Abstract
Background
Feedback from active locomotor muscles contributes to the exercise pressor response in healthy humans, and is thought to be more prominent in heart failure (HF). The purpose of this study was to examine the influence of metaboreflex stimulation on arterial pressure in HF.
Methods
Eleven HF patients (51±15yrs, NYHA Class I/II, LVEF 32±9%) and 11 controls (CTL) (42±9yrs) were recruited. Participants completed two exercise tests on separate days: 1) symptom limited graded exercise test; and 2) constant work rate cycling (60% peak oxygen consumption, V̇O2) for 4 min with 2 min passive recovery. Recovery was randomized to normal or locomotor muscle regional circulatory occlusion (RCO). V̇O2, mean, systolic, and diastolic blood pressure (MAP, SBP, and DBP) and heart rate (HR) were measured at rest, end-exercise and recovery. O2 pulse (V̇O2 /HR) and the rate pressure product (RPP = HR × SBP) were calculated.
Results
In response to RCO, MAP and SBP increased in HF compared with CTLs (6.8±5.8% vs −3.0±7.8%, p<0.01 and 3.4±6.4% vs −12.7±10.4%, p<0.01, respectively), with no difference in diastolic pressure (p=0.61). HF patients had a smaller reduction in HR and RPP, but also displayed a larger decrease in O2 pulse consequent to locomotor metaboreflex stimulation (p<0.05, for all).
Conclusion
RCO resulted in a markedly increased pressor response in HF relative to CTL, due primarily to an increase of SBP and attenuated cardiac recovery as noted by the persistent elevation in HR.
Keywords: circulatory occlusion, group III and IV muscle afferents, pressor response, exercise
INTRODUCTION
Systolic heart failure (HF), initiated by left ventricular dysfunction1, results in the dysregulation of multiple organ systems that subsequently progresses to a condition of exercise intolerance2, characterized by symptoms of muscle fatigue and dyspnea. Although the causes of exercise intolerance are not completely elucidated, it is accepted that impaired exercise cardiac function is a poor predictor of exercise capacity in this population2. Recent evidence suggests that hyperactivation of the locomotor muscle metaboreflex, secondary to peripheral skeletal myopathy, may play an important role in mediating reduced exercise capacity in HF3, 4.
During heavy-intensity exercise, metabolites (e.g., lactic acid, adenosine, potassium, etc.) accumulate within the muscle. These metabolic by-products are thought to stimulate group III-IV (mechano- and metabo-receptors) muscle afferent neurons5, which project to the central nervous system and elicit a reflex-mediated increase in sympathetic activity and subsequently blood pressure via sympathetically mediated vasoconstriction5. When demand for blood flow to active skeletal muscle during exercise increases in the setting of sufficient cardiac reserve (as in healthy subjects), metaboreflex activity will also lead to increased cardiac output to maximize blood flow to the skeletal muscle6.
Patients with HF present with elevated sympathetic vasoconstrictor drive, limited cardiac reserve, impaired muscle perfusion, and global deconditioning, which together leads to a generalized muscle myopathy. For example, these patients experience muscle atrophy, loss of oxidative muscle fibers, with an increase in glycolytic fibers resulting in greater anaerobically derived metabolic activity at relatively lower workloads7. Consequently, the accumulation of intramuscular metabolites is accelerated during exercise in HF. This results in greater stimulation of locomotor muscle group III-IV afferent fibers and leads to further sympathoexcitation, which has been suggested as a key mechanism for exercise intolerance (i.e. the ‘muscle’ hypothesis)8. As such, our laboratory has shown that stimulation of the metaboreflex (via regional circulatory occlusion, RCO) after submaximal cycling in HF leads to increased ventilatory drive compared to healthy controls (CTL)9.
The pressor response to metaboreceptor stimulation in HF is more controversial. During rhythmic handgrip exercise, muscle sympathetic nerve activity (MSNA) increased earlier in HF compared to CTLs, and remained elevated during post-exercise RCO10. Similarly, Shoemaker et al., (1998) reported that mean arterial pressure (MAP) increases more in HF than controls with RCO to the active forearm11. Both of these studies identified a greater increase in metabolic byproducts in HF10, 11. In contrast, other investigators observed an attenuated MSNA response to metaboreflex stimulation with no change in MAP in HF after static handgrip exercise compared with CTLs12. The reason for these contrasting results is unclear, but may be related to between-study differences in disease severity (varying NYHA class), exercise-intensity (low-intensity and long-duration vs. high-intensity and short-duration) and exercise mode (rhythmic versus static handgrip.
