Abstract
Background
Acinetobacter baumannii is increasingly recognized as being a significant pathogen associated with nosocomial outbreaks in both civilian and military treatment facilities. Current analyses of these outbreaks frequently describe patient-to-patient transmission. To date, occupational transmission of A. baumannii from a patient to a health care worker (HCW) has not been reported. We initiated an investigation of an HCW with a complicated case of A. baumannii pneumonia to determine whether a link existed between her illness and A. baumannii–infected patients in a military treatment facility who had been entrusted to her care.
Methods
Pulsed-field gel electrophoresis and polymerase chain reaction/electrospray ionization mass spectrometry, a form of multilocus sequencing typing, were done to determine clonality. To further characterize the isolates, we performed a genetic analysis of resistance determinants.
Results and Conclusions
A “look-back” analysis revealed that the multidrug resistant A. baumannii recovered from the HCW and from a patient in her care were indistinguishable by pulsed-field gel electrophoresis. In addition, polymerase chain reaction/electrospray ionization mass spectrometry indicated that the isolates were similar to strains of A. baumannii derived from European clone type II (Walter Reed Army Medical Center strain type 11). The exposure of the HCW to the index patient lasted for only 30 min and involved endotracheal suctioning without use of an HCW mask. An examination of 90 A. baumannii isolates collected during this investigation showed that 2 major and multiple minor clone types were present and that the isolates from the HCW and from the index patient were the most prevalent clone type. Occupational transmission likely occurred in the hospital; HCWs caring for patients infected with A. baumannii should be aware of this potential mode of infection spread.
Acinetobacter baumannii is an aerobic gram-negative pleomorphic coccobacillus commonly found in soil, skin flora, and the hospital environment [1–3]. The ability of A. baumannii to survive on environmental surfaces for extended periods of time, along with its capacity to demonstrate antimicrobial resistance genes, make it exceptionally well suited to emerge as a successful nosocomial pathogen [4–8]. Generally speaking, this organism is responsible for pulmonary, urinary tract, bloodstream, or surgical wound infections in immunocompromised patients with multiple comorbidities [4, 9]. Numerous reports also describe A. baumannii outbreaks of bloodstream infections, osteomyelitis, and complicated skin and soft-tissue infections in US military and civilian personnel who were wounded while serving in Iraq and Afghanistan [10, 11]. Scott et al. [12] traced an outbreak of A. baumannii infection in wounded soldiers to the environmental contamination of field hospitals in Iraq and Kuwait. Transporting these injured personnel who are colonized or infected with A. baumannii back to the United States is also linked to the nosocomial transmission of these strains from wounded US service personnel to civilian patients in military treatment facilities (MTFs) [6]. Additionally, an analysis of the complex transmission dynamics of 1 major outbreak showed that multiple clones with multidrug-resistant phenotypes were circulating at the same time [6, 12].
A. baumannii rarely cause community-acquired pneumonia and sepsis. These uncommon manifestations of A. baumannii infection are found among patients who reside in tropical climates and who have chronic obstructive pulmonary disease and/or who abuse alcohol [13]. In June 2006, a 55-year-old woman presented to a teaching medical center with pneumonia, bacteremia, and sepsis due to A. baumannii. At the time of this illness, she was employed as an intensive care unit (ICU) health care worker (HCW) at an MTF for US service personnel wounded in Iraq and Afghanistan. Of further note, a nosocomial outbreak of A. baumannii infection was occurring in the MTF at the same time. We investigated the possibility that her infection was related to occupational exposure of patients infected or colonized with A. baumannii. We found that the strain of A. baumannii that infected her matched a strain from the sputum of a wounded US serviceman. We also discovered that this strain was the most common clone type isolated within the MTF at the time the HCW became infected, was related to previously described strains circulating in Europe, and was also present at another MTF, the Walter Reed Army Medical Center (WRAMC).
CASE REPORT
The HCW had noninsulin dependent diabetes mellitus. Her diabetes was managed with metformin (500 mg per day), and she did not abuse tobacco or alcohol. Her hemoglobin A1C level was 8.7% at hospital admission. She had worked for the previous 15 years as an ICU HCW at the National Naval Medical Center (NNMC) in Maryland and cared for both US service personnel wounded in Iraq or Afghanistan and civilian patients. She initially presented to the emergency department of a nonmilitary teaching hospital complaining of a 2-day history of hemoptysis, fever, and shortness of breath, and she was admitted to the hospital.
