Abstract
Major depression is characterized by low mood, a reduced ability to experience pleasure and frequent cognitive, physiological and high anxiety symptoms. It is also the leading cause of years lost due to disability worldwide in women and men, reflecting a lifelong trajectory of recurring episodes, increasing severity and progressive treatment resistance. Yet, antidepressant drugs at best treat only one out of every two patients and have not fundamentally changed since their discovery by chance >50 yr ago. This status quo may reflect an exaggerated emphasis on a categorical disease classification that was not intended for biological research and on oversimplified gene-to-disease models for complex illnesses. Indeed, genetic, molecular and cellular findings in major depression suggest shared risk and continuous pathological changes with other brain-related disorders. So, an alternative is that pathological findings in major depression reflect changes in vulnerable brain-related biological modules, each with their own aetiological factors, pathogenic mechanisms and biological/environment moderators. In this model, pathological entities have low specificity for major depression and instead co-occur, combine and interact within individual subjects across disorders, contributing to the expression of biological endophenotypes and potentially clinical symptom dimensions. Here, we discuss current limitations in depression research, review concepts of gene-to-disease biological scales and summarize human post-mortem brain findings related to pyramidal neurons, γ-amino butyric acid neurons, astrocytes and oligodendrocytes, as prototypical brain circuit biological modules. Finally we discuss nested aetiological factors and implications for dimensional pathology. Evidence suggests that a focus on local cell circuits may provide an appropriate integration point and a critical link between underlying molecular mechanisms and neural network dysfunction in major depression.
Keywords: Corticolimbic, dimensional, major depression, microcircuitry, post-mortem
A categorical (vs. biological) definition of major depression
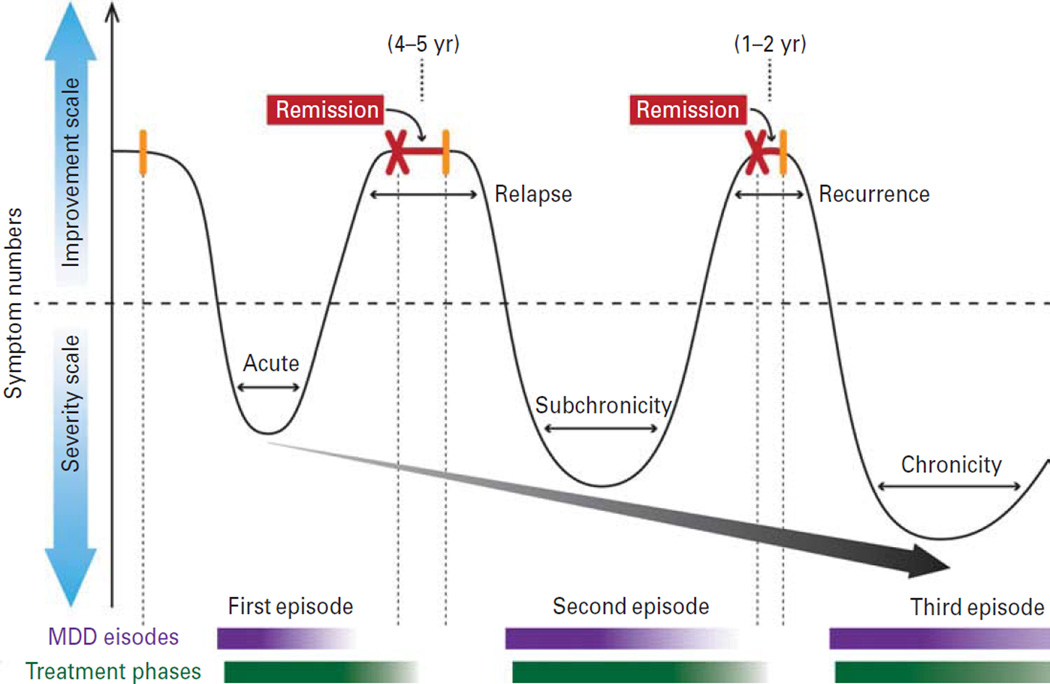
The classification of mental illnesses, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) in the USA and the International Statistical Classification of Diseases and Related Health Problems internationally, was borne out of the necessity to provide a common language for descriptive purposes and delivery of care (Fischer, 2012). This categorical system is broad and somewhat arbitrary, but has been successful in clinical practices and overall for the health care system. Yet, although a biological basis was not a factor in the initial classification, the system has fostered a general attitude that psychiatric disorders are biologically distinct. As defined by DSM-IV, major depressive disorder (MDD) is diagnosed by a variable set of five symptoms for a continuous 2-wk period (APA, 2000). Depressed mood or reduced interest in activities previously enjoyable (anhedonia) represent core symptoms, but other cognitive (attention, concentration, recurrent thoughts of suicide) and physiological (weight, locomotor and sleep pattern changes) symptoms need to co-occur. Depression is a biological syndrome that affects the brain and possibly peripheral organs also (Musselman et al., 1998; Ciechanowski et al., 2000; Steptoe and Whitehead, 2005; McIntyre et al., 2007), but whether its clinical definition corresponds to a coherent biological entity and whether ‘categorical’ is the appropriate model for characterizing the biological underpinnings of depression, remain open questions. The lifelong trajectory of depression does provide strong evidence for a biological basis (Fig. 1). For many patients, this includes recurring episodes with increasing symptom severity, longer duration, shorter or partial remission period and increasing resistance to antidepressant modalities, together leading to states of chronic and treatment-resistant depression (Kessler et al., 2005b; Moylan et al., 2012). This suggests a progressive strengthening of biological underpinnings over time, although the nature of these lifelong pathological changes is not known. Finally, although most research implicitly assumes a framework based on depression being a single entity, the primary evidence of biological disturbances observed in depressed subjects suggests a more equivocal picture. For general reviews on depression, see Pariante (2009) and López-Muñoz and Álamo González (2012).
Fig. 1.
Schematic representation of a common trajectory towards chronic recurrent depression. Major depression frequently follows a lifelong and recurrent trajectory, with many features of a neuroprogressive disease, such as recurring episodes of increasing severity, reduced therapeutic response and shorter remission period. Depressive episodes are represented by the valleys in the curve (high on severity scale), whereas each peak represents periods of remission (high on improvement scale). MDD, Major depressive disorder.
