Abstract
Herein we review the use of non-nephrotoxic perfluorocarbon nanoparticles (PFC NP) for noninvasive detection and therapy of kidney diseases, and provide a synopsis of other related literature pertinent to anticipated clinical application. Recent reports indicate that PFC NP allow quantitative mapping of kidney perfusion, and oxygenation after ischemia-reperfusion injury with the use of a novel multi-nuclear 1H/19F magnetic resonance imaging (MRI) approach,. Furthermore, when conjugated with targeting ligands, the functionalized PFC NP offer unique and quantitative capabilities for imaging inflammation in the kidney of atherosclerotic ApoE-null mice. Additionally, PFC NP can facilitate drug delivery for treatment of inflammation, thrombosis, and angiogenesis in selected conditions that are comorbidities for to kidney failure. The excellent safety profile of PFC NP with respect to kidney injury positions these nanomedicine approaches as promising diagnostic and therapeutic candidates for treating and following acute and chronic kidney diseases.
Keywords: Nanoparticles, Molecular Imaging, Perfluorocarbon, Perfusion, Oxygenation
A. Introduction
In this brief review, we introduce the topic of nanomedicine approaches for detection and potential therapy of acute and chronic kidney diseases. The prevalence and severe consequences of ischemia, hypoperfusion, and hypoxia on renal function mark these pathological substrates as clear targets for new approaches to ameliorate or preserve kidney function, especially in acute kidney injury where diagnosis is challenging and therapy primarily supportive. Because the kidney is a highly metabolic organ, protracted bouts of ischemia or hypoxia can initiate an injury program leading to extensive cellular necrosis and/or apoptosis, especially under circumstances of mixed acute and chronic disease, or in the face of comorbidities such as diabetes or atherosclerosis or sepsis. Accordingly, sensitive noninvasive methods to detect and monitor intrarenal blood flow and tissue oxygenation could be a boon to emerging therapeutic regimens designed more specifically to interdict the progression of renal dysfunction at its earliest stages.
Unfortunately, quantitative methods for assessing kidney hypoxia are neither well developed nor commonly deployed in the clinic. Traditional blood and urine markers, such GFR or kidney injury molecules, do not provide direct measures of kidney hypoxic injury. Even such metrics as total oxygen consumption based on the difference in arterial and venous pO2 appears insensitive to kidney hypoxia because less than 10% of delivered oxygen is needed for kidney metabolism.1 Although contrast-enhanced CT or MRI could provide multiple indexes (e.g. time of peak perfusion, blood volume) for assessing kidney perfusion,2–4 the increased risk of contrast agent induced kidney failure or nephrogenic systemic fibrosis limits the application of these imaging methods for patients with existing kidney diseases.5–8
In the last two decades, non-invasive BOLD and arterial spin labeling MRI have been applied to image kidney hypoxia and perfusion, which might obviate the need for exogenous contrast agents.9–13 The BOLD MRI approach maps blood oxygenation based on the increased ratio of paramagnetic deoxyhemoglobin in the capillary bed of hypoxic tissue that elicits shortening of proton T2* relaxation times.2 However, BOLD readouts are sensitive to many factors such as the blood volume, flow rate, magnetic field inhomogeneity, or image "susceptibility" artifacts induced by hemorrhage. Moreover, the BOLD readout may not be precisely correlated with the severity of kidney dysfunction at various stages of chronic kidney disease 14. The arterial spin labeling approaches have been proposed to elucidate kidney perfusion by quantifying the intra-renal signal derived from magnetically prepared or "labeled" segments of flowing blood.15, 16 Although the technique might provide an accurate and reproducible readout of renal perfusion, its low signal-to-noise features are problematic for clinical application. Given these limitations, ultimately it may be the case that multi-parametric MRI with the use of BOLD, spin labeling, and/or other techniques could improve detection and quantification of intrarenal perfusion defects but at the cost of time and expense required to perform the linked studies.11, 17, 18
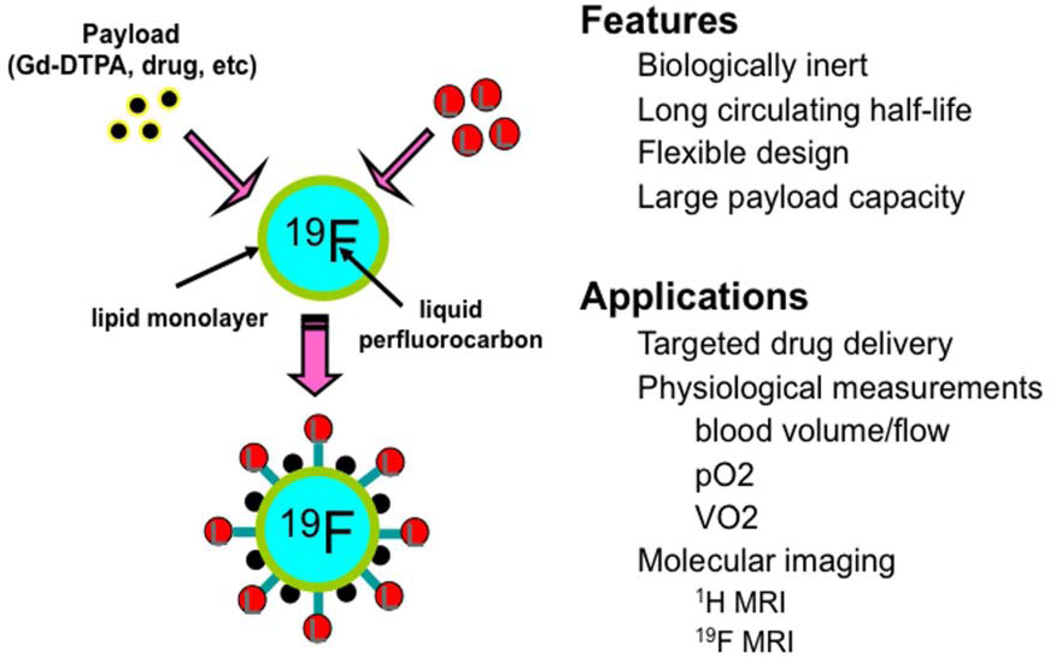
Multinuclear 1H/19F MRI coupled with perfluorocarbon (PFC) based nanoparticles (NP) represent an alternative readout applicable for imaging kidney hypoperfusion and associated injury (Figure 1).19, 20 PFC NP are a class of clinically approved vehicles with no apparent toxic effect on the kidney.21, 22 As a blood-pool agent, the linear relationship between the 19F signal intensity and PFC NP’ concentration allows direct measure of blood volume in tissue.23–25 In addition, the relationship between the 19F relaxation time (R1= 1/T1) of PFC NP and dissolved O2 that alters 19F relaxation linearly according to pO2, has been utilized for in vivo assessment of pO2 in blood and tissue26–28. Finally, functionalized PFC NP with the use of selected targeting ligands that can bind to and detect inflammatory markers (e.g. VCAM-1) may also allow early detection of inflammatory kidney diseases. This review offers an overview of diagnostic 1H/19F MRI with the use of PFC NP, as well as insights into combined therapeutics with the same 19F nanosystem carrying drug and gene cargoes.
Figure 1.
Paradigm for physiological and molecular imaging with perfluorocarbon nanoemulsions. Reprinted from Wickline et al.20 with permission.
B. PFC NP For Imaging
PFCs are a group of 19F-containing compounds derived from hydrocarbons by partial or complete substitution of 1H with 19F.29 PFCs are biologically stable, inert, well tolerated, and can carry a significant payload of O2 in dissolved form, which established their original use as blood substitutes. Liquid PFCs also have been employed as an organ preservation media for kidney transplant.30 31, 32
For in vivo applications, PFCs typically are emulsified into a nanoparticle form with a surfactant coating for stabilization and functionalization (Figure 1). A typical PFC NP emulsion contains 40% (v/v) PFOB, 2% (w/v) safflower oil, 2% (w/v) of surfactant commixture, 1.7% (w/v) glycerin, and water balance.33 Thus the final formulated PFC NP comprises principally a liquid PFC core encapsulated by a lipid monolayer with a diameter of 150–250 nm and a variable surface charge depending on the types of phospholipids and surfactants used to construct it (e.g., −40 to +20 mV).
The use of PFC NP for examining tumor hypoxia has been pursued for some time and recent excellent reviews are available on the subject from pioneers in this area such as Mason.26, 34–37 In most of these prior applications, targeting ligands have not been included as direct injections were often required to provide sufficient signal for detection of the 19F signal in order to generate proof of concept for pO2 measurements. However, as interest in the broad field of "molecular imaging" was emerging around that time, our group was the first to explore the large signal generating capacity of a nanoparticle when linked to chelated lanthanides to formulate a robust paramagnetic "bright spot" molecular MRI contrast agent.38, 39 Because the PFC NP technology was well known and offered a ready substrate for functionalization with its surrounding lipid-surfactant surface layer, it was relatively straightforward to consider adding gadolinium complexes to the surface in large quantities (e,g., 100,000–200,000 Gd per particle), which would provide substantial signal amplification upon localization to molecular binding sites. In addition to the large cargo of Gd, a molecular targeting ligand could be added as well, where both imaging agent and targeting ligand were covalently coupled to a phospholipid anchor that was directly formulated into the particle in a single emulsification step (see specific descriptions below).
Furthermore, this PFC NP construct also was developed as the first reported ultrasound molecular imaging agent due to special features of its composition that render it visible to mechanical waves at clinically relevant imaging frequencies.40 Fluorescence and SPECT imaging capabilities also exist for the PFC NP system when it is coupled to contrast moieties appropriate to those modalities.41, 42
As compared with iodinated or Gd contrast agents, PFC NP could be well suited to patients with kidney disease. PFC NP exhibit a good safety profile with minor dose dependent flu-like symptoms or fever in 24 hours after dosing. 43, 44 No renal toxicity was reported in animals and human.22 With a nominal diameter of 250 nm, PFC NP are not susceptible to glomerular filtration but rather are removed from the circulation primarily by the reticuloendothelial system and then the PFC component ultimately is vaporized through respiration.21 The blood clearance half-life of PFC NP ranges from 3 to 42 hours depending the exact preparation method,41 thereby providing sufficient time for MRI detection.
