Abstract
Background
All patients with atrial fibrillation (AF) require optimization of their ventricular rate. Factors leading to use of additional rhythm control in clinical practice have not been thoroughly defined.
Methods
The ORBIT-AF registry enrolled patients with AF from a broad range of practice settings and collected data on rate versus rhythm control, as indicated by the treating physician. Multivariable logistic regression analysis was performed to identify factors associated with each strategy.
Results
Of 10,061 patients enrolled, 6,859 (68%) were managed with rate only control versus 3,202 (32%) with rhythm control. Patients managed with rate control were significantly older and more likely to have hypertension, heart failure, prior stroke, and gastrointestinal bleeds. They also had fewer AF-related symptoms (41% with no symptoms vs 31% for rhythm control). Systemic anticoagulation was prescribed for 5,448 (79%) rate-control patients versus 2,219 (69%) rhythm-control patients (P < .0001). After multivariable adjustment, patients with higher symptom scores (severe symptoms vs. none, OR 1.62, 95% CI 1.41–1.87) and those referred to electrophysiologists (OR 1.64, 95% CI 1.45–1.85) were more likely to be managed with a rhythm control strategy.
Conclusions
In this outpatient registry of US clinical practice, the majority of patients with AF were managed with rate control alone. Patients with more symptoms and who were treated by an electrophysiologist were more likely to receive rhythm-control therapies. A significant proportion of AF patients, regardless of treatment strategy, were not treated with anticoagulation for thromboembolism prophylaxis.
Atrial fibrillation (AF) represents the most common dysrhythmia in the United States, and contributes significantly to healthcare expenditures. Management of AF varies and may include medical and interventional therapies to maintain sinus rhythm (“rhythm control”), as well as strategies to control the ventricular rate. While many patients managed with rhythm control also receive medications to control ventricular rate, there is a significant percentage of patients managed only with rate-controlling therapies (hereafter referred to as “rate control”). Clinical trials in selected patients have failed to demonstrate a survival benefit or lower complications with a rhythm control strategy,1 yet contemporary observational data suggest a long-termbenefit.2 These discrepant findings may be related to clinical features that determine selection of management strategy in practice, such as symptomatology, quality of life, and other patient or provider preferences.3 The appropriate criteria for selecting a management strategy in patients with AF have not been well-defined; therefore, it is largely left to providers to determine which patients are suitable for rhythm versus rate control alone.
To date, the use of rhythm versus rate control strategies has not been well-characterized in US community practice. International data, as well as the AFFECTS registry in the United States, have suggested significant differences in the population of patients selected for rate versus rhythm control, as well as differences in outcomes across a broad spectrum of AF patient types.4–6 Furthermore, contemporary medical therapy for both rate control and stroke prevention across management strategies remains unclear. We used data from the ORBIT-AF registry to address the following aims: (1) to measure the rates of use of different management strategies in AF patients in the United States; (2) to identify factors associated with the selection of a rhythm control strategy, versus rate control only; and (3) to describe the medical management of patients with rhythm versus rate control, including antiarrhythmic and anticoagulant therapies.
Methods
The ORBIT-AF study is a contemporary registry of outpatients in the United States with AF managed by a variety of providers, including internists, cardiologists, and electrophysiologists. A nationally representative sample of sites was invited to participate, with diversity across practice-type and geography. An adaptive design was used to ensure provider and geographic heterogeneity. However, enrollment was not formally stratified.7 Site selection and management was performed by the Duke Clinical Research Institute. Site investigators enrolled consecutive patients with AF meeting inclusion and exclusion criteria. Eligible patients included those 18 years of age or older, with electrocardiographic evidence of AF, providing informed consent, and able to follow-up. Patients with life expectancy of less than 6months or AF secondary to reversible conditions were excluded. The medical record served as the primary source of data, which was entered into a web-based case report form. Data collection focused on demographics, past medical history, type of AF and prior interventions, ongoing antithrombotic therapy (with monitoring), vital signs, laboratory studies, electrocardiographic findings, and echocardiographic findings. Prior and incident electrophysiology interventions are also captured, including both catheter-based and surgical ablations for AF and atrial flutter. It is important to note that the inclusion criteria mandated a diagnosis of atrial fibrillation. Patients with atrial flutter only were not eligible for ORBIT AF. Details about the ORBIT-AF registry have been described previously.7
The ORBIT-AF case report form specifically asked each treating physician to state the management strategy for each patient, as indicated by a mutually-exclusive check box (rate control vs. rhythm control). For the purpose of this analysis, patients were stratified by strategy (rate control or rhythm control), regardless of the type of AF (new onset, paroxysmal, persistent, or longstanding persistent AF). Baseline characteristics were compared between the two groups, including demographics, medical history, procedures, medical therapies, vital signs, and laboratory studies. Contraindications to antic- oagulation were also collected. Risk scores for stroke (eg, CHADS2) were calculated from baseline clinical data. The data are presented as frequencies and percentages for categorical variables and medians (interquartile range) for continuous variables (except where appropriate). The chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables were used for univariate comparisons.