Importantly, experimental studies exploring the influence of lower-limb muscle metaboreflex activation on the pressor response in HF is lacking. Clinically, the involvement of locomotor muscles is important as it dictates a patient’s ability to engage in functional activities of daily living as well as higher intensity tasks (i.e. an exercise program)8. Therefore, the purpose of this study was to examine the pressor response to metaboreflex stimulation of the locomotor muscles after moderate-intensity cycling in HF. We hypothesized that HF patients would demonstrate a greater pressor response to metaboreflex stimulation compared with healthy CTLs. The findings of this study may have significant clinical utility providing greater insight to the mechanism(s) contributing to blood pressure regulation and exercise tolerance in HF.
METHODS
Population Characteristics
Eleven systolic HF patients (51±15yrs, NYHA Class I/II, LVEF 32±9%) completed this study. Inclusion criteria: ischemic or dilated cardiomyopathy, stable HF symptoms (>3 months), duration of HF >1 year, left ventricular ejection fraction ≤ 35%, BMI<35 kg/m2, and current non-smoking status with a smoking history of < 15 pack-years. Patients were treated with standard medications at the time of the study (Table 1).
TABLE 1.
Participant Characteristics
| HF | CTL | P | |
|---|---|---|---|
| Age (yrs) | 51 ± 5 | 43 ± 3 | 0.13 |
| Sex (M/F) | 7/4 | 7/4 | |
| Height (in) | 68.0 ± 1.0 | 69.5 ± 0.7 | 0.27 |
| Weight (lbs) | 192.2 ± 12.3 | 172.3 ± 7.2 | 0.18 |
| BMI (kg·m2) | 29.1 ± 1.8 | 25.2 ± 1.1 | 0.08 |
| VO2peak mL·Kg−1min−1 | 17.4 ± 1.4 | 36.3 ± 1.7 | <0.001 |
| LVEF, % | 32.1 ± 2.8 | ||
| CHF etiology (ischemic/idiopathic) | 4/7 | ||
| NYHA class (I/II) | 4/7 | ||
| Medications | |||
| ACE Inhibitors | 6 (55) | ||
| Angiotensin II receptor blockers | 4 (36) | ||
| Aspirin | 7 (64) | ||
| β – blockers | 10 (91) | ||
| Digitalis | 4 (36) | ||
| Diuretics | 7 (64) |
Data are presented as mean ± SEM or as n (%). ACE indicates angiotensin-converting enzyme; BMI: Body Mass index LVEF: Left Ventricular Ejection Fraction, NYHA: New York Heart Association
Eleven healthy CTL participants (42±9yrs) were recruited through local advertisement with attempts to match for age and sex. Control participants had no evidence of cardiopulmonary or neuromuscular disease.
All participants gave written informed consent after being provided a description of study requirements. This study was approved by the Mayo Clinic Institutional Review Board and conducted in accordance with the Declaration of Helsinki: all procedures followed institutional and Health Insurance Portability and Accountability Act (HIPAA) guidelines. Portions of the participant characteristics and raw blood pressure data have been published previously under a different working hypothesis9. This work was supported by: American Heart Association, National Center for Advancing Translational Science and National Institute of Health. The authors are solely responsible for the design and conduct of this study; all study analyses, drafting and editing of the paper, and its final contents.
Experimental Protocol
Participants completed two days of testing, separated by a minimum of 48 hours but not more than two weeks, in an environmentally controlled laboratory. The first day of testing consisted of exercise to volitional fatigue (symptom limited peak oxygen consumption [V̇O2 peak]). The second testing day consisted of 3 separate submaximal exercise sessions at 60% V̇O2 peak. For both days, participants were asked to avoid strenuous activity for 24 hours and refrain from eating/consuming caffeine for 3 hours prior to testing. All gas-exchange and electrocardiogram data were measured continuously during exercise. Blood pressure was measured by manual sphygmomanometry. Participants were fitted with a nose clip and standard mouthpiece attached to a PreVent Pneuomotach (Medgraphics CPX/C). For peak exercise testing, participants were verbally encouraged to continue the exercise to maximal exertion, which was identified as a rating of perceived exertion ≥ 17 (Borg scale, 6 to 20) and/or respiratory exchange ratio of ≥ 1.10.
