Abstract
Newly formed microvessels in most solid tumors show an abnormal morphology and thus do not fulfil the metabolic demands of the growing tumor mass. Due to the chaotic and heterogeneous tumor microcirculation, a hostile tumor microenvironment develops, that is characterized inter alia by local hypoxia, which in turn can stimulate the HIF-system. The latter can lead to tumor progression and may be involved in hypoxia-mediated radioresistance of tumor cells. Herein, cellular and molecular mechanisms in tumor angiogenesis are discussed that, among others, might impact hypoxia-related radioresistance.
Keywords: tumor angiogenesis, tumor microcirculation, tumor hypoxia, hypoxia-inducible factor (HIF), radioresistance, hypoxia modifiers
1. Introduction
Endothelial cells build up the first “barrier” between the blood, interstitial space (and stroma) and parenchymal cells. A dense network of blood vessels is necessary to provide an adequate supply of oxygen and nutrients, and efficient drainage of waste products. Although the turnover rate of endothelial cells is generally slow in adult organs, endothelial cell growth can be induced under (patho-)physiological conditions like wound healing, menstrual cycle or placenta formation. As shown for normal tissues also growth of solid tumors depends on blood vessels. Vasculogenesis, arteriogenesis and angiogenesis are three major principles to build up new vessels. Vasculogenesis is a process that involves undifferentiated progenitor cells in order to form a vascular network. Vasculogenesis is required for the de novo formation of a vascular network in embryogenesis and growth [1]. In contrast to vasculogenesis, arteriogenesis refers to the remodelling of pre-existing arterioles to form arteries upon, e.g., increased shear stress. Arteriogenesis is based on chemokine/growth factor-induced growth processes and enlargement of vascular wall structures at larger shear stress that is induced by increased blood flow rates in arteries [2]. During angiogenesis vessels are formed from the existing microvasculature. The mechanism of angiogenesis involves either sprouting from pre-existing vessels or splitting through intussusception [3,4]. Apart from the female reproductive organs, during pregnancy and in wound healing [5,6], the vasculature rarely forms new branches in adults. However, endothelial cells retain their plasticity to sense and to respond to angiogenic signals during their whole life-time. In general, angiogenesis is tightly regulated by a fine balance of activating and inhibiting signals. Cytokines, hormones, circulating progenitor cells, whose role is not completely understood, endothelial cell migration and destabilization of the vessel wall, the basal lamina, and the interstitial matrix can impact on angiogenesis. Apart from physiological parameters, microenvironmental factors such as hypoxia and nutrient deficiencies can also trigger the angiogenic switch. Angiogenesis is also a crucial player in the pathogenesis of autoreactive diseases such as age-related macular degeneration, rheumatoid diseases, inflammation, arteriosclerosis, vascular restenosis and different vasculopathies. A close link of inflammation and angiogenesis is indicated by hallmark factors of acute and chronic inflammation such as VEGF-A and angiopoietins [7].
2. Vessel Formation in Malignant Tumors
Tumor angiogenesis involves the production and release of growth factors, permeability regulating factors, migration stimulating factors, proteolytic enzymes, extracellular matrix and adhesion molecules. These factors can be released either by tumor, stromal and/or inflammatory cells that are located within or in close proximity to the tumor. Growth factors of tumor angiogenesis can either involve specific vascular endothelium factors (i.e., vascular endothelial growth factor (VEGF), angiopoietin and ephrin family members), or non-specific factors (i.e., platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF) or tumor necrosis factor-α (TNF-α)) [8]. In principle, the progression of tumor growth is critically dependent on oxygen and nutrient supply and the drainage of metabolites [9], since diffusion without the involvement of blood vessels allows transport processes only over very short distances of less than 500 µm.
The physiology of tumors is different from that of normal tissues. It is characterized inter alia by O2 depletion (hypoxia or anoxia), extracellular acidosis, high lactate and adenosine levels, glucose and bicarbonate deprivation, energy impoverishment, significant interstitial fluid flow, interstitial hypertension, and other adverse conditions characterizing the metabolic tumor microenvironment [10,11,12,13,14]. This hostile microenvironment is largely determined by an abnormal tumor microcirculation. When considering the continuous and indiscriminate formation of a vascular network in growing tumors, the following pathogenetic mechanisms can be involved either alone or in combination:
-
(a)
Angiogenesis by endothelial sprouting from pre-existing venules [15,16].
-
(b)
Co-option of existing vessels [17].
-
(c)
Vasculogenesis (de novo vessel formation) through incorporation of circulating endothelial precursor cells [17].
-
(d)
Intussusception (splitting of the lumen of a vessel into two).
-
(e)
Formation of pseudo-vascular channels lined by tumor cells rather than endothelial cells (“vascular mimicry”).
-
(f)
Microvessel formation by a subset of bone marrow-derived myeloid cells infiltrating the tumor [17].