In order to determine factors associated with rhythm control (versus rate control), a multivariable logistic regression model was constructed for the binary outcome of AF management strategy (with rate control as the reference group). Candidate variables included demographics, medical history, echocardiographic assessment, physician-assessed stroke and bleeding risks, vital signs, laboratory studies, functional status, provider care specialty, and enrolling site region, but not current therapies or symptoms. Identification of provider specialty was not mutually exclusive—patients managed by a primary care provider and multiple specialists were identified as having multiple providers.
Missing data was multiply-imputed and final estimates and standard errors reflect the combined analysis over five imputed datasets (all the candidate variables were missing < 5% except for the following: electrocardiographic evidence of left ventric- ular hypertrophy [7%], serum creatinine [7%], hemoglobin [10%], hematocrit [11%], left ventricular ejection fraction [20%], and left atrial diameter [26%], and posterior wall thickness [38%]). Model selection using backward selection with a stay criteria of 0.05 was used to obtain a set of factors in which each factor was independently associated with AF management strategy. The model was fit using logistic generalized estimating equations method with exchangeable working correlation matrix to account for within-site clustering because patients at the same site are more likely to have similar responses relative to patients at other sites (ie, within-center correlation for responses). The resulting model was subsequently used to adjust for confounders, and identify factors associated with treatment strategy selection.
The above-described multivariable model was also used to derive adjusted rates of rate-control therapies, including β- blockers, calcium-channel blockers, and digoxin.
All statistical analyses of the aggregate, de-identified data were performed by the Duke Clinical Research Institute using SAS software (version 9.2 and 9.3, SAS Institute, Cary, NC). All P values were 2 sided. The ORBIT-AF Registry is approved by the Duke Institutional Review Board, and all participating sites obtained institutional review board approval pursuant to local requirements. All subjects provided written, informed consent. The authors had access to the primary data, and take full responsibility for the validity.
Results
The entire baseline ORBIT-AF population included 10,098 patients enrolled between June 29, 2010 and August 09, 2011. The current analysis excluded 37 patients: 1 due to missing AF type/diagnosis and 36 for missing AF management strategy. This yielded a final study population of 10,061 patients from 174 sites. Baseline characteristics of the study cohort are shown in Table I according to rate or rhythm control. Over two- thirds were managed with a primary strategy of rate only control (n = 6,859, 68%) and nearly one-third with rhythm control (n = 3,202, 32%). Those managed with rhythm control were younger and had less medical co- morbidity. They also had a higher body mass index, calculated creatinine clearance, and left-ventricular ejection fraction, but lower resting heart rate.
Table I.
Baseline characteristics by treatment strategy
| Overall (n = 10,061) |
Rate control (n = 6859) |
Rhythm control (n = 3202) |
P | |
|---|---|---|---|---|
| Age (years) | 75 (67–82) | 76 (68–82) | 72 (63–79) | <.0001 |
| Female | 42 | 42 | 42 | .9 |
| Race | <.0001 | |||
| White | 89 | 88 | 91 | |
| Black or African-American | 5 | 5.3 | 4.4 | |
| Hispanic | 4.1 | 4.9 | 2.5 | |
| Other | 1.4 | 1.4 | 1.4 | |
| Medical history | ||||
| Smoking | 48 | 48 | 49 | .9 |
| Hypertension | 83 | 85 | 80 | <.0001 |
| Hyperlipidemia | 72 | 73 | 71 | .05 |
| Diabetes | 29 | 31 | 26 | <.0001 |
| Obstructive sleep apnea | 18 | 17 | 20 | .004 |
| Coronary artery disease | 32 | 33 | 30 | .003 |
| Heart failure | 32 | 35 | 27 | <.0001 |
| Implanted device | 27 | 29 | 25 | .0002 |
| Moderate/severe mitral stenosis | 1.4 | 1.6 | 0.8 | .002 |
| Prior cerebrovascular events | 16 | 17 | 14 | .0006 |
| Stroke (all-cause) | 8.9 | 9.5 | 7.6 | .002 |
| Non-hemorrhagic | 8 | 8.4 | 7 | .01 |
| Hemorrhagic | 0.8 | 0.9 | 0.5 | .02 |
| Other intracranial bleeding | 0.9 | 1.0 | 0.9 | .7 |
| Gastrointestinal bleeding | 9 | 9.6 | 7.9 | .006 |
| Cognitive impairment or dementia | 3.1 | 3.4 | 2.2 | .0009 |
| Frailty | 5.7 | 6.8 | 3.5 | <.0001 |
| BMI (kg/m2) | 29 (25–34) | 29 (25–34) | 30 (26–35) | .0001 |
| Heart rate (bpm) | 70 (63–80) | 72 (64–80) | 68 (60–76) | <.0001 |
| Systolic blood pressure (mm Hg) | 126 (116–138) | 125 (116–137) | 126 (116–138) | .15 |
| Diastolic blood pressure (mm Hg) | 72 (66–80) | 72 (66–80) | 72 (68–80) | .006 |
| Calculated creatinine clearance (mL/min per 1.73m2) | 70 (50–97) | 67 (48–93) | 76 (54–105) | <.0001 |
| LVEF (%) | 55 (50–61) | 55 (50–60) | 58 (50–63) | <.0001 |
| LA diameter (cm) | 4.4 (3.9–5.0) | 4.5 (4.0–5.1) | 4.2 (3.8–4.8) | <.0001 |
Values presented as percentage or median (interquartile range). Coronary artery disease includes any history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting. Creatinine clearance calculated by Cockcroft-Gaul formula.
