Abstract
Background
Severe obesity (SO) in pediatrics has become increasing prevalent in recent decades.
The objective of our study was to examine differences in demographic, anthropometric, cardiometabolic, and lifestyle variables in children and youth with SO versus their less overweight/obese (OW/OB) peers.
Methods
A retrospective medical record review of 6-19 year old participants enrolled in an outpatient pediatric weight management clinic was conducted. SO (body mass index [BMI] ≥99th percentile) and OW/OB (BMI ≥85th and <99th percentile) groups were created according to Centers for Disease Control and Prevention definitions. Demographic, anthropometric, cardiometabolic and lifestyle data reported at baseline (pre-intervention) were retrieved.
Results
Of the 345 participants, most were girls (56.2%), Caucasian (78.7%), and had family incomes > $50,000/year (65.7%). The SO group (n = 161) had lower HDL-cholesterol and higher liver enzymes, insulin resistance and blood pressure than the OW/OB group (n = 184; all p < 0.01). They also reported higher total energy intakes, fewer steps/day, less moderate-to-vigorous physical activity, and more leisure time screen time (all p < 0.02) than their leaner peers. Compared to the OW/OB group, a higher proportion of individuals in the SO group possessed cardiometabolic risk factors, including high triglycerides (45.8% vs 58.5%), alanine aminotransferase (55.4% vs 81.4%), insulin resistance (55.6% vs 82.1%), systolic blood pressure (11.5% vs 27.3%), diastolic blood pressure (17.8% vs 30.0%), and low HDL-cholesterol (44.6% vs 64.6%; all p < 0.02). Aside from the ~75% of participants (groups combined) who met the daily recommended intakes of grain and meat products, <50% of boys and girls met any of the remaining nutrition and physical activity-related recommendations. Compared to the OW/OB group, greater proportions of children and youth in the SO group failed to meet moderate-to-vigorous physical activity (48.4% vs 31.9%) and leisure-time-screen-time recommendations (43.4% vs 28.3%; both p < 0.05).
Conclusion
Children and youth with SO have a worse cardiometabolic profile and less favorable lifestyle habits than their OW/OB peers. These differences emphasize the heightened obesity-related health risks associated with SO in the pediatric years.
Keywords: Severe obesity, Pediatric, Cardiometabolic risk, Nutrition, Physical activity, Canada
Background
Obesity is one of the most common chronic disorders affecting the health and well-being of children and youth and its prevalence remains high [1,2]. Recent data from the Canadian Health Measures Survey revealed that almost one-third of Canadian 5 – 17 year-olds are either overweight or obese [3]. Even more striking is recent evidence from the US indicating an increasing prevalence of severe obesity (SO). For example, between the National Health and Nutrition Examination Survey (NHANES) 1988-1994 and NHANES 1999-2004, the prevalence of SO in children and youth increased by 72% from 2.2% to 3.8% [4]. With evidence suggesting that the overall prevalence of pediatric overweight and obesity has remained stable [2], these data are cause for concern because SO is associated with even worse cardiometabolic risk factors profiles, including elevated total cholesterol, triglycerides, serum glucose, and systolic blood pressure and low HDL-cholesterol [2,5,6]. Compounding these health risks is the observation that SO tends to be more challenging for clinicians and families to manage successfully. For instance, a lower degree of obesity predicts successful weight loss during pediatric weight management [7,8], suggesting that as the degree of obesity in children and youth increases, it becomes more entrenched and resistant to therapeutic interventions.
Recent reports have highlighted a growing research and clinical focus on SO in pediatrics, with data characterizing high prevalence levels [2,4-9] and cardiometabolic health risks [10-14]. This information is highly relevant from a health services perspective because many children and youth referred to weight management clinics present with SO and because most clinical programs use the severity of obesity and/or presence of obesity-related co-morbidities as referral criteria [11]. Clinical practice guidelines [12] and expert recommendations [13] highlight the importance of making positive lifestyle (nutrition and physical activity) changes in order to manage obesity. With this in mind, the objectives of our research were to characterize and examine differences in demographic, anthropometric, cardiometabolic and lifestyle variables in children and youth with SO versus their less overweight and obese (OW/OB) peers among a cohort of individuals referred for weight management.
Methods
Participants, setting, and procedures
A cross-sectional study design was used to assess demographic, anthropometric, cardiometabolic and lifestyle variables of children and youth. We included 6- to 19-year-olds with an age- and sex-specific BMI ≥85th percentile [15]. Data from individuals were excluded if they were (i) outside of our defined age range or (ii) not ambulatory (i.e., in a wheelchair). Only data collected before weight management was initiated were included. Participants were ambulatory and otherwise healthy individuals who were referred by physicians to an outpatient, multidisciplinary weight management clinic (Pediatric Centre for Weight and Health, Stollery Children’s Hospital, (PCWH), Edmonton, AB) between April 2005 and December 2011. Family-centered interventions offered at the PCWH take a lifestyle and behavioral focus and include both individual and group-based programming, the details of which have been reported [16-18]. All data were retrieved retrospectively via medical record review, which included a systematic protocol for data capture, entry, management, and audit [14]. Site approval for this research was received by Alberta Health Services and research ethics approval was granted by the Health Research Ethics Board at the University of Alberta (Edmonton, AB).
