs (Figure 3d).
Figure 3. TEM images of (a) graphene oxied, (b) pure TiO2 nanorods, and (c, d) 0.1-TiO2-x/GR composite.
To further characterize the composition of the as-prepared samples, we performed Raman spectroscopy (Supplementary Figure S1). The samples exhibited strong peaks at g = 1.978 and g = 1.959, characteristic of Ti3+28,29. The peak corresponding to surface Ti3+ is difficult to observe at room temperature due to its instability but it can be inferred that the signal arising from paramagnetic Ti3+ centers belongs to bulk Ti3+. Additionally, we observed no peaks indicative of surface Ti3+ (g = 2.02–2.03) further confirming the interaction between Ti3+ and O2 to form O2−30,31. Thus, it can be concluded that sufficient amounts of Ti3+ exist in the bulk under conditions using NaBH4 as the reducing agent during hydrothermal processing.
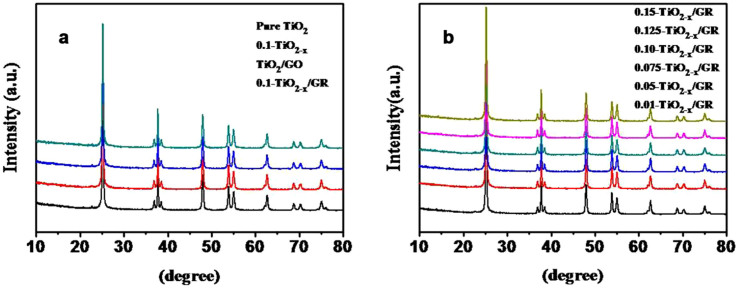
XRD patterns of TiO2-x/GR composites prepared using different amounts of NaBH4 are shown in Figure 4. Well-defined diffraction peaks of the anatase phase structure of TiO2 are clearly visible. Diffraction peaks are located at 25.3°, 37.8°, 48.0°, 53.9°, 54.9°, 62.9° and 68.8°, corresponding to the (101), (004), (200), (105), (201), (204) and (116) facets of anatase TiO2, respectively (JCPDS No. 21-1272). It can be observed for all composites that increasing amounts of NaBH4 do not alter the polymorph of TiO2. In all cases, the polymorph can be described as fine anatase crystallites, confirming that the graphene supports are not affecting the phase or structure of TiO2. Compared to pure TiO2 in Figure 4b, the crystallinity of samples prepared with NaBH4 is weakened. This is likely because a large amount of hydrogen gas was evolved during the reaction, resulting in the reduction of Ti4+ on the surface to Ti3+ and oxygen vacancies during the hydrothermal treatment. These defects inhibited the growth of TiO2 nanoparticles, decreasing the crystallinity.
Figure 4. XRD patterns of (a) n-TiO2-x/GR samples with adding different amount of NaBH4 and (b) 0.1-TiO2-x/GR and control blank samples.
The average crystal size and d-spacing of different samples were determined by XRD using the Scherrer equation as shown in Supplementary Table S1. It can be seen that Ti3+ self-doping does not change the phase, however, there is a slight increase in particle size after reduction. It has been reported that boron doping into the lattice tends to lead to lattice distortion32, suppressing crystal growth and thereby diminishing the particle size of the catalyst5. Therefore, it can be inferred that boron is not introduced into the TiO2 lattice here by using NaBH4 as the reducing agent. Additionally, “d” space values are similarly unchanged, implying that the doping modification does not change the dimensions of the average unit cell.
XPS techniques were adopted in order to detect the different chemical states present in TiO2/GO and the interaction between GO and TiO2. In the C1s core level spectrum (Figure 5), there are six main peaks corresponding to TiO2-x/GR composites7,33,34, including: (i) the C of the Ti-O-C corresponding to the interaction of TiO2 and graphene (283.1 eV); (ii) sp2 C bonds of the graphene skeleton (283.5 eV); (iii) adventitious carbon impurities adsorbed on the surface of sample (284.6 eV); (iv) the C of the C-OH bonds (285.9 eV); (v) the C of the epoxy group (C-O-C, 287.3 eV); and (vi) the C of the carboxyl group (O = C-OH, 288.3 eV). There are large changes in the low field peaks of C1s and the appearance of a new peak at 283.9 eV assigned to the sp2 B-C bond35. These results indicate that NaBH4 was introduced as a reducing agent as well as a boron dopant in the graphene.