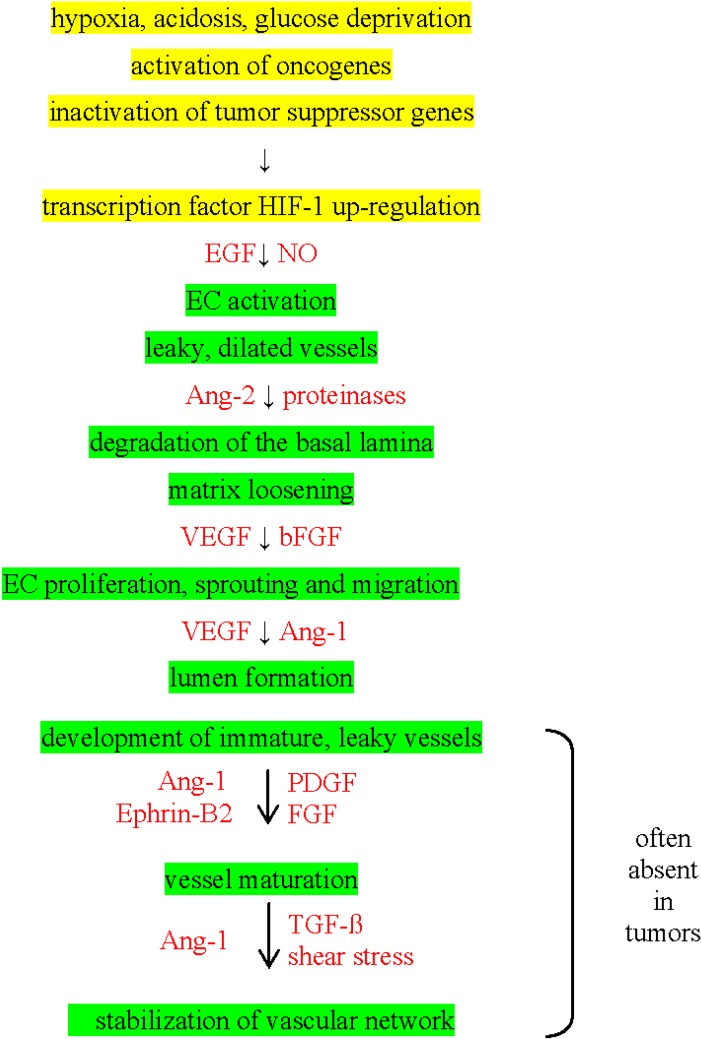
Despite these various possibilities for the formation of tumor microvessels, the tumor vasculature often lacks the signals to mature and therefore, tumor vasculature is also termed “aberrant monster” [18]. Tumor vessels are characterized by vigorous proliferation which leads to immature, structurally defective and, in terms of perfusion, ineffective microvessels (Figure 1). Consequently, tumor blood flow is chaotic and heterogeneous, the vascular supply and the metabolic microenvironment are inadequate and hostile. However, due to the spatio-temporal heterogeneity of pro-angiogenic signals, not all vessels are totally immature in clinical cancers. Some of them actually retain contractile properties [19].
Figure 1.
“Chronological” steps in tumor angiogenesis (marked in green). Causative mechanisms are marked in yellow and pro-angiogenic factors are marked in red.
3. Angiogenic Switch in Tumors
Small tumors can stay dormant until the so-called angiogenic switch occurs. Neovascularization is driven by pro-angiogenic factors that facilitate the formation of new microvessels from pre-existing blood vessels. This angiogenic switch by which an avascular tumor nodule is converted into a fast growing, vascularized and aggressive tumor is important in tumor growth and dissemination [20].
The angiogenetic switch is regulated by various pro-angiogenic factors such as VEGF, IL-8, bFGF, EGF, PDGF, MMP-2/-9, uPA, Notch-1/-4, osteopontin, and angiogenin and anti-angiogenic factors such as angiostatin, thrombospondin, IFN-γ, IL-1/-4/-12/-18/-21. Among them, members of the VEGF family (VEGF-A, B, C, D) have been identified as the dominant players in tumorigenesis. VEGF-C has been shown to activate the VEGFR-3 and Notch signalling pathways [21]. Under normoxic conditions, VEGF is mainly regulated by hypoxia-inducible factor 1 (HIF-1) that can be activated by pro-inflammatory cytokines such as IL-1 and TNF via PI3K and NF-κB [22,23], or indirectly by IL-6 and PGE2 in an autocrine manner [24,25]. In human tumors the VEGF expression is frequently upregulated [26]. High VEGF levels promote the production of abnormal vessels as mentioned before [27] and binding of VEGF to its corresponding receptor leads to the activation of the PI3K/Akt/mTOR and Ras/Raf/MAPK pathways that in turn promote not only angiogenesis but also proliferation, differentiation and survival of tumor cells [28]. Human osteosarcoma and pancreatic adenocarcinoma cells have been found to spontaneously release high amounts of VEGF, MMP-2, and IL-8 that enhance the invasiveness of tumors [29,30,31]. Via an auto-regulatory loop, the pro-inflammatory cytokine IL-1 further up-regulates the secretion of the pro-angiogenic factor IL-8 and thus supports the growth of tumors [30]. In SW1353 chondrosarcoma cells IL-1 induces a massive release of the pro-angiogenic factors MMP-1 and MMP-13. These findings highlight the crucial impact of inflammatory mediators in tumor angiogenesis [32]. Epidermal growth factor receptor (EGFR) that is also frequently overexpressed in tumors is associated with poor prognosis, high resistance to radiochemotherapy and increased metastatic spread [32]. EGFR predominantly induces the Ras/Raf/MAPK and the PI3K/Akt pathway that are both responsible for anti-apoptotic and pro-survival signals. Pro-angiogenic factors orchestrating the complex angiogenesis in solid tumors also include metabolites/catabolites such as lactate, 3-hydroxybutyrate, succinate and fumarate [33]. The role of irradiation in angiogenesis is still a matter of debate. Whereas some groups speculate that radiation can induce angiogenesis others report on a repression of angiogenesis by ionizing irradiation [34,35,36,37].
4. Tumor Microcirculation
As already mentioned, newly formed microvessels in most solid tumors do not conform to the normal morphology of the host tissue vasculature. The tumor vasculature can be described as a system that is maximally stimulated, yet only minimally fulfils the metabolic demands of the growing tumor that it supplies.
Microvessels in solid tumors exhibit a large series of severe structural and functional abnormalities. They are often dilated, tortuous, elongated, and saccular. It is of note that not only the quantity of microvessels counts, but also—or even more so—the quality of vascular function in terms of the tumor tissue supply or drainage [37,38]. There is significant arterio-venous shunt perfusion accompanied by a chaotic vascular organization that lacks any regulation matched to the metabolic demands or functional status of the tissue [11]. Excessive branching is a common finding, often coinciding with blind vascular endings. Incomplete or even missing endothelial lining and interrupted basement membranes result in an increased vascular permeability with extravasation of blood plasma and/or red blood cells expanding the interstitial fluid space and drastically increasing the hydrostatic pressure in the tumor interstitium (interstitial fluid pressure).