Anthropometry and demography
Wearing only light clothing and without shoes, weight was measured to the nearest 0.1 kg with a medical digital balance scale (SECA 644, Hanover, MD) and height was measured to the nearest 0.1 cm using a digital stadiometer (SECA 242 stadiometer, Hanover, MD). Subsequently, weight and height data were entered into EpiInfo™ (version 5.3.1, 2008; Centers for Disease Control and Prevention; Atlanta, GA) to calculate body mass index (BMI), BMI percentile, and BMI z-score. Waist circumference was measured to the nearest 0.1 cm at the iliac crest using a spring-loaded Gulick anthropometric tape (FitSystems; Calgary, AB). A standardized questionnaire was used to collect demographic data from parents who provided information regarding their family income as well as their sons’ and daughters’ dates of birth, gender, and ethnicity.
Cardiometabolic measurements
Although samples were taken at several clinic locations in the Edmonton-area, all analyses were completed at the University of Alberta Hospital outpatient laboratory. Glucose was analyzed with a Beckman LX20 analyzer; all other variables were measured with electrochemiluminescence (Elecsys 2010, Roche, Basel, Switzerland). After a 10 – 12 hour fast, participants provided a blood sample in order to determine a panel of cardiometabolic risk factors that included total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, alanine aminotransferase (ALT), glucose, and insulin. The total cholesterol/HDL-cholesterol ratio was calculated. Similarly, the homeostatic model assessment of insulin resistance (HOMA-IR) was used to derive a measure of insulin resistance (IR) according to the following formula: fasting insulin (mU/L) × fasting glucose (mM)/22.5 [19]. Systolic and diastolic blood pressures (SBP and DBP, respectively) were measured manually while participants were seated, after a 5-minute rest, by a clinician using a sphygmomanometer and an appropriately-sized arm cuff, all of which were in accordance with Canadian guidelines [20]. Five minutes later, another measure was taken; when SBP differed ≥10 mm Hg between the two measurements, the lower value of the two was recorded.
Nutrition and physical activity assessments
Prospective measurements of dietary intake were made using 4-day food records, which included 1-2 weekend days. Families completed the records, which were reviewed by the clinic dietitian in order to reduce the likelihood of food and beverage omissions and to verify brand names and portion sizes. The Food Processor Diet Analysis Software SQL (version 10.0.0, ESHA Research; Salem, OR) program was used to analyze the data and calculate average daily intakes. The software was not able to provide food group data in accordance with Canada’s Food Guide[21], so the clinic dietitian manually calculated the number of daily servings of Vegetables and Fruit, Grain Products, Milk and Alternatives, and Meat and Alternatives.
The New Lifestyles Digi-Walker SW-200 pedometer (Lee’s Summit, MO) was used to assess physical activity over a 7-day period including 1-2 weekend days. During the first visit, the PCWH exercise specialist instructed participants and parents on correct pedometer placement, calibration, and data-monitoring procedures. In advance, all pedometers underwent a 100 step accuracy test. Only those units that recorded ≥99% accuracy were used for physical activity assessment. On the second visit, pedometer log books were reviewed with families for accuracy and completeness. Since pedometers provide an overall estimate of physical activity that does not include intensity, the 7-day physical activity recall interview [22] was used to assess the amount of time participants spent engaged in moderate -to- vigorous physical activity (MVPA); MVPA represents higher- intensity physical activities (e.g., brisk, walking, skating, bike riding, running, basketball, soccer), which may be particularly important for pediatric weight management [23,24]. Accordingly, moderate, hard, and very hard physical activity were calculated for each participant and summed to derive MVPA. The exercise specialist conducted all interviews, during which information regarding screen time (including leisure time television viewing, movie rental and (or) theatre viewing, video game playing, and computer time) as a proxy measure of sedentary activity and sleep duration over the previous seven days was also collected. Children and parents were interviewed together, whereas adolescents completed the interviews independently. The exercise specialist reviewed all records with families after they were completed to verify responses and ensure the accuracy of the information they contained.
Weight status, cardiometabolic risk factor, and lifestyle habit definitions
Overweight/obesity (OW/OB) was defined as an age- and sex-specific BMI ≥85th to <99th percentile whereas SO was defined as an age- and sex-specific BMI ≥99th percentile [15]. A number of recent reports have used the same criteria to define SO in children and adolescents [4,25-27]. Criteria for dichotomizing cardiometabolic risk factors and lifestyle habits to determine relatively high/low health risk are summarized in Table 1.
Table 1.
Cardiometabolic risk factor cut-offs and lifestyle recommendations
| Variable | Cut-off | Variable | Recommendation |
|---|---|---|---|
| WC |
≥90th percentile [28] |
V & F |
5-8 servings/day [29] |
| TC |
≥5.2 mmol/L [30] |
Grains |
4-7 servings/day [29] |
| TG |
≥1.24 mmol/L [28] |
Milk |
2-4 servings/day [29] |
| HDL-C |
≤1.03 mmol/L [28] |
Meat |
1-3 servings/day [29] |
| LDL-C |
≥3.4 mmol/L [31] |
Steps |
Children [32]: |
| Boys: 13,000-15,000 steps/d | |||
| Girls: 11,000-12,000 steps/d | |||
| Adolescents [30]: | |||
| 10,000-11,700 steps/d | |||
| ALT |
<20 U/L [33] |
MVPA |
≥60 minutes/day [34] |
| HOMA-IR |
≥3.16 mmol/L [19] |
LTST |
≤2 hours/day [35] |
| SBP |
≥90th percentile [36] |
Sleep |
Children: ≥10 hr/night [37] |
| DBP | ≥90th percentile [36] | Adolescents: ≥9 hr/night [37] |
WC (waist circumference), TC (total cholesterol), TG (triglycerides), HDL-C (high density lipoprotein cholesterol), LDL-C (low density lipoprotein cholesterol), ALT (alanine aminotransferase), HOMA-IR (homeostatic model assessment of insulin resistance), SBP (systolic blood pressure), DBP (diastolic blood pressure), V & F (Vegetables & Fruit), Grains (Grain Products), Milk (Milk & Alternatives), Meat (Meat & Alternatives), Steps, MVPA (moderate-to-vigorous physical activity), LTST (leisure time screen time), Sleep (sleep duration).