Figure 5. XPS analysis.
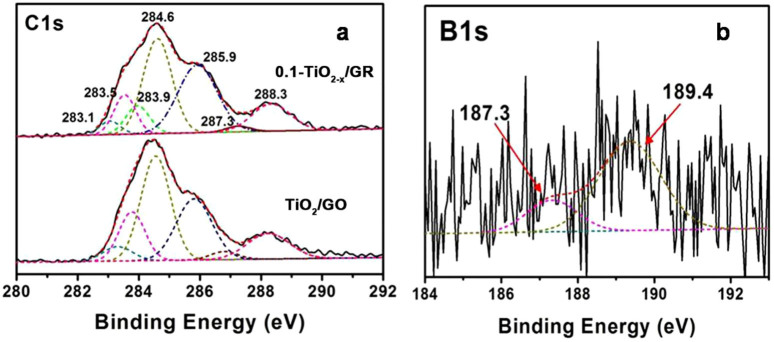
(a) C1s for 0.1-TiO2-x/GR and TiO2/GO, and (b) B1s for 0.1-TiO2-x/GR composites.
The results of the high resolution B1s XPS spectra of the 0.1-TiO2-x/GR composite are displayed in Figure 5b, further confirming that the boron has been doped into the lattice of graphene rather than into TiO2. The peak at 187.3 eV can be associated with a boron carbide such as C3B with boron atoms substituting carbon atoms in the graphene structure26. Additionally, there is another new peak at 189.4 eV attributed to C-B bonds resulting from boron supplanting hydroxyl groups on the edges of graphene. It is noteworthy that no peak corresponding to Ti-B bonds appears between 186.0–187.0 eV, demonstrating the absence of boron doping into TiO2. The above result is consistent with our previous work12.
In order to investigate the presence of Ti3+ in TiO2 after the addition of NaBH4, we performed room-temperature electron paramagnetic resonance (EPR) on NaBH4 reduced samples (see Supplementary Figure S2). Strong peaks were observed at g = 1.978 and g = 1.959, characteristic of Ti3+ 28,29. The peak of surface Ti3+ does not appear at room temperature because of its instability, therefore it can be inferred that the signal of the paramagnetic Ti3+ centers belongs to bulk Ti3+. In addition, there is no signal peak at g = 2.02–2.03 indicative of surface Ti3+, further confirming the interaction between Ti3+ and O2 to form O2−30,31. It can be concluded that a large amount of Ti3+ exists in the bulk when NaBH4 is used as the reducing agent during the hydrothermal process.
Supplementary Figure S3 represents the FTIR spectra of TiO2/GO and TiO2 before and after addition NaBH4. The peak at around 3400 cm−1 can be assigned to the vibration of the O-H groups of adsorbed water and Ti-OH groups on the catalyst surface36. The intensity of this band is obviously enhanced after the addition of NaBH4. The release of hydrogen from NaBH4 gives rise to oxygen defects on the TiO2 surface during the solvothermal process, helping absorb -OH and H2O and thus concentrating hydroxyl groups at the catalyst surface. We also observe peaks corresponding to carbon impurities including saturated and unsaturated C-H and C = O bonds in the range of 2300–3300 cm−1. These impurities likely result from solvents present on the sample surface arriving there during the solvothermal process12.
The band appearing at about 1600 cm−1 of the FTIR spectrum of GO and GR (Figure S3b) can be attributed to the skeletal vibration of the GR sheets33, confirming the reduction of GO to GR. By comparison, after reduction, no obvious signals characteristic of oxygen-containing functional groups such as C-O alkoxy, O = C-O carboxyl or -OH hydroxyl can be observed for GR. The peak in the range of 2500–3700 cm−1 is sharper and broader for GO compared to GR, likely resulting from residual unreduced -OH and adsorbed water molecules. The curve of GO shows two sharp absorption bands in the range of 1500–2000 cm−1 corresponding to the stretching vibration of C = O (1750 cm−1) and the bending vibration of O-H (1620 cm−1), respectively, but they are not obvious for GR. This indicates that hydrothermal treatment in the presence of NaBH4 can effectively result in the reduction of carboxyl and hydroxyl groups and thus the reduction of GO to GR.