At medical examination, her temperature was 39.1°C, her pulse rate was 129 beats per min, and her oxygen saturation was 89% on room air. She was in respiratory distress, and it was noted that she had decreased breath sounds over the right lower lung field. The findings of the remainder of her examination were normal. Evidence of skin breaks was absent. Laboratory testing showed a WBC count of 1400 cells/mm3, and her chest radiograph revealed a right lower lobe infiltrate with an effusion. She received a diagnosis of community-acquired pneumonia, and treatment with intravenous levofloxacin was initiated. On the following day, blood, sputum, and pleural fluid culture samples grew a gram-negative organism.
Her hospital course was complicated by hypotension that required vasopressor support and by respiratory failure (i.e., adult respiratory distress syndrome) that necessitated intubation. By the second hospital day, the organism growing in the blood, sputum, and pleural fluid was identified as A. baumannii. Levofloxacin was changed to imipenem-cilistatin and tigecycline (which was replaced by amikacin when susceptibility data were available). Antibiotics were administered for 3 weeks. Convalescence was complicated by the need for ventilator support, a left-sided pleural space decortication, and hemodialysis. After her 2-month recovery in the ICU, another month in a rehabilitation facility was required before being discharged home.
METHODS
In 2003, the NNMC initiated an intensive infection-control effort to prevent nosocomial transmission of Acinetobacter species. To document colonization or infection, all ICU and non-ICU patients admitted from the Middle East theater of operations had routine cultures performed of specimens obtained from the axillae, groin, and wounds. Patients were admitted under strict contact precautions until the results of surveillance cultures were known. Patients found to be colonized were maintained with contact precautions. All collected Acinetobacter species isolates were stored at −70°C. The medical records of each patient colonized or infected with Acinetobacter species were reviewed.
Antimicrobial susceptibility testing
We performed antimicrobial identification and susceptibility testing of the A. baumannii isolates with the VITEK 2 (bioMérieux) automated system with use of Clinical and Laboratory Standards Institute guidelines [14].
Clonal analysis
We extracted genomic DNA from A. baumannii isolates and digested it with ApaI (New England BioLabs). PFGE was performed using a CHEF DRIII apparatus (Bio-Rad), according to an established method [12]. Gel images were analyzed using BioNumerics software (Applied Maths). A. baumannii strains with >90% similarity, determined on the basis of Dice coefficient, were considered to be related.
In addition to PFGE, we determined genetic relatedness by multilocus PCR/electrospray ionization mass spectrometry (PCR/ESI-MS), according to the methods described elsewhere [6].
Determination of resistance genes
The identification of genes responsible for resistance to antibiotics was performed as described by Hujer et al. [6]. PCR primers, controls, and conditions for amplification were reported in table 1 of the work of Hujer et al. [6].
Ethical approval
This work was conducted as a public health activity and under a study approved by the Institutional Review Board of the NNMC, under protocol 2006.0136.
RESULTS
Once the identity of the pathogen causing severe pneumonia, adult respiratory distress syndrome, and sepsis in the HCW was known, a molecular epidemiological investigation was undertaken.
Epidemiological and molecular analyses
A retrospective chart review was performed to identify MTF ICU patients who were in the care of the affected HCW. The bloodstream isolate recovered from the HCW was compared with A. baumannii isolates recovered from patients 33 days before the HCW became ill. PFGE analysis revealed a match between the isolate recovered from the HCW’s blood and an isolate from the sputum of a patient whom she had cared for 2 days before becoming ill (figure 1). A match was not found with A. baumannii isolates collected from other patients with whom the HCW had contact during the previous month (figure 2).
Figure 1.
Days on which the health care worker provided direct care to 5 different National Naval Medical Center patients colonized or infected with Acinetobacter baumannii. Above each arrow is the designated PFGE lane shown in figure 2 that corresponds to the A. baumannii clonal types recovered from these patients.
Figure 2.
PFGE analysis of the Acinetobacter baumanii isolate recovered from the health care worker, compared with A. baumannii patient isolates from the National Naval Medical Center. Lanes 1 and 8 are molecular weight standards. Lanes 4 and 7 represent the genomic digests of the isolates from the health care worker (lane 4) and the wounded US serviceman (lane 7). Lanes 2, 3, 5, and 6 are genomic digests of A. baumannii isolated from patients under the direct care of the health care worker and represent additional clone types circulating at the National Naval Medical Center.