BMI, Body mass index; LVEF, left-ventricular ejection fraction; LA, left atrium.
Patients managed with a rhythm control strategy were significantly more likely to be in sinus rhythm on their most recent electrocardiogram (Table II). They were more likely to have paroxysmal AF, higher symptom scores, and had lower CHADS2 scores. Almost one-third of patients in the rate control group had current or prior antiarrhythmic drug use, whereas 82% of patients managed with rhythm control were previously (or currently) taking antiarrhythmic therapy. Nearly one-third of the population had a prior cardioversion, and 11% had a prior electrophysiology intervention. These were both more common in the rhythm control group (P < .0001 for each).
Table II.
Atrial fibrillation history by treatment strategy
| Overall (n = 10,061) |
Rate control (n = 6859) |
Rhythm control (n = 3202) |
P | |
|---|---|---|---|---|
| AF type | <.0001 | |||
| First detected/new onset | 4.7 | 4.4 | 5.3 | |
| Paroxysmal | 50 | 41 | 70 | |
| Persistent | 17 | 17 | 17 | |
| Longstanding persistent | 28 | 38 | 7.2 | |
| Median (IQR) duration of AF diagnosis (months) | 47 (18–94) | 51 (20–98) | 41 (15–85) | <.0001 |
| Sinus rhythm on most recent ECG | 33 | 24 | 55 | <.0001 |
| EHRA symptom level | <.0001 | |||
| No symptoms | 38 | 41 | 31 | |
| Mild | 45 | 44 | 47 | |
| Severe | 14 | 13 | 18 | |
| Disabling | 1.8 | 1.5 | 2.6 | |
| CHADS2 risk groups | <.0001 | |||
| 0 | 6.5 | 5.1 | 9.5 | |
| 1 | 22 | 20 | 27 | |
| ≥2 | 71 | 75 | 63 | |
| Prior treatment with antiarrhythmic drug | 45 | 32 | 74 | <.0001 |
| Prior cardioversions | 30 | 26 | 40 | <.0001 |
| Prior electrophysiology interventions | 11 | 9 | 16 | <.0001 |
| Catheter ablation of AF | 5.5 | 3.4 | 9.8 | <.0001 |
| Atrial flutter ablation | 2.6 | 2 | 3.9 | <.0001 |
| AV node/HIS bundle ablation | 2.2 | 2.7 | 1.2 | <.0001 |
| Any surgical intervention | 1.9 | 1.6 | 2.7 | <.0001 |
| Treating provider specialty* | ||||
| Cardiology | 80 | 81 | 76 | <.0001 |
| Internal medicine/primary care | 67 | 70 | 60 | <.0001 |
| Electrophysiology | 17 | 13 | 24 | <.0001 |
| Neurology | 2.1 | 2.5 | 1.3 | .0003 |
| Site investigator specialty* | <.0001 | |||
| Cardiology | 65 | 67 | 61 | |
| Electrophysiology | 15 | 12 | 21 | |
| Internal medicine/primary care | 19 | 20 | 18 |
Values presented as %, except where noted.
ECG, Electrocardiogram; AV, atrioventricular; IQR, interquartile range.
Provider specialty is not mutually exclusive; however, site investigator specialty is mutually exclusive for each patient.