The UV-visible diffuse reflectance spectra of pure TiO2, 0.1-TiO2-x, TiO2/GO and TiO2-x/GR composites with varying amounts of boron doping demonstrate that the absorption intensity of samples in the visible region modified with Ti3+ self-doping is clearly enhanced in comparison to that of pure TiO2 (Supplementary Figure S4). This result agrees with our previous work12 which also demonstrated that the conversion of Ti3+ into TiO2 using the vacuum-activated process or NaBH4 increased the absorption intensity in the visible region. It should be noted there is an obvious red shift to longer wavelengths in the UV-vis absorption spectra. Considering that band gap narrowing can allow more absorption of visible light and more efficient photogenerated electron transfer, the prepared TiO2-x/GR composites are expected to have enhanced photocatalytic performance under visible light irradiation.
Discussion
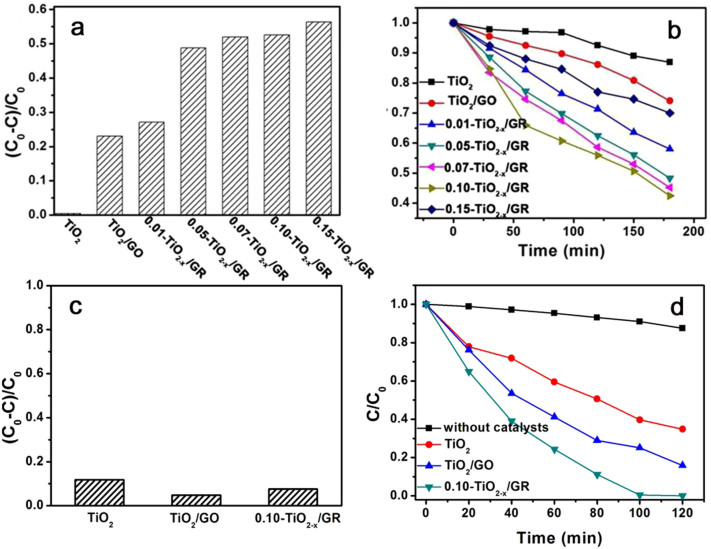
The photocatalytic activity of catalysts in the visible light specturm was investigated for the purpose of demonstrating potential applications. Figure 6a shows the concentration of methylene blue (MB) solution after reaching the adsorption-desorption equilibrium in the dark. Note that the catalyst containing graphene exhibited improved MB adsorption compared to pure TiO2. This is likely due to the large π-conjugation system and 2D planar structure of graphene34,36. Interestingly, with inceased amounts of NaBH4, the adsorption capacity of composites was enhanced accordingly. When boron was incorporated into the graphene lattice, the negative surface charge was increased, resulting in a different isoelectric point. This effect is likely to enhance the adsorption of cationic dye molecules. The photocatalytic activities of pure TiO2 and composites with different weight ratios of NaBH4 were explored by photodegration of 20 mg/L of MB under visible light irradiation (Figure 6b). The photocatalytic activity of TiO2 nanorods anchored to B-GR nanosheets was greater than pure TiO2. Because of the hydrothermal reduction, TiO2 interacted with the graphene surface –OH hydroxyl groups to form Ti-O-C bonds, ultimately resulting incovalently bound TiO2-x/GR composites17. Several reports found that MB is not appropriate as a model compound for testing visible light induced photocatalytic activity37,38. In order to fully understand the photocatlytic activity of Ti3+-TiO2 nanorods/B-graphene composite, the photo-degradation of colorless phenol was measured under simulated solar light irradiation (using an AM 1.5 air mass filter). The results are shown in Figure 6c,d. Unlike the adsorption of MB, the 0.10-TiO2-x/GR composite cannot enhance the adsorption of phenol in absense of light (Figure 6c). In addition to the conjugated structure, the surface charges may be another important factor affecting the adsorption of organic molecules on the GR. The phenol's absence of surface charges may explain the poor adsorption onto the surface of TiO2-x/GR composites. Recently, many efforts have been made towards the exploitation of TiO2-based photocatalysts under intense simulated solar light conditions for industrial purposes. Exmaples include black hydrogen-doped TiO239, yellow-vacuumed TiO240, and TiO2/graphene aerogels41. Here, the solar light photocatalytic activities of Ti3+-TiO2 nanorods/B-graphene composites are investigated to further confirm their photocatalytic performance (Figure 6d). The solar light photocatalytic activity of 0.10-TiO2-x/GR for the degradation of phenol is greater than other similar catalysts such as TiO2 and TiO2/GO. In fact, 0.10-TiO2-x/GR can completely decompose of phenol in 100 min under solar light irradiation; a far faster rate than other catalysts. The impurity level between TiO2 and GR narrows the band gap and is responsible for the enhanced photocatalytic activity of the Ti3+-TiO2 nanorods/B-graphene composites. Additionally, the oxygen vacancy and Ti3+ produced in the TiO2 bulk also decrease the bandgap of TiO2.