Condition 1 included 3 minutes of rest, 4 minutes of steady-state submaximal cycle ergometry, and 5 minutes of passive normal recovery (NR). Condition 2 was identical except immediately following exercise RCO was induced via rapid inflation of bilateral upper-thigh pressure tourniquets for 2 minutes. Tourniquets were inflated to ~20 mmHg above the highest exercise systolic blood pressure (SBP) measured during peak exercise. Condition 3 was identical to condition 2 with carbon dioxide (CO2) added to the inspired air during RCO to clamp end-exercise end tidal partial pressure of CO2(PETCO2) (RCO+CO2). The clamping of end-exercise PETCO2 served to minimize the impact of reduced CO2 returning from the limbs to the central chemoreceptors, an effect which has the potential to alter both ventilation and cardiovascular function9. After the baseline session, the remaining 2 sessions were conducted in random order. Heart rate (HR) was measured via a 12-lead ECG. Data were averaged over the last minute of rest and the last 30 s of each minute during exercise and recovery. Manual blood pressure was measured at the end of rest, exercise, and immediately prior to releasing the pressure tourniquets. Rate pressure product (RPP) was calculated as HR multiplied by SBP. RPP is a surrogate for myocardial oxygen consumption13.
Measurement of Gas-exchange
Oxygen consumption (V̇O2), carbon dioxide production (V̇O2), and PETCO2 were measured with a metabolic measurement system through a mouth piece and pneumotach while wearing a nose clip (MedGraphics CPX/C). Manual volume calibration was performed with a 3–L syringe, and gas calibration was performed with manufacturer-recommended gases of known concentration immediately before each testing protocol.
Statistical Analysis
A sample size estimate was conducted a priori, using an α level of 0.05, means and standard deviations (SD) from the existing literature. These data indicated a need for 11 participants in each group, based on a 2-tailed hypothesis14, 15. No dropouts or test failures occurred during this study and all data were included in analyses. Percent difference was calculated from end exercise to 2 minutes of passive recovery for all conditions (NR, RCO and RCO+CO2). One-way ANOVA was performed to determine differences in the percent change between groups (CTL and HF). Repeated measures ANOVA (time x condition) with group (HF vs. CTL) as a between-subjects factor was performed to determine response to exercise from baseline for both conditions. An additional paired t-test was used to compare condition (NR vs. RCO, NR vs. RCO+CO2, RCO vs. RCO+CO2). Statistical analysis and graphic representation were accomplished with SPSS v19 (IBM, Armonk, New York) and GraphPad Prism version 6 (GraphPad Software, LaJolla, CA). Data are presented as mean±S.E.M.
RESULTS
Population characteristics are provided in Table 1.
End Tidal CO2
Baseline values of PETCO2 for CTLs and HF were 36.4±1.2 and 33.8±1.4 (p<0.05). PETCO2 was decreased at the second minute of RCO for both CTLs and HF (33.2 ± 1.5 and 30.2 ± 1.0, respectively, p<0.05). With CO2 added to the inspirate, PETCO2 increased when compared to RCO (36.6±1.2 and 31.7±1.3, respectively, p<0.05) but was not different from NR (35.1±1.3)16.
Blood Pressure
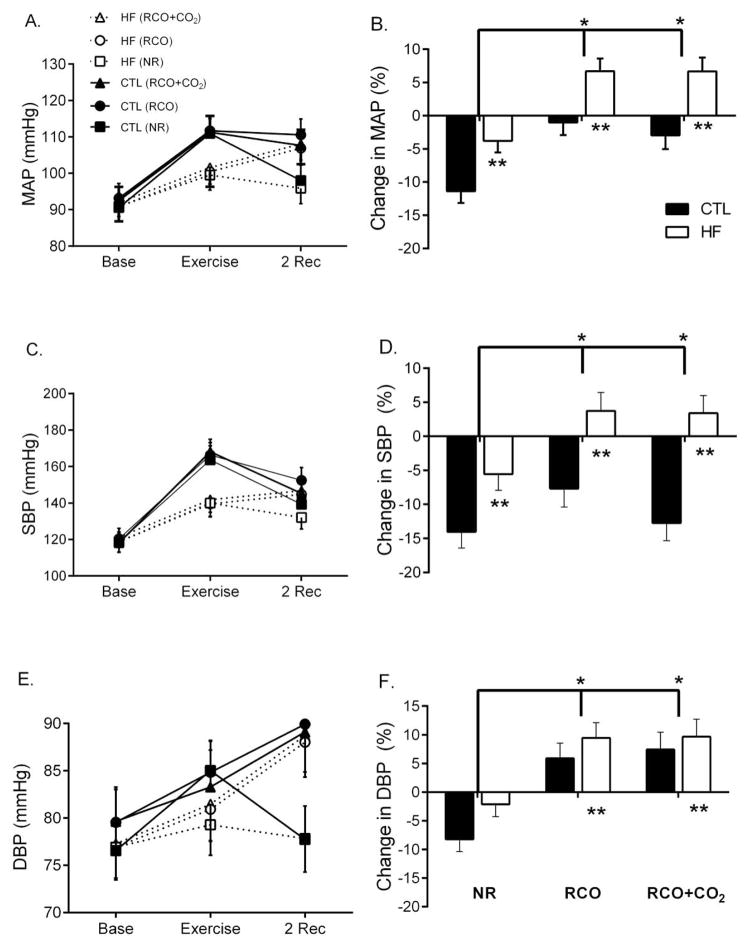
Baseline systolic (SBP), diastolic (DBP) and MAP were similar at rest between HF and CTL (p>0.05). MAP increased more in CTL than HF immediately after exercise (time × group, p<0.001, Figure 1A). The increase in MAP was not different across sessions with exercise (p>0.05). During NR, however, the return of MAP to baseline levels was attenuated in HF (p=0.006). In response to post-exercise RCO and RCO+CO2, MAP continued to increase in HF, but decreased in CTLs (p<0.01, Figure 1B) with no difference between RCO and RCO+CO2 (p=0.51).
