Abstract
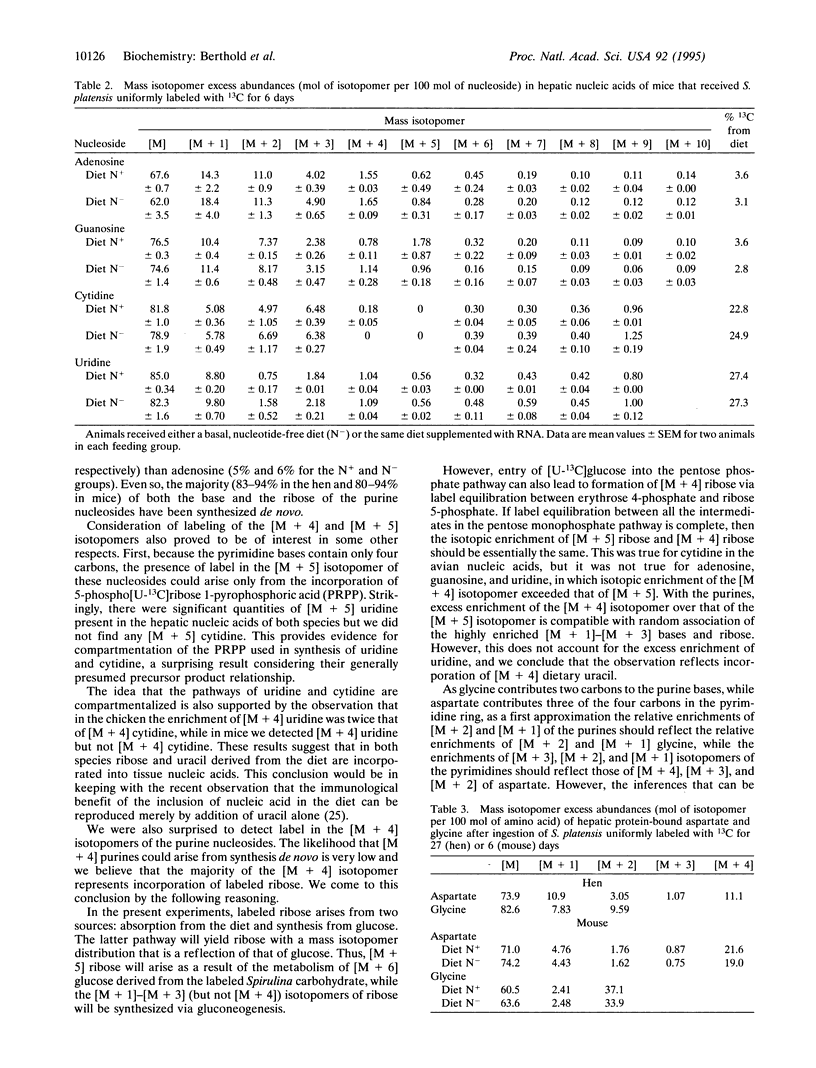
The absorption and metabolism of dietary nucleic acids have received less attention than those of other organic nutrients, largely because of methodological difficulties. We supplemented the rations of poultry and mice with the edible alga Spirulina platensis, which had been uniformly labeled with 13C by hydroponic culture in 13CO2. The rations were ingested by a hen for 4 wk and by four mice for 6 days; two mice were fed a normal diet and two were fed a nucleic acid-deficient diet. The animals were killed and nucleosides were isolated from hepatic RNA. The isotopic enrichment of all mass isotopomers of the nucleosides was analyzed by selected ion monitoring of the negative chemical ionization mass spectrum and the labeling pattern was deconvoluted by reference to the enrichment pattern of the tracer material. We found a distinct difference in the 13C enrichment pattern between pyrimidine and purine nucleosides; the isotopic enrichment of uniformly labeled [M + 9] isotopomers of pyrimidines exceeded that of purines [M + 10] by > 2 orders of magnitude in the avian nucleic acids and by 7- and 14-fold in the murine nucleic acids. The purines were more enriched in lower mass isotopomers, those less than [M + 3], than the pyrimidines. Our results suggest that large quantities of dietary pyrimidine nucleosides and almost no dietary purine nucleosides are incorporated into hepatic nucleic acids without hydrolytic removal of the ribose moiety. In addition, our results support a potential nutritional role for nucleosides and suggest that pyrimidines are conditionally essential organic nutrients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONO V. H., Jr, WEISSMAN S. M., FREI E., 3rd THE EFFECT OF 6-AZAURIDINE ADMINISTRATION ON DE NOVO PYRIMIDINE PRODUCTION IN CHRONIC MYELOGENOUS LEUKEMIA. J Clin Invest. 1964 Jul;43:1486–1494. doi: 10.1172/JCI105025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold H. K., Hachey D. L., Reeds P. J., Thomas O. P., Hoeksema S., Klein P. D. Uniformly 13C-labeled algal protein used to determine amino acid essentiality in vivo. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8091–8095. doi: 10.1073/pnas.88.18.8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciferri O. Spirulina, the edible microorganism. Microbiol Rev. 1983 Dec;47(4):551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément G., Giddey C., Menzi R. Amino acid composition and nutritive valve of the alga Spirulina maxima. J Sci Food Agric. 1967 Nov;18(11):497–501. doi: 10.1002/jsfa.2740181101. [DOI] [PubMed] [Google Scholar]