Despite the apparent utility of nanostructures carrying large payloads of Gd for T1 weighted MRI, it was also clear that the fluorine core of the PFC NP might provide an alternative approach for magnetic resonance detection. This could represent a unique imaging signature because there is no detectable 19F in the body at typical clinical field strengths. For imaging there would be no background signal as is the case for other contrast agents that rely on their effect on proton relaxation to create contrast.45 In the case of 19F imaging, quantification of the amount of PFC NP in the imaging field of view is possible by directly assessing the spectral energy contained within the 19F readout, which is proportional to the 19F concentration, and thus the number of PFC NP containing them.46–49 Thus, the non-targeted PFC NP agents have been developed for blood pool imaging and perfusion,46–48, 50 cellular (stem cell, dendritic cell, etc.) labeling and tracking,49, 51–55 cellular and tissue uptake for imaging inflammation and allograft rejection, among others. 56, 57
Elucidation of Kidney Function and Injury with PFC NP
Hypoxic/ischemic injury is a common pathology in acute and chronic kidney diseases.35, 58–60 Some contributing factors include: interstitial fibrosis that increases the diffusion distance of O2 from capillaries to tubular epithelial cells,61 vessel rarefraction that reduces the capillary density,62 arterial stenosis that can reduce the overall amount of blood delivered to the kidney,63 and acute thrombosis that blocks regional blood flow. Hypoxic/ischemic injury may develop regionally, e.g. in the cortex or medulla,61 but nevertheless still affect the global renal function. This regional manifestation of the disease process can pose a challenge to the precise elucidation of the disease process and stage when only global kidney functional readouts are available such as GFR or blood/urine markers.64 We therefore have proposed that non-invasive regional mapping of kidney oxygenation could be beneficial for understanding the disease process and for clinical management that may be stage and molecular pathway specific.
Quantitative 19F MRI using PFCs or PFC NP has been extensively exploited for in vivo imaging of hypoxia.29, 35 PFCs can carry a high payload of O2 that exhibit a linear dissociation curve, which renders them potential reporters for local pO2 such that at a given temperature, the partial pressure of dissolved paramagnetic O2 in PFCs is linearly correlated with the 19F relaxation rate.65 The exchange of O2 between PFCs and surrounding media occurs through free diffusion operating on a millisecond timescale.66 It is straightforward to generate a calibration curve specifying the relationship between 19F R1 and pO2 at a given field strength in order to read out the pO2 to provide a non-invasive measure of tissue oxygenation.67 In solid tumor where tissue temperature is heterogeneous, hexafluorobenzene and perfluoropolyether that exhibit minimal sensitivity of 19F R1 to temperature changes between 30 and 42°C are typically used for 19F MRI oximetry.68 The precision of 19F MRI oximetry may be within 1–3 mmHg and the absolute readout of pO2 is comparable to that measured with fiberoptic,69 and near-infrared spectroscopy70, 71 in tumor tissues.
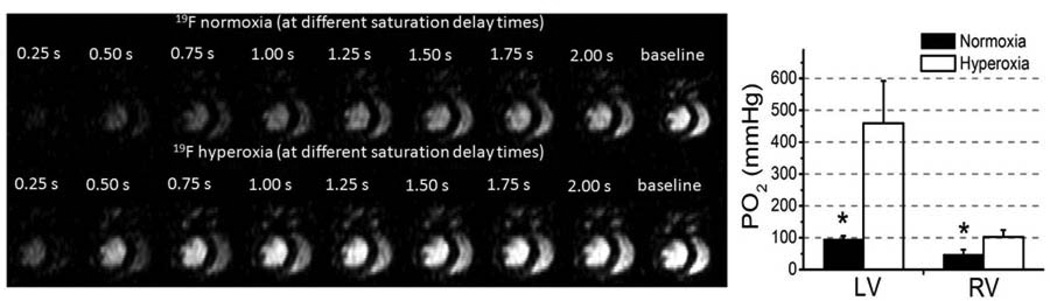
Recently, fast 19F MRI techniques like FREDOM (Fluorocarbon Relaxometry using Echo planar imaging for Dynamic Oxygen Mapping) and BESR (Blood Enhanced Saturation Recovery) have been developed to facilitate in vivo mapping of pO2,72–74 which should be applicable for kidney imaging and pO2 measurements. Our approach using the BESR method mirrors some existing methods for defining proton relaxivity in vivo but takes advantage of flowing blood to quantize the 19F signal that is moving rather than stationary, which facilitates correlation of the detected signal with blood volume, blood flow, and regional pO2.74 Figure 2 shows the effect on the MRI signal intensity for 19F in circulating PFC NP as it is affected by blood pO2. The presaturated (darker cardiac chambers at left side of panels) 19F spins in the heart recover (i.e., brighten) more quickly as the signal is measured over 2 seconds under conditions of hyperoxia (lower panels) than of normoxia (upper panels). Moreover, the signal in the RV recovers more slowly than that in the LV due to the inherently lower pO2 in the RV chamber under all conditions. The quantification of LV and RV pO2 matches well with the expected values under each condition. These types of measurements in the kidney should now be possible in vivo in actual patients due to the recent implementation of similar approaches on clinical 3T MRI scanners that can acquire simultaneous 1H and 19F signals for mapping PFC NP concentrations, regional pO2,75 arterio-venous differences in pO274 or blood volume fraction,76, therefore to derive global renal oxygen consumption.
Figure 2.
Left19F BESR images of the mouse heart under hyperoxia and normoxia illustrating signal due to 19F PFC NP that is responsive to blood pO2. Right: Measured blood pO2 based on measured 19F T1 of PFC NP showed pO2 difference between LV and RV, and between normoxic and hyperoxic conditions for each ventricle. Reprinted from Hu et al. 74 with permission.
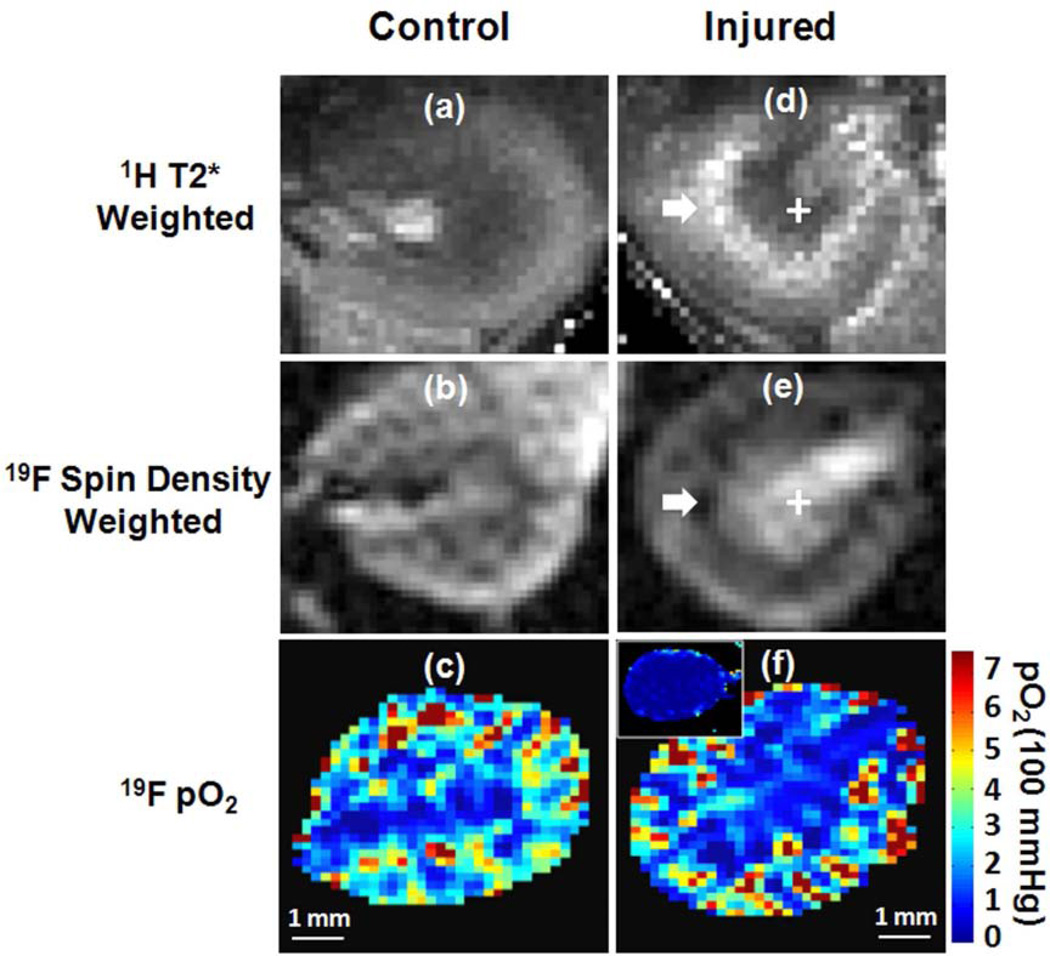
Moving on to the kidney itself, we have employed an acute kidney injury model and applied 19F MRI in conjunction with PFC NP to assess ischemic injury and the consequences of regional hypoperfusion.77 The objective here is to develop a multi-nuclear 1H/19F MRI approach for functional renal imaging. We first demonstrated the feasibility of 19F MR mapping of renal blood volume and pO2 in healthy mouse kidneys (Figure 3. Left panel). Subsequently, a unilateral renal ischemia-reperfusion model of AKI78 to demonstrate that transient ischemia damages endothelial and tubular cells causing sustained medullary microvessel non-perfusion79 and tubular ischemia.80–82 The result of this study showed that 19F MRI sensitively detected decreased renal blood volume in the cortical-medullary (CM) junction, where 1H BOLD MRI also detected increased T2* reflecting reduced susceptibility effect from blood flow. (Figure 3. Right panel). The follow-up histology analysis confirmed a reduced density of perfused blood vessels in the CM junction of injured kidneys. The intrarenal blood pO2, however, remained unchanged, which may attributed to the capability of kidney to auto-regulate perfusion to maintain oxygenation despite the reduction of blood flow.1, 83
Figure 3.
Representative 1H BOLD T2*-weighted image, 19F spin density weighted image and 19F T1-mapping derived pO2 map in the left control (A–C) and right injured (D–F) kidneys of the same mouse. The inserted panel in (F) shows 19F MRI detected pO2 map of another mouse kidney during ischemia. White arrow points to the CM junction of the injured kidney where abnormally increased 1H T2* and decreased 19F signal intensity were detected. Reprinted from Hu et al.77 with permission.
Our previous work have demonstrated the feasibility of in vivo 19F MRI angiography in rabbits on a 1.5T Philips clinical MR system.50 Given the high blood volume in the kidney and the availability of 3T clinical MR consoles, 19F MRI assessment of kidney blood volume and pO2 should be achievable in clinical settings.
Potential Molecular Imaging Applications For Targeted PFC NP
The panoply of molecular imaging applications for kidney disease that can be addressed with the use of targeted contrast agents is extensive. Here we review some indications for targeted PFC NP that are directly related to or associated with important comorbid conditions that apply to progressive renal disease.