The source patient was a 45-year-old US Navy man wounded by an improvised explosive device while serving in Iraq. He suffered massive injuries that included multiple fractures (an open left tibia-fibula, right fibular, open right femur, unstable pelvic, and 5 vertebral fractures), splenic artery rupture, and a retroperitoneal hematoma. He was initially treated at a combat-support hospital in Balad, Iraq, where he was intubated and resuscitated. On the following day, he was flown to Landstuhl Regional Medical Center in Germany and was still intubated and sedated when he arrived at NNMC. On the night of the source patient’s arrival, the HCW spent ~30 min providing routine ventilator care, including endotracheal suctioning. The HCW was wearing gloves and a gown but not a mask.
On admission to NNMC, the source patient was afebrile and clinically stable. His medications included cefazolin and levofloxacin. Surveillance culture results indicated that he was colonized with A. baumannii in his sputum, abdominal wound, axilla, and nares. The A. baumannii isolate collected from his sputum on the day of arrival to our MTF was a match to that found in the HCW’s blood 2 days later (figures 1 and 2). Several days after admission to NNMC, the source patient developed a fever, and an infiltrate was seen on a chest radiograph. The patient received a diagnosis of A. baumannii ventilator-associated pneumonia and received meropenem for 14 days.
Characterization of the isolates
Testing of the A. baumannii isolates collected from the HCW and source patient demonstrated susceptibility only to meropenem, imipenemcilistatin, amikacin, and colistin. PCR amplification of the A. baumannii isolate from theHCWidentified 3 β-lactamase genes (blaTEM, blaADC, and blaOXA-51/69–like), 2 genes encoding aminoglycoside-modifying enzymes (aacC1 and aadA1), an integrase gene (int), and mutations conferring quinolone resistance (single-point mutations in the quinolone resistance–determining regions of gyrA [at amino acid S83] and parC [at amino acid S80]). These genetic determinants confer resistance to ceftazidime (blaADC), gentamicin (aacC1), and ciprofloxacin (changes in quinolone resistance–determining regions of topoisomerase genes). The blaOXA-51/69 genes are housekeeping genes in A. baumannii that do not confer carbapenem resistance [15].
Analysis of occupational transmission in the context of the outbreak at NNMC
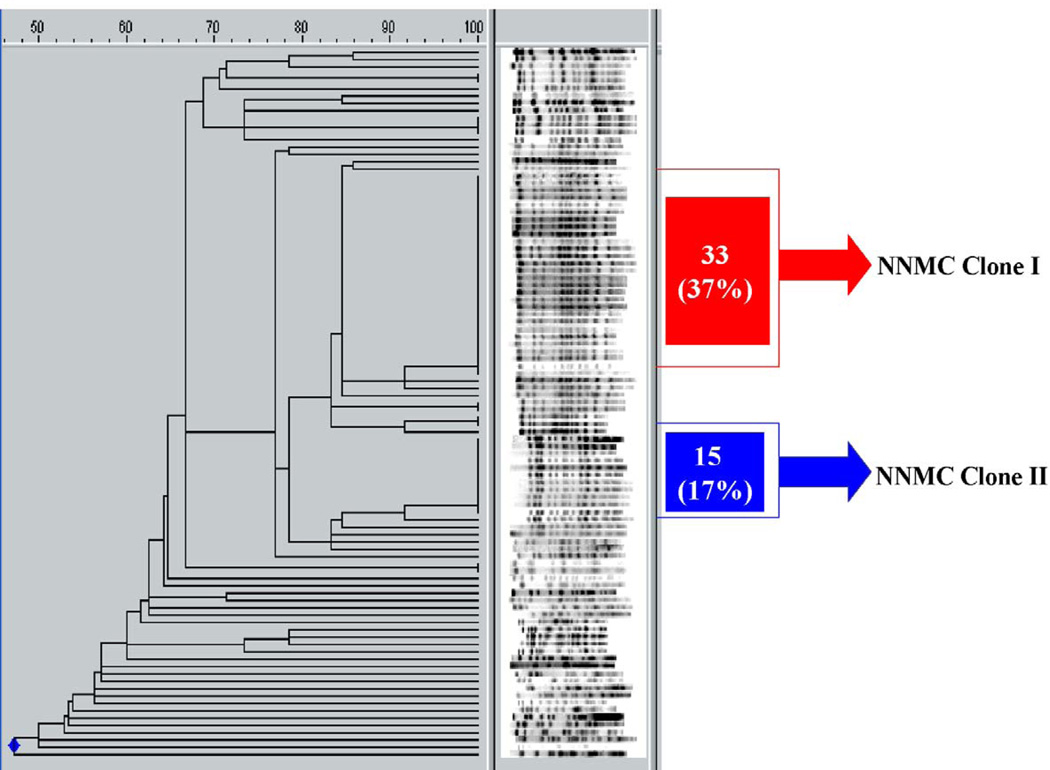
Analyzing the isolates of A. baumannii collected from 67 inpatients at the NNMC during March–November 2006, we discovered that 2 major clones were present (figure 3; online only). We found 31 isolates (34%) of pulsotype I (labeled “NNMC clone I”) and 15 isolates (17%) of the second pulsotype (“NNMC clone II”; figure 3; online only). The strains infecting the HCW and the source patient belonged to NNMC clone I. In addition to the 2 main clone types, the remaining isolates collected at NNMC comprised >30 different clone types. A summary of the dissemination of these isolates is represented in figure 4.