Figure 1.
Mean arterial pressure (MAP), systolic pressure (SBP) and diastolic pressure (DBP) at baseline, end-exercise and 2 minutes of normal recovery (NR-square), regional circulatory occlusion (RCO-circular), or RCO+CO2 (triangular). Heart failure (HF-open) had less of an increase in blood pressure compared with controls (CTL-closed) during exercise (p<0.05, A, C, E). In response to post-exercise RCO and RCO+CO2, MAP and SBP (B and D) increased in HF compared with controls (p<0.05), however, DBP (F) increased in both groups. *Significant across conditions (NR vs. RCO or RCO+CO2). **Significant between groups (HF and CTL)
Similar to MAP, SBP increased more for CTLs than HF immediately after exercise (time × group, p<0.001, Figure 1C) and continued to increase in HF during post-exercise RCO and RCO+CO2, but decreased in CTLs (p<0.01, Figure 1D). There was no difference in SBP between RCO and RCO+CO2 (p=0.11). The SBP return to baseline was attenuated in HF compared to CTL during NR (p<0.05).
Diastolic pressure increased for CTLs and HF (time effect, p=0.001) similarly (time × group, p=0.12, Figure 1E) and continued to increase for both groups in response to RCO compared to NR (p<0.001, Figure 1F) with no difference between groups (p>0.05), nor where there differences between RCO and RCO+CO2 (p<0.05). Although, not significant, DBP return to baseline was attenuated in HF compared with CTL during NR (p=0.06).
Heart Rate, O2 Pulse and Rate Pressure Product
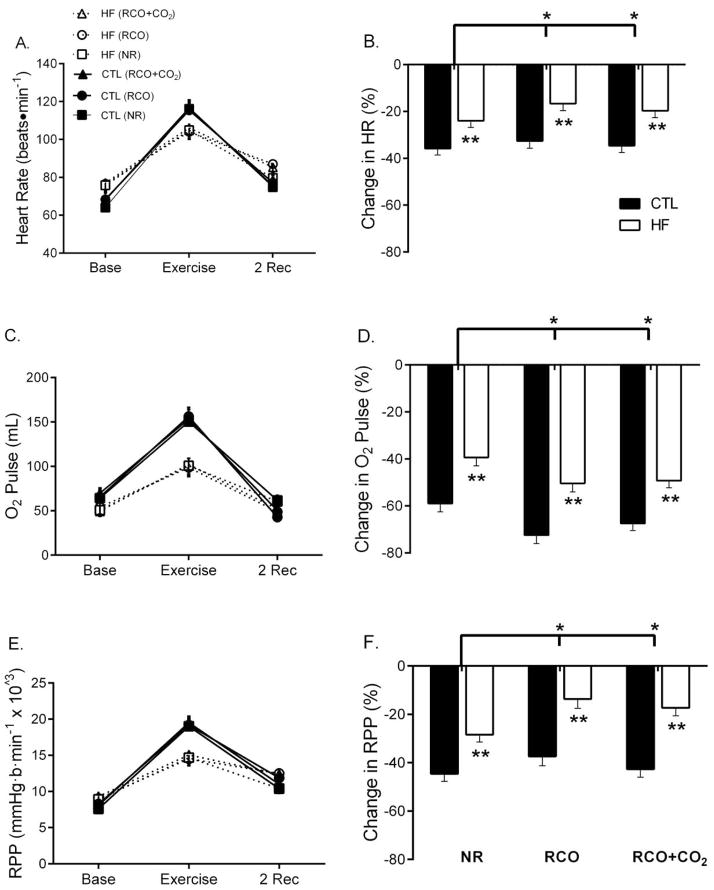
HR increased more in CTL than HF after exercise (time × group, p<0.001, Figure 2A). In response to RCO, HR had an attenuated return to baseline in HF, compared with CTLs, p=0.002, Figure 2B). HR return to baseline was greater during RCO+CO2 compared with RCO (p=0.01).