Molecular vs. syndromal specificity of pathological mechanisms from a multi-scale biological perspective
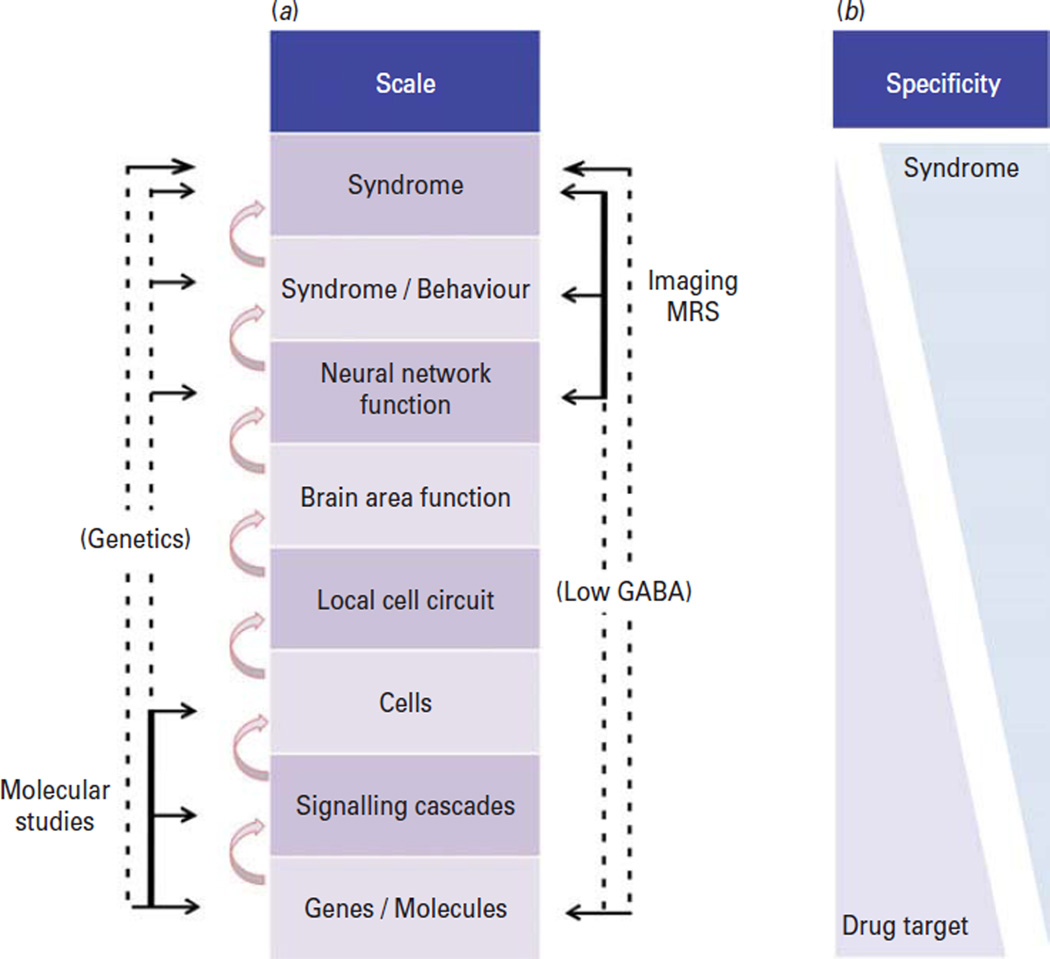
Neuropsychiatric disorders are unique in the span of affected biological scales (Fig. 2a). Characterized in a top-down fashion, they are defined at the highest level by complex behavioural symptoms, differing from diseases of other organ systems, which are defined at lower biological scales by aberrations in gene function, signal transduction and cellular growth (e.g. cancer) or peptide/hormone production and release (e.g. insulin function in diabetes) and for which any behavioural syndromes associated with the diseases are secondary to their clinical pathophysiological core. In depression, clinical and neuroimaging studies operate at the top of biological scales, whereas molecular and cellular research uses bottom-up approaches (Fig. 2a). Accordingly, the former has the highest syndromal specificity and the latter displays highest specificity for genes, molecules, signalling cascades and cells. Therapeutic agents, such as serotonin reuptake inhibitors (SSRI), similarly have high specificity for molecules and low specificity for complex heterogeneous clinical syndromes (Fig. 2b), displaying moderate efficacy for a range of psychiatric and systemic disorders (Wong and Licinio, 2001; Warden et al., 2007). To move forward in therapeutic discovery, it will be critical to develop fully integrated models, informed by detailed knowledge of the human pathology within respective biological scales, that bridge top-down clinical with bottom-up molecular findings and that are predictive across biological scales.
Fig. 2.
A multi-scale biological perspective on mechanisms of depression and its relationship to specificity of drug treatment and diagnoses. (a) The functionality of complex biological systems emerges from the integration of events occurring across hierarchical biological scales, starting from the bottom with genes, molecules, cells, microcircuits, and neural networks. Each scale depends on the function of level below, and determines the function of the level above. Feedback and modulation occur across levels and from the environment (not shown). Brain functions, behaviours, and endophenotypes emerge at the neural network levels, which then define individual symptoms and syndromes. Molecular and brain imaging studies investigate opposite ends of the spectrum (b) and can only speculate on mechanisms underlying causal links between those opposite ends (dashed lines). More direct links are provided by molecular genetics and imaging magnetic resonance spectroscopy (MRS) studies, but these studies do not inform on changes at intermediate scales. This results in inverse correlations between specificities of findings for disease and molecular/pharmacological studies (b). Therapeutic drugs have high specificities for their molecular targets, but lowest specificities in terms of treating complex behavioural syndromes; conversely, imaging and associated studies have high syndromal specificity (due to the Diagnostic and Statistical Manual of Mental Disorders-based clinical diagnosis), but low molecular target specificity (due to low consideration of biological phenotypes in diagnosis). GABA, γ-amino butyric acid.
Keeping biological complexity in mind
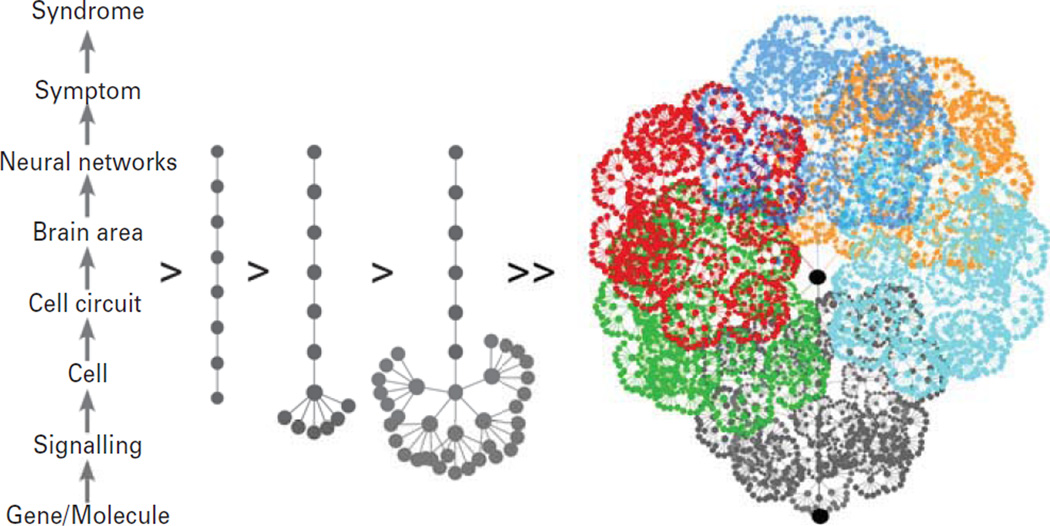
Given the opposing scales of diagnosis and treatment, it is not surprising that major depression has proven difficult to treat and that novel treatments have been rare. Reductionist approaches convert a multi-scale perspective into a linear pathway and posit that the disease is caused by an as-yet unknown biological factor (e.g. gene; Fig. 3), which, once identified and targeted, may treat the illness and associated symptoms. However, this gives the false impression of a straightforward linear pathway from gene to syndrome, whereas the reality is that molecular and cellular components assemble into local integrated functional units, defined here as biological modules, which are then combined and re-used in intricate networks, with the complexity increasing with each biological scale (Fig. 3, right). Biological modules differ from endophenotypes (Gottesman and Gould, 2003), which emerge behaviourally at the neural network level and above and therefore still functionally integrate various and complex sets of underlying biological modules. The gene-to-disease linear approach is supported by forward genetic studies in rodents, where single gene manipulations can cause complex rodent behavioural changes. However, neuropsychiatric disorders are complex diseases implicating large number of genes, which do not strictly follow classic Mendelian structure (Kendler, 2012) and screening for genetic impact on behavioural outcomes in rodent systems does not equate to identifying primary pathological changes in complex human brain disorders. Moreover, current rodent preclinical models used for screening and testing gene-to-behaviour paradigms and novel compounds are often oversimplified, streamlined and validated by monoaminergic pharmacological compounds with moderate efficacy in human subjects.
Fig. 3.