Statistical analysis
Continuous variables were reported using means and standard deviations whereas categorical data were reported as proportions. Group comparisons of continuous variables were conducted using independent samples t-tests (OW/OB vs SO groups). The Chi-square test was used to compare the proportions of participants (OW/OB vs SO groups) (i) across high/low cardiometabolic risk factors and (ii) between individuals who met/did not meet nutrition and physical activity-related recommendations. For descriptive purposes, also we completed stratified analyses using multivariable analysis of variance across all (i) cardiometabolic risk factors and (ii) nutrition and physical activity-related variables to examine weight status (OW/OB vs SO groups) by (i) gender (boys vs girls), (ii) age group (children [<12 years old] vs youth [12 years old]), and (iii) ethnicity (Caucasian vs non-Caucasian) interaction effects. Group differences were considered statistically significant at a p-value <0.05 and all data were analyzed using SPSS (version 19; IBM SPSS Statistics).
Results
In total, 345 participants were included in this report, the majority of whom were girls (56.2%), Caucasian (78.7%), and had family incomes > $50,000 CDN/year (65.7%). The anthropometric characteristics of children and youth grouped according to OW/OB and SO categories are presented in Table 2. By design, the SO group was both heavier and more overweight than their OW/OB peers. We also completed exploratory analyses between OW (n = 26) and OB (n = 158) sub-groups. Among all of the demographic, cardiometabolic, and lifestyle variables, the only group differences related to systolic blood pressure (OW: 119 ± 13mmHg versus OB: 110 ± 11 mmHg; p < 0.05) and dietary energy from saturated fat (OW: 203 ± 78 kcal/d versus OB: 254 ± 119 kcal/d; p < 0.05). These findings highlighted the homogeneity within the OW/OB group and justified our decision to combine these sub-groups.
Table 2.
Anthropometric characteristics of children and youth categorized as overweight/obese (OW/OB) and severely obese (SO)
| OW/OB | SO | p-value | |
|---|---|---|---|
| Age (y) |
12.8 ± 2.8 |
12.3 ± 2.9 |
0.07 |
| |
(n = 184) |
(n = 161) |
|
| Height (cm) |
155.9 ± 13.0 |
157.9 ± 14.5 |
0.2 |
| |
(n = 184) |
(n = 161) |
|
| Weight (kg) |
71.2 ± 19.8 |
94.4 ± 29.3 |
<0.001 |
| |
(n = 184) |
(n = 161) |
|
| BMI (kg/m2) |
28.7 ± 4.1 |
36.9 ± 6.7 |
<0.001 |
| |
(n = 184) |
(n = 161) |
|
| BMI Percentile |
97.1 ± 2.3 |
99.5 ± 0.2 |
<0.001 |
| |
(n = 184) |
(n = 161) |
|
| BMI Z-Score |
2.0 ± 0.3 |
2.6 ± 0.2 |
<0.001 |
| |
(n = 184) |
(n = 161) |
|
| Waist Circumference (cm) |
88.0 ± 11.0 |
104.6 ± 15.6 |
<0.001 |
| (n = 184) | (n = 153) |
Data are presented as mean ± standard deviation. BMI (body mass index).
Note: Sample sizes vary variable-to-variable because of an incomplete dataset.
Comparisons of cardiometabolic risk factors (continuous data) between OW/OB and SO groups are shown in Table 3, with lower TC and HDL-C as well as higher ALT, fasting insulin, HOMA-IR, SBP, and DBP in the SO vs OW/OB group. After we dichotomized cardiometabolic risk factors (see Table 1 for thresholds), we found that the proportions of children and youth with cardiometabolic risk factors were higher in the SO group compared to the OW/OB group for most variables, including WC, TG, HDL-C, ALT, HOMA-IR, SBP and DBP (see Figure 1).
Table 3.