Figure 2.
Heart rate (HR), O2 pulse and rate pressure product (RPP) at baseline, end-exercise and 2 minutes of normal recovery (NR-square), regional circulatory occlusion (RCO-circular), or RCO+CO2 (triangular). Heart failure (HF) had less of an increase in HR, O2 pulse and RPP compared with CTLs during exercise (p<0.05, A, C, E). In all three conditions, HF had an attenuated return of HR, O2 Pulse and RPP compared with controls (p<0.05). The recovery of HR and RPP (B and F) was attenuated during post-exercise RCO and RCO+CO2 (p<0.05). In contrast, the recovery of O2 pulse was greater during post-exercise RCO and RCO+CO2 compared with NR (p<0.05). *Significant across conditions (NR vs. RCO or RCO+CO2). **Significant between groups (HF and CTL)
O2 pulse, an indicator of stroke volume17, increased more for CTLs than HF with exercise (time × group, p=0.004, Figure 2C) and was lower in HF (group effect, p=0.003). O2 pulse had less of a return to baseline in HF compared with CTLs during NR (p<0.001). In response to RCO, O2 pulse had a greater return to baseline compared with NR in HF (p<0.01, Figure 2D). O2 pulse recovered more during RCO compared with RCO+CO2 in HF (p=0.07).
HF had less of an increase in RPP from baseline to end exercise compared with CTLs (p<0.001, Figure 2E). During NR, HF had less of a return of RPP to baseline compared with CTLs (p=0.001, Figure 1F). This attenuated response was greater in RCO for both HF and CTLs (p<0.001), although had a greater return to baseline when CO2 was added to the inspirate (p=0.002).
DISCUSSION
The novel finding from this study is that HF patients display an exaggerated pressor response to metaboreflex stimulation after moderate-intensity lower-extremity dynamic exercise. Specifically, both MAP and SBP continued to rise throughout the post-exercise RCO in HF, in contrast to the CTLs who had an attenuated recovery. Despite these differences between HF and CTLs, DBP continued to increase in both groups in response to metaboreflex stimulation during post-exercise RCO.
Further, HR, O2 pulse and RPP had an attenuated return to baseline during NR in HF compared with CTLs. In response to metaboreflex stimulation (RCO) however, HR and RPP remained elevated compared with NR and more so in HF compared with CTLs. O2 pulse, in contrast, had a greater return to baseline in response to metaboreflex stimulation, and more so with CO2 added to the inspirate. Overall, O2 pulse recovery was less for HF in all conditions.
The majority of the work investigating the exercise pressor reflex has been in upper extremity models11, 18, 19. The metaboreflex response in HF patients demonstrates non-uniformity between muscle groups20 and therefore the lower and upper extremity most likely do not elicit similar responses to muscle afferent stimulation. As such, the pressor response was found to be lower with post-exercise RCO in the plantar flexors compared with the forearm muscles, a finding that was similar in HF and CTLs20. Interestingly, the faster relaxation rates found in the plantar flexor muscles of HF patients suggest a greater proportion of type II muscle fibers. Therefore, greater glycolytic activity with exercise in HF and a greater stimulus for the pressor response might have been expected in that study21. In this context, we found that with cycling, which involves coordinated activation of muscle groups, the post-exercise pressor response was greater in HF compared with CTLs.
Perfusion pressure during exercise
In order to maintain adequate blood pressure, peripheral vasculature dilation is precisely linked to cardiac output. If peripheral blood flow capacity is greater than cardiac output22 then some controlling influence must be interposed between the cardiac and vasculature systems to maintain blood pressure, thereby ensuring that cerebral, myocardial and skeletal muscle perfusion (particularly during exercise) is preserved. This regulatory function is achieved via the complex interplay between efferent sympathetic activation (vasoconstrictor drive) and local autoregulatory processes (vasodilator drive) leading to redistribution of blood flow to match supply and demand9. As such, the sympathetic nervous system is increased during exercise. This contributes to an increase in HR, peripheral vasoconstriction and subsequently higher blood pressure23. In response to metaboreflex stimulation in this study, MAP increased by ~7% in HF and decreased by ~3% in CTLs. Importantly, the pressor response was somewhat maintained in response to metaboreflex stimulation in CTLs, but consistent with our hypothesis it was exaggerated in HF.