Complex biological pathways from gene to syndrome. In a linear gene-to-disease pathway (left), single genes code for syndromes in direct step-by-step upward trajectory, across all biological scales. However, in reality the biological complexity greatly increases with each higher scale. From the bottom-up, genes have multiple functions and coalesce in signalling pathways and structural entities in various combinations across cell types (left-to-right arrows). Cells assemble in local circuits, which are re-used, modified and repeated across brain regions. In turn, assemblies of these biological modules form the functional bases of brain areas and neural networks. The result is a massively interlinked and multi-scale network, whose output contributes to the expression of symptom dimensions, including mood, cognition and physiological changes in major depression (illustrated as various shades of colour). This redundancy in the use of genes and biological modules, combined with their putative distal impact on neural networks and putative symptom dimensions represents a core challenge in research on complex heterogeneous neuropsychiatric disorders, in which dominant roles of genes are rare and ambiguous, compared to neurodegenerative or peripheral organ diseases.
How do we move forward in identifying and characterizing biological bases of depression? Here, we focus on selected findings on the primary pathology of the illness, as directly identified in brains of human subjects with major depression. We place an emphasis on cells and local circuitry, as integrated functional units with intrinsic biological vulnerabilities implicated across psychiatric disorders. After briefly reviewing genetic findings in major depression, we focus on four cell-based biological modules emerging from post-mortem studies: altered pyramidal neuron structure; altered γ-amino butyric acid (GABA) circuitry; astrocyte dysfunction; oligodendrocyte dysfunction. Finally, we discuss disease-specificities and aetiological factors implicated in affected biological modules. Given the relevance of corticolimbic structures to low mood and affect dysregulation in depression (Seminowicz et al., 2004; Phillips et al., 2008; Price and Drevets, 2012) we limit the scope of the review to these structures.
Genetic findings in major depression
Unbiased large-scale studies seeking to identify conserved DNA variants across a population of affected individuals have provided only weak evidence for a core biological underpinning of major depression. In a recent mega-analysis of genome-wide association studies (GWAS) of 1.2 million single nucleotide polymorphisms in 9249 major depression cases and 9518 control subjects, no single nucleotide polymorphisms achieved genome-wide significance, nor did exploratory approaches provide robust leads for further studies (MDD GWAS Consortium, 2012). The negative findings were recently replicated by meta-analysis of GWAS using measures of depressive symptoms in large epidemiological cohorts (n = 34549; Hek et al., 2013). The interpretation of these and similar previous findings (Wray et al., 2010) is often a lack of statistical power due to the large number of variants investigated or the engagement of too many genes with very small effects, suggesting that sample sizes in the tens of thousands of subjects will be needed to detect significant gene variant effects (Hek et al., 2013). Alternative explanations are that the ‘soft’ nature of the disease characterization, together with a high heterogeneity and a robust environmental component, may prevent the identification of consistent biological findings (Wray et al., 2010). Yet this reflects circular reasoning, as the clinical cohorts that are chosen for these studies are based on the broad symptomatic definition of the illness, rather than on evidence of biological homogeneity. An alternative interpretation is that the association with depression as a distinct illness is weakened, either by the presence of behavioural endophenotypes or subtypes of depression, or at a lower level by the recruitment of various biological modules which are often similarly affected across other major mental disorders, albeit in different combinations, together contributing to the apparent low (or diluted) heritability of depression (~40%; Kessler et al., 2005a). Indeed, there is some GWAS-based evidence for shared genetic vulnerability across major mental illnesses, consistent with a dimensional basis of risk (Schulze et al., 2012).
Studies of variants associated with single genes are mostly equivocal in terms of effect sizes and specificity. For instance, studies of the promoter polymorphism of the serotonin transporter (SERT) suggest a weak moderating effect on major depression and associated traits and more robust effects on neural endophenotypes that relate to general risk for psychopathology and vulnerability to affective disorders (Canli and Lesch, 2007). Conversely, rare coding or gain-or-function mutation in genes such as SERT (Ozaki et al., 2003), monoamine oxidase A (Brunner et al., 1993) and disrupted in schizophrenia (Millar et al., 2005) implicate single genes in severe major mental illnesses, but the observed phenotypes are complex, mixed and often severe, including combinations of depression, obsessive-compulsive disorder, substance abuse disorders, with aggression, schizoaffective and psychotic features, and in some cases suggesting strong developmental contributions. Together these studies significantly contributed to lowering boundaries between major depression and other categorical psychiatric disorders and provided leads for investigating pathophysiological mechanisms with implications beyond their original areas of investigation (Brandon and Sawa, 2011).
Altered cortical neuronal structure in major depression
In contrast to neurodegenerative disorders, depression does not include major cell loss. However, studies have reported reduced density of neuronal cell bodies with large cell body size in cortical layers 2–5 of the orbitofrontal cortex (OFC) and in layers 2, 3 and 6 of the dorsolateral prefrontal cortex (dlPFC), concurrent with increased density of small body size neurons in layer 3 (OFC) and layers 3 and 6 (dlPFC; Rajkowska et al., 1999; Rajkowska, 2000). Region-specific reports of decreased mean neuronal cell body size include layers 3 and 6 (dlPFC), layers 2 and 3 (OFC) and layer 6 [anterior cingulate cortex (ACC); Rajkowska et al., 1999; Cotter et al., 2001]. These findings are consistent with lower combined neuron density in both dorsal and ventral PFC in depressed suicides (Underwood et al., 2012) and reduced neuronal size in layer 6 of the dlPFC in major depression (Cotter et al., 2002a). In a study designed for more focused analysis of cell-specific changes, lower density of N200-positive pyramidal neurons was found in the dlPFC [Brodmann area (BA) 9, 32, 46] in major depression compared to bipolar disorder and schizophrenia, although post hoc comparisons between controls and depressed subjects were not significant (Law and Harrison, 2003). No difference in packing density of pyramidal neurons in layer 3 of the BA 9 between depressed and control subjects was found in a separate study using NF200 as a pyramidal marker (Miguel-Hidalgo et al., 2005). For cohorts of elderly MDD or older mood-disorder subjects, reduced pyramidal neuron density in layers 3 and 5 of the OFC (Rajkowska et al., 2005) and layer 5b of the ACC (Gittins and Harrison, 2011) but not in the dlPFC (Van Otterloo et al., 2009), was reported.
Although it appears unlikely that neuronal loss underlies these changes, whether decreased neuronal density reflects changes in neuropil and dendritic complexity is not known. Using Golgi staining of neuronal processes, reduced numbers of third-order basilar dendritic branches were observed in ACC layer 6 of depressed suicide victims, although the subgroup with major depression demonstrated only a trend towards reduction (Hercher et al., 2010). Furthermore, a decrease in total dendritic length and somal area was observed in deep and superficial layer 3, respectively, in dlPFC in a cohort enriched in MDD patients (Glantz and Lewis, 2000). Reduced density of synapses was also observed by electron microscopy in frontal cortex in a small cohort of MDD patients (Kang et al., 2012), together supporting the hypothesis of altered neuronal densities and potentially reduced dendritic complexity in depression. However, further studies are needed in larger post-mortem cohorts to confirm and firmly establish these findings.