Atherosclerosis
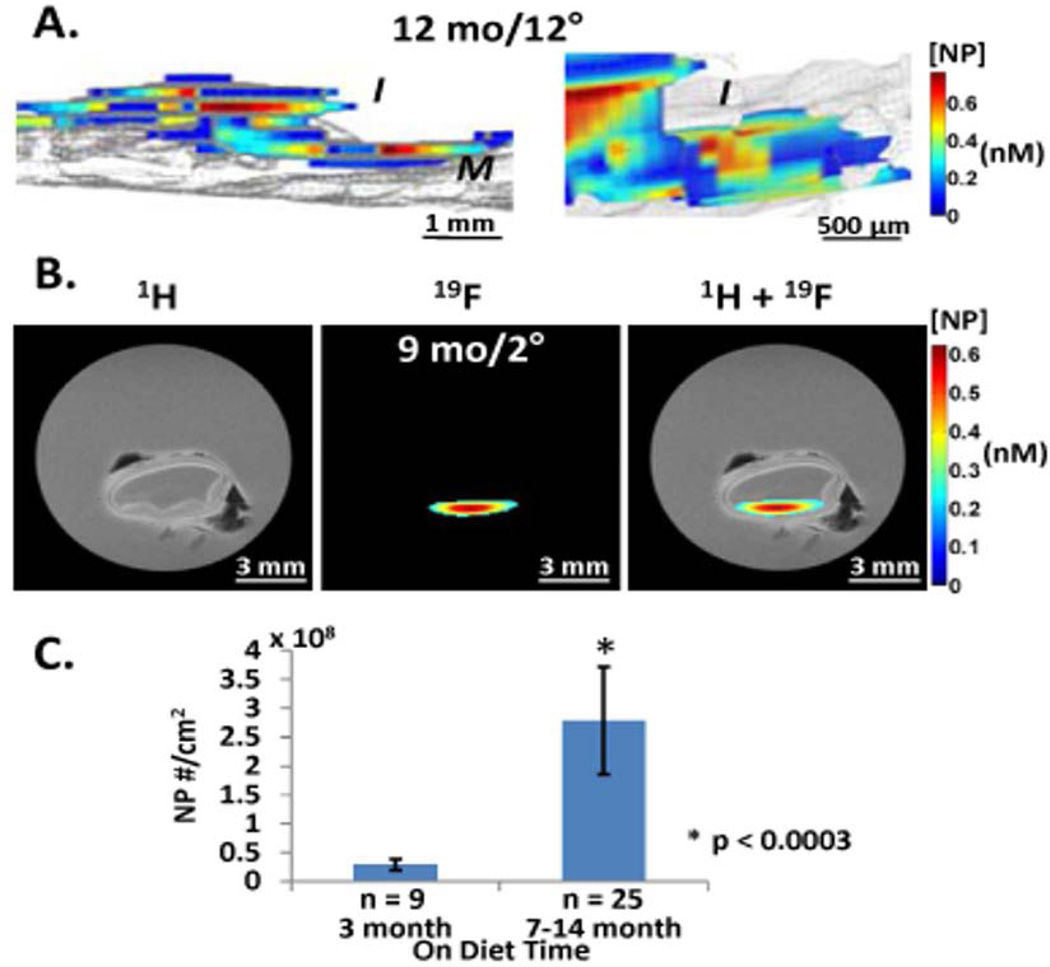
The role of atherosclerosis in predisposing to acute and chronic renal failure under many conditions is clear.60, 84 Moreover, the inflammatory milieu in both early and advanced plaques can be addressed with 19F imaging in several ways. We have shown recently that non-targeted PFC NP can diffuse passively into the core of intimal plaques where vascular barrier disruption has occurred over time as a consequence of endothelial damage, apoptosis, and cellular sloughing.56 Indeed, in the later stages of the disease process in fat fed rabbits for example, massive infiltration of nanoparticles into the plaque extending up to the medial boundary can occur in vivo, and be detected with 19F MRI, indicating the ready access of blood coagulation elements to a hypercoagulable inflamed plaque milieu, predisposing to acute and/or chronic thrombosis (Figure 4). Similar passive penetration into human carotid endarterectomy samples excised for clinical indications in symptomatic patients mimicked the experimental detection of endothelial barrier disruption. These data suggest that vascular endothelial disruption in acute kidney injury might similarly be amenable to quantification with 19F MRI, as we showed above for nanoparticles passively penetrating the renal interstitium in ischemic injury.
Figure 4.
MRI imaging and quantification of NP signal in cholesterol plaques (A) Left: 3D saggital rendering of nanoparticle signals from aorta of 12 mo cholesterol fed rabbit after 12 hours circulation time in vivo.19F MR image registering nanoparticle fluorine cores (color) overlaid on 1H MR image (gray) of aorta show intimal (I) location of particles trapped in thickened plaque (color) atop the medial (M) layer (gray). Particle concentration per voxel is coded in nM (scale bar). Right: Close up of intimal layer. (B) 1H, 19F and overlay MR transverse images of aortic rings with nanoparticles trapped in intima of thickened plaque from 9 mo cholesterol fed rabbit after 2 hour circulation in vivo. Black artifacts are small air bubbles. Note lack of 19F signal from more normal adjacent tissue sections. (C) Comparison of normalized CE NP number to the endothelial surface area between 3 month diet and >7 month diet rabbit aorta samples showing 10 fold greater accumulation in older plaques. Reprinted from Zhang et al. 56 with permission.
Alternatively, angiogenesis imaging is possible with a number of modalities including MRI, PET, SPECT, and ultrasound.85–87 The role of plaque neovasculature that is driven by tissue/macrophage hypoxia is known to be required for early plaque growth and predisposes to late plaque rupture and subsequent thrombosis and acute vessel occlusion.88–92 For example, in experimental atherosclerosis in fat fed rabbits, the neovasculature emerging from the inflamed adventitial vasa vasorum can be detected and quantified by MRI with Gd-conjugated NP targeted to upregulated endothelial integrins (Figure 5) at clinical field strengths.39, 93–95 The same agents have been used for tumor angiogenesis imaging, which also have been detected in vivo with 19F MRI.96
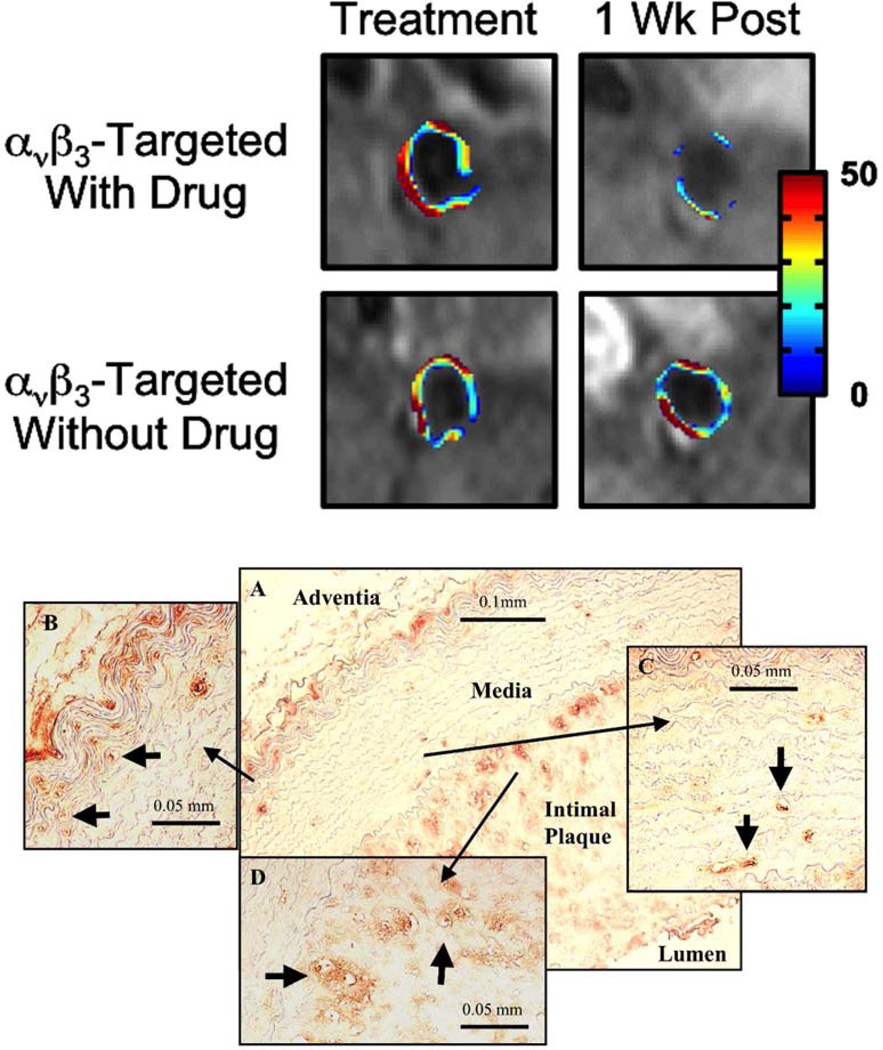
Figure 5.
Top Panel: MRI of neovasculature in rabbit plaque with αvβ3-targeted nanoparticles. Significantly lower angiogenesis one week after treatment with targeted fumagillin particles. Rabbits treated with non-targeted fumagillin nanoparticles show high levels of angiogenesis one week post treatment, similar to particles without fumagillin. Bottom Panel: Angiogenesis in rabbit atherosclerotic plaque. (A) Histological section of abdominal aorta from control animal demonstrating intimal plaque and strong staining for αvβ3-integrin (LM-609) at the media-adventia and media-intima interfaces. Angiogenic vessels (arrows) are observed in the vasa vasorum (B), media (C) and intimal plaque (D). Reprinted from Winter et al. 120 with permission.