Cardiometabolic risk factors of children and youth categorized as overweight/obese (OW/OB) and severely obese (SO)
| OW/OB | SO | p-value | |
|---|---|---|---|
| Triglycerides (mmol/L) |
1.4 ± 0.8 |
1.5 ± 0.6 |
0.2 |
| (n = 168) |
(n = 147) |
||
| Total cholesterol (mmol/L) |
4.5 ± 0.9 |
4.3 ± 0.8 |
0.03 |
| (n = 168) |
(n = 147) |
||
| HDL-cholesterol (mmol/L) |
1.1 ± 0.3 |
1.0 ± 0.2 |
<0.001 |
| (n = 168) |
(n = 147) |
||
| LDL-cholesterol (mmol/L) |
2.7 ± 0.8 |
2.6 ± 0.7 |
0.1 |
| (n = 168) |
(n = 147) |
||
| Total cholesterol/HDL ratio |
4.3 ± 1.2 |
4.5 ± 1.1 |
0.2 |
| (n = 168) |
(n = 147) |
||
| ALT (U/L) |
24.9 ± 16.0 |
31.6 ± 17.8 |
0.001 |
| (n = 157) |
(n = 145) |
||
| Fasting glucose (mmol/L) |
4.9 ± 0.4 |
4.9 ± 0.4 |
0.7 |
| (n = 159) |
(n = 138) |
||
| Fasting insulin (mU/L) |
18.5 ± 15.8 |
31.3 ± 20.4 |
0.02 |
| (n = 154) |
(n = 130) |
||
| HOMA-IR |
4.0 ± 3.4 |
6.8 ± 4.6 |
0.03 |
| (n = 151) |
(n = 123) |
||
| Systolic BP (mmHg) |
108 ± 9 |
115 ± 11 |
0.01 |
| (n = 174) |
(n = 150) |
|
|
| Diastolic BP (mmHg) | 69 ± 8 |
72 ± 9 |
0.01 |
| (n = 174) | (n = 150) |
Data are presented as mean ± standard deviation. HDL-C (high density lipoprotein cholesterol), LDL-C (low density lipoprotein cholesterol), ALT (alanine aminotransferase), HOMA-IR (homeostatic model assessment of insulin resistance).
Note 1: Sample sizes vary variable-to-variable because dataset was incomplete.
Note 2: Group comparisons for fasting glucose, fasting insulin, HOMA-IR, systolic BP and diastolic BP were completed using non-parametric analyses since these variables were not normally distributed.
Figure 1.
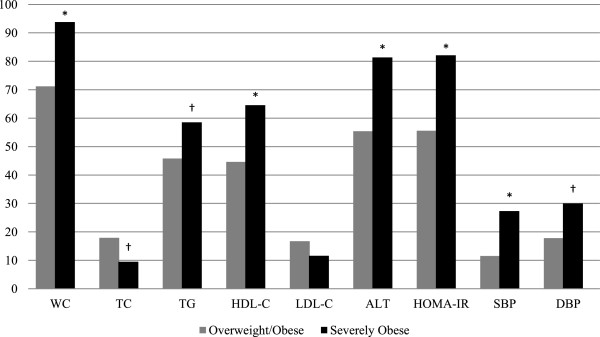
The prevalence of abnormal cardiometabolic risk factors in overweight/obese (OW/OB) and severely obese (SO) groups. WC (waist circumference), TC (total cholesterol), TG (triglycerides), HDL-C (high density lipoprotein cholesterol), LDL-C (low density lipoprotein cholesterol), ALT (alanine aminotransferase), HOMA-IR (homeostatic model assessment of insulin resistance), SBP (systolic blood pressure), DBP (diastolic blood pressure). *p ≤ 0.001. † p ≤ 0.05.
Comparisons of nutrition- and physical activity-related behaviors between the SO and OW/OB groups are shown in Table 4. Overall, the SO group consumed a greater amount of total energy, which was reflected in their higher intakes of all macronutrients (carbohydrate, protein, and fat) as well as servings/day of Grain Products and Meat and Alternatives. In addition, in relation to their OW/OB peers, the SO group reported accumulating fewer daily steps, less MVPA, and more LTST.
Table 4.
Nutrition- and physical activity-related habits of children and youth categorized as overweight/obese (OW/OB) and severely obese (SO)
| OW/OB | SO | p-value | |
|---|---|---|---|
| Energy intake (kcal/d) |
1955 ± 551 |
2333 ± 857 |
<0.001 |
| (n = 166) |
(n = 139) |
||
| Carbohydrate intake (g/d) ‡ |
261 ± 83 |
294 ± 110 |
0.004 |
| (n = 166) |
(n = 139) |
||
| Protein intake (g/d) ‡ |
77 ± 32 |
93 ± 43 |
<0.001 |
| (n = 166) |
(n = 139) |
||
| Fat intake (g/d) ‡ |
68 ± 25 |
89 ± 42 |
<0.001 |
| (n = 166) |
(n = 139) |
||
| Vegetables and Fruit (servings/d) |
3.6 ± 1.9 |
3.6 ± 2.4 |
0.8 |
| (n = 156) |
(n = 132) |
||
| Grain Products (servings/d) |
7.7 ± 2.6 |
8.8 ± 4.0 |
0.01 |
| (n = 156) |
(n = 132) |
||
| Milk and Alternatives (servings/d) |
2.0 ± 1.0 |
2.2 ± 1.3 |
0.4 |
| (n = 156) |
(n = 132) |
||
| Meat and Alternatives (servings/d) |
1.9 ± 0.8 |
2.6 ± 1.4 |
0.01 |
| (n = 155) |
(n = 132) |
||
| Steps/d |
8012 ± 3456 |
7049 ± 3127 |
0.02 |
| (n = 154) |
(n = 120) |
||
| MVPA (min/d) |
69.1 ± 52.8 |
50.5 ± 41.8 |
0.007 |
| (n = 159) |
(n = 119) |
||
| Active video game time (min/d) |
2.9 ± 14.1 |
1.8 ± 7.8 |
0.3 |
| (n = 158) |
(n = 117) |
||
| Leisure time screen time (min/d) |
163.5 ± 117.8 |
217.6 ± 131.2 |
0.04 |
| (n = 159) |
(n = 120) |
||
| Sleep duration (hr/night) | 9.6 ± 1.0 |
9.5 ± 1.0 |
0.4 |
| (n = 163) | (n = 125) |
Data are presented as mean ± standard deviation. MVPA (moderate-to-vigorous physical activity).