In solid tumors, there is a rise in viscous resistance to flow mainly caused by the hemoconcentration (hematocrit increase ranging from 5 to 14%) [39,40]. Aberrant vascular morphology and a decrease in vessel density are responsible for an increase in geometric resistance to flow, which can lead to an inadequate perfusion. Substantial spatial heterogeneity in the distribution of tumor vessels and significant temporal heterogeneity in the microcirculation within a tumor [41,42,43,44] may result in a considerably anisotropic distribution of tumor tissue oxygenation and a number of other factors, which are usually closely linked and which define the so-called pathophysiological microenvironment. Variations in these relevant parameters in different tumors are often more pronounced than differences occurring between different locations or microareas within a tumor [12,45,46].
5. Tumor Blood Flow Rates
A number of studies on blood flow through human tumors have been reported. Some of them are anecdotal reports rather than systematic investigations, and therefore, definite conclusions cannot be drawn partly due to the use of non-validated techniques to measure flow in volume flow rate units. Considering the presently available data, the following conclusions can be drawn when flow data derived from different reports are pooled (for reviews see [11,14,17,47]):
-
(a)
Blood flow can vary considerably despite similar histological classification and primary site (0.01–2.9 mL/g/min; [17,48,49].
-
(b)
Tumors can have flow rates which are similar to those measured in organs with a high metabolic rate such as liver, heart or brain.
-
(c)
Some tumors exhibit flow rates which are even lower than those of tissues with a low metabolic rate such as skin, resting muscle or adipose tissue.
-
(d)
Blood flow in human tumors can be higher or lower than that of the tissue of origin, depending on the functional state of the latter tissue (e.g., average blood flow in breast cancers is substantially higher than that of postmenopausal breast and significantly lower than flow data obtained in the lactating, parenchymal breast).
-
(e)
The average perfusion rate of carcinomas does not deviate substantially from that of tissue sarcomas.
-
(f)
Metastatic lesions exhibit a blood supply which is comparable to that of the primary tumor [11].
-
(g)
In some tumor entities, blood flow in the periphery is distinctly higher than in the center whereas in others, blood flow is significantly higher at the tumor center compared to the tumor edge.
-
(h)
Flow data from multiple sites of measurement show marked heterogeneity within individual tumors. In cervical cancer, the intra-tumor heterogeneity was similar to the inter-tumor heterogeneity [50].
-
(i)
There is substantial temporal flow heterogeneity on a microscopic level within human tumors as shown by multichannel laser Doppler flowmetry [51,52].
-
(j)
There is no association between tumor size and blood flow in many cancers [48,53].
-
(k)
Tumor blood flow is not regulated according to the metabolic demand as is the case in normal tissues.
With regard to the efficacy of radiotherapy the effectiveness of blood flow greatly influences the oxygen supply of tumors. Therefore, the responsiveness of solid tumors to radiotherapy (and chemotherapy) profoundly depends on blood perfusion [54].
6. Arterio-Venous Shunt Perfusion in Tumors
First rough estimations concerning the arterio-venous shunt flow in malignant tumors showed that at least 30% of the arterial blood can pass through experimental tumors without participating in the microcirculatory exchange processes [55,56,57]. In patients receiving intra-arterial chemotherapy for head and neck cancer, shunt flow is reported to be 8% to 43% of total tumor blood flow, the latter consistently exceeding normal tissue perfusion on the scalp [58]. The mean fractional shunt perfusion of tumors was 23% ± 13% in studies utilizing 99mTc-labeled micro-aggregated albumin (diameter of the particles, 15–90 µm). The significance of this shunt flow on local, intra-tumoral pharmacokinetics, on the development of hypoxia, and on other relevant metabolic phenomena has not yet been systematically studied and remains speculative.
High amounts of shunt flow through solid tumors not only impact on pharmacokinetics of anti-cancer agents, but also limit the effectiveness of radiotherapy due to the development of diffusion-limited, chronic hypoxia [44].
7. Tumor Hypoxia and HIF
Aberrant microcirculation is a major causative factor for the development of hypoxia in solid tumors [59]. Hypoxia is strongly associated with radio-resistance of malignant tumors, tumor recurrence after radiation therapy, and poor prognosis in patients subjected to radiation therapy [50,60]. On the one hand, free radicals that are produced by radiation, either directly or indirectly from an interaction with other molecules such as water, can react with H+ in the absence of oxygen and thus the target can be chemically restored to its original form. On the other hand, hypoxia can stimulate the HIF system which in turn can lead to tumor progression. It is hypothesized that the heterodimeric transcription factor HIF-1 is also involved in hypoxia-mediated radioresistance of tumor cells [61,62]. However, in vitro data from our group indicate that high HIF-1α levels in lung cancer cell lines are not associated with radioresistance [63].
Apart from its role in the development of radioresistance, HIF-1α is crucially involved in tumor angiogenesis, invasion, survival, and growth [64]. Harada et al. have demonstrated that irradiation causes an up-regulation of intra-tumoral HIF-1α protein and activity in regions of radiation-induced re-oxygenation of the solid tumor via the PI3K/Akt/mTOR pathway which is responsible for synthesis, stabilization and accumulation of HIF-1α, the oxygen-regulated subunit of HIF-1 [62]. From these results it can be concluded that Akt/mTOR-dependent translation of HIF-1α plays a critical role in the post-irradiation up-regulation of intra-tumoral HIF-1 activity in response to radiation-induced alterations of oxygen availability in solid tumors. Stability of HIF-1α can also be regulated in an oxygen-independent manner by RACK-1 (receptor of activated protein kinase C) through competition with Hsp90 and recruitment of the elongin-C/B ubiquitin ligase complex [65]. As stated by Yoshimura et al., the transactivational activity of HIF-1 is critically regulated by the MAPK/ERK pathway [66]. HIF-1 transactivational activity was found as being suppressed by FIH-1 (factor inhibiting HIF-1) under normoxic conditions via HIF-1α hydroxylation and concomitant blockage of adapter molecule binding [67]. Furthermore, the irradiation-induced HIF-1 activation can also depend on the availability of nitric oxide [68].