Neovasculature expressing heterodimeric molecular epitopes such as αvβ3 integrins also has been detected sensitively with ultrasound imaging in experimental models.97–99 In a genetic model of squamous cell carcinoma, extremely limited extents of inflammatory neovasculature could be demonstrated in the mouse pinna with high resolution ultrasound imaging systems and special signal processing algorithms that harbor quantitative potential based on the "information content" of the data stream.98–100 Both pathological (e.g., atherosclerosis, cancer, inflammation) and tissue reparative processes (e.g., recovery from ischemic injury) invoke significant angiogenesis that might be candidates for renal imaging,
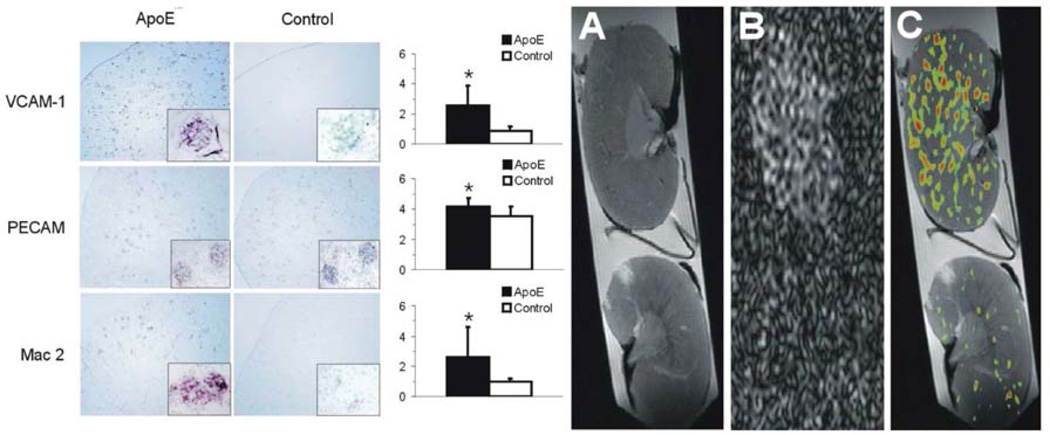
An array of other inflammatory molecules involved in atherosclerosis might be amenable to noninvasive molecular imaging. For example, we have used 19F MRI to detect the upregulation of renal vascular VCAM-1 in mice fed a Western diet for 35 weeks (Figure 6).101 Because VCAM and other related adhesion molecules are responsible for trafficking of immune effector cells such as monocytes and neutrophils to areas of damage and inflammation, assessment of the potential for cellular recruitment in kidney injury might be useful disease staging and optimization of therapeutic approaches. Ultrasound approaches with targeted microbubbles containing a gaseous perfluorocarbon also have been demonstrated for renal VCAM imaging,102 although there is lesser potential for quantification.
Figure 6.
The ApoE−/− kidney is characterised by significant up-regulation of VCAM-1 expression and a marked infiltration of macrophages into the glomeruli. A representative 1H MR image (A) showing structural detail, 19F MR image (B), and a composite 1H/19F image (C) showing VCAM-1-targeted nanoparticle accumulation in atherosclerotic ApoE−/− (top) and wild-type control (bottom) kidneys, imaged at 12T. Voxels with a signal intensity greater than twice the standard deviation were defined as positive for 19F to produce a distribution map of 19F throughout each kidney, which corresponds to nanoparticle binding via VCAM targeting. Data represent mean (n=6±SD). *represents p<0.05. Reprinted from Southworth et al.101 with permission.
Thrombosis
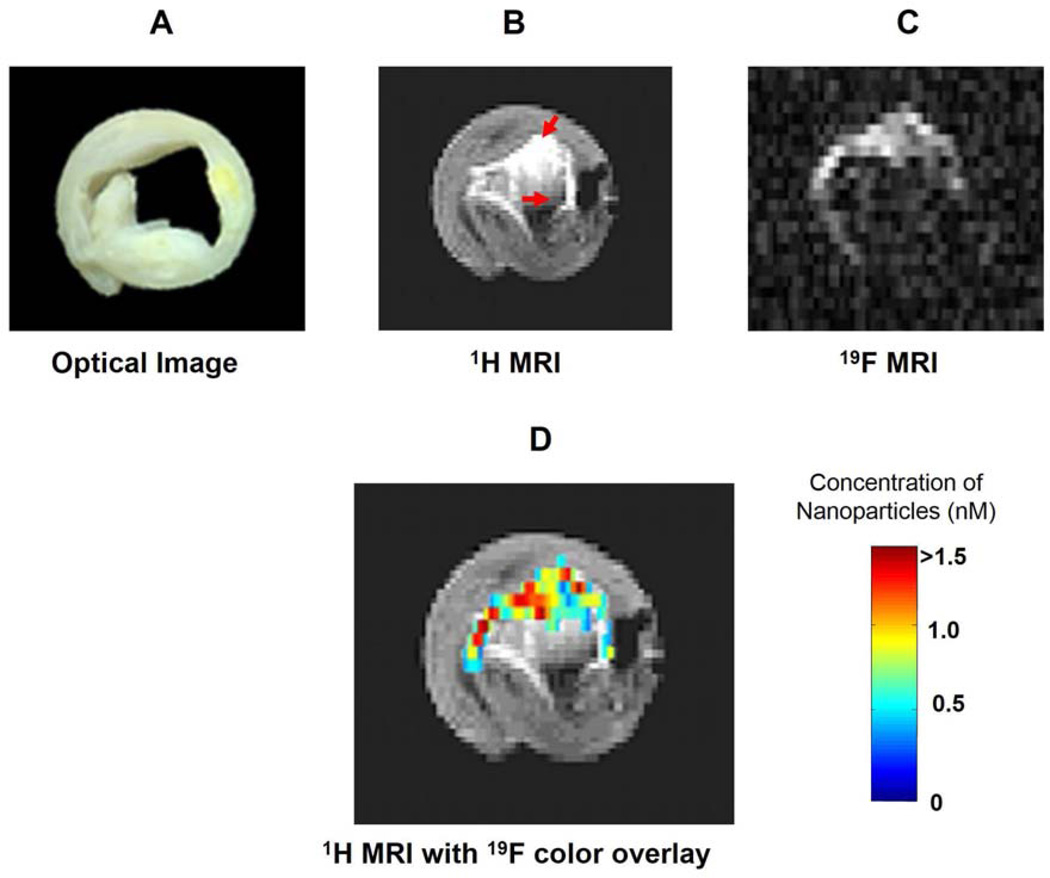
Fibrin detection in formed clots also has been demonstrated in vivo with ultrasound at clinical frequencies after binding of PFC NP targeted to selected fibrin epitopes, again with a monoclonal antibody.38, 40, 103 Because fibrin clots are barely echogenic and therefore difficult to detect with clinical ultrasound, this could be useful for renal diseases that are prone to thrombosis such as hemolytic uremic syndrome, AKI, sepsis, atherosclerosis, etc. Additional examples of fibrin imaging have been reported with the use of MRI by detection of either the 1H or 19F signatures of PFC NP (Figure 7) 47, 104–106 As compared with standard ultrasound molecular imaging, the MRI readouts for 19F are quantitative and can be expressed in molar concentrations of bound nanoparticles or fluorine atoms (see Figure 7) permitting longitudinal determination of the local concentration of targeted molecular epitopes.
Figure 7.
Fibrin targeted PFC NP binding to human carotid endarterectomy specimen in vitro. (A) Optical image of a 5 mm cross-section of a human carotid endarterectomy sample. This section showed moderate lumenal narrowing as well as several atherosclerotic lesions and areas of calcification. (B) A 1H image acquired at 4.7 T at the same location shows signal enhancement due to the presence of gadolinium on the targeted particles (red arrows). (C) A 19F projection image acquired at 4.7 T through the entire carotid artery sample shows high signal in the same areas due to nanoparticles bound to fibrin. (D) 1H image in B with a false color overlay of the quantified nanoparticle concentration in the carotid as derived from the 19F image. Reprinted from Morawski et al.33 with permission.
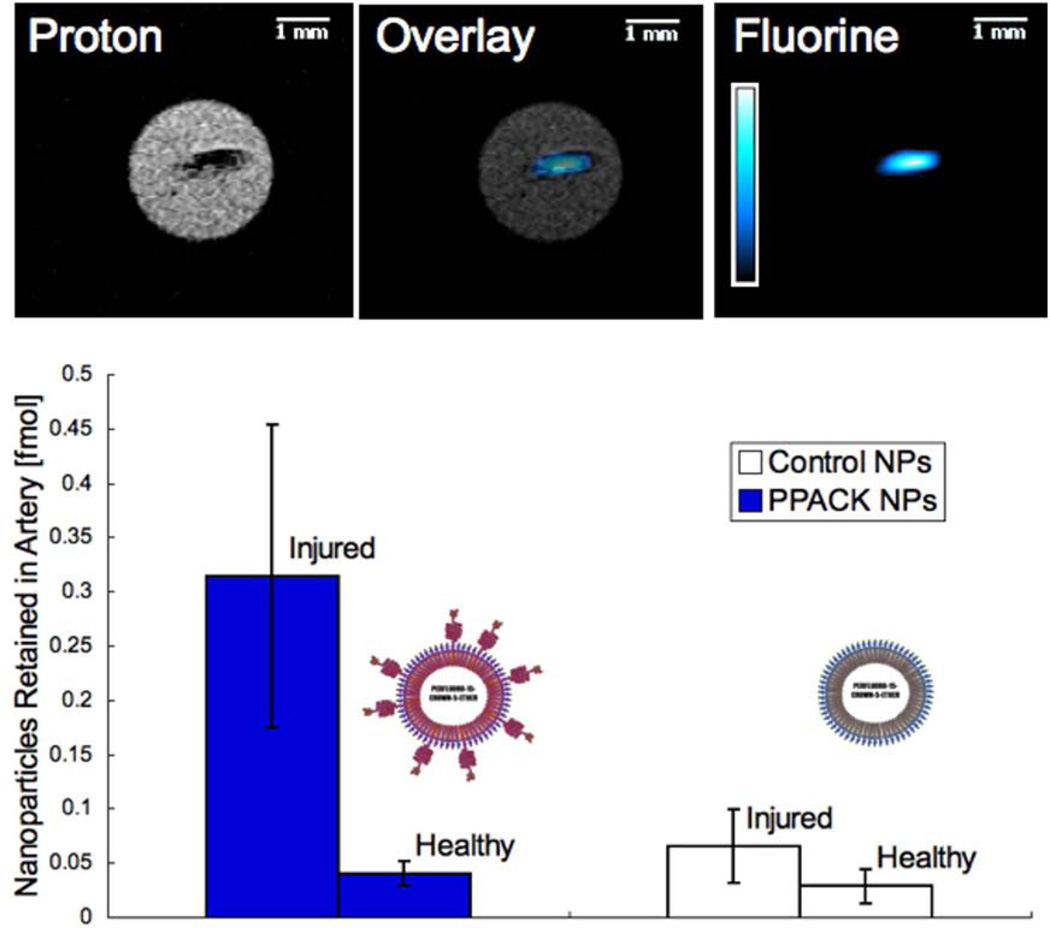
The presence of thrombin itself in newly forming clots can be delineated with thrombin targeted PFC NP imaged with MRI.107 Thrombin is the key regulator of clot formation because it converts fibrinogen to fibrin locally after being activated on an acute clotting surface by factor Xa. Nanoparticles bearing a small, modified peptide, PPACK (phenylalanine-proline-arginine-chloromethylketone), can covalently couple to the active pocket of thrombin molecules within forming clots and can be imaged dynamically and quantified with 19F MRI (Figure 8). Another advantage of this approach concerns the role of thrombin as a signaling molecule operating through protease activated receptors (e.g., PAR-1) that participate in signaling events in inflammation, atherosclerosis, and thrombosis, potentially conferring the capability to noninvasively delineate certain molecular regulatory pathways that might attend selected renal pathologies.