Note 1: Sample sizes vary variable-to-variable because dataset was incomplete.
Note 2: Group comparisons for Vegetables and Fruit, Grain Products, Milk and Alternatives, Meat and Alternatives, MVPA, Active video game time, and Leisure time screen time were completed using non-parametric analyses since these variables were not normally distributed.
‡Differences in macronutrients (carbohydrate, protein, and fat) remained significant after adjustment for total energy intake.
The proportions of participants in the OW/OB and SO groups that met each of the nutrition- and physical activity-related recommendations (defined in Table 1) are shown in Figure 2. While we failed to observe any significant group differences according to the food groups, in general, a large proportion (70 – 80%) of our sample met the recommendations for daily intakes of Grain Products and Meat and Alternatives whereas a small proportion (10 – 25%) satisfied the Vegetables and Fruit and Milk and Alternatives guidelines. Similar to the nutrition-related recommendations, we observed that most children and youth in our study did not satisfy the physical activity-related recommendations. However, almost twice as many OW/OB participants met the MVPA recommendation compared to their SO peers (χ2 = 7.6; p < 0.01). Similar differences were revealed with a greater proportion of the OW/OB group meeting LTST recommendations (χ2 = 6.7; p < 0.05) than the SO group.
Figure 2.
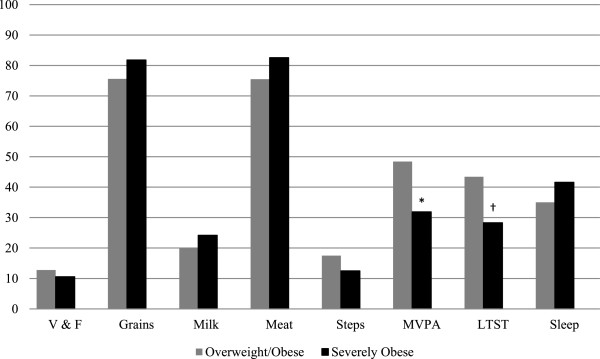
The proportion of participants in overweight/obese (OW/OB) and severely obese (SO) groups that achieved recommendations for nutrition and physical activity behaviours. V & F (Vegetables and Fruit), MVPA (moderate-to-vigorous physical activity), LTST (leisure time screen time). *p ≤ 0.01. † p ≤ 0.05.
Our stratified analyses revealed no significant (i) weight status (OW/OB vs SO) × gender (boys vs girls), (ii) weight status × age group (children vs youth), or (iii) weight status × ethnicity (Caucasian vs non-Caucasian) interactions across our cardiometabolic risk factors and nutrition and physical activity-related habits (all interaction effects >0.05).
Discussion
In our study of children and youth referred for weight management, we showed that (i) those with SO were at increased cardiometabolic health risk compared to their OW/OB peers, and (ii) while the study sample as a whole had sub-optimal nutrition and physical activity habits, those with SO tended to have poorer lifestyle behaviors than their OW/OB counterparts.
Our observations are consistent with those of Skelton et al.[4] who applied the same classification system to define weight status categories in a sample (n ~ 12,000) of boys and girls from the National Health and Nutrition Examination Surveys II, III, and 1999-2004 and also reported a higher degree of cardiometabolic risk among the SO group in relation to their OW/OB peers. To our knowledge, we are the first to report differences in lifestyle behaviors between children and youth with SO versus their OW/OB peers. This is a noteworthy finding and emphasizes the point that a one-size-fits-all approach to weight management is inappropriate since those with SO appear to have greater potential to improve their lifestyle habits, which may have led to their higher degree of obesity. Viewed another way, the less favorable lifestyle habits of the SO group provides an opportunity for them to make positive improvements to nutrition and physical activity that can contribute to weight management success and improvements in other health outcomes, independent of substantial weight loss. For instance, a recent review of 22 studies of immersion programs examined treatments that combined a controlled diet, physical activity, nutrition education and therapy and/or education supportive of behavioral change. The participants in the reviewed treatments lost an average of 23.9% of their overweight during treatment and 20.6% during follow up [38]. Although there are exceptions [39], we recently reported [16] that it is challenging for most boys and girls with obesity to improve their weight status by making modest lifestyle changes; for many individuals, preventing further weight gain by making nutrition and physical activity changes that lead to weight stabilization is an important and achievable goal, albeit one that may or may not satisfy the expectations and desires of families [40].
Despite the differences we observed between SO and OW/OB groups, overall, most children and youth in our study did not achieve current lifestyle recommendations. In addition to supporting previous findings from our clinic [41], these results are aligned with other larger, population-based studies. For instance, Shields showed low levels of vegetable and fruit intakes as a reflection of poor diet quality that may increase the risk of pediatric obesity [42]. Similarly, Garriguet and colleagues found that a substantial number of children and youth do not meet the recommendations for vegetables and fruit, and milk and alternatives intake [21,43]. He et al. suggested that increased consumption of fruits and vegetables might reduce long-term weight gain and obesity risk [44]. Lowering the amount of added fructose in diets may also benefit children and youth with obesity by improving their markers for cardiometabolic dysfunction, which may be particularly beneficial for those at risk for developing type 2 diabetes or nonalcoholic fatty liver dysfunction [45]. Although our data are cross-sectional, our observations suggest that interventions that emphasize improvements in diet quality and quantity are likely to be important strategies for managing pediatric obesity [12].