8. Therapeutic Interventions to Overcome Hypoxia-Related Radioresistance
Since hypoxia is known to protect tumor cells from standard radiation therapy, several strategies have been developed to interfere with hypoxia-related radioresistance of solid tumors [66]. Hyperbaric oxygen therapy, carbogen, nicotinamide and other “flow modifiers” as well as modification of the hemoglobin-O2 affinity have been tested to facilitate oxygen delivery to hypoxic regions. Nitroimidazole derivatives such as misonidazole and nimorazole have been used to sensitize tumors to radiation by mimicking the effect of oxygen. Hypoxic cytotoxins (tirapazamine and analogues) are meant to directly kill tumor cells by hydroxyl radicals or oxidizing radicals. Combined treatment strategies consisting of tirapazamine analogues and HIF-1 inhibitors, such as YC-1, have been tested to increase the radio-responsiveness of tumors [69,70,71,72,73]. However, due to the chaotic vascularization in most solid tumors none of these approaches could significantly improve the sensitivity towards ionizing irradiation.
Anti-angiogenesis is another approach that may affect the radiosensitivity of tumors. The key angiogenic factor VEGF is facilitating tumor growth and survival, and therefore most anti-angiogenic strategies aim to interrupt the VEGF pathway. This idea has led to the development of several anti-VEGF reagents including anti-VEGF antibody bevacizumab, anti-VEGF receptor antibody ramucirumab and VEGF antagonist aflibercept. Despite some promising results in clinical trials, the blockade of VEGF signalling also exerts adverse effects such as resistance to VEGF inhibitors as well as hemorrhagic and thrombotic events due to the damage of healthy vessels [71]. In molecular biology a small molecule is defined as a reagent with a low molecular weight of approximately less than 900 Da. These molecules harbour the capacity to rapidly diffuse across cell membranes and thus can enter cells. Small molecule drugs in pharmacology frequently serve as signalling molecules. A wealth of evidence indicates that small-molecule tyrosine kinase inhibitors such as axitinib, brivanib, cediranib, imatinib, motesanib, pazopanib, sorafenib, sunitinib as well as vatalanib and vandetanib harbor promising activity and safety in certain cancer subtypes (for reviews see [66,74,75]).
Attempts to target the tumor microenvironment in order to improve the effects of radiotherapy also comprise the endogenous angiogenesis inhibitors angiostatin [76] and endostatin [77,78,79,80]. Preclinical results of Ke et al. demonstrated that the recombinant human endostatin, endostar, can increase the radiation sensitivity of nasopharyngeal carcinomas in a nude mouse model by lowering the VEGF expression [79]. Interestingly, in patients with advanced cervical cancer the combination of endostar with standard chemoradiotherapy was found to improve the early therapy outcome with acceptable adverse effects [79]. Due to the small sample size and the relatively short follow-up period further investigations are needed with respect to long-term effects.
Despite its history as a human teratogen, thalidomide was tested as a putative drug to disrupt tumor angiogenesis. Although thalidomide monotherapy in patients with therapy-resistant uterine carcinomas prolonged the progression-free survival in a phase II trial [81], a phase III trial did not reveal any survival benefit for patients with brain metastases that have been treated with thalidomide in combination with radiotherapy compared to radiotherapy alone [82]. A meta-analysis of eight randomized trials with 2,317 patients with brain tumors confirmed this observation. Whole brain radiotherapy (WBRT) combined with the potential “radiosensitizer” thalidomide did not significantly improve the overall survival, local control and tumor response compared to WBRT alone [83].
Novel approaches in enhancing tumor radiosensitivity include inhibitors of distinct molecular pathways and key signalling factors such as Ras/Raf/MAPK, PI3K/Akt/mTOR (rapalogs, NVP-BEZ235, NVP-BGT226), c-Kit (imatinib, amuvatinib—also known as MP470), EGFR (cetuximab, erlotinib, sunitinib), PDGFR (sunitinib), and Hsp90 (NVP-AUY922). Cetuximab plus radiotherapy significantly improved the 5-year overall survival compared to radiotherapy alone in patients with locoregionally advanced head and neck tumors [84]. Preclinical studies with the multi-tyrosine kinase inhibitor eunitinib indicate that this drug enhances the radiosensitivity of human prostate cancer [85].
Targeting tumor cells with the EGFR inhibitor erlotinib followed by radiation delayed tumor re-growth to a greater extent than radiation alone [85]. The increase in radiosensitivity by erlotinib was accompanied by a down-regulation of HIF-1 and VEGF, decreased vascular permeability, an increase in tumor blood flow, and a decrease in hypoxia. In a phase I trial, the safety and tolerability of therapy with the mTOR inhibitor everolimus in combination with radiation and temozolomide (TMZ) was evaluated in patients with newly diagnosed glioblastoma multiforme (GBM) [86]. As demonstrated in this study, the combination of everolimus with a standard chemoradiotherapy in patients with GBM was reasonably well tolerated. Moreover, early FDG-PET imaging one week after an everolimus monotherapy revealed a partial metabolic response in a subset of the patients. The efficacy of adding the anti-VEGF antibody bevacizumab and everolimus to standard radiation therapy plus TMZ in the first-line therapy of patients with glioblastoma has been shown to be feasible and safe [87]. The progression-free survival was improved compared to standard radiation therapy plus TMZ. These data are in line with results achieved in other phase II trials in which bevacizumab was used as a fist-line therapy [87]. At present, phase III clinical trials are ongoing to clarify the role of bevacizumab in glioblastoma patients.