Figure 3.
PFGE analysis of 90 patient isolates of A. baumannii collected from March 2006 to November 2006.
Figure 4.
Flow diagram representing the dissemination of different clone types identified at the National Naval Medical Center (NNMC). The isolates from the US serviceman and the HCW are added to clone type I to arrive at 31 total isolates. OIF, Operation Iraqi Freedom; WRAMC, Walter Reed Army Medical Center.
Clonal determination with use of PCR/ESI-MS, an analysis based on the DNA sequence of 6 housekeeping genes, identified the isolate as being related to European clone type II and PCR/ESI-MS strain type 11 from WRAMC [6, 16, 17].
DISCUSSION
To our knowledge, this work describes the first reported case of an HCW acquiring A. baumannii infection from a strain present in her work environment. Occupational transmission likely occurred in the setting of a nosocomial outbreak of this same clonal type of A. baumannii among other patients at NNMC. As a result of infection, the HCW experienced a life-threatening pneumonia complicated by an empyema and bacteremia that were precipitants of septic shock. This case report raises several important questions. How did this isolate infect this HCW, and why was her illness so severe? Why did 1 clonal type predominate in nosocomial transmission? How should this affect infection-control practices for HCWs in similar epidemic circumstances?
We postulate that the HCW became ill after she inhaled A. baumannii that was aerosolized from the source patient’s sputum during endotracheal suctioning. Despite extensive knowledge that this organism is aerosolized, HCWs do not routinely wear masks when performing ventilator care for patients whose sputum is colonized or infected with A. baumannii [18–21]. An alternative explanation is that this HCW became colonized by an environmental isolate at NNMC and that her illness was coincident with this patient exposure. The methods of genetic analysis that are available to us do not distinguish between these 2 possibilities. The factors that lead to respiratory colonization and enhance the virulence of A. baumannii in various hosts are still being debated. The only predisposing condition for infection identified for this HCW was diabetes.
With consideration of the wide variety of A. baumannii clone types isolated from patients at NNMC, it is unclear why this strain seemed to be the most prevalent and, therefore, more transmissible to both the HCW and patients within the ICU. Of note, this clone was present in MTFs since combat operations began in 2003 and was recently found to be the second most common strain circulating in another MTF, the WRAMC [6]. Because this isolate was susceptible to a variety of antimicrobial agents, we suspect that its ability to spread in hospitals was not attributable to inherent resistance mechanisms or selective pressure by 1 antibiotic agent. Likely, the environmental conditions at NNMC at the time of the outbreak and possibly a temporary lapse in infection control favored this 1 particular clone type. Alternatively, this clone type may be more resistant to desiccation, better at forming biofilms, or better at adhering to fomites. Of most concern is the fact that our analysis with use of PCR/ESI-MS indicates that this particular strain is still being transported from Iraq and Landstuhl into the United States [6]. Clearly, further research is needed to elucidate the characteristics of specific strains of A. baumannii that predominate in an outbreak setting.
The Centers for Disease Control and Prevention established specific guidelines to avoid potential exposure to influenza, tuberculosis, and other respiratory pathogens in the work place. In contrast, recommendations, at least about droplet precautions, for working with patients infected with A. baumannii, Serratia marcescens, and Pseudomonas aeruginosa are not published.
We report, to our knowledge, the first occupational transmission of severe multidrug-resistant A. baumannii infection (European clone type II, WRAMC strain type 11) to an HCW in the setting of a nosocomial outbreak. Studies are needed to investigate whether droplet precautions should be in place for patients with A. baumannii in their sputum or whether more-frequent attention should be paid to ventilator maintenance and management.