It is clear that a physically active lifestyle has numerous health promoting benefits (beyond weight management) [46], but it remains a challenge for most boys and girls to achieve current recommendations. The Canadian Physical Activity and Sedentary Behaviour Guidelines[34] suggest that children and youth should accumulate ≥60 minutes of daily MVPA and limit their sedentary time to <2 hours daily [47]. Following these guidelines can help improve body composition, cardiorespiratory and musculoskeletal health, social behaviors, self-esteem and academic success [48]. A relatively high proportion (30 – 40% according to the self-reported data) of our sample met the physical activity recommendations; however, based on objective physical activity data collected using accelerometers, <10% of Canadian children and youth accumulate ≥60 minutes of daily MVPA [49]. The difference in the proportion of individuals meeting MVPA recommendations in our sample versus national normative data suggests boys and girls in our study may have overestimated their physical activity levels. Our observation that a lower percentage of our sample achieved the daily steps recommendation versus self-reported MVPA provides indirect evidence in support of this point, but is validated by other data showing that young individuals may overestimate their physical activity using self-report tools [50].
Our study has several limitations to acknowledge. First, since all of the participants were referred by physicians to a weight management clinic, our findings may not apply to all children and youth with SO, especially those who do not enroll in health services for obesity management. Notably, our clinic is one of eight that is participating in a new Canadian multi-center study to characterize children, youth and families receiving health services for managing pediatric obesity [51]. Evidence generated from this registry of ~1,600 participants will show whether our findings are generalizable to other similar settings. Second, given our cross-sectional study design, we cannot comment on whether our observed differences between SO and OW/OB groups remain consistent over time or whether changes in response to therapy are similar across groups. Third, while our analyses included objective measurements of anthropometric variables and cardiometabolic risk factors, self-reported nutrition and physical activity data are subjective in nature, which can reduce measurement reliability and accuracy. The fact that we still observed group differences based on these self-reported data provides some confidence that real differences exist between SO and OW/OB groups. Finally, a recent report [52] highlighted the need for consistent terms and definitions of SO in pediatrics. While the current manuscript applied one of several different definitions of SO in the literature, applying the newer recommended criteria in future studies is needed to establish its superiority.
Conclusions
In conclusion, our study revealed that children and youth with SO were at increased cardiometabolic health risk and tended to have less healthy nutrition and physical activity habits compared to their OW/OB counterparts. In their discussions with families, clinicians can use this information to highlight the health risks associated with a higher degree of obesity. By applying family- and client-centered counseling strategies [53,54], they can also emphasize the potential that exists for boys and girls with obesity to make healthy changes since most do not satisfy current lifestyle recommendations.
Competing interest
The authors declare that they have no competing interest.
Authors’ contributions
HS analyzed the data and co-authored the first draft of the manuscript with GDCB; KAA assisted with data collection and management; RSP, DRM, and CC provided critical feedback and edits on data analysis, data interpretation, and manuscript presentation; GDCB conceived the study, assisted with data analysis and interpretation, and co-authored the first draft of the manuscript with HS. All authors contributed to writing the manuscript. All authors reviewed and approved the final draft of the manuscript prior to submission.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Hebah A Salawi, Email: salawi@ualberta.ca.
Kathryn A Ambler, Email: kambler@med.ualberta.ca.
Rajdeep S Padwal, Email: rajdeep.padwal@ualberta.ca.
Diana R Mager, Email: diana.mager@ualberta.ca.
Catherine B Chan, Email: cathy.chan@ualberta.ca.
Geoff D C Ball, Email: gdball@ualberta.ca.
Acknowledgements
The authors wish to thank the children and youth whose data are included in this report as well as the team of professionals at the Pediatric Centre for Weight and Health (Stollery Children’s Hospital, Edmonton, AB), Mr. Rick Watts (Clinical Research Informatics Core, Women and Children’s Health Research Institute, University of Alberta), and Ms. Alli Rasquinha (Research Assistant, Department of Pediatrics, University of Alberta) for their administrative and research support. HS was supported by a scholarship awarded by the King Abdullah Scholarship Program (Saudi Arabia).