A novel approach to radiosensitize tumors is the use of the Hsp90 inhibitor NVP-AUY922. This compound was found to radiosensitize cervical, colorectal and head and neck squamous cell carcinoma (HNSCC) cell lines with a greater potency than any other tested Hsp90 inhibitor in vitro and in vivo [88]. Moreover, NVP-AUY922 in combination with radiotherapy resulted in a delayed growth of human prostate cancer cells in a mouse model in a supra-additive manner [89]. This effect is due to an oxygen-independent degradation of HIF-1α [65]. These data indicate that the interference of signalling pathways related to hypoxia might improve the radiosensitivity of tumors.
9. Conclusions
As summarized in this review, the dynamic and complex tumor microenvironment is largely determined by an aberrant tumor microcirculation characterized, among others, by hypoxia leading to radioresistance of malignant tumors and promoting tumor progression via stimulation of HIF-1. However, more detailed information is needed to characterize the dynamic aspects of tumor hypoxia during treatment. A better understanding of signalling pathways related to hypoxia in the tumor microenvironment should help to develop clinical approaches that address radioresistant hypoxic tumors.
Acknowledgment
This work was supported by the HelmholtzZentrum münchen (Clinical Cooperation Group—“Innate Immunity in Tumor Biology”), Deutsche Forschungsgemeinschaft (SFB 824/2 subproject B4; Cluster of Excellence: Munich-Centre for Advanced Photonics (MAP); INST 95/980-1 FUGG, Gulmay irradiation device), BMBF (Kompetenzverbund Strahlenforschung, 03NUK007E; Leading Edge Cluster m4—Personalized Medicine and Targeted Therapies, 01EX1021C).
Abbreviations
- Akt
protein kinase B (PKB)
- Ang
Angiopoietin
- EC
endothelial cell
- EGF
epidermal growth factor
- EGFR
EGF receptor
- bFGF
basic fibroblast growth factor
- ERK
extracellular signal-regulated kinase
- FIH-1
factor inhibiting HIF-1
- FDG PET
fluorine-18-fluorodeoxyglucose positron emission tomography
- HIF
hypoxia-inducible factor
- HSP
heat shock protein
- IL
Interleukin
- IFN
Interferon
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappa-B
- PDGF
platelet-derived growth factor
- PDGFR
PDGF receptor
- PGE2
prostaglandin E2
- PI3K
phosphatidylinositol 3-kinase
- RACK-1
receptor of activated protein kinase C
- Raf
rapidly growing fibrosarcoma protein
- Ras
rat sarcoma protein
- TGF-β
transforming growth factor beta
- TMZ
temozolomide
- TNF
tumor necrosis factor
- uPA
urokinase-type plasminogen activator
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Risau W., Sariola H., Zerwes H.G., Sasse J., Ekblom P., Kemler R., Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 2.Patel-Hett S., D’amore P.A. Signal transduction in vasculogenesis and developmental angiogenesis. Int. J. Dev. Biol. 2011;55:353–363. doi: 10.1387/ijdb.103213sp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J., Bach M., Rowe J.W., Davidoff F., Lambert P., Hirsch C., Goldberg A., Hiatt H.H., Glass J., Henshaw E. Tumor angiogenesis - Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Fagiani E., Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Arnold F., West D.C. Angiogenesis in wound healing. Pharmacol. Ther. 1991;52:407–422. doi: 10.1016/0163-7258(91)90034-J. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds L.P., Redmer D.A. Angiogenesis in the placenta. Biol. Reprod. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- 7.Grunewald M., Avraham I., Dor Y., Bachar-Lustig E., Itin A., Jung S., Chimenti S., Landsman L., Abramovitch R., Keshet E. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland R.M. Cell and environment interactions in tumor microregions: The multicell spheroid model. Science. 1988;240:177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 11.Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 12.Vaupel P., Höckel M. Blood supply, oxygenation status and metabolic micromilieu of breast cancers: Characterization and therapeutic relevance. Int. J. Oncol. 2000;17:869–879. doi: 10.3892/ijo.17.5.869. [DOI] [PubMed] [Google Scholar]
- 13.Vaupel P. Blood flow and metabolic microenvironment of brain tumors. J. Neurooncol. 1994;22:261–267. doi: 10.1007/BF01052931. [DOI] [PubMed] [Google Scholar]
- 14.Vaupel P. Physiological properties of malignant tumours. NMR Biomed. 1992;5:220–225. doi: 10.1002/nbm.1940050505. [DOI] [PubMed] [Google Scholar]
- 15.Reinhold H.S., van den Berg-Blok A. Vascularization of experimental tumours. Ciba Found. Symp. 1983;100:100–119. doi: 10.1002/9780470720813.ch7. [DOI] [PubMed] [Google Scholar]
- 16.Reinhold H.S., van den Berg-Blok A. Circulation physiology of tumors. In: Kallmann R.F., editor. Rodent Tumor Models in Experimental Cancer Therapy. Pergamon Press; New York, NY, USA: 1987. [Google Scholar]
- 17.Vaupel P. Abnormal microvasculature and defective microcirculatory function in solid tumors. In: Siemann D.W., editor. Vascular-Targeted Therapies in Oncology. John Wiley & Sons, Ltd.; Chichester, UK: 2006. pp. 9–29. [Google Scholar]
- 18.Shchors K., Evan G. Tumor angiogenesis: Cause or consequence of cancer? Cancer Res. 2007;67:7059–7061. doi: 10.1158/0008-5472.CAN-07-2053. [DOI] [PubMed] [Google Scholar]