Mechanisms contributing to the exercise pressor reflex
There are two main theoretical mechanisms detailing neural control of the cardiovascular system during exercise. The first, central command, suggests that central motor drive and sympathetic activation occur in parallel. This suggests a link to skeletal muscle metabolic need via the pattern of motor unit recruitment, i.e. as muscle fatigues, more motor units are recruited and a commensurate increase in sympathetic outflow occurs24.
During exercise, HR was lower for HF compared with CTLs. This is likely due to the blunting effect of pharmacotherapy (beta-blockers) and/or due to the fact that the patients were exercising at a lower absolute workload (HF: 55±17 watts, and CTL: 132±44 watts). In response to metaboreflex stimulation, however; HR remained at greater levels in HF compared with CTLs. The CTLs had a near full recovery of HR during metaboreflex stimulation. This response in the CTLs is most likely due to the rise in sympathetic activity being masked by the concomitant return of parasympathetic outflow. This is consistent with the reduction of central command and activation of the arterial baroreflex post-exercise, which responds to the metaboreflex-induced increase in blood pressure by buffering the elevated sympathetic drive to the cardiac and arteriolar vessels25. Because HF patients have reduced baroreflex sensitivity26, parasympathetic activity was likely attenuated and HR remained elevated during metaboreflex stimulation.
The second theory suggests that stimulation of mechanically and metabolically afferents in the exercising muscle results in increased afferent feedback which modulates central command and evokes the “exercise pressor reflex”27, 28. The mechanoreceptor is thought to be located within the interstitial space, in close proximity to collagen bundles, within and between muscle fibers, within the muscle tendon, and within the adventia of the vasculature 18, 29, 30. Therefore, the mechanoreceptor is sensitive to fiber recruitment, muscle length, rate of length change and relative tension applied to the muscle. In contrast, the metaboreceptors are located in close proximity to lymphatic and blood vessels of muscle and tendons, making these receptors ideal for chemotransduction. These two arms of the exercise pressor reflex have been identified in both animal and human models28.
In humans, the influence of this reflex is clearly recognized as an important component of cardiovascular and ventilatory control during exercise28. For example, there is a greater contribution of the mechano- and metaboreflex to exercise ventilation in HF9, 31. A compensatory adaptation to reduced cardiac output in HF is the development of intrinsic skeletal muscle abnormalities, specifically, loss of oxidative capacity and gain of glycolytic capacity. Overall, skeletal muscle atrophy and fibrosis occurs, reducing mitochondrial density, volume and mitochondrial cristae surface area32. These changes lead to early onset fatigue and excessive cardiovascular and ventilatory responses to exercise, ultimately contributing to exercise intolerance in HF2.
In the present study, consistent with the increased exercise pressor reflex, MAP remained elevated in CTLs during metaboreflex stimulation. It appears that this elevation was predominately due to increased DBP since SBP began to decline towards resting levels. The differential response between systolic and diastolic pressure in the CTLs is not clear. This may reflect more rapid return of HR and O2 pulse towards baseline during systole with maintenance of tonic vasoconstrictor activity during diastole. In HF, however; we observed an increase in blood pressure with RCO despite the interaction with blood pressure attenuating medications suggesting that the pressor response may be underestimated in this patient group. With the exception of the findings reported by Carrington et al., (2004), the MAP response to metaboreflex stimulation in HF patients has only been demonstrated in the upper extremity19, 31, 33. Carrington et al., did not show differences between CTLs and HF in the pressor response to for the plantar flexor muscles20. The lower extremity is functionally relevant to activities of daily living (e.g. walking and climbing stairs); therefore, the contribution of the exercise pressor reflex for the knee extensor muscles during exercise in HF is important. The differential findings between Carrington et al. and the present study may be a result of differences in methodology and muscle groups stimulated. Importantly, the somatosensory feedback from large locomotor muscles during exercise may be one mechanism contributing to exercise intolerance in HF.