- Crain P. F. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol. 1990;193:782–790. doi: 10.1016/0076-6879(90)93450-y. [DOI] [PubMed] [Google Scholar]
- DEUTSCH A., NILSSON R. [On acid-soluble nucleotides of human milk]. Hoppe Seylers Z Physiol Chem. 1960 Dec 2;321:246–251. doi: 10.1515/bchm2.1960.321.1.246. [DOI] [PubMed] [Google Scholar]
- FUJII K., MIZOTA S., TAKAMA T., MIYAMOTO S., OZAKI T. High voltage paper electrophoresis of free and conjugated estrogens. J Biochem. 1962 Feb;51:167–168. doi: 10.1093/oxfordjournals.jbchem.a127514. [DOI] [PubMed] [Google Scholar]
- Gil A., Sanchez-Medina F. Acid-soluble nucleotides of human milk at different stages of lactation. J Dairy Res. 1982 May;49(2):301–307. doi: 10.1017/s0022029900022391. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. D., Fanslow W. C., Rudolph F. B., Van Buren C. T. Immunohemopoietic effects of dietary nucleotide restriction in mice. Transplantation. 1992 Feb;53(2):467–472. doi: 10.1097/00007890-199202010-00038. [DOI] [PubMed] [Google Scholar]
- Phillipson D. W., Edmonds C. G., Crain P. F., Smith D. L., Davis D. R., McCloskey J. A. Isolation and structure elucidation of an epoxide derivative of the hypermodified nucleoside queuosine from Escherichia coli transfer RNA. J Biol Chem. 1987 Mar 15;262(8):3462–3471. [PubMed] [Google Scholar]
- Rudolph F. B., Kulkarni A. D., Fanslow W. C., Pizzini R. P., Kumar S., Van Buren C. T. Role of RNA as a dietary source of pyrimidines and purines in immune function. Nutrition. 1990 Jan-Feb;6(1):45–62. [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J. Active transport of some pyrimidines across the rat intestinal epithelium. J Pharmacol Exp Ther. 1960 Feb;128:115–121. [PubMed] [Google Scholar]
- Scharrer E., Stubenhofer L., Tiemeyer W., Bindl C. Active pyrimidine absorption by chicken colon. Comp Biochem Physiol A Comp Physiol. 1984;77(1):85–88. doi: 10.1016/0300-9629(84)90016-1. [DOI] [PubMed] [Google Scholar]
- Smith L. H., Jr Pyrimidine metabolism in man. N Engl J Med. 1973 Apr 12;288(15):764–771. doi: 10.1056/NEJM197304122881505. [DOI] [PubMed] [Google Scholar]
- Sonoda T., Tatibana M. Metabolic fate of pyrimidines and purines in dietary nucleic acids ingested by mice. Biochim Biophys Acta. 1978 Nov 21;521(1):55–66. doi: 10.1016/0005-2787(78)90248-4. [DOI] [PubMed] [Google Scholar]
- Uauy R., Stringel G., Thomas R., Quan R. Effect of dietary nucleosides on growth and maturation of the developing gut in the rat. J Pediatr Gastroenterol Nutr. 1990 May;10(4):497–503. doi: 10.1097/00005176-199005000-00014. [DOI] [PubMed] [Google Scholar]



