Abstract
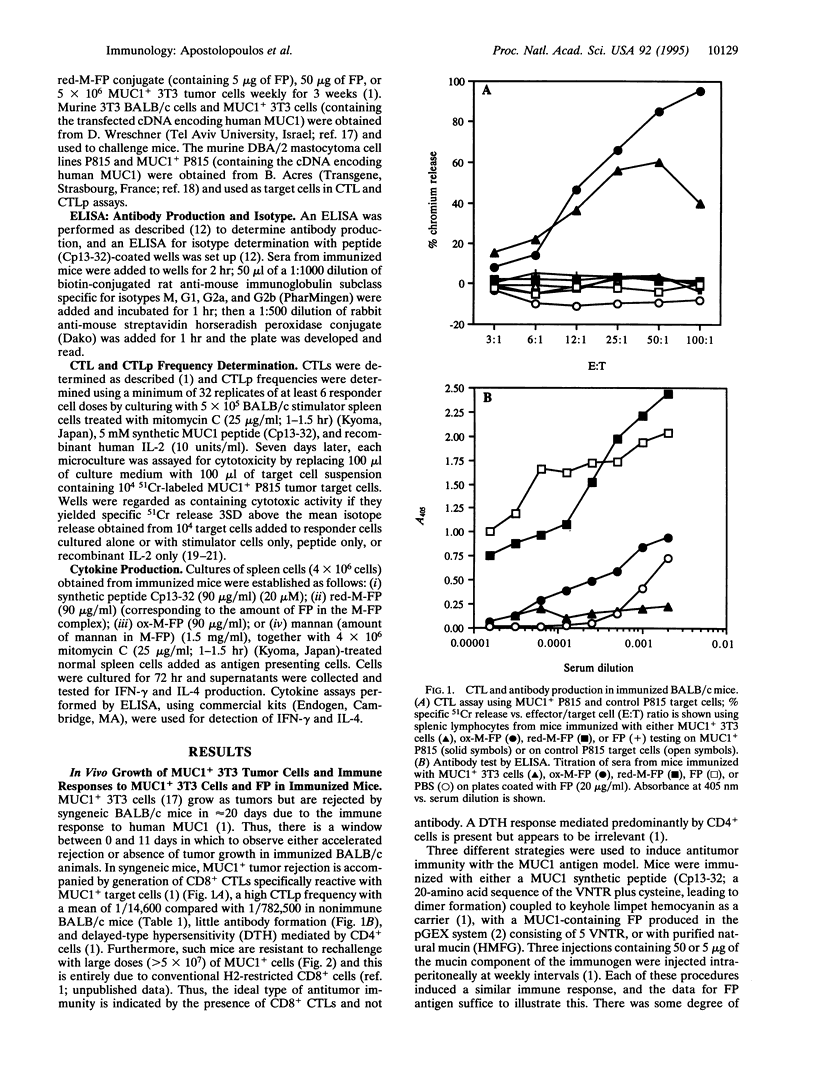
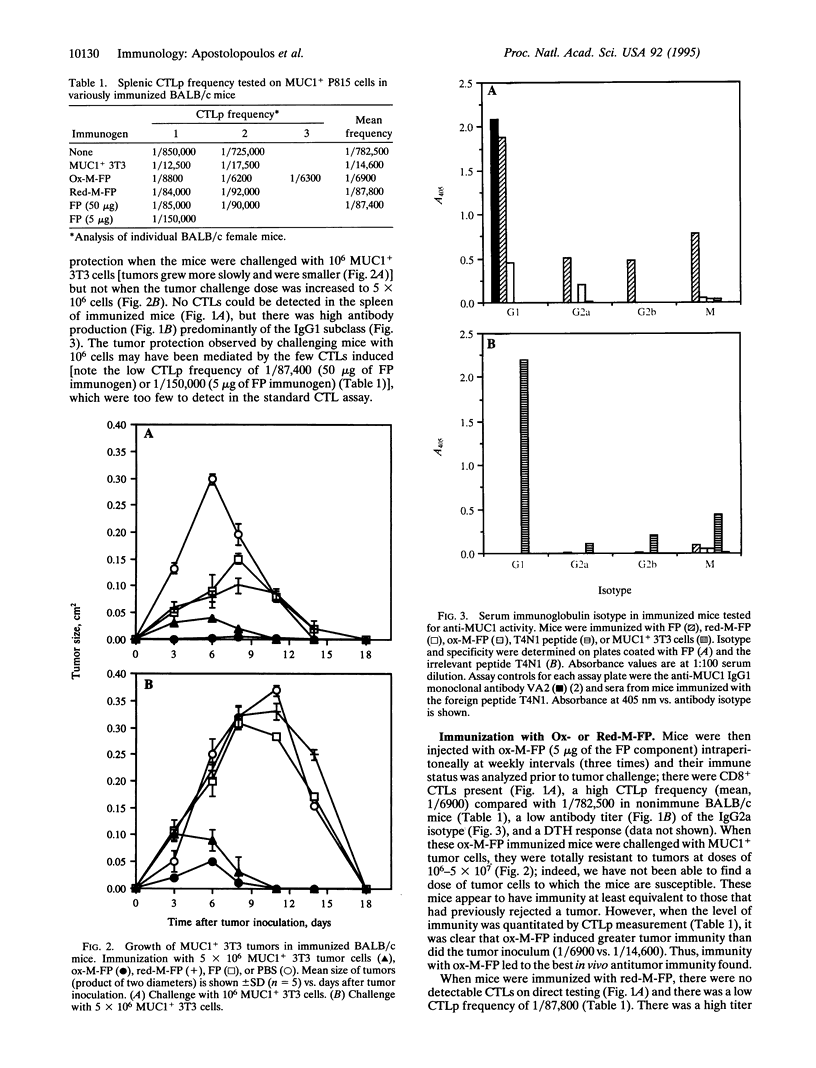
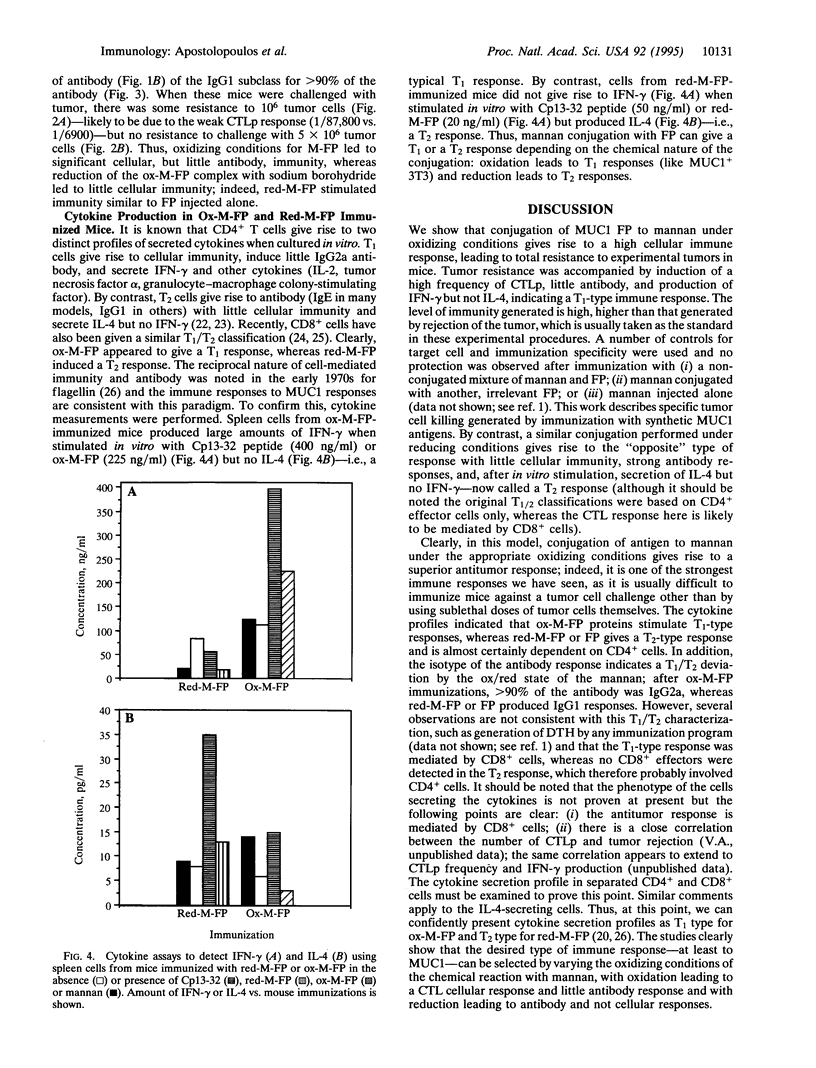
The induction of CD8+ cytotoxic T lymphocytes (CTLs) is desirable for immunization against many diseases, and recombinant-synthetic peptide antigens are now favored agents to use. However, a major problem is how to induce CTLs, which requires a T1-type response to such synthetic antigens. We report that T1-type (generating high CTL, low antibody) or T2-type (the reciprocal) responses can be induced by conjugation of the antigen to the carbohydrate polymer mannan: T1 responses are selected by using oxidizing conditions; T2 responses are selected by using reducing conditions for the conjugation. Using human MUC1 as a model antigen in mice, immunization with oxidized mannan-MUC1 fusion protein (ox-M-FP) led to complete tumor protection (challenge up to 5 x 10(7) MUC1+ tumor cells), CTLs, and a high CTL precursor (CTLp) frequency (1/6900), whereas immunization with reduced mannan-MUC1 FP (red-M-FP) led to poor protection after challenge with only 10(6) MUC1+ tumor cells, no CTLs, and a low CTLp frequency (1/87,800). Ox-M-FP selects for a T1 response (mediated here by CD8+ cells) with high interferon gamma (IFN-gamma) secretion, no interleukin 4 (IL-4), and a predominant IgG2a antibody response; red-M-FP selects for a T2-type response with IL-4 production and a high predominant IgG1 antibody response but no IFN-gamma.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acres R. B., Hareuveni M., Balloul J. M., Kieny M. P. Vaccinia virus MUC1 immunization of mice: immune response and protection against the growth of murine tumors bearing the MUC1 antigen. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):136–143. [PubMed] [Google Scholar]