Figure 6. Photocatalytic activities of different catalysts.
(a) Adsorption changes in the dark and (b) photocatalytic degradation of MB under visible light of different catalysts. (c) Adsorption changes in the dark and (d) photocatalytic degradation of phenol under the simulated solar light (with an AM 1.5 air mass filter) of different catalysts.
However, graphene has excellent electron accepting and transporting properties and effectively allows for the transfer of photogenerated electrons from TiO2 to the graphene surface. Additionally, since boron atoms have three valence electrons26, boron-doped graphene, a kind of p-type semiconductor, could produce abundant photogenerated vacancies for the capture of more electrons and would exhibit clearly improved reduction effectiveness. It can be concluded that the photocatalytic activities of composites depend on the amount of NaBH4 added and that the dopant at 0.10 g is optimal. However, when a gross excess of NaBH4 was added, the surfaces of TiO2 and GR became covered with boron oxide, thus decreasing the number of available active sites on the surface12.
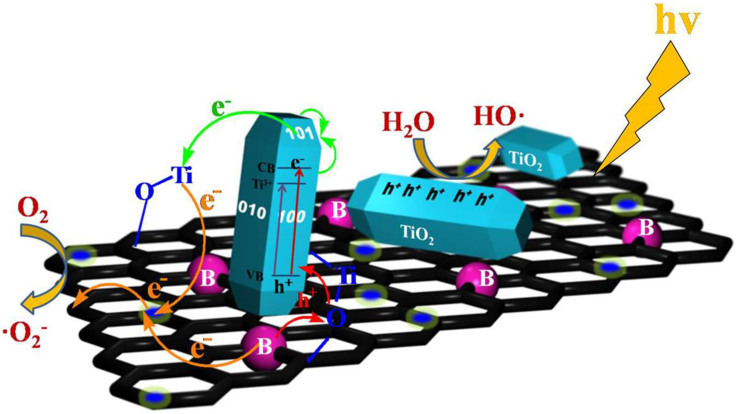
We also explored the mechanism of the photocatalytic activity described here. The impurities introduced by Ti3+ self-doping enabled TiO2 to respond to visible-light, as shown in Figure 7. As previoulsy mentioned, NaBH4 was used as a boron dopant on graphene and the unique p-type semiconductor properties of B-GR enhance hole transfer and effective charge separation. Upon solar-light irradiation, the composite exhibited a significant synergistic effect between Ti3+ doping on TiO2 and boron doping on graphene. That is, the photogenerated electrons were transfered from the valence band of TiO2 to the Ti3+ impurity level, narrowing the bandgap of TiO2. Also, given that the surface energy of the exposed (100) or (001) facet is relatively high compared to that of the (101) facet, the electrons have the tendency to transfer from (100) or (001) facet to the (101) facet. Finally, the electrons of the nanorod transfered to the graphene surface via the Ti-O-C bonds. Meanwhile, the incremental holes on the B-GR surface were transfered to the valence band, resulting in the effective separation of electron-hole pairs. As a result, electrons were left lying on the graphene sheet and holes on TiO2 surface. The electrons can be scavenged by O2, in turn producing the superoxide O2− while the positive holes can be trapped by OH− or H2O species to produce reactive hydroxyl radicals42. All of the reactive radicals are induced by the synergistic effects of Ti3+ doping on TiO2 and boron doping on graphene, resulting in powerful oxidizing agents for the degradation of dyes and phenols.