Technical considerations
The advantages and disadvantages of using RCO to stimulate metaboreflex have been discussed elsewhere34. Briefly, RCO allows for stimulation of the metaboreflex via trapping of metabolic by-products in the vicinity of the metaboreceptors. This is typically done immediately at cessation of exercise, after metabolite accumulation, while limiting the influence of central command and mechanoreflex activity. There is, however, evidence to suggest that certain metabolic products may stimulate mechanoreceptors in rats with HF35. In this context, it is difficult to rule out any mechanoreflex influence on the increase in MAP with RCO.
When trapping metabolites from large locomotor muscle groups, normal feedback of CO2 from these muscles to traditional CO2 sensitive central chemoreceptors is inhibited due to lack of venous return. Thus, during one of our recovery sessions, CO2 was added to the inspirate to clamp PETCO2 at end-exercise levels to maintain normal CO2 delivery to the lungs. In doing so, we mitigated the influence of any reduction in ventilation and potential effects on the pressor response from the lack of traditional chemoreceptor stimulation. We found no differences for either HF or CTLs in the pressor response to RCO and RCO+CO2 as has often been found in ventilation and PETCO2. There were, however, small but significant differences in HR (25% reduction for RCO and 27% reduction for RCO+CO2 for HF and CTLs combined) and RPP (25% reduction for RCO and 30% reduction for RCO+CO2 for HF and CTLs combined) when CO2 was added to the inspirate during RCO for both groups, suggesting HR and RPP recovered more quickly during RCO+CO2. The PETCO2 was reduced in RCO compared with RCO+CO2, with no difference between NR and RCO+CO216. This highlights the interaction between the chemoreflex and metaboreflex. The hypocapnic response during RCO may have slightly enhanced metaboreflex activation of HR and RPP. Therefore, these findings suggest that during hypocapnia, the metaboreflex may cause further sympathoexcitation in both HF and CTLs. This has significant clinical implications for HF patients who often demonstrate mild hyperventilation with chronically reduced arterial CO2 levels (PaCO2).
Finally, despite the modestly reduced ejection fraction in this cohort, the patients in this study were of NYHA classes I and II and thus may underrepresent the impact of metaboreceptor stimulation on blood pressure compared with sicker patients. Further, despite being adequately powered, a potential limitation is the small sample size with a slight, but non-significant difference in age between groups; thus, further studies with larger sample sizes are encouraged to ensure the underlying assumptions of the analyses hold true.
CONCLUSION
The findings from this study suggest metaboreflex stimulation of the lower-extremity contributes to the exercise pressor response in HF. This was accompanied by an elevated HR, most likely due to a lack of buffering from the baroreflex and subsequently reduced parasympathetic activity. The overall impact of this response may result in greater myocardial work, excessive vasoconstriction, and contribute to early onset fatigue in HF patients. This study is clinically relevant because it employs a more functional lower-extremity model with exercise-intensities equivalent to those that are performed during activities of daily living. In addition, this study provides greater insight into the mechanisms contributing to the pressor response in HF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–49. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JR, Mancini DM. Factors contributing to the exercise limitation of heart failure. J Am Coll Cardiol. 1993;22(4 Suppl A):93A–98A. doi: 10.1016/0735-1097(93)90469-h. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JR, Martin JL, et al. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984;69(6):1079–87. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]
- 4.Belli JF, Bacal F, et al. Ergoreflex activity in heart failure. Arquivos brasileiros de cardiologia. 2011;97(2):171–8. doi: 10.1590/s0066-782x2011005000072. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JH, Kaufman MP, et al. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–42. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 6.Ichinose MJ, Sala-Mercado JA, et al. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol. 2010;298(1):H245–50. doi: 10.1152/ajpheart.00909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancini DM, Walter G, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85(4):1364–73. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 8.Piepoli M, Ponikowski P, et al. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. American heart journal. 1999;137(6):1050–6. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- 9.Olson TP, Joyner MJ, et al. Effects of respiratory muscle work on blood flow distribution during exercise in heart failure. J Physiol. 2010;588(Pt 13):2487–501. doi: 10.1113/jphysiol.2009.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silber DH, Sutliff G, et al. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol. 1998;84(5):1551–9. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 11.Shoemaker JK, Kunselman AR, et al. Maintained exercise pressor response in heart failure. J Appl Physiol. 1998;85(5):1793–9. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- 12.Sterns DA, Ettinger SM, et al. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84(5):2034–9. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 13.Wasmund WL, Westerholm EC, et al. Interactive effects of mental and physical stress on cardiovascular control. J Appl Physiol. 2002;92(5):1828–34. doi: 10.1152/japplphysiol.00019.2001. [DOI] [PubMed] [Google Scholar]