- Apostolopoulos V., Xing P. X., McKenzie I. F. Murine immune response to cells transfected with human MUC1: immunization with cellular and synthetic antigens. Cancer Res. 1994 Oct 1;54(19):5186–5193. [PubMed] [Google Scholar]
- Apostolopoulos V., Xing P. X., Trapani J. A., McKenzie I. F. Production of anti-breast cancer monoclonal antibodies using a glutathione-S-transferase-MUC1 bacterial fusion protein. Br J Cancer. 1993 Apr;67(4):713–720. doi: 10.1038/bjc.1993.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arklie J., Taylor-Papadimitrious J., Bodmer W., Egan M., Millis R. Differentiation antigens expressed by epithelial cells in the lactating breast are also detectable in breast cancers. Int J Cancer. 1981 Jul 15;28(1):23–29. doi: 10.1002/ijc.2910280105. [DOI] [PubMed] [Google Scholar]
- Barnd D. L., Lan M. S., Metzgar R. S., Finn O. J. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume E. Time of truth for cancer vaccines. J Natl Cancer Inst. 1994 Mar 2;86(5):330–331. doi: 10.1093/jnci/86.5.330. [DOI] [PubMed] [Google Scholar]
- Ding L., Lalani E. N., Reddish M., Koganty R., Wong T., Samuel J., Yacyshyn M. B., Meikle A., Fung P. Y., Taylor-Papadimitriou J. Immunogenicity of synthetic peptides related to the core peptide sequence encoded by the human MUC1 mucin gene: effect of immunization on the growth of murine mammary adenocarcinoma cells transfected with the human MUC1 gene. Cancer Immunol Immunother. 1993;36(1):9–17. doi: 10.1007/BF01789125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner R. E., Childress A. M., Human L. G., Domer J. E. Characterization of Candida albicans mannan-induced, mannan-specific delayed hypersensitivity suppressor cells. Infect Immun. 1990 Aug;58(8):2613–2620. doi: 10.1128/iai.58.8.2613-2620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch F. G., Uhlenbruck G., Peter-Katalinic J., Egge H., Dabrowski J., Dabrowski U. Structures of neutral O-linked polylactosaminoglycans on human skim milk mucins. A novel type of linearly extended poly-N-acetyllactosamine backbones with Gal beta(1-4)GlcNAc beta(1-6) repeating units. J Biol Chem. 1989 Jan 15;264(2):872–883. [PubMed] [Google Scholar]