Figure 7. Structural model of energy states.
Schematic diagram of the charge transfer of TiO2-x/GR composite.
In summary, TiO2-x/GR composite photocatalysts consisting of Ti3+ self-doped TiO2 nanorods decorated on boron doped graphene sheets were successfully synthesized via a simple, one-step, hydrothermal method in which low-cost NaBH4 was introduced as a reducing agent while simultaneously affording boron as a dopant. The prepared TiO2 nanorods were about 200 nm in length with exposed (100) and (010) facets. The prepared catalysts were anatase crystallites with high photocatalytic activity under visible or solar light irradiation. The samples containing 0.10 g NaBH4 exhibited better MB adsorption and displayed the overall greatest efficiency in the degradation of MB and phenol. The high solar light-dependant activity was attributed to the synergistic effect between Ti3+ self-doped TiO2 and boron-doped graphene.
Methods
Preparation of graphene oxide (GO). GO was synthesized from natural graphite flakes by a modified Hummers method43,44. The synthesis method is as follows: Flake graphite (1 g) and NaNO3 (0.5 g) were added into cold (0°C) concentrated H2SO4 (23 mL) in a flask. 3 g of KMnO4 was slowly added to the flask under vigorous stirring and the temperature was kept below 20°C. The mixture was stirred at 35°C for 30 min and then diluted with de-ionized water (40 mL), causing a gradual increase in temperature to 98°C. The suspension was kept at 98°C for 15 min. Subsequently, 140 mL of deionized water and 10 mL of 30 wt% H2O2 solution were slowly added into the mixture, after which the suspension turned bright yellow and evolved bubbles. The mixture was filtered and washed several times with 5% HCl solution to remove residual salt and impurities45,46,47. The resulting solid was dried in vacuo at 60°C overnight and finally ground into powdered GO.
Preparation of TiO2 nanorods. 40 mL of deionized water was added to 2.0 g of titanium sulfate in a cylindrical vessel and stirred for 30 min before the slow addition of 20 mL of ammonia under vigorous stirring. Stirring was continued for 1 h. Then the cylindrical vessel was sealed in a Teflon-lined autoclave and hydrothermally treated at 180°C for 24 h. As the autoclave cooled to room temperature under ambient conditions, the resulting suspension was centrifuged before being washed with deionized water for five times. TiO2 nanorods were obtained by drying at 60°C in a vacuum oven.
In order to remove impurities, the TiO2 powder was calcinated at 500°C for 60 min with a heating rate of 2°C/min and the final sample was denoted as Pure TiO2.
Preparation of Ti3+ doped TiO2 nanorods/boron doped graphene composite photocatalyst. 0.03 g of GO was mixed with 70 mL of deionized water before ultrasonic dispersion for 1 h. Before adding specific and different amounts of NaBH4, 0.5 g of pure TiO2 was added and the suspension was stirred for 2 h. Subsequently, the mixture was hydrothermally treated at 150°C for 12 h. After it cooled to the room temperature, the precipitate was collected by centrifugation for 40 min before the addition of 50 mL hydrochloric acid (1 M), followed by stirring for an additional for 3 h. The HCl solution was used to remove the by-products of boron oxides12. The resulting solution was washed with deionized water five times and the solid was dried in vacuo at 60°C for 12 h. The final sample was denoted as n-TiO2-x/GR, where n is the weight of NaBH4, chosen as 0.01 g, 0.05 g, 0.075 g, 0.1 g, 0.125 g and 0.15 g.
For comparison, control samples were prepared in the absence of NaBH4 or GO according to the above procedure. These were denoted as TiO2/GO and 0.1-TiO2-x (“0.1” denoted the weight of NaBH4), respectively.