Figure 8.
In mice receiving treatment with control nanoparticles (n=3) or PPACK nanoparticles (n=3), both carotid arteries were excised following induction of occlusive thrombi in the right carotid artery. (A) 19F MRI at 11.7T exhibited coregistration of 19F signal from PPACK nanoparticles with 1H images depicting the occlusive clot in the artery. 19F MRS was used to quantify retention of nanoparticles in the injured right carotid artery (RA) and the unharmed left carotid artery (LA) for the two tested nanoparticle treatments. (B) Retained are represented in particles mean ± standard error. Reprinted from Myerson et al.121 with permission.
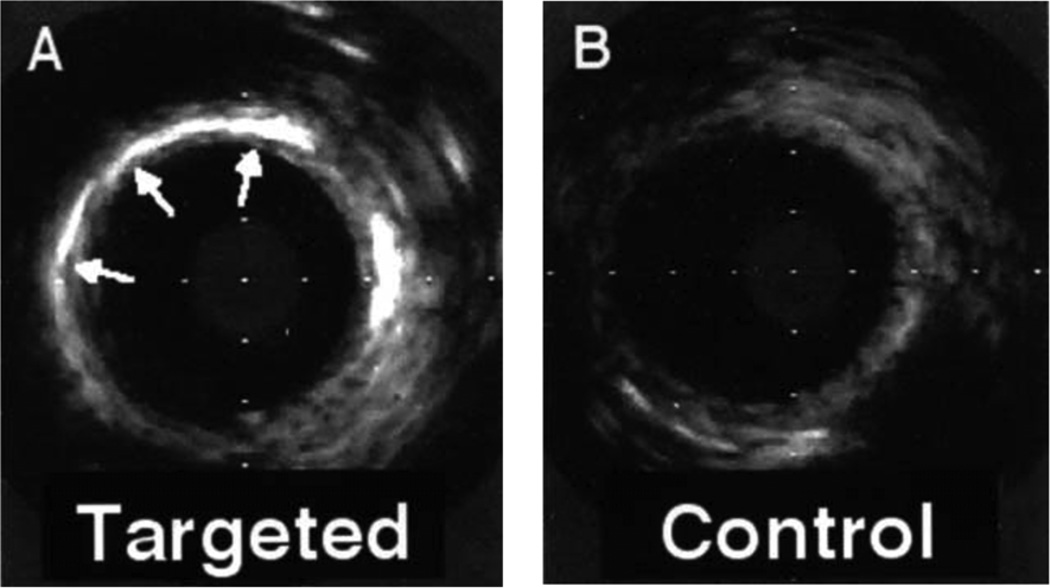
Ultrasound molecular imaging with targeted liquid PFC NP has been reported to detect other constituents of thromboembolic disease. For example, Tissue Factor (TF), which is the proximate cause of thrombosis during plaque rupture and is also associated with microangiopathic renal thrombosis in AKI and other kidney diseases, is detectable in vascular segments exposed to balloon injury that incites inflammation and upregulation of TF in the media layer (Figure 9).108–111 In this case, the selective binding and deposition of PFC NP are targeted to TF with the use of a specific conjugated monoclonal antibody fragment.
Figure 9.
High-frequency intravascular ultrasonic images of carotid arteries exposed to tissue factor–targeted or control emulsion nanoparticles after angioplasty. (A) Bright acoustic enhancement (arrows) in a portion of the vessel wall consistent with nonuniform overstretch injury with the balloon. (B) Lack of acoustic enhancement in the vessel wall after control contrast. Similar results were observed in each of 3 animals. Reprinted from Lanza et al.108 with permission.
Cellular imaging with PFC NP labeled immune, stem, and vascular precursor cells represents another opportunity for addressing inflammatory pathologies or regenerative approaches for therapy. A variety of cell types are capable of being isolated, subjected to PFC NP labeling by endocytosis in vivo, and then reinjected either locally into tissues or systemically where they can be tracked by 19F MRI.49, 51, 52, 55, 112–114 Figure 10 shows an example of two distinct PFC labels in endothelial precursor cells that contribute to tumor angiogenesis, and illustrates the opportunity for tracking multiple cell labels simultaneously. In other example, Ahrens and collaborators have mapped acute allograft rejection with 19F labeled immune cells.57 Such strategies might exhibit clear significance for kidney transplantation or for defining immunologically driven kidney pathologies.
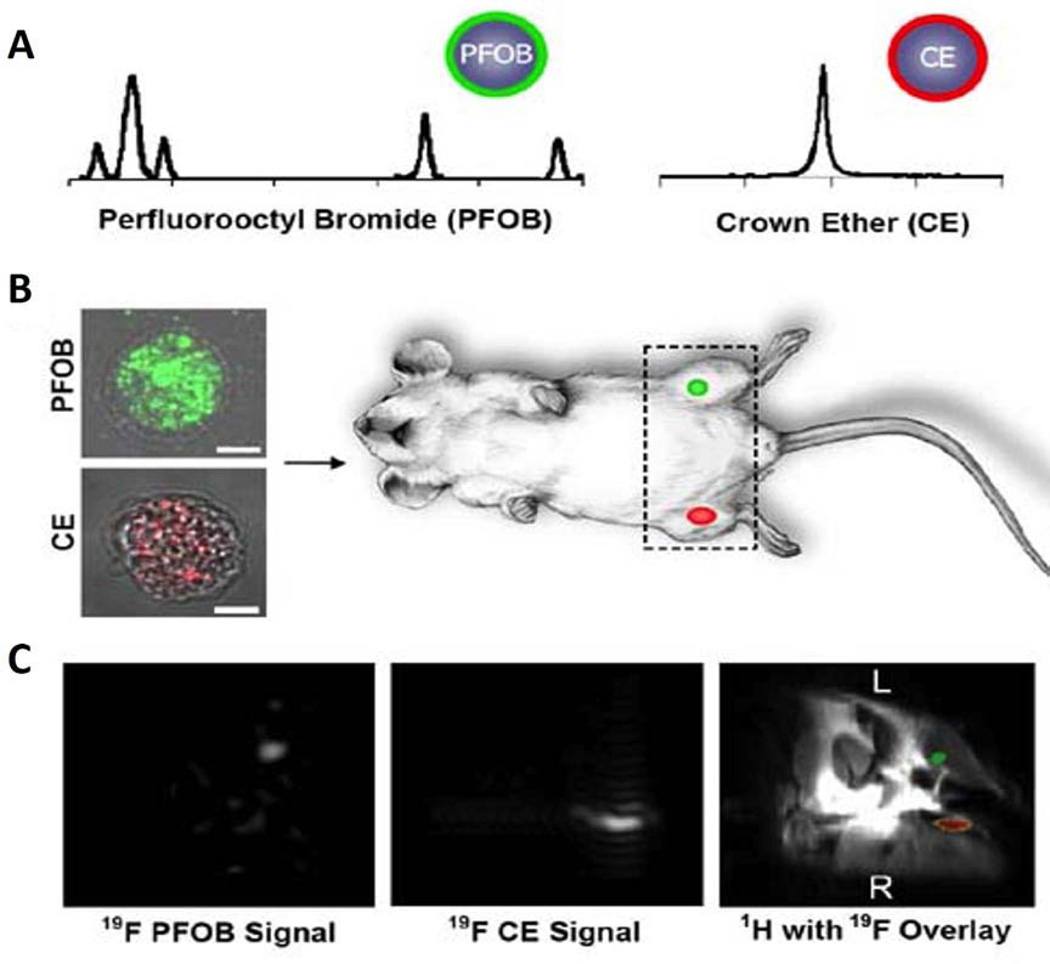
Figure 10.
(A) The different 19F spectrospic features between PFOB and CE allows selective detection of PFOB or CE NP labeled cells. (B) To determine the feasibility of detecting cells at specific tissue sites after local delivery, one million cells (2×104/µL) loaded with CE or PFOB NP were injected into mouse thigh skeletal muscle. At 11.7T, the PFC labeled cells were imaged rapidly in approximately 4 minutes by selectively tuning to PFOB or CE. (C) Overlaying the fluorine images atop a conventional matched proton image reveals the 19F PFOB and CE signals are located in the left and right mouse thigh, respectively. Reprinted from Partlow et al. 49 with permission.
A limitation for molecular 19F MRI is the low signal from targeted/localized PFC NP. To reduce imaging time in clinical settings, Gd-DTPA conjugated PFC NP maybe used for fast molecular 1H MRI to detect targeted/localized PFC NP, while a low resolution 19F MRI or MRS can be used to quantify the amount of PFC NP. Since Gd-DTPA is associated with increased frequency of acute adverse reactions, the safety profile and optimal dosage of Gd-DTPA loaded PFC NP in patients with kidney injury needs to be evaluated.
Therapeutics with PFC NP
A brief mention of the therapeutic utility of PFC NP is warranted, as both imaging and therapy can be combined to good effect with this system due to the ability to quantify drug deposition based on 19F spectroscopy. In other words, by knowing how much drug or gene product is present per nanoparticle and measuring the concentration of NP by 19F MRI as per reported methodologies,47, 48, 115 one can estimate the dose of drug deposited at any given time in an imaging voxel. A selected listing of some of the agents that have been used in various diseases in conjunction with therapeutic PFC NP is shown in Table 1. Moreover, it is worth noting that most of the formulations have been molecularly targeted to enhance local deposition and retention in the selected tissues with the use of preselected targeting ligands that are prepared in a one step process along with the therapeutic agent. However, we have demonstrated that it is feasible to prepare a base imaging and/or therapeutic agent and choose the targeting ligand later for "postformulation targeting" with a membrane inserting peptide cargo carrier,116–118 which allows great flexibility in selection of targets based on changing conditions as diseases progress through different stages. This feature is expected to facilitate more personalized approaches to nanomedicine in the future.
Table 1.