- Ioannides C. G., Fisk B., Jerome K. R., Irimura T., Wharton J. T., Finn O. J. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993 Oct 1;151(7):3693–3703. [PubMed] [Google Scholar]
- Jerome K. R., Domenech N., Finn O. J. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993 Aug 1;151(3):1654–1662. [PubMed] [Google Scholar]
- Lett E., Gangloff S., Zimmermann M., Wachsmann D., Klein J. P. Immunogenicity of polysaccharides conjugated to peptides containing T- and B-cell epitopes. Infect Immun. 1994 Mar;62(3):785–792. doi: 10.1128/iai.62.3.785-792.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Okawa Y., Howard C. R., Steward M. W. Production of anti-peptide specific antibody in mice following immunization with peptides conjugated to mannan. J Immunol Methods. 1992 Apr 27;149(1):127–131. doi: 10.1016/s0022-1759(12)80057-3. [DOI] [PubMed] [Google Scholar]
- Parish C. R. Immune response to chemically modified flagellin. I. Induction of antibody tolerance to flagellin by acetoacetylated derivatives of the protein. J Exp Med. 1971 Jul 1;134(1):1–20. doi: 10.1084/jem.134.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podzorski R. P., Gray G. R., Nelson R. D. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990 Jan 15;144(2):707–716. [PubMed] [Google Scholar]
- Powrie F., Coffman R. L. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993 Jun;14(6):270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Erythrocyte rosettes provide an analogue for Schiff base formation in specific T cell activation. J Immunol. 1990 Jul 15;145(2):463–469. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Boulay J. L., Finkelman F., Barbier S., Ben-Sasson S. Z., Le Gros G., Paul W. E. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992 Mar 15;148(6):1652–1656. [PubMed] [Google Scholar]