Characterization. X-ray diffraction (XRD) measurements were performed with a Rigaku Ultima IV (Cu Kα radiation, λ = 1.5406Å) in the range of 10–80° (2θ). The morphologies were characterized by transmission electron microscopy (TEM, JEM2000EX) and scanning electron microscopy (SEM, JEOL JSM-6360 LV). The instrument employed for X-ray photoelectron spectroscopy (XPS) studies was a Perkin-Elmer PHI 5000C ESCA system with Al Kα radiation. The shift of the binding energy was referenced to the C1s level at 284.6 eV as an internal standard. The X-band EPR spectra were recorded at room temperature (Varian E-112). The Fourier transform infrared (FTIR) spectra were recorded with KBr disks containing the powder sample with an FTIR spectrometer (Nicolet Magna 550). Raman spectra measurements were recorded with an inVia Reflex Raman spectrometer with 524.5 nm laser excitation. UV-vis diffuse reflectance spectra (DRS) were obtained with a SHIMADZU UV-2450 spectroscope equipped with an integrating sphere assembly and using BaSO4 as reflectance sample.
Photocatalytic Measurements. The visible light photocatalytic activity was measured by analyzing the degradation of methyl blue (MB) (20 mg/L). Solar light photocatalytic activity was measured by analyzing the degradation of phenol (10 mg/L). 0.06 g of prepared sample was added into a 100 mL quartz photoreactor containing 60 mL of MB/phenol solution. After ultrasonication for 1 min, the suspension was stirred in the dark for an hour to achieve adsorption-desorption equilibrium on the catalyst surface. A 500 W tungsten halogen lamp equipped with a UV cutoff filter (λ>420 nm) was used as a visible light source and the distance between the light and the reaction tube was fixed at 10 cm. The lamp was cooled with flowing water in a quartz cylindrical jacket around the lamp, and the ambient temperature was maintained during the photocatalytic reaction. A 300 W Xe lamp with an AM 1.5 air mass filter was used as a simulated solar light source. The mixture was stirred for 60 min in the dark in order to reach the adsorption–desorption equilibrium. At regular irradiation intervals, the dispersion was sampled (ca.5 mL), centrifuged, and subsequently filtered to remove the photocatalyst. The resulting solution was analyzed by checking the maximum absorbance of the residual MB/phenol solution with a UV-vis spectrophotometer (Varian Cary 100) at 660/270 nm.
Author Contributions
M.X. and J.Z. conceived and designed the experiments. M.X. and X.L. prepared the samples and performed characterization. M.X., X.L. and J.Z. were mainly responsible for preparing the manuscript. All the authors discussed the results and reviewed the manuscript.
Supplementary Material
Supplementary information
Acknowledgments
This work has been supported by the National Nature Science Foundation of China (21173077, 21377038, 21203062 and 21237003); the National Basic Research Program of China (973 Program, 2013CB632403), the Project of International Cooperation of the Ministry of Science and Technology of China (No. 2011DFA50530); Science and Technology Commission of Shanghai Municipality (12230705000, 12XD1402200); the Research Fund for the Doctoral Program of Higher Education(20120074130001) and the Fundamental Research Funds for the Central Universities.