Acknowledgments
We thank Leigh Ann Sanders, for her dedication to our program and technical assistance with the molecular analysis; Karen Cromwell and Ear-line Vasquez, for their tireless efforts in infection control; Kyle Peterson and David You, for their assistance with the manuscript; Kurt John, for his contribution to the molecular analysis; and Dr. David Ecker, from Ibis Biosciences, for his helpful assistance with data analysis and manuscript preparation.
Financial support. The Veterans Affairs Merit Review Program and the National Institutes of Health (RO1 AI072219) supported the work of R.A.B. Molecular analysis was partially funded by a grant from the US Department of Defense Global Emerging Infectious Surveillance and Response System.
Footnotes
The manuscript was reviewed at the National Naval Medical Center and is being published without objection. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting true views of the Department of Defense, the US Navy, or other organizations listed.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henriksen SD. Moraxella, Acinetobacter and the Mimeae. Bacteriol Rev. 1973;37:522–561. doi: 10.1128/br.37.4.522-561.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeana C, Larson E, Sahni J, Bayuga SJ, Wu F, Della-Latta P. The epidemiology of multidrug-resistant Acinetobacter baumannii: does the community represent a reservoir? Infect Control Hosp Epidemiol. 2003;24:275–279. doi: 10.1086/502209. [DOI] [PubMed] [Google Scholar]
- 4.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 5.Fournier PE, Vallenet D, Barbe V, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hujer KM, Hujer AM, Hulten EA, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richet H, Fournier PE. Nosocomial infections caused by Acinetobacter baumannii: a major threat worldwide. Infect Control Hosp Epidemiol. 2006;27:645–646. doi: 10.1086/505900. [DOI] [PubMed] [Google Scholar]
- 9.Husni RN, Goldstein LS, Arroliga AC, et al. Risk factors for an outbreak of multi-drug–resistant Acinetobacter nosocomial pneumonia among intubated patients. Chest. 1999;115:1378–1382. doi: 10.1378/chest.115.5.1378. [DOI] [PubMed] [Google Scholar]
- 10.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen K, Riddle MS, Danko JR, et al. Trauma-related infections in battlefield casualties from Iraq. Ann Surg. 2007;245:803–811. doi: 10.1097/01.sla.0000251707.32332.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott P, Deye G, Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 13.Anstey NM, Currie BJ, Hassell M, Palmer D, Dwyer B, Seifert H. Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii with carriage in the throat in at-risk groups. J Clin Microbiol. 2002;40:685–686. doi: 10.1128/JCM.40.2.685-686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Performance Standards for Antimicrobial Susceptibility Testing. Suppl 16. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. p. M100-S16. [Google Scholar]
- 15.Heritier C, Poirel L, Fournier PE, Claverie JM, Raoult D, Nordmann P. Characterization of the naturally occurring oxacillinase of Acinetobacter baumannii. Antimicrob Agents Chemother. 2005;49:4174–4179. doi: 10.1128/AAC.49.10.4174-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijkshoorn L, Aucken H, Gerner-Smidt P, et al. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol. 1996;34:1519–1525. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dessel H, Dijkshoorn L, van der Reijden T, et al. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res Microbiol. 2004;155:105–112. doi: 10.1016/j.resmic.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Akalin H, Ozakin C, Gedikoglu S. Epidemiology of Acinetobacter baumannii in a university hospital in Turkey. Infect Control Hosp Epidemiol. 2006;27:404–408. doi: 10.1086/503349. [DOI] [PubMed] [Google Scholar]
- 19.Siegel JD, Rhinehart E, Jackson M, Chiarello L the Healthcare Infection Control Practices Advisory Committee. [Accessed 18 March 2008];Guidelines for isolation precautions: preventing transmission of infectious agents in healthcare settings 2007. doi: 10.1016/j.ajic.2007.10.007. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Isolation2007.pdf. [DOI] [PMC free article] [PubMed]
- 20.Jawad A, Heritage J, Snelling AM, Gascoyne-Binzi DM, Hawkey PM. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol. 1996;34:2881–2887. doi: 10.1128/jcm.34.12.2881-2887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulin B, Rouget C, Clement C, et al. Association of private isolation rooms with ventilator-associated Acinetobacter baumanii pneumonia in a surgical intensive-care unit. Infect Control Hosp Epidemiol. 1997;18:499–503. doi: 10.1086/647655. [DOI] [PubMed] [Google Scholar]