Altered glutamate homeostasis in major depression
Large cortical neurons contain glutamate and are excitatory, but glutamatergic function cannot be measured under post-mortem conditions. In vivo glutamate levels can be measured using proton magnetic resonance spectroscopy (1H-MRS). MRS studies frequently report glutamate-related metabolite levels using the combined term Glx (Valentine and Sanacora, 2009; Maddock and Buonocore, 2012). As reviewed in (Yuksel and Ongur, 2010), MRS studies have shown reduced Glx and/or glutamate concentrations across multiple cortical and subcortical brain regions, including ACC (Auer et al., 2000; Pfleiderer et al., 2003; Zhang et al., 2012); PFC (Michael et al., 2003a; Hasler et al., 2007), amygdala (Michael et al., 2003b) and hippocampus (Block et al., 2009), although others have found no change in the hippocampus (Milne et al., 2009) and occipital cortex (Price et al., 2009) and even increased glutamate levels (Sanacora et al., 2004) in occipital cortex. In several of these studies, both Glx (Michael et al., 2003a; Pfleiderer et al., 2003) and glutamate (Zhang et al., 2012) were shown to increase with electroconvulsive therapy (ECT) treatment response. Although these findings present strong evidence that glutamate, glutamine and related metabolites are altered in brain tissue of depressed patients, given that only a small amount of measured metabolites are synaptic, any conclusions about altered glutamatergic neurotransmission on the basis of these studies would be premature (Sanacora et al., 2012).
Together, evidence of reduced neuronal density and dendritic morphology, altered glutamatergic signalling, reduced neurotrophic support through low brain-derived neurotrophic factor (BDNF) function (Duman et al., 2000) and of the rapid antidepressant effects of ketamine (Berman et al., 2000; Zarate et al., 2006), a glutamatergic NMDA receptor antagonist, have contributed to the neurotrophin hypothesis of major depression. Note that this first potential major breakthrough for novel and efficacious antidepressant modalities in >50 yr, i.e. ketamine-related NMDA antagonism, does not rely on direct evidence for dysregulated NMDA function in major depression, but rather on clinical observations and recently on a growing number of rodent pre-clinical studies (Li et al., 2010; Autry et al., 2011). This long delay between identifying novel therapeutic modalities highlights the need to focus on characterization of the primary pathology of the illness, in order to accelerate target and mechanism identifications.
Low GABA and reduced markers of dendritic-targeting GABA neurons in major depression
Studies using cellular markers for small cortical neurons have reported a reduction in the density of GABA neurons immunoreactive for specific calcium binding proteins in the dlPFC in major depression (Rajkowska et al., 2007), but for negative findings, see also Beasley et al., (2002) and Cotter et al., (2002b). The density of calbindin (CB)-positive neurons was reduced by 50% in dlPFC, but no difference in parvalbumin (PV)-positive neurons were observed. Recently, our group has reported reduced somatostatin (SST), a modulatory neuropeptide, in the dlPFC (Sibille et al., 2011), subgenual (sg) ACC (Tripp et al., 2011, 2012) and amygdala (Guilloux et al., 2011) of subjects with major depression. These studies are consistent with the prior CB-related findings since SST is mostly expressed in CB-positive cells (Hayes et al., 1991; Conde et al., 1994; DeFelipe, 1997; reviewed in Viollet et al., 2008). SST GABA neurons target pyramidal neuron dendrites. Interestingly, neuropeptide Y (NPY) and cortistatin, two neuropeptides that also target dendrites, showed similar low expression in the sgACC and amygdala (Guilloux et al., 2011; Tripp et al., 2012). In contrast, other GABAergic cellular markers, including cholecystokinin (CCK) and calretinin (CR) were unaffected in ACC and amygdala in depression, although PV expression was lower in ACC but not in dlPFC (Sibille et al., 2011; Tripp et al., 2012). PV and CCK neurons target pyramidal cell bodies and axon initial segment, while CR neurons target other GABA neurons. Expression of genes coding for GABA-producing enzyme (GAD65 and GAD67) has been reported either unchanged or reduced in combined grey matter samples in major depression (Sibille et al., 2011; Tripp et al., 2012). These results suggest selective changes affecting the CB/SST-positive and potentially other dendritic targeting GABA neuron subtypes, although studies using double markers or laser captured cells will need to confirm the extent of cell type-specific deficits.
The findings are consistent with reports of decreased GABA concentration in subjects with major depression as observed by 1H-MRS in occipital and frontal cortices or by transcranial magnetic stimulation (Hasler et al., 2007; Levinson et al., 2010; Hasler and Northoff, 2011; Gabbay et al., 2012), which was reversed after therapy with SSRI or ECT (Sanacora et al., 2002, 2003), hence correlating with the depressed state. Reduced ACC GABA levels also correlated with measures of anhedonia across depressed and control adolescents (Gabbay et al., 2012). Studies combining functional imaging and resting-state MRS suggest that the concentration of GABA in the ACC mediates default-mode network negative responses during emotion processing (Northoff et al., 2007). Hence, converging evidence from several biological scales, supported by early studies showing low GABA in the plasma of depressed subjects (Gold et al., 1980) and genetic manipulation studies in rodents (Earnheart et al., 2007), have together formed the basis of a GABA hypothesis of emotion dysregulation in depression (Brambilla et al., 2003; Luscher et al., 2011).
Astrocyte-related findings in major depression
Observations of a marked 24% decrease in number of sgACC (BA 24) glial cells, concurrent with decreased cell density, in patients with familial major depression provided early evidence for glial changes in depression (Ongur et al., 1998). Several studies have reported reduced glial cell density in the dlPFC and ACC in major depression (Rajkowska et al., 1999; Cotter et al., 2001, 2002a) and low glial numbers in the amygdala (Bowley et al., 2002); whereas others identified no changes in overall glial cells in OFC and dlPFC in late-life depression (Khundakar et al., 2009, 2011). Interestingly, a meta-analysis of 174 subjects revealed increased levels of serum S100B, a glial marker protein expressed in astrocytes, but also in oligodendrocytes (Hachem et al., 2005), in major depression and bipolar disorder; higher levels were also observed in older subjects compared to younger subjects with mood disorders (Schroeter et al., 2011).
Astrocytes are glial cells that primarily regulate the neuronal chemical environment and extracellular clearance of glutamate and partly GABA (Brodal, 2010). Investigations of astrocyte-specific glial pathologies suggest cellular hypertrophy in ACC white matter (Torres-Platas et al., 2011). However, decreases in glial fibrillary acidic protein (GFAP), another astrocytic marker, and in glutamate clearance transporters (EAAT1, EAAT2) expressed in astrocytes have also been observed in PFC of subjects with major depression (Miguel-Hidalgo et al., 2000, 2010; Si et al., 2004; Choudary et al., 2005). GFAP expression increases with age (Nichols et al., 1993) and diagnostic-based differences in GFAP levels were not observed in older depressed subjects (Si et al., 2004). Expression of astrocyte connexins 43 and 30 were down-regulated in suicide victims with a range of psychiatric diagnoses, including bipolar disorder, schizophrenia and major depression (Ernst et al., 2011). Connexins 43 and 30 mediate gap junction-based direct communication between astrocytes and also participate in astrocyte–oligodendrocyte junctions (Orthmann-Murphy et al., 2008). Together, these findings suggest dysregulated astrocytic function in depression, leading to a model in which alterations in glutamate/GABA neurotransmission and loss of GABA-mediated inhibition, may be partially explained by astrocytic deficits, specifically related to neurotransmitter recycling and homeostasis (Valentine and Sanacora, 2009; Oh et al., 2012).
Reduced oligodendrocyte numbers in major depression
Using morphological cell-type determination, reports have suggested that the decreased glial cell numbers in amygdala and PFC may be attributed to reduced oligodendrocytes (Hamidi et al., 2004; Uranova et al., 2004). Oligodendrocytes are glial cells that form myelin sheaths around axons, thereby facilitating axonal conduction (Brodal, 2010). A gene array study in the amygdala of male subjects with major depression from our laboratory showed reduced expression of numerous genes related to oligodendrocyte structure and function (Sibille et al., 2009), consistent with reduced expression of similar transcripts in the PFC in other studies in major depression (Klempan et al., 2009; Kim and Webster, 2010). Decreased oligodendrocytes were also reported by flow cytometry, using fluorescently labelled suspended nuclei from the frontopolar cortex in major depression (Hayashi et al., 2011). However, down-regulation of oligodendrocyte transcripts was not reported for the ACC (Sibille et al., 2009) or amygdala in female subjects (Guilloux et al., 2011). Due to their role in supporting axonal conduction and of specific dysregulation of genes coding for proteins located at the nodes of Ranvier, dysregulated oligodendrocyte function has been suggested to mediate impaired communication and altered integrity of neuronal information transfer in major depression (Edgar and Sibille, 2012).