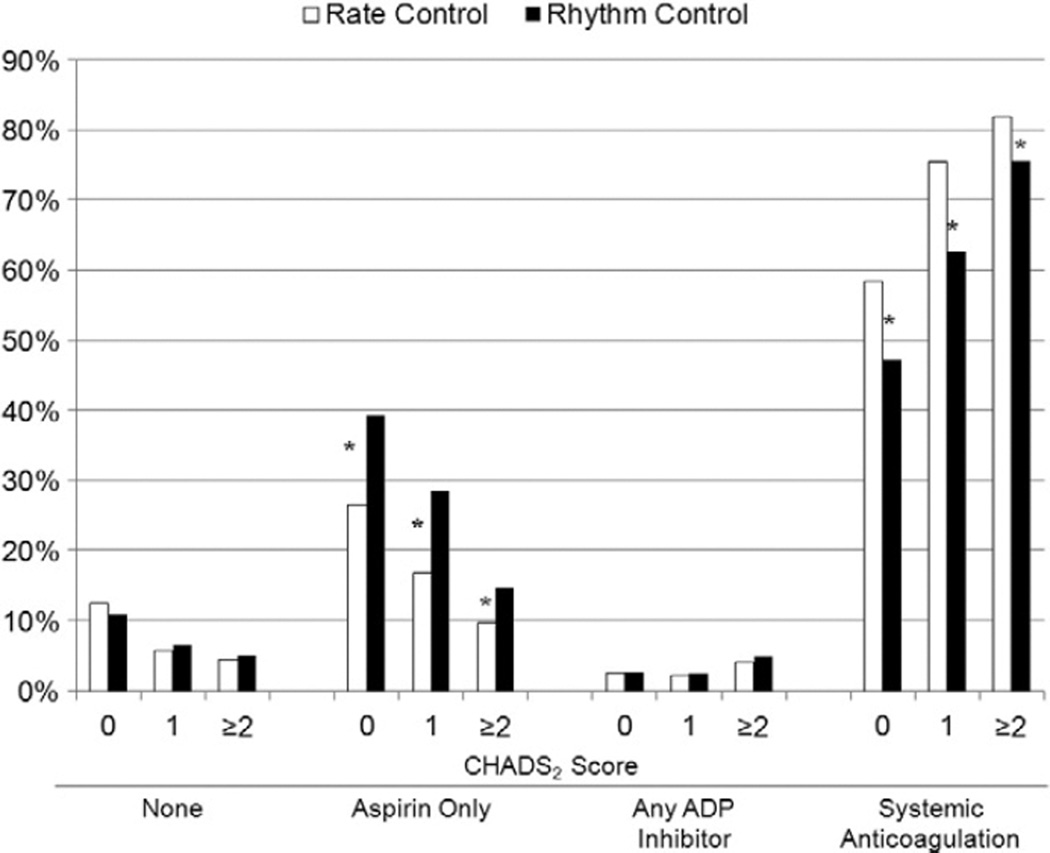
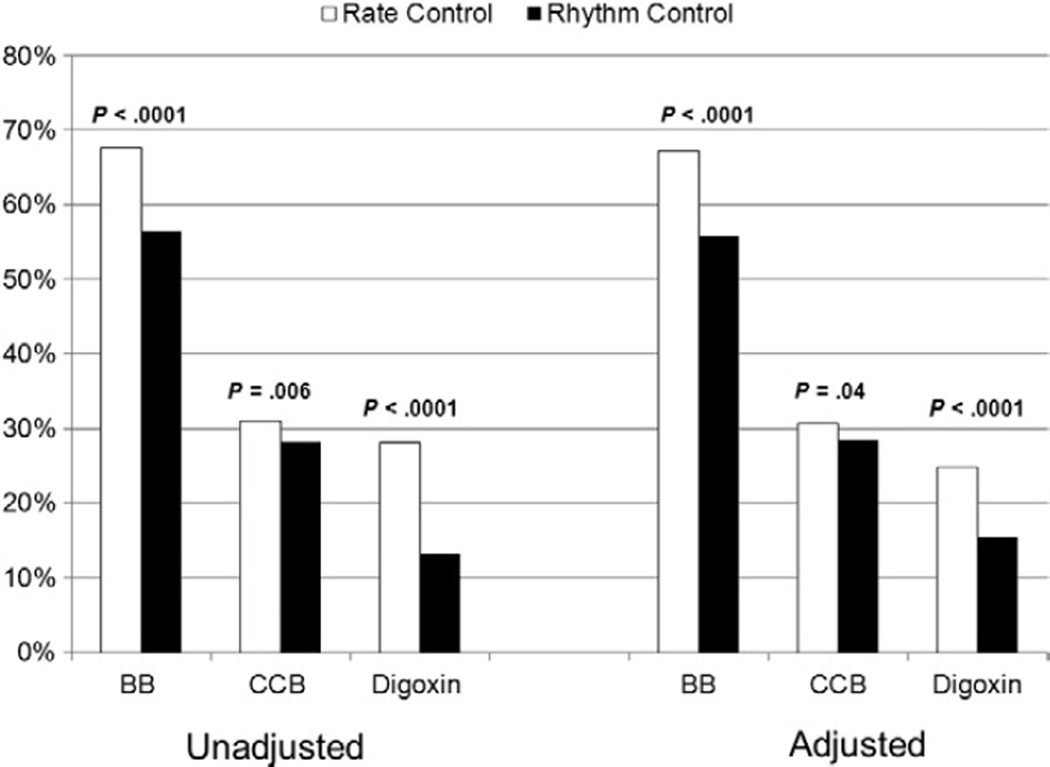
Unadjusted rates of medical therapies for AF are shown in Table III. A significant proportion of rhythm-control patients were also taking pure rate-controlling medications, yet to a lesser extent compared with rate-control patients. Strategies for the prevention of thromboembolism in each group, stratified by CHADS2 score, are shown in Figure 1. Overall, rhythm-controlled patients were more likely to be taking aspirin alone (21% vs. 12%, P < .0001), and less likely to be treated with oral anticoagulation (69% vs. 79%, P < .0001), despite a lower rate of contraindication to systemic anticoagulation (12% vs 15%, P = .002). As CHADS2 score increased, use of lone aspirin therapy decreased in favor of systemic anticoagulation. However, at all levels of risk, patients managed with rate control were significantly less likely to be prescribed aspirin alone and more likely to be on systemic anticoagulation. Therapies used to control heart rate, in unadjusted and adjusted analyses, are displayed in Figure 2. The use of nodal-blocking agents significantly favored the rate control group.