References
- Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJ. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KC, Shields M, de Groh M, Aziz A, Gilbert JA. Overweight and obesity in children and adolescents: results from the 2009-2011 Canadian Health Measures Survey. Health Rep. 2012;23(3):37–41. [PubMed] [Google Scholar]
- Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr. 2009;9(5):322–329. doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon TS, Rofey DL, Ryan CM, Clapper DA, Chakravorty S, Arslanian SA. Relationships among obstructive sleep apnea, anthropometric measures, and neurocognitive functioning in adolescents with severe obesity. J Pediatr. 2012;160(5):732–735. doi: 10.1016/j.jpeds.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff MB, Jenkins TM, Reiter-Purtill J, Noll JG, Zeller MH. Risk-taking behaviors of adolescents with extreme obesity: normative or not? Pediatrics. 2011;127(5):827–834. doi: 10.1542/peds.2010-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens E, Braet C, Van Winckel M. An 8-year follow-up of treated obese children: children’s, process and parental predictors of successful outcome. Behav Res Ther. 2010;48(7):626–633. doi: 10.1016/j.brat.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity. 2006;14(1):148–155. doi: 10.1038/oby.2006.18. [DOI] [PubMed] [Google Scholar]
- Wang YC, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. Int J Pediatr Obes. 2011;6(1):12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- van Emmerik NM, Renders CM, van de Veer M, van Buuren S, van der Baan-Slootweg OH, Kist-van Holthe JE, HiraSing RA. High cardiovascular risk in severely obese young children and adolescents. Arch Dis Child. 2012;97(9):818–821. doi: 10.1136/archdischild-2012-301877. [DOI] [PubMed] [Google Scholar]
- Ball GD, Ambler KA, Chanoine JP. Pediatric weight management programs in Canada: where, what and how? Int J Pediatr Obes. 2011;6(2Part2):e58–e61. doi: 10.3109/17477166.2010.512390. [DOI] [PubMed] [Google Scholar]
- Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary] CMAJ. 2007;176(8):S1–S13. doi: 10.1503/cmaj.061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SE. Expert Committee. Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120 Suppl 4(120 Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Gearing RE, Mian IA, Barber J, Ickowicz A. A methodology for conducting retrospective chart review research in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2006;15(3):126. [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- Avis J, Ambler KA, Jetha MM, Boateng H, Ball G. Modest treatment effects and high program attrition: The impact of interdisciplinary, individualized care for managing paediatric obesity. Paediatr Child Health. 2013;18(10):e59–e63. doi: 10.1093/pch/18.10.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GD, Ambler KA, Keaschuk RA, Rosychuk RJ, Holt NL, Spence JC, Jetha MM, Sharma AM, Newton AS. Parents as Agents of Change (PAC) in pediatric weight management: The protocol for the PAC randomized clinical trial. BMC Pediatr. 2012;12(1):114. doi: 10.1186/1471-2431-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GD, Mackenzie-Rife KA, Newton MS, Alloway CA, Slack JM, Plotnikoff RC, Goran MI. One-on-one lifestyle coaching for managing adolescent obesity: Findings from a pilot, randomized controlled trial in a real-world, clinical setting. Paediatr Child Health. 2011;16(6):345. doi: 10.1093/pch/16.6.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–e503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- Canadian Society for Exercise Physiology (CSEP) Canadian Physical Activity, Fitness & Lifestyle Approach: CSEP-Health & Fitness Program’s Health-Related Appraisal and Counselling Strategy. 3. Ottawa: Sport Information and Resource Centre (SIRC); 2003. [Google Scholar]
- Garriguet D. Canadians’ eating. Health Rep. 2007;18(2):17. [PubMed] [Google Scholar]
- Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25(1):99. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Gordon‒Larsen P, Adair LS, Popkin BM. Ethnic differences in physical activity and inactivity patterns and overweight status. Obes Res. 2002;10(3):141–149. doi: 10.1038/oby.2002.23. [DOI] [PubMed] [Google Scholar]
- Trost SG, Kerr L, Ward DS, Pate RR. Physical activity and determinants of physical activity in obese and non-obese children. Int J Obes Relat Metab Disord. 2001;25(6):822–829. doi: 10.1038/sj.ijo.0801621. [DOI] [PubMed] [Google Scholar]
- Selewski DT, Collier DN, MacHardy J, Gross HE, Pickens EM, Cooper AW, Bullock S, Earls MF, Pratt KJ, Scanlon K. Promising insights into the health related quality of life for children with severe obesity. Health Qual Life Outcomes. 2013;11(1):29. doi: 10.1186/1477-7525-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KA, Weedn AE, Crawford PB. Disparities in peaks, plateaus, and declines in prevalence of high BMI among adolescents. Pediatrics. 2010;126(3):434–442. doi: 10.1542/peds.2009-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenders CM, Wright JA, Apovian CM, Hess DT, Shukla RR, Adams WG, Lee K. Weight loss surgery eligibility according to various BMI criteria among adolescents. Obesity. 2009;17(1):150–155. doi: 10.1038/oby.2008.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145(4):439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Health Canada. Eating well with Canada’s Food Guide. http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/order-commander/eating_well_bien_manger-eng.php.
- Lauer R, Barness L, Clark R, Deckelbaum R, Finberg L, Kwiterovich P, McBride P, Schieken R, Scott L, Strong J. National Cholesterol Education Program (NCEP)-Highlights of the Report of the Expert Panel on blood cholesterol levels in children and adolescents. Am Acad Pediatrics. 1992;89(3):495–501. [PubMed] [Google Scholar]
- O’Loughlin J, Lauzon B, Paradis G, Hanley J, Levy E, Delvin E, Lambert M. Usefulness of the American Academy of Pediatrics recommendations for identifying youths with hypercholesterolemia. Pediatrics. 2004;113(6):1723–1727. doi: 10.1542/peds.113.6.1723. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Cameron C, Griffiths JM. Canadian children’s and youth’s pedometer-determined steps/day, parent-reported TV watching time, and overweight/obesity: The CANPLAY Surveillance Study. Int J Behav Nutr Phys Act. 2011;8:1–10. doi: 10.1186/1479-5868-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Wu CY, Wang YJ, Hung HH, Yang HI, Kao WY, Su CW, Wu JC, Chan WL, Lin HC. Updated thresholds for serum alanine aminotransferase level in a large‒scale population study composed of 34 346 subjects. Aliment Pharmacol Ther. 2012;36(6):560–568. doi: 10.1111/j.1365-2036.2012.05224.x. [DOI] [PubMed] [Google Scholar]
- CSEP. Canadian physical activity guidelines: 2011 science statements. http://www.csep.ca/CMFiles/Guidelines/CanadianPhysicalActivityGuidelinesStatements_E.pdf.