- Swallow D. M., Griffiths B., Bramwell M., Wiseman G., Burchell J. Detection of the urinary 'PUM' polymorphism by the tumour-binding monoclonal antibodies Ca1, Ca2, Ca3, HMFG1, and HMFG2. Dis Markers. 1986 Dec;4(4):247–254. [PubMed] [Google Scholar]
- Takahashi T., Makiguchi Y., Hinoda Y., Kakiuchi H., Nakagawa N., Imai K., Yachi A. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol. 1994 Sep 1;153(5):2102–2109. [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Weston S. A., Parish C. R. Evidence that mannose recognition by splenic sinusoidal cells plays a role in the splenic entry of lymphocytes. Eur J Immunol. 1992 Aug;22(8):1975–1981. doi: 10.1002/eji.1830220804. [DOI] [PubMed] [Google Scholar]
- Weston S. A., Parish C. R. Modification of lymphocyte migration by mannans and phosphomannans. Different carbohydrate structures control entry of lymphocytes into spleen and lymph nodes. J Immunol. 1991 Jun 15;146(12):4180–4186. [PubMed] [Google Scholar]
- Wreschner D. H., Hareuveni M., Tsarfaty I., Smorodinsky N., Horev J., Zaretsky J., Kotkes P., Weiss M., Lathe R., Dion A. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur J Biochem. 1990 May 20;189(3):463–473. doi: 10.1111/j.1432-1033.1990.tb15511.x. [DOI] [PubMed] [Google Scholar]
- Xing P. X., Prenzoska J., Quelch K., McKenzie I. F. Second generation anti-MUC1 peptide monoclonal antibodies. Cancer Res. 1992 Apr 15;52(8):2310–2317. [PubMed] [Google Scholar]
- Xing P. X., Tjandra J. J., Stacker S. A., Teh J. G., Thompson C. H., McLaughlin P. J., McKenzie I. F. Monoclonal antibodies reactive with mucin expressed in breast cancer. Immunol Cell Biol. 1989 Jun;67(Pt 3):183–195. doi: 10.1038/icb.1989.29. [DOI] [PubMed] [Google Scholar]