The application of therapeutic PFC NP in various diseases
| Therapeutic Agent | Application | Molecular Target | Reference |
|---|---|---|---|
| PPACK (phenyalanine-proline-arginine-chloromethylketone | anti-thrombosis | thrombin | 121 |
| Bivalirudin | anti-thrombosis | thrombin | 107 |
| Urokinase | thrombolysis | fibrin | 122 |
| Fumagillin | anti-angiogenesis in atherosclerosis | Endothelial aminopeptidase | 120, 123 |
| Fumagillin | anti-angiogenesis in cancer | Endothelial aminopeptidase | 95, 124, 125 |
| Fumagillin | anti-angiogenesis in arthritis | Endothelial aminopeptidase | 126–128 |
| Rapamycin | Restenosis prevention, anti-inflammatory | mTOR | 129 |
| Rapamycin | Muscular dystrophy | Autophagy enhancer (via mTOR) | 130 |
| Paclitaxel/Doxorubicin | Vascular inflammation, smooth muscle cell inhibition | Tissue Factor | 131 |
| Anti NFκB peptides (NBD) | Inflammation, cancer | NFκB (p65) | 132 |
| Melittin peptides | cancer | Cell lipid membrane disruptor | 116, 133 |
| siRNA | various | mRNA | 134 |
Limitations and Challenges
For imaging renal perfusion, 19F MRI requires longer imaging time (minutes to tens of minutes) than 1H MRI techniques like ASL and DCE enhanced MRI (takes seconds to a few minutes). The use of PFC NP as a contrast agent will also incur extra cost. However, the unique advantage of 19F MRI is its capability to directly mapping intrarenal blood volume since the 19F signal intensity is linearly correlated with the quantity of PFC NP in each voxel. When a 19F inversion-recovery gradient echo sequence was employed to measuring pO2, the 19F signal from PFC NP are contributed by both artery and veins. 74 Alternatively, the signal from fast moving PFC NP in arterial blood flow can be suppressed by employing spin echo acquisitions. 77 The detected 19F signal are primary contributed by PFC NP in capillaries and veins. Similar to the limitation of BOLD MRI, the limitation of 19F MRI is that it detects blood pO2. It is yet unknown how accurately blood pO2 reflects tissue pO2.119 Finally, translating PFC NP to clinical settings is limited by the low sensitivity of 19F signal on the “low field” (e.g. 3T) clinical MR scanner, which may require prolonged imaging time (tens of minutes) to achieve satisfactory signal to noise ratio. This will pose practical problems to image AKI patients who are in critical condition and require intensive care. The motion artifact from bowel movement and respiration during image acquisition should be also corrected for image reconstruction. 96
Clinical Summary.
Perfluorocarbon nanoparticles (PFC NP) are a non-nephrotoxic molecular imaging agent for noninvasive quantitative assessment of kidney function and injury.
Functionalized PFC NP allow site-targeted drug delivery as a novel therapeutic modality for treating acute and chronic kidney diseases.
Acknowledgements
We acknowledge the financial support from NIH grants R01 HL073646 and R21 DK095555 to Wickline SA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure:
Wickline, SA: equity in Kereos, Inc.
Lanza, GM: equity in Kereos, Inc.
References
- 1.Gloviczki ML, Glockner JF, Lerman LO, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55(4):961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol. 2006;290(5):F958–F974. doi: 10.1152/ajprenal.00114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantarinia K. Novel imaging techniques in acute kidney injury. Curr Drug Targets. 2009;10(12):1184–1189. doi: 10.2174/138945009789753246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsushima Y. Functional CT of the kidney. Eur J Radiol. 1999;30(3):191–197. doi: 10.1016/s0720-048x(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 5.Briguori C, Marenzi G. Contrast-induced nephropathy: pharmacological prophylaxis. Kidney Int Suppl. 2006;(100):S30–s38. doi: 10.1038/sj.ki.5000372. [DOI] [PubMed] [Google Scholar]
- 6.Briguori C, Visconti G, Focaccio A, et al. Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II): RenalGuard System in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124(11):1260–1269. doi: 10.1161/CIRCULATIONAHA.111.030759. [DOI] [PubMed] [Google Scholar]
- 7.Gleeson TG, Bulugahapitiya S. Contrast-induced nephropathy. AJR Am J Roentgenol. 2004;183(6):1673–1689. doi: 10.2214/ajr.183.6.01831673. [DOI] [PubMed] [Google Scholar]
- 8.Wong PC, Li Z, Guo J, Zhang A. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012;158(2):186–192. doi: 10.1016/j.ijcard.2011.06.115. [DOI] [PubMed] [Google Scholar]
- 9.Grenier N, Basseau F, Ries M, et al. Functional MRI of the kidney. Abdom Imaging. 2003;28(2):164–175. doi: 10.1007/s00261-001-0183-8. [DOI] [PubMed] [Google Scholar]
- 10.Maril N, Rosen Y, Reynolds GH, et al. Sodium MRI of the human kidney at 3 tesla. Magn Reson Med. 2006;56(6):1229–1234. doi: 10.1002/mrm.21031. [DOI] [PubMed] [Google Scholar]
- 11.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging. 2003;17(1):104–113. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 12.Rossi C, Sharma P, Pazahr S, et al. Blood Oxygen Level-Dependent Magnetic Resonance Imaging of the Kidneys: Influence of Spatial Resolution on the Apparent R2* Transverse Relaxation Rate of Renal Tissue. Invest Radiol. 2013 doi: 10.1097/RLI.0b013e31828b9830. [DOI] [PubMed] [Google Scholar]
- 13.Ritt M, Janka R, Schneider MP, et al. Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant. 2010;25(4):1126–1133. doi: 10.1093/ndt/gfp639. [DOI] [PubMed] [Google Scholar]
- 14.Pruijm M, Hofmann L, Vogt B, et al. Renal tissue oxygenation in essential hypertension and chronic kidney disease. Int J Hypertens. 2013;2013:696598. doi: 10.1155/2013/696598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detre JA, Zhang WG, Roberts DA, et al. Tissue specific perfusion imaging using arterial spin labeling. NMR in Biomedicine. 1994;7(1–2):75–82. doi: 10.1002/nbm.1940070112. [DOI] [PubMed] [Google Scholar]
- 16.Liu YP, Song R, Liang C, Chen X, Liu B. Arterial spin labeling blood flow magnetic resonance imaging for evaluation of renal injury. Am J Physiol Renal Physiol. 2012;303(4):F551–F558. doi: 10.1152/ajprenal.00288.2011. [DOI] [PubMed] [Google Scholar]
- 17.Wu WC, Su MY, Chang CC, Tseng WY, Liu KL. Renal perfusion 3-T MR imaging: a comparative study of arterial spin labeling and dynamic contrast-enhanced techniques. Radiology. 2011;261(3):845–853. doi: 10.1148/radiol.11110668. [DOI] [PubMed] [Google Scholar]
- 18.Chandarana H, Lee VS. Renal Functional MRI: Are We Ready for Clinical Application? American Journal of Roentgenology. 2009;192(6):1550–1557. doi: 10.2214/AJR.09.2390. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Lanza GM, Wickline SA. Quantitative magnetic resonance fluorine imaging: today and tomorrow. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):431–440. doi: 10.1002/wnan.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickline SA, Mason RP, Caruthers SD, et al. Fluorocarbon agents for multimodal molecular imaging and targeted therapeutics. In: Weissleder R, et al., editors. MOLECULAR IMAGING: PRINCIPLES AND PRACTICE. Shelton, CT: Peoples Medical Publishing House-USA; 2010. pp. 542–573. [Google Scholar]
- 21.Cohn CS, Cushing MM. Oxygen therapeutics: perfluorocarbons and blood substitute safety. Crit Care Clin. 2009;25(2):399–414. doi: 10.1016/j.ccc.2008.12.007. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 22.Spahn DR. Blood substitutes. Artificial oxygen carriers: perfluorocarbon emulsions. Crit Care. 1999;3(5):R93–R97. doi: 10.1186/cc364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin NJ, Wang Y, Ng TC. In situ 19F MRS measurement of RIF-1 tumor blood volume: corroboration by radioisotope-labeled [125I]-albumin and correlation to tumor size. Magn Reson Imaging. 1996;14(3):275–280. doi: 10.1016/0730-725x(95)02080-d. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Mason R, Liu H. Estimated fraction of tumor vascular blood contents sampled by near infrared spectroscopy and 19F magnetic resonance spectroscopy. Opt Express. 2005;13(5):1724–1733. doi: 10.1364/opex.13.001724. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C, Counsell C, Wood P, Adams GE. Use of fluorine-19 nuclear magnetic resonance spectroscopy and hydralazine for measuring dynamic changes in blood perfusion volume in tumors in mice. J Natl Cancer Inst. 1992;84(3):174–180. doi: 10.1093/jnci/84.3.174. [DOI] [PubMed] [Google Scholar]
- 26.Mason RP. Noninvasive physiology: 19F NMR of perfluorocarbon. Art. Cells, Blood Sub. & Immob. Biotech. 1994;22(4):1141–1153. doi: 10.3109/10731199409138809. [DOI] [PubMed] [Google Scholar]
- 27.Mason RP, Antich PP, Babcock EE, et al. Noninvasive determination of tumor oxygen tension and local variation with growth. Int J Radiat Oncol Biol Phys. 1994;29(1):95–103. doi: 10.1016/0360-3016(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhao D, Jiang L, Mason RP. Measuring changes in tumor oxygenation. Methods Enzymol. 2004;386:378–418. doi: 10.1016/S0076-6879(04)86018-X. [DOI] [PubMed] [Google Scholar]
- 29.Spiess BD. Perfluorocarbon emulsions as a promising technology: a review of tissue and vascular gas dynamics. J Appl Physiol. 2009;106(4):1444–1452. doi: 10.1152/japplphysiol.90995.2008. [DOI] [PubMed] [Google Scholar]
- 30.Kaneda MM, Caruthers S, Lanza GM, Wickline SA. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 2009;37(10):1922–1933. doi: 10.1007/s10439-009-9643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosgood SA, Nicholson ML. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89(10):1169–1175. doi: 10.1097/TP.0b013e3181da6064. [DOI] [PubMed] [Google Scholar]
- 32.Marada T, Zacharovova K, Saudek F. Perfluorocarbon improves post-transplant survival and early kidney function following prolonged cold ischemia. Eur Surg Res. 2010;44(3–4):170–178. doi: 10.1159/000280438. [DOI] [PubMed] [Google Scholar]
- 33.Morawski AM, Winter P, Yu X, et al. Quantitative Magnetic Resonance Immunohistochemistry with Ligand-Targeted 19F Nanoparticles. Magn Reson Med. 2004;52:1255–1262. doi: 10.1002/mrm.20287. [DOI] [PubMed] [Google Scholar]
- 34.Mason RP, Antich PP, Babcock EE, Gerberich JL, Nunnally RL. Perfluorocarbon imaging in vivo: An 19F MRI study in tumor-bearing mice. Mag Res Imaging. 1989;7:475–485. doi: 10.1016/0730-725x(89)90402-5. [DOI] [PubMed] [Google Scholar]