Disease-independent and module-specific cellular pathologies
The post-mortem evidence summarized above suggests cellular alterations that include altered neuronal densities and glial pathologies in major depression, which may affect specific aspects of glutamate and GABA homeostasis, although many of these findings are not specific to major depression and have been observed in other brain disorders. For instance, morphometric studies show reductions in dlPFC neuronal size, dendrite and spines in schizophrenia and in Alzheimer’s and Huntington’s diseases (Rajkowska et al., 1998, 1999; Glantz and Lewis, 2000; Cotter et al., 2002b), as well as layer specific reductions in neuronal density (layers 5 and 6) in dlPFC in bipolar disorder (Cotter et al., 2002a). Changes in the same SST GABA neuron marker are observed in schizophrenia (Morris et al., 2008), bipolar disorder (Konradi et al., 2004, 2010) and in Huntington’s (Timmers et al., 1996), Alzheimer’s (reviewed in Davies et al., 1980; Epelbaum et al., 2009) and Parkinson’s diseases (reviewed in Epelbaum et al., 1989), although in the context of additional pathologies, such as robust down-regulation of PV in perisomatic targeting GABA neurons in schizophrenia (Hashimoto et al., 2003; Volk et al., 2012), suggesting different functional outcomes despite similar changes in some of the individual components (see later sections). Similarly, oligodendrocyte abnormalities were reported in schizophrenia (Hof et al., 2003; Uranova et al., 2004; Kolomeets and Uranova, 2008) and to some extent in bipolar disorder (reviewed in Edgar and Sibille, 2012; Fields, 2008). These changes appear widespread in schizophrenia (Haroutunian et al., 2007) but neuropathological evidence in bipolar disorder is primarily from PFC (Uranova et al., 2004). Notably, the same oligodendrocyte-related genes as in major depression have been implicated in older subjects with schizophrenia, prompting a related hypothesis of reduced oligodendrocyte axonal support as the basis of the disconnectivity syndrome in schizophrenia (Roussos et al., 2012). Finally, reduced astrocyte densities were reported in multiple brain regions in schizophrenia (Katsel et al., 2011; Williams et al., 2012). Together, this brief survey of the non-selectivity of gross cellular findings across brain disorders illustrates the main points that pathological entities observed in depression often display a continuum of changes with other brain-related syndromes and occur in combinatorial patterns across cohorts and syndromes.
Integrating cell-based findings suggests altered information processing at the local circuit level in major depression
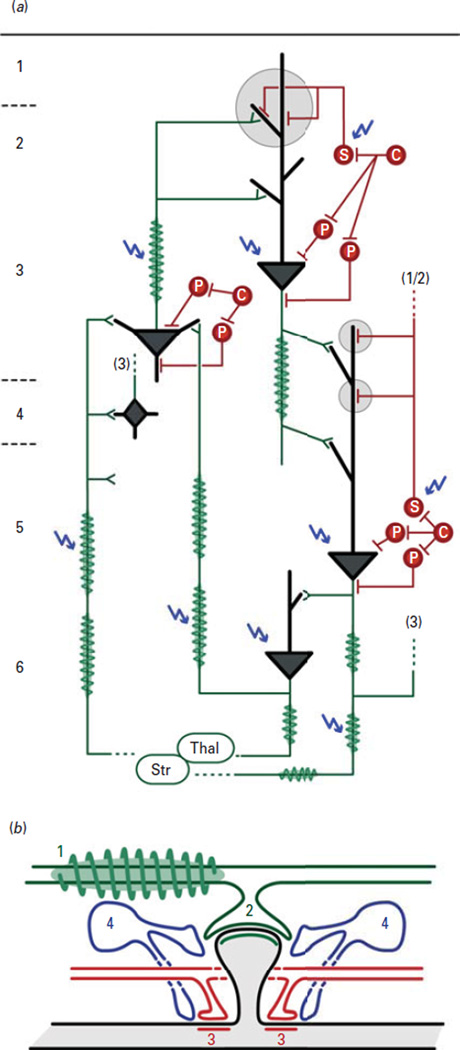
The cell-based findings in major depression described herein suggest the presence of deregulated biological modules that are integral components of canonical local cell circuits, at least in cortical and related structures (i.e. amygdala basolateral complex) (Fig. 4). Altered integrity of information transfer and processing, where ‘information’ is defined as excitatory output of pyramidal or principal cells, could occur as follows (Fig. 4a): (1) altered integrity of incoming and outgoing information, through decreased oligodendrocyte support of axonal function; (2) altered synaptic transfer of information, through altered glutamate availability; (3) altered sharpening of glutamatergic signalling-coded information through deficient fine-tuning of excitatory post-synaptic signals by SST-positive GABAergic neuron dendritic targeted inhibition; (4) maintenance of a dysregulated glutamate/GABA homeostasis through altered astrocyte recycling of neurotransmitters. Anatomical studies suggest that these deregulated modules may occur in various cortical layers, overall affecting information flow within and across cortical areas (Fig. 4a). As this speculative set of events is based on corticolimbic findings, its impact on higher biological scales may include altered sensing (by the amygdala) and processing (by the sgACC and dlPFC) of emotionally-salient information. Hence, reduced GABA-mediated inhibition on incoming information onto pyramidal dendrites may represent a putative local circuit-level phenotype underlying the increased sgACC and amygdala activation frequently reported in major depression (Mayberg et al., 1999; Siegle et al., 2007; Suslow et al., 2010), potentially tipping the balance towards bias for negative salience detection, rumination and overall low affect in depressed subjects. Local circuit changes may also impact the function of brain regions in overlapping neural networks implicated in cognitive symptoms (dlPFC) and emotion dysregulation (medial PFC, ACC and amygdala) in depression (Kupfer et al., 2012). Moreover, restoring dendritic inhibitory function may reduce pyramidal cell activation and excitatory tone and contribute to reduced ACC activation with positive response to therapeutic intervention (e.g. deep brain stimulation, antidepressants; Mayberg et al., 2000, 2005; Agid et al., 2007).
Fig. 4.
Local circuit summary of depression-related pathological findings in human post-mortem brain cortex. (a) Human post-mortem studies suggest reduced oligodendrocyte (green spindles) number and/or integrity, reduced size, density or altered dendritic branching of pyramidal cells (grey triangles) in layers 3, 5 and 6, and reduced number and/or functionally-altered calbindin/somatostatin (SST)-positive γ-amino butyric acid (GABA) neurons (S-labelled red circle and grey circles). Calretinin- and parvalbumin-positive (C- and P-labelled red circles) GABA neurons are mostly unaffected. A close-up schematic of the large grey circle in (a) is shown in (b), representing synapses between an excitatory axonal terminal (green, with myelin sheath) and a GABAergic inhibitory terminal (red) onto a pyramidal dendritic spine (black). The close-up also depicts intercalated astrocytes (blue), which show evidence of deregulated function in depression. Sites of putative pathology are marked by blue arrows. The integrity of information transfer and processing, where ‘information’ is defined as excitatory output of pyramidal or principal cells, could be compromised at several levels: (1) decreased oligodendrocyte support of axonal function leading to suboptimal conduction of action potentials along the axon; (2) disruption of synaptic transfer of information, due to changes in the structure of pyramidal neurons and availability of glutamate; (3) suboptimal modulation or ‘fine-tuning’ of excitatory post-synaptic signals onto dendritic spines due to reduced SST-positive GABAergic dendritic targeted inhibition; (4) impaired astrocyte function resulting in decreased extracellular neurotransmitter clearance, affecting the homeostatic GABA/glutamate balance. Together, the various alterations may manifest as deregulated information transfer in corticocortical [thalamus (Thal)→layer 3/4→layer 5/6→layer 3], thalamocortical (Thal→layer 3/4→layer 5/6→Thal) and corticostriatal circuit loops within corticolimbic brain areas, leading to altered processing of emotion-salient information, and affect and mood symptoms. Structural variants of this schematic loop include lack of layer 4 in the anterior cingulate cortex and lack of cortical structure in the basolateral complex of the amygdala. Str, Striatum.