References
- Fujishima A. & Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 238, 37–38 (1972). [DOI] [PubMed] [Google Scholar]
- Tryk D. A., Fujishima A. & Honda K. Recent topics in photoelectrochemistry: achievements and future prospects. Electrochim. Acta 45, 2363–2376 (2000). [Google Scholar]
- Chen X. & Mao S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007). [DOI] [PubMed] [Google Scholar]
- Hoffmann M. R., Martin S. T., Choi W. & Bahnemann D. W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 95, 69–96 (1995). [Google Scholar]
- Xing M., Wu Y., Zhang J. & Chen F. Effect of synergy on the visible light activity of B, N and Fe co-doped TiO2 for the degradation of MO. Nanoscale 2, 1233–1239 (2010). [DOI] [PubMed] [Google Scholar]
- Wu X.-F., Song H.-Y., Yoon J.-M., Yu Y.-T. & Chen Y.-F. Synthesis of Core−Shell Au@TiO2 Nanoparticles with Truncated Wedge-Shaped Morphology and Their Photocatalytic Properties. Langmuir 25, 6438–6447 (2009). [DOI] [PubMed] [Google Scholar]
- Khalid N. R., Ahmed E., Hong Z., Sana L. & Ahmed M. Enhanced photocatalytic activity of graphene–TiO2 composite under visible light irradiation. Curr. Appl. Phys. 13, 659–663 (2013). [Google Scholar]
- Xing M., Zhang J., Chen F. & Tian B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 47, 4947–4949 (2011). [DOI] [PubMed] [Google Scholar]
- Jun Y.-W. et al. Surfactant-assisted elimination of a high energy facet as a means of controlling the shapes of TiO2 nanocrystals. J. Am. Chem. Soc. 125, 15981–15985 (2003). [DOI] [PubMed] [Google Scholar]
- Li J. & Xu D. Tetragonal faceted-nanorods of anatase TiO2 single crystals with a large percentage of active {100} facets. Chem. Commun. 46, 2301–2303 (2010). [DOI] [PubMed] [Google Scholar]
- Sasikala R. et al. Highly dispersed phase of SnO2 on TiO2 nanoparticles synthesized by polyol-mediated route: Photocatalytic activity for hydrogen generation. Int. J. Hydrogen. Energ 34, 3621–3630 (2009). [Google Scholar]
- Xing M. et al. Self-doped Ti3+-enhanced TiO2 nanoparticles with a high-performance photocatalysis. J. Catal. 297, 236–243 (2013). [Google Scholar]
- Rusu C. N. & Yates J. T. Defect Sites on TiO2 (110). Detection by O2 Photodesorption. Langmuir 13, 4311–4316 (1997). [Google Scholar]
- Sato S. Photocatalytic activity of NOx-doped TiO2 in the visible light region. Chem. Phys. Lett. 123, 126–128 (1986). [Google Scholar]
- Yu J., Zhao X. & Zhao Q. Photocatalytic activity of nanometer TiO2 thin films prepared by the sol–gel method. Mater. Chem. Phys. 69, 25–29 (2001). [Google Scholar]
- Novoselov K. S. et al. Electric Field Effect in Atomically Thin Carbon Films. Science 306, 666–669 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang H., Lv X., Li Y., Wang Y. & Li J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 4, 380–386 (2009). [DOI] [PubMed] [Google Scholar]
- Hu H., Zhao Z., Wan W., Gogotsi Y. & Qiu J. Ultralight and Highly Compressible Graphene Aerogels. Adv. Mater. 25, 2219–2223 (2013). [DOI] [PubMed] [Google Scholar]
- Lee J. S., You K. H. & Park C. B. Highly Photoactive, Low Bandgap TiO2 Nanoparticles Wrapped by Graphene. Adv. Mater. 24, 1084–1088 (2012). [DOI] [PubMed] [Google Scholar]
- Williams G., Seger B. & Kamat P. V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2, 1487–1491 (2008). [DOI] [PubMed] [Google Scholar]
- Bekyarova E. et al. Chemical Modification of Epitaxial Graphene: Spontaneous Grafting of Aryl Groups. J. Am. Chem. Soc. 131, 1336–1337 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang W. L. & Choi H. J. Fast and facile fabrication of a graphene oxide/titania nanocomposite and its electro-responsive characteristics. Chem. Commun. 47, 12286–12288 (2011). [DOI] [PubMed] [Google Scholar]
- Lambert T. N. et al. Synthesis and Characterization of Titania−Graphene Nanocomposites. J. Phys. Chem. C 113, 19812–19823 (2009). [Google Scholar]
- Liu S., Sun H., Liu S. & Wang S. Graphene facilitated visible light photodegradation of methylene blue over titanium dioxide photocatalysts. Chem. Eng. J. 214, 298–303 (2013). [Google Scholar]