- 35.Mason RP, Ran S, Thorpe PE. Quantitative assessment of tumor oxygen dynamics: Molecular Imaging for Prognostic Radiology. J. Cell. Biochem. 2002;87(suppl):45–53. doi: 10.1002/jcb.10404. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011;24(2):114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu JX, Hallac RR, Chiguru S, Mason RP. New frontiers and developing applications in 19F NMR. Prog Nucl Magn Reson Spectrosc. 2013;70:25–49. doi: 10.1016/j.pnmrs.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanza GM, Lorenz CH, Fischer SE, et al. Enhanced detection of thrombi with a novel fibrin-targeted magnetic resonance imaging agent. Academic Radiology. 1998;5:S173–S176. doi: 10.1016/s1076-6332(98)80097-4. [DOI] [PubMed] [Google Scholar]
- 39.Winter P, Athey P, Kiefer G, et al. Improved paramagnetic chelate for molecular imaging with MRI. Journal of Magnetism and Magnetic Materials. 2005;293(1):540–545. [Google Scholar]
- 40.Lanza GM, Wallace KD, Scott MJ, et al. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1996;94(12):3334–3340. doi: 10.1161/01.cir.94.12.3334. [DOI] [PubMed] [Google Scholar]
- 41.Hu G, Lijowski M, Zhang H, Partlow KC, Caruthers SD, Kiefer G, Gulyas G, Athey P, Scott MJ, Wickline SA, Lanza GM. Imaging of Vx-2 rabbit tumors with αvβ3-integrin-targeted 111In nanoparticles. International Journal of Cancer. 2007;120:1951–1957. doi: 10.1002/ijc.22581. [DOI] [PubMed] [Google Scholar]
- 42.Lijowski M, Caruthers S, Hu G, et al. High sensitivity: High-resolution SPECT-CT/MR molecular imaging of angiogenesis in the Vx2 model. Investigative Radiology. 2009;44(1):15–22. doi: 10.1097/RLI.0b013e31818935eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keipert PE, Otto S, Flaim SF, et al. Influence of perflubron emulsion particle size on blood half-life and febrile response in rats. Artif Cells Blood Substit Immobil Biotechnol. 1994;22(4):1169–1174. doi: 10.3109/10731199409138812. [DOI] [PubMed] [Google Scholar]
- 44.Noveck RJ, Shannon EJ, Leese PT, et al. Randomized safety studies of intravenous perflubron emulsion. II. Effects on immune function in healthy volunteers. Anesth Analg. 2000;91(4):812–822. doi: 10.1097/00000539-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Wickline SA, Lanza GM. Quantitative Magnetic Resonance Fluorine Imaging: Today and tomorrow. WIRE Nanomed Nanobiotechnol. 2010 doi: 10.1002/wnan.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morawski AM, Lanza GA, Wickline SA. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr Opin Biotechnol. 2005;16(1):89–92. doi: 10.1016/j.copbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Morawski AM, Winter PM, Yu X, et al. Quantitative “magnetic resonance immunohistochemistry” with ligand-targeted 19F nanoparticles. Magn Reson Med. 2004;52:1255–1262. doi: 10.1002/mrm.20287. [DOI] [PubMed] [Google Scholar]
- 48.Morawski AM, Winter PM, Crowder KC, et al. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn Reson Med. 2004;51:480–486. doi: 10.1002/mrm.20010. [DOI] [PubMed] [Google Scholar]
- 49.Partlow KC, Chen J, Brant JA, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007;21(8):1647–1654. doi: 10.1096/fj.06-6505com. Epub 2007 Feb 6. [DOI] [PubMed] [Google Scholar]
- 50.Neubauer AM, Caruthers SD, Hockett FD, et al. Fluorine cardiovascular magnetic resonance angiography in vivo at 1.5 T with perfluorocarbon nanoparticle contrast agents. J Cardiovasc Magn Reson. 2007;9(3):565–573. doi: 10.1080/10976640600945481. [DOI] [PubMed] [Google Scholar]
- 51.Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol. 2005;23(8):983–987. doi: 10.1038/nbt1121. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Cabello J, Walczak P, Kedziorek DA, et al. In vivo "hot spot" MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med. 2008;60(6):1506–1511. doi: 10.1002/mrm.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonetto F, Srinivas M, Heerschap A, et al. A novel (19)F agent for detection and quantification of human dendritic cells using magnetic resonance imaging. Int J Cancer. 2011;129(2):365–373. doi: 10.1002/ijc.25672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helfer BM, Balducci A, Nelson AD, et al. Functional assessment of human dendritic cells labeled for in vivo (19)F magnetic resonance imaging cell tracking. Cytotherapy. 2010;12(2):238–250. doi: 10.3109/14653240903446902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janjic JM, Ahrens ET. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(5):492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Zhang L, Myerson J, et al. Quantifying the evolution of vascular barrier disruption in advanced atherosclerosis with semipermeant nanoparticle contrast agents. PLoS One. 2011;6(10):e26385. doi: 10.1371/journal.pone.0026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hitchens TK, Ye Q, Eytan DF, et al. 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn Reson Med. 2011;65(4):1144–1153. doi: 10.1002/mrm.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49(Suppl 2):29S–48S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 59.Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112(9):1362–1374. doi: 10.1161/CIRCULATIONAHA.104.492348. [DOI] [PubMed] [Google Scholar]
- 60.Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis. 2009;52(3):196–203. doi: 10.1016/j.pcad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palm F, Nordquist L. Renal tubulointerstitial hypoxia: cause and consequence of kidney dysfunction. Clin Exp Pharmacol Physiol. 2011;38(7):474–480. doi: 10.1111/j.1440-1681.2011.05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindenmeyer MT, Kretzler M, Boucherot A, et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18(6):1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- 63.Gloviczki ML, Glockner JF, Crane JA, et al. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension. 2011;58(6):1066–1072. doi: 10.1161/HYPERTENSIONAHA.111.171405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22(8):1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mason RP, Shukla H, Antich PP. In vivo oxygen tension and temperature: simultaneous determination using 19F NMR spectroscopy of perfluorocarbon. Magn Reson Med. 1993;29(3):296–302. doi: 10.1002/mrm.1910290304. [DOI] [PubMed] [Google Scholar]
- 66.O'Brien RN, Langlais AJ, Seufert WD. Diffusion coefficients of respiratory gases in a perfluorocarbon liquid. Science. 1982;217(4555):153–155. doi: 10.1126/science.6806902. [DOI] [PubMed] [Google Scholar]
- 67.Zhang W, Ito Y, Berlin E, Roberts R, Berkowitz BA. Role of hypoxia during normal retinal vessel development and in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44(7):3119–3123. doi: 10.1167/iovs.02-1122. [DOI] [PubMed] [Google Scholar]
- 68.Kodibagkar VD, Wang X, Mason RP. Physical principles of quantitative nuclear magnetic resonance oximetry. Front Biosci. 2008;13:1371–1384. doi: 10.2741/2768. [DOI] [PubMed] [Google Scholar]
- 69.Zhao D, Constantinescu A, Hahn EW, Mason RP. Tumor oxygen dynamics with respect to growth and respiratory challenge: investigation of the Dunning prostate R3327-HI tumor. Radiat Res. 2001;156(5 Pt 1):510–520. doi: 10.1667/0033-7587(2001)156[0510:todwrt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 70.Kim JG, Zhao D, Song Y, et al. Interplay of tumor vascular oxygenation and tumor pO2 observed using near-infrared spectroscopy, an oxygen needle electrode, and 19F MR pO2 mapping. J Biomed Opt. 2003;8(1):53–62. doi: 10.1117/1.1527049. [DOI] [PubMed] [Google Scholar]
- 71.Xia M, Kodibagkar V, Liu H, Mason RP. Tumour oxygen dynamics measured simultaneously by near-infrared spectroscopy and 19F magnetic resonance imaging in rats. Phys Med Biol. 2006;51(1):45–60. doi: 10.1088/0031-9155/51/1/004. [DOI] [PubMed] [Google Scholar]
- 72.Hunjan S, Zhao D, Constantinescu A, et al. Tumor oximetry: demonstration of an enhanced dynamic mapping procedure using fluorine-19 echo planar magnetic resonance imaging in the Dunning prostate R3327-AT1 rat tumor. Int J Radiat Oncol Biol Phys. 2001;49(4):1097–1108. doi: 10.1016/s0360-3016(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 73.Jordan BF, Cron GO, Gallez B. Rapid monitoring of oxygenation by 19F magnetic resonance imaging: Simultaneous comparison with fluorescence quenching. Magn Reson Med. 2009;61(3):634–638. doi: 10.1002/mrm.21594. [DOI] [PubMed] [Google Scholar]
- 74.Hu L, Chen J, Yang X, et al. Rapid quantification of oxygen tension in blood flow with a fluorine nanoparticle reporter and a novel blood flow-enhanced-saturation-recovery sequence. Magn Reson Med. 2012 doi: 10.1002/mrm.24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shukla HP, Mason RP, Bansal N, Antich PP. Regional myocardial oxygen tension: 19F MRI of sequestered perfluorocarbon. Magn Reson Med. 1996;35(6):827–833. doi: 10.1002/mrm.1910350607. [DOI] [PubMed] [Google Scholar]
- 76.Duong TQ, Kim SG. In vivo MR measurements of regional arterial and venous blood volume fractions in intact rat brain. Magn Reson Med. 2000;43(3):393–402. doi: 10.1002/(sici)1522-2594(200003)43:3<393::aid-mrm11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 77.Hu L, Chen J, Yang X, et al. Assessing Intrarenal Non-perfusion and Vascular Leakage in Acute Kidney Injury with multi-nuclear 1H/19F MRI and Perfluorocarbon Nanoparticles. Magn Reson Med. 2013;2013 doi: 10.1002/mrm.24851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paller MS, Murray BM. Renal dysfunction in animal models of cyclosporine toxicity. Transplant Proc. 1985;17(4 Suppl 1):155–159. [PubMed] [Google Scholar]
- 79.Mason J, Torhorst J, Welsch J. Role of the medullary perfusion defect in the pathogenesis of ischemic renal failure. Kidney Int. 1984;26(3):283–293. doi: 10.1038/ki.1984.171. [DOI] [PubMed] [Google Scholar]
- 80.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 81.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72(2):151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 82.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 83.Leong CL, Anderson WP, O'Connor PM, Evans RG. Evidence that renal arterial-venous oxygen shunting contributes to dynamic regulation of renal oxygenation. Am J Physiol Renal Physiol. 2007;292(6):F1726–F1733. doi: 10.1152/ajprenal.00436.2006. [DOI] [PubMed] [Google Scholar]
- 84.Stinghen AE, Pecoits-Filho R. Vascular damage in kidney disease: beyond hypertension. Int J Hypertens. 2011;2011:232683. doi: 10.4061/2011/232683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lanza GM, Winter PM, Caruthers SD, et al. Nanomedicine opportunities for cardiovascular disease with perfluorocarbon nanoparticles. Nanomedicine (London, England) 2006;1(3):321–329. doi: 10.2217/17435889.1.3.321. [DOI] [PubMed] [Google Scholar]