This emerging framework suggests a core biological disruption in depression onto which one can integrate additional biological findings (i.e. phenotypic moderators), but it also needs multiple qualifiers. (1) Cellular findings were not systematically reported in the same subjects and cohorts, supporting a combinatorial basis for pathogenesis, which may also form a cellular basis for variable phenotypic severity. (2) This integrated set of findings displays potential phenotypic specificity in depression (altered local circuit-mediated incoming information processing), but interactions with various ‘pathophysiological modifiers’ in other categorical diseases will affect the phenotypic presentation. Such ‘modifiers’ may include reduced PV-mediated GABAergic inhibitory control on pyramidal cell body and axon initial segment in schizophrenia (Hashimoto et al., 2003), or reduced cortical input from affected subcortical cell population in neurodegenerative diseases (i.e. cholinergic neurons in Alzheimer’s; McGeer et al., 1984; Lehericy et al., 1993; Mesulam, 2012). (3) Local circuit modules and associated pathological entities have their own aetiological factors (see next section), but also interact with neuromodulatory input, including monoaminergic (Arango et al., 2002) and neuropeptidergic systems (Griebel and Holsboer, 2012), neuroendocrine and other hormonal factors, inflammatory factors and the immune system, for which ample evidence suggest roles in depression (reviewed in Nestler et al., 2002; Belmaker and Agam, 2008; Moylan et al., 2012). Notably, since cellular changes are mediated by changes in gene expression and function, cell-specific gene and molecular studies in human post-mortem subjects may help identify adaptive changes and relative contributions of these neuromodulatory systems onto cortical local circuits. (4) Whereas post-mortem studies are essential in characterizing the primary brain pathology, the sequence of pathological events cannot be determined and information on time-dependent trajectories are sparse. However, one can argue that post-mortem studies are more than simple snapshots in time, since they inform on stable biological adaptations that sustained the pre-morbid state or that mediated the cumulative and neuroprogressive pathology of the illness. This is an important consideration to have in mind for studies of cellular pathology, as models propose a trajectory affecting glial and neuronal cells at different time-points throughout the progression of the illness, suggesting varying cellular pathology based on both age of the subject and stage of the illness. As proposed by Rajkowska and Miguel-Hidalgo (2007), early glial reductions as a result of a combination of stress-related biological insults and genetic vulnerability may lead to neuronal damage due to dysfunctional or insufficient support and clearance of extracellular glutamate. In this model, compensatory increases in glia in response to damage to neurons may occur with increasing age and illness progression. Subjects with late-life depression may enter at the stage of neuronal damage and loss through other pathways, such as vascular lesions or neuronal atrophy associated with age. (5) Local circuit-mediated changes in the function of specific brain regions and neural networks will feedback on its cellular components, through activity- and hormone-dependent transcriptional changes. Indeed, post-mortem studies preclude detection of short-term effects (h), yet gene expression is under the control of multiple rhythms across several time-frames, from circadian clock to hormonal fluctuations, including metabolic (insulin, thyroid), sex and stress hormones, often through nuclear hormone receptor-orchestrated changes in transcriptional programmes. Moreover, genes are also influenced by acute ‘out-of-sync’ effects of hormones during stress or inflammation, which will contribute to altered synchronous cell function. For instance, there is indirect evidence for multiple hormonal systems deregulation in depression, using corticolimbic gene synchrony as an assay for common upstream regulation (Gaiteri et al., 2010).
Module-specific and nested aetiological factors: the example of SST
As referred to in this review, biological modules and endophenotypes are not interchangeable. Endophenotypes, as originally described by Gottesman and Gould (2003) encompassed biological (neurophysiological, biochemical, endocrinological), neuroanatomical and behavioural (cognitive, neuropsychological; i.e. working memory, sensory motor gating) categories, which in fact reflect functions over a large panel of biological scales (Fig. 2). Conversely, GABAergic and glutamatergic synapses, with their associated glial cells (Fig. 4) participate in biological modules that are repeated across cortical layers and throughout the brain. Moreover, biological modules have their own aetiological pathways, meaning that pathologies in these modules often do not show specificity to categorically-defined brain disorders. Rather, various risk factors may together orchestrate pathological changes across sets of vulnerable modules. Here we use pathological findings and known modulators of SST expression and function to illustrate module-specific factors and frequent lack of adherence to a categorically-defined disorder.
In MDD, reports of low SST expression are more robust in female subjects (Tripp et al., 2011, 2012). SST expression also appears lower in aged-matched normal healthy women compared to male subjects (Tripp et al., 2012), consistent with a moderating, rather than interacting effect of sex on SST and potentially contributing to the heightened female vulnerability to develop episodes of depression. Similarly, normal subjects lose on average 50% SST expression between ages 20 and 70 yr (Glorioso et al., 2011), consistent with age-related changes in brain neurotrophic environment (Erraji-Benchekroun et al., 2005). SST expression also depends on BDNF (Glorioso et al., 2006), a neuropeptide responsible for maintaining neuroplasticity, which itself shows age-dependent decreased expression in control subjects (Webster et al., 2002; Erraji-Benchekroun et al., 2005) and reduced expression in depression (Dwivedi, 2009; Guilloux et al., 2011) and other brain-related disorders (Lu and Martinowich, 2008; Rakofsky et al., 2012). The extent of depression-related decreases in SST and other neuropeptides expressed in dendritic-targeting GABAergic neurons (cortistatin and NPY) varies based on brain region and aspects of BDNF signalling (i.e. activity-dependent vs. constitutive function; B vs. BDNF decreases; Guilloux et al., 2011; Sibille et al., 2011; Tripp et al., 2011, 2012). More proximal cell-specific risk factors include vulnerability to oxidative stress, since neuronal nitric oxide synthase, a source of cytotoxic nitric oxide, is mostly expressed in SST and NPY neurons (Jaglin et al., 2012). These cell-specific factors may also be sex-dependent, in view of human–mouse conserved sex-bias in expression of mitochondria-related genes (Lin et al., 2011). Together, these observations suggest that SST expression and potentially dendritic inhibition, is subject to sets of nested regulatory pathways (i.e. occurring on consecutive biological scales), which include cell-specific (oxidative stress), neuron and circuit factors (BDNF), regional (ACC vs. amygdala), individual (sex) and general (age) factors. Finally, individual genetic liabilities, environmental factors and longitudinal trajectories (developmental and age-dependent changes) will impinge on most factors discussed.
Conclusions and implications of a modular perspective on the biological substrates underpinning diagnosis of major depression
Molecular and cellular evidence suggest that several components of local canonical cortical (or related) cell circuits are deregulated in major depression, affecting the structure and function of glutamatergic neurons and dendritic-targeting GABAergic neurons, as well as oligodendrocytes and astrocytes. The data suggest that cumulative pathological changes in each of these cell types may affect the integrity of information transfer across these local cell circuits, in turn affecting the processing capacity across cortical layers, brain regions and neural networks (summarized in Fig. 4). The fact that these changes have been identified in brain regions participating in sensing and assessment of emotional salience suggests a direct contribution to core symptoms of depression, such as low affect and anhedonia. The assumption is that deregulated cell circuits may affect the functional balance of individual brain regions, ‘pushing’ the corticolimbic neural network towards maladaptive states that favour negative bias, rumination and lack of pleasure/reward. This latter link is however speculative and illustrates the inherent limitations of bridging bottom-up cellular and molecular post-mortem studies with top-down clinical and brain imaging studies in subjects with major depression.