- 19.Sonveaux P. Provascular strategy: Targeting functional adaptations of mature blood vessels in tumors to selectively influence the tumor vascular reactivity and improve cancer treatment. Radiother. Oncol. 2008;86:300–313. doi: 10.1016/j.radonc.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/S0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 21.Tammela T., Zarkada G., Nurmi H., Jakobsson L., Heinolainen K., Tvorogov D., Zheng W., Franco C.A., Murtomaki A., Aranda E., et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing notch signalling. Nat. Cell Biol. 2011;13:1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung Y.J., Isaacs J.S., Lee S., Trepel J., Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 23.Jiang B.H., Liu L.Z. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim. Biophys. Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Eibl G., Bruemmer D., Okada Y., Duffy J.P., Law R.E., Reber H.A., Hines O.J. PGE2 is generated by specific COX-2 activity and increases VEGF production in COX-2-expressing human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2003;306:887–897. doi: 10.1016/S0006-291X(03)01079-9. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N., Gerber H.P., Lecouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Salven P., Lymboussaki A., Heikkila P., Jaaskela-Saari H., Enholm B., Aase K., von Euler G., Eriksson U., Alitalo K., Joensuu H. Vascular endothelial growth factors VEGF-b and VEGF-c are expressed in human tumors. Am. J. Pathol. 1998;153:103–108. doi: 10.1016/S0002-9440(10)65550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roskoski R., Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit. Rev. Oncol. Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Claesson-Welsh L., Welsh M. VEGFa and tumour angiogenesis. J. Intern. Med. 2013;273:114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann J., Junker H., Schmieder A., Venz S., Brandt R., Multhoff G., Falk W., Radons J. EGCG downregulates IL-1R expression and suppresses IL-1-induced tumorigenic factors in human pancreatic adenocarcinoma cells. Biochem. Pharmacol. 2011;82:1153–1162. doi: 10.1016/j.bcp.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 30.Hardtner C., Multhoff G., Falk W., Radons J. (−)-epigallocatechin-3-gallate, a green tea-derived catechin, synergizes with celecoxib to inhibit IL-1-induced tumorigenic mediators by human pancreatic adenocarcinoma cells colo35. Eur. J. Pharmacol. 2012;684:36–43. doi: 10.1016/j.ejphar.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Honicke A.S., Ender S.A., Radons J. Combined administration of EGCG and IL-1 receptor antagonist efficiently downregulates IL-1-induced tumorigenic factors in U-2 OS human osteosarcoma cells. Int. J. Oncol. 2012;41:753–758. doi: 10.3892/ijo.2012.1498. [DOI] [PubMed] [Google Scholar]
- 32.Radons J., Falk W., Schubert T.E. Interleukin-10 does not affect IL-1-induced interleukin-6 and metalloproteinase production in human chondrosarcoma cells, SW1353. Int. J. Mol. Med. 2006;17:377–383. [PubMed] [Google Scholar]
- 33.Murray B., Wilson D.J. A study of metabolites as intermediate effectors in angiogenesis. Angiogenesis. 2001;4:71–77. doi: 10.1023/A:1016792319207. [DOI] [PubMed] [Google Scholar]
- 34.Sonveaux P., Brouet A., Havaux X., Gregoire V., Dessy C., Balligand J.L., Feron O. Irradiation-induced angiogenesis through the up-regulation of the nitric oxide pathway: Implications for tumor radiotherapy. Cancer Res. 2003;63:1012–1019. [PubMed] [Google Scholar]
- 35.Maddirela D.R., Kesanakurti D., Gujrati M., Rao J.S. Mmp-2 suppression abrogates irradiation-induced microtubule formation in endothelial cells by inhibiting alpha-v-beta3-mediated SDF-1/CXCR4 signaling. Int. J. Oncol. 2013;42:1279–1288. doi: 10.3892/ijo.2013.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asuthkar S., Velpula K.K., Nalla A.K., Gogineni V.R., Gondi C.S., Rao J.S. Irradiation-induced angiogenesis is associated with an MMP-9-MIR-494-Syndecan-1 regulatory loop in medulloblastoma cells. Oncogene. 2013 doi: 10.1038/onc.2013.151. [DOI] [PubMed] [Google Scholar]
- 37.Multhoff G., Vaupel P. Radiation-induced changes in microcirculation and interstitial fluid pressure affecting the delivery of macromolecules and nanotherapeutics to tumors. Front. Oncol. 2012 doi: 10.3389/fonc.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaupel P. Pathophysiology of solid tumors. In: Molls M., Vaupel P., Nieder C., Anscher M.S., editors. The Impact of Tumor Biology on Cancer Treatment and Multidisciplinary Strategies. Springer; Berlin, Heidelberg, Germany; New York, NY, USA: 2009. pp. 51–92. [Google Scholar]
- 39.Butler T.P., Grantham F.H., Gullino P.M. Bulk transfer of fluid in the interstitial compartment of mammary tumors. Cancer Res. 1975;35:3084–3088. [PubMed] [Google Scholar]
- 40.Sevick E.M., Jain R.K. Viscous resistance to blood flow in solid tumors: Effect of hematocrit on intratumor blood viscosity. Cancer Res. 1989;49:3513–3519. [PubMed] [Google Scholar]
- 41.Gillies R.J., Schornack P.A., Secomb T.W., Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1:197–207. doi: 10.1038/sj.neo.7900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto S., Yasui H., Mitchell J.B., Krishna M.C. Imaging cycling tumor hypoxia. Cancer Res. 2010;70:10019–10023. doi: 10.1158/0008-5472.CAN-10-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardenas-Navia L.I., Mace D., Richardson R.A., Wilson D.F., Shan S., Dewhirst M.W. The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 2008;68:5812–5819. doi: 10.1158/0008-5472.CAN-07-6387. [DOI] [PubMed] [Google Scholar]
- 44.Vaupel P., Mayer A. Imaging tumor hypoxia: Blood-borne delivery of imaging agents is fundamentally different in hypoxia subtypes. J. Innov. Opt. Health Sci. 2014 doi: 10.1142/S179354581330005X. [DOI] [Google Scholar]