- Tremblay MS, Warburton DE, Janssen I, Paterson DH, Latimer AE, Rhodes RE, Kho ME, Hicks A, LeBlanc AG, Zehr L. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011;36(1):36–46. doi: 10.1139/H11-009. [DOI] [PubMed] [Google Scholar]
- National high blood pressure education program working group on hypertension control in children and adolescents. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics. 1996;98:649. 658.c. [PubMed] [Google Scholar]
- Owens JA, Witmans M. Sleep Problems. Curr Probl Pediatr Adolesc. 2004;34:154–179. doi: 10.1016/j.cppeds.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kelly K, Kirschenbaum D. Immersion treatment of childhood and adolescent obesity: the first review of a promising intervention. Obes Rev. 2011;12(1):37–49. doi: 10.1111/j.1467-789X.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- Reinehr T, Temmesfeld M, Kersting M, De Sousa G, Toschke A. Four-year follow-up of children and adolescents participating in an obesity intervention program. Int J Obes. 2007;31(7):1074–1077. doi: 10.1038/sj.ijo.0803637. [DOI] [PubMed] [Google Scholar]
- Skelton JA, Irby MB, Geiger AM. A Systematic Review of Satisfaction and Pediatric Obesity Treatment: New Avenues for Addressing Attrition. Health Qual Life Outcomes. 2013. http://dx.doi.org/10.1111/jhq.12003. [DOI] [PMC free article] [PubMed]
- Ball GD, Lenk JM, Barbarich BN, Plotnikoff RC, Fishburne GJ, Mackenzie KA, Willows ND. Overweight children and adolescents referred for weight management: are they meeting lifestyle behaviour recommendations? Appl Physiol Nutr Metab. 2008;33(5):936–945. doi: 10.1139/H08-088. [DOI] [PubMed] [Google Scholar]
- Shields M. Measured obesity: overweight Canadian children and adolescents. Nutrition: findings from the Canadian Community Health Survey. 2005;1:1–34. [Google Scholar]
- Garriguet D. Nutrition: Findings from the Canadian Community Health Survey-Overview of Canadians’ Eating Habits. http://www.statcan.gc.ca/pub/82-620-m/82-620-m2006002-eng.pdf.
- He K, Hu F, Colditz G, Manson J, Willett W, Liu S. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes. 2004;28(12):1569–1574. doi: 10.1038/sj.ijo.0802795. [DOI] [PubMed] [Google Scholar]
- Mager DR, Iñiguez IR, Gilmour S, Yap J. The Effect of a Low Fructose and Low Glycemic Index/Load (FRAGILE) Dietary Intervention on Indices of Liver Function, Cardiometabolic Risk Factors, and Body Composition in Children and Adolescents With Nonalcoholic Fatty Liver Disease (NAFLD) JPEN J Parenter Enteral Nutr. 2013. http://dx.doi.org/10.1177/0148607113501201. [DOI] [PubMed]
- Reinehr T, Schaefer A, Winkel K, Finne E, Toschke A, Kolip P. An effective lifestyle intervention in overweight children: findings from a randomized controlled trial on “Obeldicks light”. Clin Nutr. 2010;29(3):331–336. doi: 10.1016/j.clnu.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Luan JA, Sherar LB, Esliger DW, Griew P, Cooper A. International Children’s Accelerometry Database (ICAD) Collaborators. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307(7):704–712. doi: 10.1001/jama.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MS, LeBlanc AG, Janssen I, Kho ME, Hicks A, Murumets K, Colley RC, Duggan M. Canadian sedentary behaviour guidelines for children and youth. Appl Physiol Nutr Metab. 2011;36(1):59–64. doi: 10.1139/H11-012. [DOI] [PubMed] [Google Scholar]
- Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian children and youth: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011;22(1):15–23. [PubMed] [Google Scholar]
- Adamo KB, Prince SA, Tricco AC, Connor-Gorber S, Tremblay M. A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: a systematic review. Int J Pediatr Obes. 2009;4(1):2–27. doi: 10.1080/17477160802315010. [DOI] [PubMed] [Google Scholar]
- Morrison KM, Damanhoury S, Berall G, Hamilton J, Buchholz A, Chanoine JP, Lambert M, Ambler KA, Tremblay MS, Ball GDC. The CANadian pediatric weight management registry (CANPWR): study protocol. BMC Pediatrics. (In Press) [DOI] [PMC free article] [PubMed]
- Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches. A scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- Keaschuk RA, Ball GD. Tailoring health services for managing pediatric obesity: A proposed, practice-based framework for working with families. Curr Nutr Rep. 2013;2(4):243–250. doi: 10.1007/s13668-013-0055-1. [DOI] [Google Scholar]
- Farnesi B-C, Newton AS, Holt NL, Sharma AM, Ball GD. Exploring collaboration between clinicians and parents to optimize pediatric weight management. Patient Educ Couns. 2012;87(1):10–17. doi: 10.1016/j.pec.2011.08.011. [DOI] [PubMed] [Google Scholar]