Evidence also suggests a modular and combinatorial model, where pathological findings do not represent biomarkers for respective categorical syndromes, but rather correspond to cellular and molecular pathological entities affecting distinct and vulnerable brain-related biological modules. The interactions between these biological modules identify multiple potential pathological routes and suggest that a network-based approach to biological modules and pathological entities may be more appropriate to characterize the biological substrates of major depression, compared to linear causal pathways (Fig. 2). However, a potential caveat of the network approach is the frequent focus on seeking affected network ‘hubs’, a masked alternative of reductionist approaches geared toward finding ‘silver bullet’ targets. Early evidence suggests that hubs are stable and not affected in psychiatric disorders, at least from the gene network perspective (Gaiteri and Sibille, 2011). Additional forms of deregulated transfer of genetic information in gene networks have not been investigated yet.
Here we have focused on depression-related cell-based findings and implications for local circuits in cortical and related structures, but additional biological subcellular modules, modulatory systems and neural network areas are involved. From a research perspective, the apparent non-selectivity of a biological module perspective should not detract from putative causal roles in major depression, as characterizing individual modules in terms of aetiology, molecular pathology and phenotypic output will contribute to our understanding of the biological complexity of brain dysfunction and their combinatorial recruitment may offer insight into mechanisms underlying the clinical presentation of depression. For instance, numerous preclinical studies demonstrate that affecting components of the described local networks can affect behavioural emotionality in rodents (Banasr and Duman, 2008; Banasr et al., 2010; Edgar et al., 2011). Similarly, one can speculate that genetic studies of behavioural endophenotypes based on knowledge of vulnerable underlying biological modules and associated pathological changes within a broader depression syndrome may yield more robust findings, informing mechanisms beyond mood disorders. A modular perspective is consistent with the emerging dimensional understanding of symptom co-occurrence across syndromes and provides a bottom-up approach to identify relevant biological modules and associated pathological entities, which is complementary to the research domain criteria top-down effort to cluster clinical symptoms into a coherent and biologically-informed endophenotype structure (Insel et al., 2010). Given the stasis and problems associated with antidepressant drug discovery, and due to its reliance on simplified assumptions and models, it is critical to develop integrated models that are predictive across biological scales. A focus on local cell circuitry integrates molecular mechanisms with neural network function, bridging the gap between molecular drug targets and clinical syndromes that has thus far impeded novel drug discovery for neuropsychiatric diseases.
Acknowledgements
This work was supported by National Institute of Mental Health (NIMH) MH084060 and MH085111 grants (to E.S.). The funding agency had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health. We thank Guy Griebel and Tija Jacob for drafts of Figs. 1 and 4b respectively.
Footnotes
Statement of Interest
None.
References
- Agid Y, Buzsaki G, Diamond DM, Frackowiak R, Giedd J, Girault JA, Grace A, Lambert JJ, Manji H, Mayberg H, Popoli M, Prochiantz A, Richter-Levin G, Somogyi P, Spedding M, Svenningsson P, Weinberger D. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007;6:189–201. doi: 10.1038/nrd2217. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th edn. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–313. doi: 10.1016/s0006-3223(99)00159-6. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Block W, Traber F, von Widdern O, Metten M, Schild H, Maier W, Zobel A, Jessen F. Proton MR spectroscopy of the hippocampus at 3T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–422. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. 715. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P. The central nervous system: structure and function. 4th edn. New York: Oxford University Press; 2010. [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002a;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002b;51:377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- Davies P, Katzman R, Terry RD. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980;288:279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–449. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar NM, Touma C, Palme R, Sibille E. Resilient emotionality and molecular compensation in mice lacking the oligodendrocyte-specific gene Cnp1. Transl Psychiatry. 2011;1:e42. doi: 10.1038/tp.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum J, Agid F, Agid Y, Beaudet A, Bertrand P, Enjalbert A, Heidet V, Kordon C, Krantic S, Leonard JF, Musset F, Moyse E, Peillon F, Slama A, Videau C. Somatostatin receptors in brain and pituitary. Horm Res. 1989;31:45–50. doi: 10.1159/000181085. [DOI] [PubMed] [Google Scholar]
- Epelbaum J, Guillou JL, Gastambide F, Hoyer D, Duron E, Viollet C. Somatostatin, Alzheimer’s disease and cognition: an old story coming of age? Prog Neurobiol. 2009;89:153–161. doi: 10.1016/j.pneurobio.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, Turecki G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312–319. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Erraji-Benchekroun L, Underwood MD, Arango V, Galfalvy H, Pavlidis P, Smyrniotopoulos P, Mann JJ, Sibille E. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biol Psychiatry. 2005;57:549–558. doi: 10.1016/j.biopsych.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BA. A review of American psychiatry through its diagnoses: the history and development of the diagnostic and statistical manual of mental disorders. J Nerv Ment Dis. 2012;200:1022–1030. doi: 10.1097/NMD.0b013e318275cf19. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiteri C, Sibille E. Differentially expressed genes in major depression reside on the periphery of resilient gene coexpression networks. Front Neurosci. 2011;5:95. doi: 10.3389/fnins.2011.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiteri C, Guilloux JP, Lewis DA, Sibille E. Altered gene synchrony suggests a combined hormone-mediated dysregulated state in major depression. PLoS ONE. 2010;5:e9970. doi: 10.1371/journal.pone.0009970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133:328–332. doi: 10.1016/j.jad.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, Mirnics K. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11:633–648. doi: 10.1038/sj.mp.4001835. [DOI] [PubMed] [Google Scholar]
- Glorioso C, Oh S, Douillard GG, Sibille E. Brain molecular aging, promotion of neurological disease and modulation by Sirtuin5 longevity gene polymorphism. Neurobiol Dis. 2011;41:279–290. doi: 10.1016/j.nbd.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BI, Bowers MB, Roth RH, Sweeney DW. Gaba levels in Csf of patients with psychiatric-disorders. Am J Psychiatry. 1980;137:362–364. doi: 10.1176/ajp.137.3.362. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012;11:462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2011;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem S, Aguirre A, Vives V, Marks A, Gallo V, Legraverend C. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia. 2005;51:81–97. doi: 10.1002/glia.20184. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Dracheva S, Stewart DG, Davis KL. Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. Int J Neuropsychopharmacol. 2007;10:565–573. doi: 10.1017/S1461145706007310. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nihonmatsu-Kikuchi N, Yu X, Ishimoto K, Hisanaga SI, Tatebayashi Y. A novel, rapid, quantitative cell-counting method reveals oligodendroglial reduction in the frontopolar cortex in major depressive disorder. Mol Psychiatry. 2011;16:1155–1158. doi: 10.1038/mp.2011.84. [DOI] [PubMed] [Google Scholar]
- Hayes TL, Cameron JL, Fernstrom JD, Lewis DA. A comparative analysis of the distribution of prosomatostatin-derived peptides in human and monkey neocortex. J Comp Neurol. 1991;303:584–599. doi: 10.1002/cne.903030406. [DOI] [PubMed] [Google Scholar]