Table III.
Medical therapies by treatment strategy
| Overall (n = 10,061) |
Rate control (n = 6,859) |
Rhythm control (n = 3,202) |
P | |
|---|---|---|---|---|
| β-Blockers | 64 | 68 | 56 | <.0001 |
| Calcium-channel blockers | 30 | 31 | 28 | .006 |
| Non-dihydropyridine | 17 | 18 | 14 | <.0001 |
| Dihydropyridine | 14 | 13 | 15 | .1 |
| Digoxin | 23 | 28 | 13 | <.0001 |
| Currently on antiarrhythmic drug | 29 | 10 | 70 | <.0001 |
| Amiodarone | 9.9 | 3.8 | 23 | <.0001 |
| Sotalol | 6.1 | 1.7 | 16 | <.0001 |
| Dronedarone | 4.6 | 1.4 | 12 | <.0001 |
| Flecainide | 2.9 | 0.73 | 7.5 | <.0001 |
| Propafenone | 2.4 | 0.67 | 5.9 | <.0001 |
| Dofetilide | 1.9 | 0.31 | 5.3 | <.0001 |
| Ranolazine | 0.3 | 0.3 | 0.4 | .1 |
| Disopyramide | 0.1 | 0 | 0.4 | <.0001 |
| Oral anticoagulation in patients with CHADS2 ≥2 and no contraindication | 88 | 90 | 82 | <.0001 |
| Contraindication to anticoagulant therapy | 14 | 15 | 12 | .002 |
Values are presented as percentage
Figure 1.
Unadjusted use of antithrombotic therapies. Aspirin only included aspirin/dipyridamole, (n = 14, 0.14%). Any ADP inhibitor included clopidogrel or prasugrel, with or without aspirin, but no oral anticoagulant. Systemic anticoagulation included warfarin or dabigatran (with any antiplatelet). *P < .05 for the comparison between rate control and rhythm control groups. ADP: adenosine diphosphate.
Figure 2.
Unadjusted and adjusted comparisons of medical therapies between strategies. Multivariable rates adjusted for age, left atrial diameter, posterior wall thickness, level of education, site region, medical history of frailty, AF type, and provider specialty. BB, β-blocker; CCB, calcium-channel blocker.
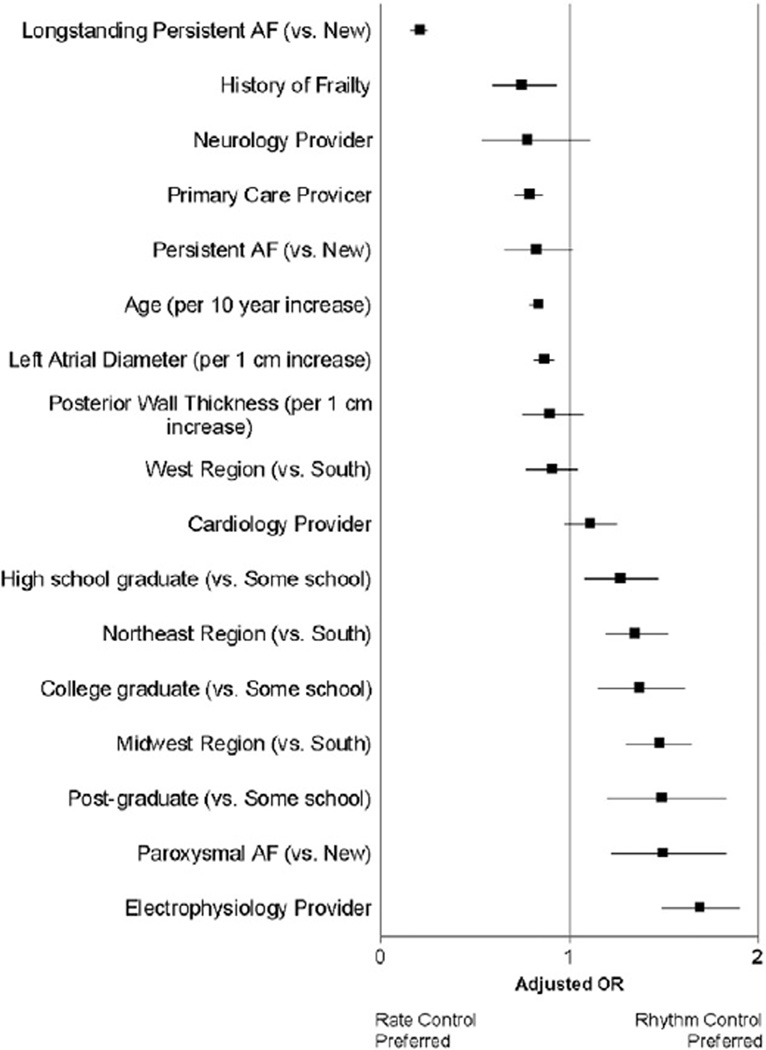
Clinical features associated with selection of rhythm control (versus rate control) after multivariable adjustment are shown in Figure 3 (c-index 0.74). Referral to an electrophysiologist (adjusted OR 1.68, 95% CI 1.49–1.90, P < .0001), paroxysmal AF (adjusted OR 1.49, 95% CI1.22–1.83, P < .0001), and more advanced educational background (post-graduate vs. some school, adjusted OR 1.48, 95% CI 1.20–1.83, P = .0002) all were associated rhythm control management. In contrast, older patients, those with longstanding AF, and those managed by primary care physicians were more likely to be treated with a rate control strategy. The European Heart Rhythm Association (EHRA) score was added to the model, and the presence of symptoms was also associated with selection of rhythm control strategy (compared to no symptoms [EHRA score I]): mild symptoms (EHRA score II) OR 1.25, 95% CI 1.12–1.38, severe symptoms (EHRA score III) OR 1.62, 95% CI 1.41–1.87, and disabling symptoms (ERHA score IV) OR 1.42, 95% CI 1.02–1.96. After adjustment for symptom burden, electrophysiology provider specialty, type of AF (paroxysmal), and younger age remained associated with a rhythm control strategy.