- Khai T. V. et al. Comparison study of structural and optical properties of boron-doped and undoped graphene oxide films. Chem. Eng. J. 211–212, 369–377 (2012). [Google Scholar]
- Niu L. et al. Pyrolytic synthesis of boron-doped graphene and its application as electrode material for supercapacitors. Electrochim. Acta 108, 666–673 (2013). [Google Scholar]
- Lv R. & Terrones M. Towards new graphene materials: doped graphene sheets and nanoribbons. Mater. Lett. 78, 209–218 (2012). [Google Scholar]
- Conesa J. & Soria J. Reversible titanium (3+) formation by hydrogen adsorption on M/anatase (TiO2) catalysts. J. Phys. Chem. 86, 1392–1395 (1982). [Google Scholar]
- Nakamura I. et al. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A: Chem. 161, 205–212 (2000). [Google Scholar]
- Heller A. Chemistry and Applications of Photocatalytic Oxidation of Thin Organic Films. Accounts. Chem. Res. 28, 503–508 (1995). [Google Scholar]
- Strunk J., Vining W. C. & Bell A. T. A Study of Oxygen Vacancy Formation and Annihilation in Submonolayer Coverages of TiO2 Dispersed on MCM-48. J. Phys. Chem. C 114, 16937–16945 (2010). [Google Scholar]
- Liu H. et al. A green and direct synthesis of graphene oxide encapsulated TiO2 core/shell structures with enhanced photoactivity. Chem. Eng. J. 230, 279–285 (2013). [Google Scholar]
- Ghasemi S. et al. Synthesis and characterization of TiO2–graphene nanocomposites modified with noble metals as a photocatalyst for degradation of pollutants. Appl. Catal. A: Gen. 462–463, 82–90 (2013). [Google Scholar]
- Zhang Y. & Pan C. TiO2/graphene composite from thermal reaction of graphene oxide and its photocatalytic activity in visible light. J. Mater. Sci. 46, 2622–2626 (2011). [Google Scholar]
- Yang X. et al. Wet-Chemistry-Assisted Nanotube-Substitution Reaction for High-Efficiency and Bulk-Quantity Synthesis of Boron- and Nitrogen-Codoped Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 133, 13216–13219 (2011). [DOI] [PubMed] [Google Scholar]
- Low W. & Boonamnuayvitaya V. A study of photocatalytic graphene–TiO2 synthesis via peroxo titanic acid refluxed sol. Mater. Res. Bull. 48, 2809–2816 (2013). [Google Scholar]
- Ohtani B. Preparing articles on photocatalysis: Beyond the illusions, misconceptions, and speculation. Chem. Lett. 37, 216–229 (2008). [Google Scholar]
- Yan X., Ohno T., Nishijima K., Abe R. & Ohtani B. Is methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 429, 606–610 (2006). [Google Scholar]
- Chen X., Liu L., Peter Y. Y. & Mao S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011). [DOI] [PubMed] [Google Scholar]
- Xia T., Zhang W., Murowchick J. B., Liu G. & Chen X. A Facile Method to Improve the Photocatalytic and Lithium-Ion Rechargeable Battery Performance of TiO2 Nanocrystals. Adv. Energy Mater. 3, 1516–1523 (2013). [Google Scholar]
- Qiu B., Xing M. & Zhang J. Mesoporous TiO2 Nanocrystals Grown In-Situ on Graphene Aerogels for High Photocatalysis and Lithium Ion Batteries. J. Am. Chem. Soc. 136, 5852–5855 (2014). [DOI] [PubMed] [Google Scholar]
- Liu S., Guo E. & Yin L. Tailored visible-light driven anatase TiO2 photocatalysts based on controllable metal ion doping and ordered mesoporous structure. J. Mater. Chem. 22, 5031–5041 (2012). [Google Scholar]
- Hummers W. S. Jr & Offeman R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958). [Google Scholar]
- Liang Y., Wu D., Feng X. & Müllen K. Dispersion of Graphene Sheets in Organic Solvent Supported by Ionic Interactions. Adv. Mater. 21, 1679–1683 (2009). [Google Scholar]
- Scott K. Freestanding sulfonated graphene oxide paper: a new polymer electrolyte for polymer electrolyte fuel cells. Chem. Commun. 48, 5584–5586 (2012). [DOI] [PubMed] [Google Scholar]
- Bo Z. et al. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan D. et al. Energetic graphene oxide: Challenges and opportunities. Nano Today 7, 137–152 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information