- Hek K, et al. A genome-wide association study of depressive symptoms. Biol Psychiatry. 2013;73:667–678. doi: 10.1016/j.biopsych.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercher C, Canetti L, Turecki G, Mechawar N. Anterior cingulate pyramidal neurons display altered dendritic branching in depressed suicides. J Psychiatr Res. 2010;44:286–293. doi: 10.1016/j.jpsychires.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jaglin XH, Hjerling-Leffler J, Fishell G, Batista-Brito R. The origin of neocortical nitric oxide synthase-expressing inhibitory neurons. Front Neural Circuits. 2012;6:44. doi: 10.3389/fncir.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology. 2011;36:1171–1177. doi: 10.1038/npp.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. The dappled nature of causes of psychiatric illness: replacing the organic-functional/hardware-software dichotomy with empirically based pluralism. Mol Psychiatry. 2012;17:377–388. doi: 10.1038/mp.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005a;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005b;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khundakar A, Morris C, Oakley A, McMeekin W, Thomas AJ. Morphometric analysis of neuronal and glial cell pathology in the dorsolateral prefrontal cortex in late-life depression. Br J Psychiatry. 2009;195:163–169. doi: 10.1192/bjp.bp.108.052688. [DOI] [PubMed] [Google Scholar]
- Khundakar A, Morris C, Oakley A, Thomas AJ. A morphometric examination of neuronal and glial cell pathology in the orbitofrontal cortex in late-life depression. Int Psychogeriatr. 2011;23:132–140. doi: 10.1017/S1041610210000700. [DOI] [PubMed] [Google Scholar]
- Kim S, Webster MJ. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol Psychiatry. 2010;15:326–336. doi: 10.1038/mp.2008.99. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, Ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Uranova NA. Pathology of oligodendroglia and myelinated fibers of the hippocampus in schizophrenia (an ultrastructural and morphometric study) Zh Nevrol Psikhiatr Im S S Korsakova. 2008;108:52–60. [PubMed] [Google Scholar]
- Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2010;68:340–350. doi: 10.1001/archgenpsychiatry.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Harrison PJ. The distribution and morphology of prefrontal cortex pyramidal neurons identified using anti-neurofilament antibodies SMI32, N200 and FNP7. Normative data and a comparison in subjects with schizophrenia, bipolar disorder or major depression. J Psychiatr Res. 2003;37:487–499. doi: 10.1016/s0022-3956(03)00075-x. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Hirsch EC, Cervera-Pierot P, Hersh LB, Bakchine S, Piette F, Duyckaerts C, Hauw JJ, Javoy-Agid F, Agid Y. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol. 1993;330:15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67:458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Lewis DA, Sibille E. A human-mouse conserved sex bias in amygdala gene expression related to circadian clock and energy metabolism. Mol Brain. 2011;4:18. doi: 10.1186/1756-6606-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Muñoz F, Álamo González C. Neurobiology of depression. Boca Raton, FL: Taylor & Francis; 2012. [Google Scholar]
- Lu B, Martinowich K. Cell biology of BDNF and its relevance to schizophrenia. Novartis Found Symp. 2008;289:119–129. doi: 10.1002/9780470751251.ch10. 129–135, 193–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2011_197. Advance online publication. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T. Aging, Alzheimer’s disease, and the cholinergic system of the basal forebrain. Neurology. 1984;34:741–745. doi: 10.1212/wnl.34.6.741. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CW, Miranda A, Fulgosi D, Kennedy SH. Should depressive syndromes be reclassified as ‘metabolic syndrome Type II’? Ann Clin Psychiatry. 2007;19:257–264. doi: 10.1080/10401230701653377. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- MDD GWAS Consortium. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2012;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. Cholinergic aspects of aging and Alzheimer’s disease. Biol Psychiatry. 2012;71:760–761. doi: 10.1016/j.biopsych.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003a;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Arolt V, Heindel W, Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003b;28:720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Dubey P, Shao Q, Stockmeier C, Rajkowska G. Unchanged packing density but altered size of neurofilament immunoreactive neurons in the prefrontal cortex in schizophrenia and major depression. Schizophr Res. 2005;76:159–171. doi: 10.1016/j.schres.2005.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Milne A, MacQueen GM, Yucel K, Soreni N, Hall GB. Hippocampal metabolic abnormalities at first onset and with recurrent episodes of a major depressive disorder: a proton magnetic resonance spectroscopy study. Neuroimage. 2009;47:36–41. doi: 10.1016/j.neuroimage.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.33. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Oh DH, Son H, Hwang S, Kim SH. Neuropathological abnormalities of astrocytes, GABAergic neurons, and pyramidal neurons in the dorsolateral prefrontal cortices of patients with major depressive disorder. Eur Neuropsychopharmacol. 2012;22:330–338. doi: 10.1016/j.euroneuro.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Understanding depression: a translational approach. London, New York: Oxford University Press; 2009. [Google Scholar]
- Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, Fiebich M, Arolt V, Heindel W. Effective electroconvulsive therapy reverses glutamate/glutamine deficit in the left anterior cingulum of unipolar depressed patients. Psychiat Res-Neuroim. 2003;122:185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakofsky JJ, Ressler KJ, Dunlop BW. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol Psychiatry. 2012;17:22–35. doi: 10.1038/mp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Katsel P, Davis KL, Bitsios P, Giakoumaki SG, Jogia J, Rozsnyai K, Collier D, Frangou S, Siever LJ, Haroutunian V. Molecular and genetic evidence for abnormalities in the nodes of Ranvier in schizophrenia. Arch Gen Psychiatry. 2012;69:7–15. doi: 10.1001/archgenpsychiatry.2011.110. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Steiner J, Mueller K. Glial pathology is modified by age in mood disorders–a systematic meta-analysis of serum S100B in vivo studies. J Affect Disord. 2011;134:32–38. doi: 10.1016/j.jad.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Akula N, Breuer R, Steele J, Nalls MA, Singleton AB, Degenhardt FA, Nothen MM, Cichon S, Rietschel M, McMahon FJ. Molecular genetic overlap in bipolar disorder, schizophrenia, and major depressive disorder. World J Biol Psychiatry. 2012 doi: 10.3109/15622975.2012.662282. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, Tseng GC, Lewis DA. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Whitehead DL. Depression, stress, and coronary heart disease: the need for more complex models. Heart. 2005;91:419–420. doi: 10.1136/hrt.2004.045310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67:155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Swaab DF, van de Nes JA, Kremer HP. Somatostatin 1–12 immunoreactivity is decreased in the hypothalamic lateral tuberal nucleus of Huntington’s disease patients. Brain Res. 1996;728:141–148. doi: 10.1016/0006-8993(96)00080-7. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonte B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36:2650–2658. doi: 10.1038/npp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux JP, Martinowich K, lewis DA, Sibille E. BDNF signaling and subgenual anterior cingulate cortex dysfunction in major depression. Am J Psychiatry. 2012;169:1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, Arango V. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol. 2012;15:435–447. doi: 10.1017/S1461145711000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Valentine GW, Sanacora G. Targeting glial physiology and glutamate cycling in the treatment of depression. Biochem Pharmacol. 2009;78:431–439. doi: 10.1016/j.bcp.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Otterloo E, O’Dwyer G, Stockmeier CA, Steffens DC, Krishnan RR, Rajkowska G. Reductions in neuronal density in elderly depressed are region specific. Int J Geriatr Psychiatry. 2009;24:856–864. doi: 10.1002/gps.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286:75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, Sampson A, Hashimoto T, Lewis DA. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res Dev Brain Res. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Williams MR, Hampton T, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2012;263:41–52. doi: 10.1007/s00406-012-0328-5. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Wray NR, et al. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2010;17:36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang J, Narr KL, Woods RP, Phillips OR, Alger JR, Espinoza RT. Glutamate normalization with ECT treatment response in major depression. Mol Psychiatry. 2012;18:268–270. doi: 10.1038/mp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]