Figure 3.
Multivariable analysis of factors associated with AF management strategy. Boxes denote adjusted OR with lines to 95% CIs.
Discussion
In this cohort of more than 10,000 outpatients with AF, over two-thirds were managed with a rate control only strategy. The rate control patients tended to be older, with more extensive medical comorbidity, and more likely to be cognitively impaired. Patients managed with rhythm control had lower resting heart rates, and generally received less aggressive thromboembolic prophylaxis strategies. In multivariable analysis, advanced age, longstanding persistent AF, and primary care management were all associated with rate control management.
Rate control was the most common management strategy in our cohort, and to a greater extent than in prior observational AF studies, including the RECORD-AF, AFFECTS, and Euro Heart Survey registries.4–6,8 The preponderance of rate control is likely due to several differences. First, ORBIT-AF included only US patients whereas the RECORD-AF and Euro Heart Survey registries drew from Europe and Asia. The only other US-based registry reporting on rate and rhythm selection, the AFFECTS registry, was more limited in scope and enrolled only patients with uncomplicated hypertension and no structural heart disease. Patients enrolled in AFFECTS were primarily managed by general cardiologists.6 Second, ORBIT-AF represents an older population of patients with AF. Third, patients in ORBIT-AF had more co-morbidities than those in RECORD-AF or the Euro Heart Survey, with higher rates of diabetes, hypertension, and prior stroke.4,8 Lastly, increased participation by electrophysiology providers in the Euro Heart Survey may have also increased the likelihood of rhythm control management. The ORBIT-AF registry was designed to capture a broad population of patients with AF, and included a wide variety of geographic regions, as well as a more diverse provider population. The participation of primary care providers likely led to the enrollment of older and/or medically-complicated patients for whom AF is a long-standing problem managed exclusively in the primary care setting. Prior registries conducted only in cardiology or electrophysiology practices likely captured a more restricted cohort.