- 86.Lanza GM, Winter PM, Caruthers SD, et al. Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis. 2010;13(2):189–202. doi: 10.1007/s10456-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lanza GM, Winter PM, Hughes MS, et al. Molecular imaging and therapy: New paradigms for 21st century medicine. Polymeric Drug Delivery I: Particulate Drug Carriers. 2006 [Google Scholar]
- 88.Moulton KS, Vakili K, Zurakowski D, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100(8):4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113(18):2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 90.Jain RK, Finn AV, Kolodgie FD, Gold HK, Virmani R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nat Clin Pract Cardiovasc Med. 2007;4(9):491–502. doi: 10.1038/ncpcardio0979. [DOI] [PubMed] [Google Scholar]
- 91.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque Hemorrhage and Progression of Coronary Atheroma. New England Journal of Medicine. 2003;349(24):2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 92.Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(10):2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 93.Winter PM, Cai K, Caruthers SD, Wickline SA, Lanza GM. Emerging nanomedicine opportunities with perfluorocarbon nanoparticles. Expert Review of Medical Devices. 2007;4(2):137–145. doi: 10.1586/17434440.4.2.137. [DOI] [PubMed] [Google Scholar]
- 94.Winter PM, Caruthers SD, Kassner A, et al. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 2003;63(18):5838–5843. [PubMed] [Google Scholar]
- 95.Winter PM, Schmieder AH, Caruthers SD, et al. Minute dosages of αvβ3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J. 2008;22:2758–2767. doi: 10.1096/fj.07-103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keupp J, Rahmer J, Grasslin I, et al. Simultaneous dual-nuclei imaging for motion corrected detection and quantification of 19F imaging agents. Magn Reson Med. 2011;66(4):1116–1122. doi: 10.1002/mrm.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hughes M, Caruthers S, Tran T, et al. Perfluorocarbon nanoparticles for molecular imaging and targeted therapeutics. Proceedings of the IEEE. 2008;96(3):397–415. [Google Scholar]
- 98.Hughes MS, Marsh JN, Arbeit JM, et al. Application of Renyi entropy for ultrasonic molecular imaging. J Acoust Soc Am. 2009;125(5):3141–3145. doi: 10.1121/1.3097489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hughes MS, McCarthy JE, Marsh JN, et al. Properties of an entropy-based signal receiver with an application to ultrasonic molecular imaging. Journal of the Acoustical Society of America. 2007;121(6):3542–3557. doi: 10.1121/1.2722050. [DOI] [PubMed] [Google Scholar]
- 100.Hughes MS, McCarthy JE, Wickerhauser MV, et al. Real-time calculation of a limiting form of the Renyi entropy applied to detection of subtle changes in scattering architecture. Journal of the Acoustical Society of America. 2009;126(5):2350–2358. doi: 10.1121/1.3224714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Southworth R, Kaneda M, Chen J, et al. Renal vascular inflammation induced by western diet in ApoE-null mice quantified by 19F NMR of VCAM-1 targeted nanobeacons. Nanomedicine: Nanotechnology, Biology, and Medicine. 2009;5(3):359–367. doi: 10.1016/j.nano.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004;11(2):215–221. doi: 10.1016/j.nuclcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Lanza GM, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy. Progress In Cardiovascular Disease. 2001;44:13–31. doi: 10.1053/pcad.2001.26440. [DOI] [PubMed] [Google Scholar]
- 104.Caruthers SD, Neubauer AM, Hockett FD, et al. In Vitro Demonstration Using 19F Magnetic Resonance to Augment Molecular Imaging With Paramagnetic Perfluorocarbon Nanoparticles at 1.5 Tesla. Invest Radiol. 2006;41:305–312. doi: 10.1097/01.rli.0000199281.60135.6a. [DOI] [PubMed] [Google Scholar]
- 105.Flacke S, Fischer S, Scott MJ, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 106.Yu X, Song S-K, Chen J, et al. High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn Reson Med. 2000;44:867–872. doi: 10.1002/1522-2594(200012)44:6<867::aid-mrm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 107.Myerson JW, He L, Lanza GM, Tollefsen DM, Wickline SA. Bivalirudin Nanoparticles Enable Simultaneous Detection and Potent Inhibition of Acute Clotting. Circulation. 2011;124:A15949. [Google Scholar]
- 108.Lanza GM, Abendschein DR, Hall CS, et al. In vivo molecular imaging of stretch-induced tissue factor in carotid arteries with ligand-targeted nanoparticles. J Am Soc Echocardiogr. 2000;13(6):608–614. doi: 10.1067/mje.2000.105840. [DOI] [PubMed] [Google Scholar]
- 109.Lanza GM, Abendschein DR, Hall CH, et al. Molecular imaging of stretch-induced tissue factor expression in carotid arteries with intravascular ultrasound. Invest Radiol. 2000;35:227–234. doi: 10.1097/00004424-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 110.Lanza GM, Abendschein DR, Hall CS, et al. In vivo molecular imaging of tissue factor in carotid arteries with a one-step ligand conjugated acoustic nanoparticle. Circulation. 1999;100(18):367. [Google Scholar]
- 111.Morawski AM, Winter PM, Caruthers SD, et al. "Magnetic resonance immunocytochemistry": Characterization of "Tissue factor" expression by smooth muscle cells with targeted paramagnetic nanoparticles. Circulation. 2003;108(17):139–139. [Google Scholar]
- 112.Ahrens ET, Young WB, Xu H, Pusateri LK. Rapid quantification of inflammation in tissue samples using perfluorocarbon emulsion and fluorine-19 nuclear magnetic resonance. Biotechniques. 2011;50(4):229–234. doi: 10.2144/000113652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kadayakkara DK, Janjic JM, Pusateri LK, Young WB, Ahrens ET. In vivo observation of intracellular oximetry in perfluorocarbon-labeled glioma cells and chemotherapeutic response in the CNS using fluorine-19 MRI. Magn Reson Med. 2010;64(5):1252–1259. doi: 10.1002/mrm.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med. 2007;58(4):725–734. doi: 10.1002/mrm.21352. [DOI] [PubMed] [Google Scholar]
- 115.Neubauer AM, Sim H, Winter PM, et al. Nanoparticle pharmacokinetic profiling in vivo using magnetic resonance imaging. Magnetic Resonance in Medicine. 2008;60(6):1353–1361. doi: 10.1002/mrm.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan H, Soman NR, Schlesinger PH, Lanza GM, Wickline SA. Cytolytic peptide nanoparticles ('NanoBees') for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(3):318–327. doi: 10.1002/wnan.126. [DOI] [PubMed] [Google Scholar]
- 117.Pan H, Marsh JN, Christenson ET, et al. Postformulation peptide drug loading of nanostructures. Methods Enzymol. 2012;508:17–39. doi: 10.1016/B978-0-12-391860-4.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pan H, Myerson JW, Ivashyna O, et al. Lipid membrane editing with peptide cargo linkers in cells and synthetic nanostructures. FASEB J. 2010;24:2928–2937. doi: 10.1096/fj.09-153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Evans RG, Leong CL, Anderson WP, O'Connor PM. Don't be so BOLD: potential limitations in the use of BOLD MRI for studies of renal oxygenation. Kidney Int. 2007;71(12):1327–1328. doi: 10.1038/sj.ki.5002321. author reply 1328. [DOI] [PubMed] [Google Scholar]
- 120.Winter PM, Neubauer AM, Caruthers SD, et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 121.Myerson J, He L, Lanza G, Tollefsen D, Wickline S. Thrombin-inhibiting perfluorocarbon nanoparticles provide a novel strategy for the treatment and magnetic resonance imaging of acute thrombosis. J Thromb Haemost. 2011;9(7):1292–1300. doi: 10.1111/j.1538-7836.2011.04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marsh JN, Hu G, Scott MJ, et al. A fibrin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine (Lond) 2011;6(4):605–615. doi: 10.2217/nnm.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Winter PM, Caruthers SD, Zhang H, et al. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1(5):624–634. doi: 10.1016/j.jcmg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan D, Sanyal N, Schmieder AH, et al. Antiangiogenic nanotherapy with lipase-labile Sn-2 fumagillin prodrug. Nanomedicine (Lond) 2012;7(10):1507–1519. doi: 10.2217/nnm.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmieder AH, Caruthers SD, Zhang H, et al. Three-dimensional MR mapping of angiogenesis with alpha5beta1(alpha nu beta3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008;22(12):4179–4189. doi: 10.1096/fj.08-112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou HF, Chan HW, Wickline SA, Lanza GM, Pham CT. Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009;23(9):2978–2985. doi: 10.1096/fj.09-129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou HF, Hu G, Wickline SA, Lanza GM, Pham CT. Synergistic effect of antiangiogenic nanotherapy combined with methotrexate in the treatment of experimental inflammatory arthritis. Nanomedicine (Lond) 2010;5(7):1065–1074. doi: 10.2217/nnm.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou HF, Yan H, Senpan A, et al. Suppression of inflammation in a mouse model of rheumatoid arthritis using targeted lipase-labile fumagillin prodrug nanoparticles. Biomaterials. 2012;33(33):8632–8640. doi: 10.1016/j.biomaterials.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cyrus T, Zhang H, Allen JS, et al. Intramural delivery of rapamycin with alphavbeta3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arterioscler Thromb Vasc Biol. 2008;28(5):820–826. doi: 10.1161/ATVBAHA.107.156281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li AJ, Bibee K, Marsh JN, Weihl CC, Wickline SA. Mdx mice have a defect in autophagy that is restored by rapamycin-loaded nanoparticle treatment. Conference Proceedings of Experimental Biology. 2012 [Google Scholar]
- 131.Lanza GM, Yu X, Winter PM, et al. Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent: implications for rational therapy of restenosis. Circulation. 2002;106(22):2842–2847. doi: 10.1161/01.cir.0000044020.27990.32. [DOI] [PubMed] [Google Scholar]
- 132.Pan H, Ivashyna O, Sinha B, et al. Post-formulation peptide drug loading of nanostructures for metered control of NF-kappaB signaling. Biomaterials. 2011;32(1):231–238. doi: 10.1016/j.biomaterials.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soman NR, Baldwin SL, Hu G, et al. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest. 2009;119(9):2830–2842. doi: 10.1172/JCI38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaneda MM, Sasaki Y, Lanza GM, Milbrandt J, Wickline SA. Mechanisms of nucleotide trafficking during siRNA delivery to endothelial cells using perfluorocarbon nanoemulsions. Biomaterials. 2010;31(11):3079–3086. doi: 10.1016/j.biomaterials.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]