- 45.Vaupel P., Kelleher D.K., Höckel M. Oxygen status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. Semin. Oncol. 2001;28:29–35. doi: 10.1016/S0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 46.Vaupel P., Mayer A., Briest S., Höckel M. Oxygenation gain factor: A novel parameter characterizing the association between hemoglobin level and the oxygenation status of breast cancers. Cancer Res. 2003;63:7634–7637. [PubMed] [Google Scholar]
- 47.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Lyng H., Vorren A.O., Sundfor K., Taksdal I., Lien H.H., Kaalhus O., Rofstad E.K. Intra- and inter-tumor heterogeneity in blood perfusion of human cervical cancer before treatment and after radiotherapy. Int. J. Cancer. 2001;96:182–190. doi: 10.1002/ijc.1019. [DOI] [PubMed] [Google Scholar]
- 49.Haider M.A., Milosevic M., Fyles A., Sitartchouk I., Yeung I., Henderson E., Lockwood G., Lee T.Y., Roberts T.P. Assessment of the tumor microenvironment in cervix cancer using dynamic contrast enhanced CT, interstitial fluid pressure and oxygen measurements. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:1100–1107. doi: 10.1016/j.ijrobp.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 50.Höckel M., Schlenger K., Aral B., Mitze M., Schäffer U., Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 51.Hill S.A., Pigott K.H., Saunders M.I., Powell M.E., Arnold S., Obeid A., Ward G., Leahy M., Hoskin P.J., Chaplin D.J. Microregional blood flow in murine and human tumours assessed using laser Doppler microprobes. Br. J. Cancer. 1996;27:S260–S263. [PMC free article] [PubMed] [Google Scholar]
- 52.Pigott K.H., Hill S.A., Chaplin D.J., Saunders M.I. Microregional fluctuations in perfusion within human tumours detected using laser Doppler flowmetry. Radiother. Oncol. 1996;40:45–50. doi: 10.1016/0167-8140(96)01730-6. [DOI] [PubMed] [Google Scholar]
- 53.Wilson C.B., Lammertsma A.A., Mckenzie C.G., Sikora K., Jones T. Measurements of blood flow and exchanging water space in breast tumors using positron emission tomography: A rapid and noninvasive dynamic method. Cancer Res. 1992;52:1592–1597. [PubMed] [Google Scholar]
- 54.Feldmann H.J., Molls M., Vaupel P. Blood flow and oxygenation status of human tumors. Clinical investigations. Strahlenther. Onkol. 1999;175:1–9. doi: 10.1007/BF02743452. [DOI] [PubMed] [Google Scholar]
- 55.Vaupel P., Manz R., Müller-Klieser W., Grunewald W.A. Intracapillary HbO2 saturations within tissue-isolated malignant-tumors during hyperoxia. Pflügers Arch. Eur. J. Physiol. 1978;373:R39. doi: 10.1007/BF00581147. [DOI] [Google Scholar]
- 56.Weiss L., Hultborn R., Tveit E. Blood-flow characteristics in induced rat mammary neoplasia. Microvasc. Res. 1979;17:S119. [Google Scholar]
- 57.Endrich B., Hammersen F., Götz A., Messmer K. Microcirculatory blood flow, capillary morphology and local oxygen pressure of the hamster amelanotic melanoma a-Mel-3. J. Natl. Cancer Inst. 1982;68:475–485. [PubMed] [Google Scholar]
- 58.Wheeler R.H., Ziessman H.A., Medvec B.R., Juni J.E., Thrall J.H., Keyes J.W., Pitt S.R., Baker S.R. Tumor blood flow and systemic shunting in patients receiving intraarterial chemotherapy for head and neck cancer. Cancer Res. 1986;46:4200–4204. [PubMed] [Google Scholar]
- 59.Vaupel P., Höckel M., Mayer A. Detection and characterization of tumour hypoxia using p02 histography. Antioxid. Redox Signal. 2007;9:1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 60.Brown J.M., Wilson W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 61.Harada H. How can we overcome tumor hypoxia in radiation therapy? J. Radiat. Res. 2011;52:545–556. doi: 10.1269/jrr.11056. [DOI] [PubMed] [Google Scholar]
- 62.Harada H., Itasaka S., Kizaka-Kondoh S., Shibuya K., Morinibu A., Shinomiya K., Hiraoka M. The Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. J. Biol. Chem. 2009;284:5332–5342. doi: 10.1074/jbc.M806653200. [DOI] [PubMed] [Google Scholar]
- 63.Schilling D., Duwel M., Molls M., Multhoff G. Radiosensitization of wildtype p53 cancer cells by the MDM2-inhibitor PXN727 is associated with altered heat shock protein 70 (Hsp70) levels. Cell Stress Chaperones. 2013;18:183–191. doi: 10.1007/s12192-012-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Multhoff G., Molls M., Radons J. Chronic inflammation in cancer development. Front. Immunol. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y.V., Baek J.H., Zhang H., Diez R., Cole R.N., Semenza G.L. Rack1 competes with hsp90 for binding to HIF-1 alpha and is required for O2-independent and Hsp90 inhibitor-induced degradation of HIF-1 alpha. Mol. Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshimura M., Itasaka S., Harada H., Hiraoka M. Microenvironment and radiation therapy. Biomed. Res. Int. 2013 doi: 10.1155/2013/685308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirota K., Semenza G.L. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem. Biophys. Res. Commun. 2005;338:610–616. doi: 10.1016/j.bbrc.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 68.Li F., Sonveaux P., Rabbani Z.N., Liu S., Yan B., Huang Q., Vujaskovic Z., Dewhirst M.W., Li C.Y. Regulation of HIF-1alpha stability through s-nitrosylation. Mol. Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yasui H., Ogura A., Asanuma T., Matsuda A., Kashiwakura I., Kuwabara M., Inanami O. Inhibition of HIF-1alpha by the anticancer drug Tas106 enhances x-ray-induced apoptosis in vitro and in vivo. Br. J. Cancer. 2008;99:1442–1452. doi: 10.1038/sj.bjc.6604720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwartz D.L., Powis G., Thitai-Kumar A., He Y., Bankson J., Williams R., Lemos R., Oh J., Volgin A., Soghomonyan S., et al. The selective hypoxia inducible factor-1 inhibitor px-478 provides in vivo in vivo radiosensitization through tumor stromal effects. Mol. Cancer Ther. 2009;8:947–958. doi: 10.1158/1535-7163.MCT-08-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harada H., Itasaka S., Zhu Y., Zeng L., Xie X., Morinibu A., Shinomiya K., Hiraoka M. Treatment regimen determines whether an HIF-1 inhibitor enhances or inhibits the effect of radiation therapy. Br. J. Cancer. 2009;100:747–757. doi: 10.1038/sj.bjc.6604939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li G., Xie B., Li X., Chen Y., Wang Q., Xu Y., Xu-Welliver M., Zou L. Down-regulation of survivin and hypoxia-inducible factor-1 alpha by beta-elemene enhances the radiosensitivity of lung adenocarcinoma xenograft. Cancer Biother. Radiopharm. 2012;27:56–64. doi: 10.1089/cbr.2011.1003. [DOI] [PubMed] [Google Scholar]