The preference for rate control is consistent with current guideline recommendations, which favor use of rate control only as an initial strategy.3 Patients who fail rate control or those with refractory symptoms are recommended for rhythm control (Class IIa recommen- dation, level of evidence B).3 These options might include antiarrhythmic therapy or catheter ablation— therapies that may have limited applicability or effectiveness in an older or medically-complex population. Additional medical comorbidities often limit the available rhythm-control therapies (eg, impaired renal function or coronary disease) or increase the challenges of maintaining sinus rhythm (eg, heart failure). Importantly, nearly one third of these ‘rate-controlled’ patients in ORBIT-AF had received an antiarrhythmic drug previously and roughly a quarter had undergone cardioversion, suggest- ing prior attempts at rhythm control. This is likely driven by the high symptom burden in our cohort, and a desire by patients and providers for improved functional capacity.9 Unfortunately, a small minority of these patients remained on rhythm-controlling drugs, indicating either failure of the therapy or poor tolerance. Both handicaps have plagued antiarrhythmic treatment for AF, leaving many patients without medical therapy to control their symptoms. Identifying and overcoming such deficiencies will be paramount, as there remains a significant unmet need for safe and effective medical therapies for rhythm control, and thereby symptom control, in AF.10
The finding that patients with more prominent symptoms of AF were more likely to be managed with rhythm control is consistent with prior data from the AFFIRM trial demonstrating improved symptom control with a rhythm control strategy in certain subgroups.9,11,12 In contrast, patients with lower functional status in our cohort were more likely to be managed with rate control. Several explanations for this discrepancy may exist. First, it may be difficult to ascertain AF symptoms in such patients, or their quality of life may be such that the treating physician feels they do not warrant more aggressive therapies. Second, this may represent a cohort of patients that failed prior attempts at rhythm control—either due to recurrence or intolerant to antiarrhythmic therapy. Lastly, the utility of rhythm control treatments for symptom management, particularly antiarrhythmic drugs, remains equivocal in several populations.9,13 However, poor functional status, particularly in patients with heart failure, may indicate the need for more aggressive rhythm control.12,14,15 Future trials of management strategy, including the use of interventional therapy, should help to evaluate the impact of rhythm control on both quality of life and cardiovascular outcomes.16
Stroke prevention with oral anticoagulation, a major component of guideline recommendations for AF, was lower than expected in this high-risk cohort. Over 70% had CHADS2 scores of greater than or equal to 2. Yet more than one quarter of patients overall were not treated with systemic anticoagulation (only 14% were noted to have a relative or absolute contraindication). These rates indicate an ongoing under-treatment of patients with AF, who are at high-risk for thromboembolic events—a finding con- firmed in the European population as well.8,17
We also observed differences in anticoagulation between the two management strategies. Aspirin-only therapy was more common for patients managed with rhythm control, whereas use of systemic anticoagulation was more common for the rate control group. While these data remain unadjusted for confounding characteristics, still nearly one third of rhythm-controlled patients were not on systemic anticoagulation while only 12% had a contraindication to its use. This suggests the possible perception that patients managed with rhythm control do not have significant risk for stroke—an observation disproved by both the AFFIRM and RACE trials.1 Investigators from the FRACTAL Registry also demonstrated that AF recurrence was a potent risk factor for the ongoing use of anticoagulation (or lack thereof).18 Yet, recent data have shown that atrial tachyarrhythmia duration as short as 6 minutes has been associated with increased risk of stroke or systemic embolism (1.69% vs 0.69% annually), a rate similar to that of rhythm- controlled patients with clinical AF (1.7%).4,19 While thromboembolic prophylaxis has yet to be tested in patients with clinically-silent arrhythmia, there remains room for improvement in the implementation of anticoagulation strategies for patients who do have a clinical diagnosis of AF.
Lastly, referral to an electrophysiologist was significantly associated with use of a rhythm-control strategy in multivariable analysis. While selection bias or referral bias may contribute to this finding, it suggests that the physicians who are more comfortable and familiar with antiarrhythmic therapies are more likely to implement them. Given the potential symptomatic and functional improvements for patients managed with rhythm control as well as the potential adverse effects of antiarrhythmic therapy), greater involvement of an electrophysiologist may be warranted for many patients with AF.
Limitations
The data presented herein are derived from a voluntary, observational study and thus are susceptible to the limitations inherent in such methods. These include both selection and reporting biases. Furthermore, the selection of management strategy is often on a continuum, with “rhythm control” patients often receiving therapies to control underlying ventricular rate as well. Additionally, past history of rhythm control failure can influence current therapy decisions, and any registry is limited by the “snapshot in time” during which it is performed, as well as the limited enrollment of ‘newly-diagnosed’ AF. Therefore, overlap in the two populations may exist. However, the aim of assessing management strategy in the current study is to capture the primary intent in caring for the patient with AF, and patterns of care that follow. The data in this study are dependent on the quality of medical record documentation and abstraction. Lastly, selection of management strategy was not randomized, therefore, despite multivariable adjustment it is possible that residual, unmeasured confounding remains.
Conclusions
In US clinical practice, the broad spectrum of patients with AF are much more commonly managed with a rate control strategy. Many patients treated with rate-control had failed prior attempts at rhythm control. Patients selected for rhythm control are younger, have less comorbidity, more recent-onset AF, higher symptom burden, and were more likely treated by electrophysiologists. Finally, stroke prophylaxis with oral anticoagulation remains underutilized among patients with AF.