- 14.Davies LC, Francis DP, et al. Abnormal temporal dynamics of blood pressure and RR interval regulation in patients with chronic heart failure: relationship to baroreflex sensitivity. Int J Cardiol. 2002;86(1):107–14. doi: 10.1016/s0167-5273(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 15.Scott AC, Francis DP, et al. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J Physiol. 2000;529(Pt 3):863–70. doi: 10.1111/j.1469-7793.2000.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson TP, Joyner MJ, et al. Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circulation Heart failure. 2010;3(2):212–9. doi: 10.1161/CIRCHEARTFAILURE.109.879684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavie CJ, Milani RV, et al. Peak exercise oxygen pulse and prognosis in chronic heart failure. Am J Cardiol. 2004;93(5):588–93. doi: 10.1016/j.amjcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 18.McClain J, Hardy C, et al. Limb congestion and sympathoexcitation during exercise. Implications for congestive heart failure. J Clin Invest. 1993;92(5):2353–9. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piepoli M, Clark AL, et al. Muscle metaboreceptors in hemodynamic, autonomic, and ventilatory responses to exercise in men. Am J Physiol. 1995;269(4 Pt 2):H1428–36. doi: 10.1152/ajpheart.1995.269.4.H1428. [DOI] [PubMed] [Google Scholar]
- 20.Carrington CA, Fisher JP, et al. Muscle afferent inputs to cardiovascular control during isometric exercise vary with muscle group in patients with chronic heart failure. Clinical science. 2004;107(2):197–204. doi: 10.1042/CS20040038. [DOI] [PubMed] [Google Scholar]
- 21.Petrofsky JS, Phillips CA, et al. Muscle fiber recruitment and blood pressure response to isometric exercise. Journal of applied physiology: respiratory, environmental and exercise physiology. 1981;50(1):32–7. doi: 10.1152/jappl.1981.50.1.32. [DOI] [PubMed] [Google Scholar]
- 22.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–49. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seals DR, Victor RG, et al. Plasma norepinephrine and muscle sympathetic discharge during rhythmic exercise in humans. J Appl Physiol. 1988;65(2):940–4. doi: 10.1152/jappl.1988.65.2.940. [DOI] [PubMed] [Google Scholar]
- 24.Kahn JF, Jouanin JC, et al. Complementary roles of central command and muscular reflex in the regulation of heart rate during submaximal isometric contraction. Electromyogr Clin Neurophysiol. 1992;32(1–2):3–10. [PubMed] [Google Scholar]
- 25.Pagani M, Pizzinelli P, et al. Baroreflex and metaboreflex control of cardiovascular system during exercise in space. Respir Physiol Neurobiol. 2009;169 (Suppl 1):S42–5. doi: 10.1016/j.resp.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Creager MA. Baroreceptor reflex function in congestive heart failure. Am J Cardiol. 1992;69(18):10G–15G. doi: 10.1016/0002-9149(92)91250-8. discussion 15G–16G. [DOI] [PubMed] [Google Scholar]
- 27.Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69(2):407–18. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 28.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224(1):173–86. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haouzi P, Hill JM, et al. Responses of group III and IV muscle afferents to distension of the peripheral vascular bed. J Appl Physiol. 1999;87(2):545–53. doi: 10.1152/jappl.1999.87.2.545. [DOI] [PubMed] [Google Scholar]
- 30.Cui J, Leuenberger UA, et al. Sympathetic and cardiovascular responses to venous distension in an occluded limb. Am J Physiol Regul Integr Comp Physiol. 2011;301(6):R1831–7. doi: 10.1152/ajpregu.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piepoli M, Clark AL, et al. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93(5):940–52. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan MJ, Green HJ, et al. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991;84(4):1597–607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- 33.Crisafulli A, Scott AC, et al. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc. 2003;35(2):221–8. doi: 10.1249/01.MSS.0000048639.02548.24. discussion 229. [DOI] [PubMed] [Google Scholar]
- 34.Piepoli MF, Dimopoulos K, et al. Cardiovascular and ventilatory control during exercise in chronic heart failure: role of muscle reflexes. Int J Cardiol. 2008;130(1):3–10. doi: 10.1016/j.ijcard.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Morales A, Gao W, et al. Muscle cyclo-oxygenase-2 pathway contributes to the exaggerated muscle mechanoreflex in rats with congestive heart failure. Exp Physiol. 2012;97(8):943–54. doi: 10.1113/expphysiol.2012.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]