- 73.Okamoto K., Ito D., Miyazaki K., Watanabe S., Tohyama O., Yokoi A., Ozawa Y., Asano M., Kawamura T., Yamane Y., et al. Microregional antitumor activity of a small-molecule hypoxia-inducible factor 1 inhibitor. Int. J. Mol. Med. 2012;29:541–549. doi: 10.3892/ijmm.2011.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quesada J., Amato R. The molecular biology of soft-tissue sarcomas and current trends in therapy. Sarcoma. 2012;2012:849456. doi: 10.1155/2012/849456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wehland M., Bauer J., Magnusson N.E., Infanger M., Grimm D. Biomarkers for anti-angiogenic therapy in cancer. Int. J. Mol. Sci. 2013;14:9338–9364. doi: 10.3390/ijms14059338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mauceri H.J., Hanna N.N., Beckett M.A., Gorski D.H., Staba M.J., Stellato K.A., Bigelow K., Heimann R., Gately S., Dhanabal M., et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 77.Itasaka S., Komaki R., Herbst R.S., Shibuya K., Shintani T., Hunter N.R., Onn A., Bucana C.D., Milas L., Ang K.K., et al. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:870–878. doi: 10.1016/j.ijrobp.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng F., Xu Z., Wang J., Chen Y., Li Q., Zuo Y., Chen J., Hu X., Zhou Q., Wang Y., et al. Recombinant human endostatin normalizes tumor vasculature and enhances radiation response in xenografted human nasopharyngeal carcinoma models. PLoS One. 2012;7:e34646. doi: 10.1371/journal.pone.0034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ke Q.H., Zhou S.Q., Huang M., Lei Y., Du W., Yang J.Y. Early efficacy of endostar combined with chemoradiotherapy for advanced cervical cancers. Asian Pac. J. Cancer Prev. 2012;13:923–926. doi: 10.7314/APJCP.2012.13.3.923. [DOI] [PubMed] [Google Scholar]
- 80.Zhou J., Wang L., Xu X., Tu Y., Qin S., Yin Y. Antitumor activity of endostar combined with radiation against human nasopharyngeal carcinoma in mouse xenograft models. Oncol. Lett. 2012;4:976–980. doi: 10.3892/ol.2012.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mcmeekin D.S., Sill M.W., Darcy K.M., Abulafia O., Hanjani P., Pearl M.L., Rubin S.C., Rose P.G., Small L., Benbrook D.M. A phase II trial of thalidomide in patients with refractory uterine carcinosarcoma and correlation with biomarkers of angiogenesis: A gynecologic oncology group study. Gynecol. Oncol. 2012;127:356–361. doi: 10.1016/j.ygyno.2012.07.095. [DOI] [PubMed] [Google Scholar]
- 82.Knisely J.P., Berkey B., Chakravarti A., Yung A.W., Curran W.J., Jr., Robins H.I., Movsas B., Brachman D.G., Henderson R.H., Mehta M.P. A phase III study of conventional radiation therapy plus thalidomide versus conventional radiation therapy for multiple brain metastases (RTOG 0118) Int. J. Radiat. Oncol. Biol. Phys. 2008;71:79–86. doi: 10.1016/j.ijrobp.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 83.Viani G.A., Manta G.B., Fonseca E.C., de Fendi L.I., Afonso S.L., Stefano E.J. Whole brain radiotherapy with radiosensitizer for brain metastases. J. Exp. Clin. Cancer Res. 2009;28:1. doi: 10.1186/1756-9966-28-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonner J.A., Harari P.M., Giralt J., Cohen R.B., Jones C.U., Sur R.K., Raben D., Baselga J., Spencer S.A., Zhu J., et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 85.Brooks C., Sheu T., Bridges K., Mason K., Kuban D., Mathew P., Meyn R. Preclinical evaluation of sunitinib, a multi-tyrosine kinase inhibitor, as a radiosensitizer for human prostate cancer. Radiat. Oncol. 2012 doi: 10.1186/1748-717X-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarkaria J.N., Galanis E., Wu W., Peller P.J., Giannini C., Brown P.D., Uhm J.H., Mcgraw S., Jaeckle K.A., Buckner J.C. North central cancer treatment group phase I trial N057k of everolimus (RAD001) and temozolomide in combination with radiation therapy in patients with newly diagnosed glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:468–475. doi: 10.1016/j.ijrobp.2010.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hainsworth J.D., Shih K.C., Shepard G.C., Tillinghast G.W., Brinker B.T., Spigel D.R. Phase II study of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin. Adv. Hematol. Oncol. 2012;10:240–246. [PubMed] [Google Scholar]
- 88.Zaidi S., Mclaughlin M., Bhide S.A., Eccles S.A., Workman P., Nutting C.M., Huddart R.A., Harrington K.J. The HSP90 inhibitor NVP-AUY922 radiosensitizes by abrogation of homologous recombination resulting in mitotic entry with unresolved DNA damage. PLoS One. 2012;7:e35436. doi: 10.1371/journal.pone.0035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gandhi N., Wild A.T., Chettiar S.T., Aziz K., Kato Y., Gajula R.P., Williams R.D., Cades J.A., Annadanam A., Song D., et al. Novel hsp90 inhibitor nvp-auy922 radiosensitizes prostate cancer cells. Cancer Biol. Ther. 2013;14:347–356. doi: 10.4161/cbt.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]