Acknowledgements
The ORBIT-AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ.
This project was supported (in part) by funding from the Agency of Healthcare Research and Quality through cooperative agreement number 1U19 HS021092. Dr. Steinberg was funded by NIH T-32 training grant #5 T32 HL 7101-37.
Footnotes
On behalf of the ORBIT-AF investigators and patients.
Disclosure information
The following relationships exist related to this presentation: BS, DNH, GCF, and PRK report no relation-ships; MDE reports speakers bureau with Boehringer Ingelheim and consultancy with Boehringer Ingelheim, ARYx Therapeutics, Pfizer, Sanofi, Bristol-Myers-Squibb, Portola, Diachi-Sanko, Medtronic, Merck, Gilead, and Janssen. KWM reports research support from AstraZeneca, Amgen, Bayer, Boehringer-Ingleheim, Bristol-Myers-Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Portola, POZEN Pharmaceutical, Schering-Plough, and The Medicines Company, and consulting agreements with Amgen, AstraZeneca, Glaxo SmithKline, Johnson & Johnson, and Merck; JR reports consultancy and speakers bureau for Janssen; GN reports research grants from Wyeth, Reliant, Medtronic, Boston Scientific, Sanofi-Aventis, and Boehringer Ingelheim, and consultancies to Wyeth, Reliant, Medtronic, Boston Scientific, Sanofi-Aventis, Boehringer Ingelheim, Xention, Pfizer, Novartis, GlaxoSmithKline, and St. Jude Medical; JAR reports research support from Boehringer Ingelheim and GlaxoSmithK-line, consultancies with Sanofi-Aventis, Gilead, CV Therapeutics, GlaxoSmithKline, Merck, Cardiome Pharma, Boehringer Ingelheim, Medtronic, and speakers’ bureau with Sanofi-Aventis, Boehringer Ingelheim; PC is an employee of Janssen; EDP reports research support from Eli Lilly & Company and Janssen, JPP reports research support from Boston Scientific Corporation and Janssen and consultancies to Forest Laboratories, Janssen, and Medtronic.
References
- 1.Saksena S, Slee A, Waldo AL, et al. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management): an assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J Am Coll Cardiol. 2011;58(19):1975–1985. doi: 10.1016/j.jacc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ionescu-Ittu R, Abrahamowicz M, Jackevicius CA, et al. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation rhythm vs rate control drug treatment. Arch Intern Med. 2012:1–8. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123(10):e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Breithardt G, Crijns H, et al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation) J Am Coll Cardiol. 2011;58(5):493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwlaat R, Prins MH, Le Heuzey JY, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29(9):1181–1189. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 6.Reiffel JA, Kowey PR, Myerburg R, et al. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry) Am J Cardiol. 2010;105(8):1122–1129. doi: 10.1016/j.amjcard.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J. 2011;162(4):606 e1–612 e1. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 9.Chung MK, Shemanski L, Sherman DG, et al. Functional status in rate- versus rhythm-control strategies for atrial fibrillation: results of the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Functional Status Substudy. J Am Coll Cardiol. 2005;46(10):1891–1899. doi: 10.1016/j.jacc.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg JS, Sadaniantz A, Kron J, et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109(16):1973–1980. doi: 10.1161/01.CIR.0000118472.77237.FA. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins LS, Brodsky M, Schron E, et al. Quality of life in atrial fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149(1):112–120. doi: 10.1016/j.ahj.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 12.Guglin M, Chen R, Curtis AB. Sinus rhythm is associated with fewer heart failure symptoms: insights from the AFFIRM trial. Heart Rhythm. 2010;7(5):596–601. doi: 10.1016/j.hrthm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 14.Henrard V, Ducharme A, Khairy P, et al. Cardiac remodeling with rhythm versus rate control strategies for atrial fibrillation in patients with heart failure: Insights from the AF-CHF echocardiographic sub-study. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.08.077. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, Alam S, Chiang CE, et al. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012;98(3):195–201. doi: 10.1136/heartjnl-2011-300550. [DOI] [PubMed] [Google Scholar]
- 16.Tsadok MA, Jackevicius CA, Essebag V, et al. Rhythm versus rate control therapy and subsequent stroke or transient ischemic attack in patients with atrial fibrillation. Rhythm Versus Rate Control Therapy and Subsequent Stroke or Transient Ischemic Attack in Patients With Atrial Fibrillation. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.112.092494. Epub. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwlaat R, Capucci A, Lip GY, et al. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27(24):3018–3026. doi: 10.1093/eurheartj/ehl015. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds MR, Shah J, Essebag V, et al. Patterns and predictors of warfarin use in patients with new-onset atrial fibrillation from the FRACTAL Registry. Am J Cardiol. 2006;97(4):538–543. doi: 10.1016/j.amjcard.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 19.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Eng J Med. 2012;366(2):120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]