Abstract
Exposure to chlorine (Cl2) gas during industrial accidents or chemical warfare leads to significant airway and distal lung epithelial injury that continues post exposure. While lung epithelial injury is prevalent, relatively little is known about whether Cl2 gas also promotes injury to the pulmonary vasculature. To determine this, rats were subjected to a sub-lethal Cl2 gas exposure (400ppm, 30min) and then brought back to room air. Pulmonary arteries (PA) were isolated from rats at various times post-exposure and contractile (phenylephrine) and nitric oxide (NO)-dependent vasodilation (acetylcholine and mahmanonoate) responses measured ex-vivo. PA contractility did not change, however significant inhibition of NO-dependent vasodilation was observed that was maximal at 24–48 hours post exposure. Superoxide dismutase restored NO-dependent vasodilation suggesting a role for increased superoxide formation. This was supported by ~2-fold increase in superoxide formation (measured using 2-hydroethidine oxidation to 2-OH-E+) from PA isolated from Cl2 exposed rats. We next measured PA pressures in anesthetized rats. Surprisingly, PA pressures were significantly (~4mmHg) lower in rats that had been exposed to Cl2 gas 24 hours earlier suggesting that deficit in NO-signaling observed in isolated PA experiments did not manifest as increased PA pressures in vivo. Administration of the iNOS selective inhibitor 1400W, restored PA pressures to normal in Cl2 exposed, but not control rats suggesting that any deficit in NO-signaling due to increased superoxide formation in the PA, is offset by increased NO-formation from iNOS. These data indicate that disruption of endogenous NO-signaling mechanisms that maintain PA tone is an important aspect of post-Cl2 gas exposure toxicity.
Keywords: halogen, lung, inflammation
Introduction
Chlorine gas (Cl2) is utilized in various manufacturing processes and is a chemical that is transported by rail in close proximity to significant population centers worldwide. High levels of Cl2 gas exposure have been documented in numerous case reports of accidental release during transport or industrial use, or after intentional release in the military arena (Cevik et al. 2009; Evans 2005; Jones et al. 2010; Matalon and Maull 2010; Van Sickle et al. 2009; Wenck et al. 2007; White and Martin 2010) which has led to recent interest into a more detailed understanding of the mechanisms of Cl2 gas induced toxicity (Hoyle 2010; Matalon and Maull 2010; Samal et al. 2010; White and Martin 2010).
Cl2 gas toxicity is dependent on the dose and length of exposure with several studies showing that it comprises an initial ‘during exposure’ injury that is likely mediated by direct reactions of Cl2 (or its hydrolysis product, hypochlorous acid) with biomolecules and a robust and chronic (days-weeks) ‘post exposure’ injury period (Donnelly and FitzGerald 1990; Gunnarsson et al. 1998; Leustik et al. 2008; Martin et al. 2003; Tian et al. 2008; Tuck et al. 2008). The latter culminates in acute lung injury (ALI), adult respiratory distress syndrome (ARDS) and reactive airway syndrome (RAS) and reflects increased permeability and inflammatory tissue injury. Several mechanisms have been identified including loss of endogenous antioxidants and surfactant, stimulation of inflammation, epithelial and alveolar ion transport dysfunction and activation of sensory neurons to name but a few (Bessac and Jordt 2010; Fanucchi et al. 2012; Lazrak et al. 2012; Leikauf et al. 2012; Song et al. 2010; Tian et al. 2008; Tuck et al. 2008).
Notably, these insights have centered on Cl2-dependent injury to airway (bronchial), and distal lung (alveolar type II) epithelial, cell function(Lazrak et al. 2012; Song et al. 2010). It is important to recognize however, that injury is not limited to the epithelial cells. Our previous studies documented significant dysfunction in systemic vascular function, illustrated by a loss of control over nitric oxide-dependent vasodilation (Honavar et al. 2011). Specifically, post-Cl2 gas toxicity led to an inhibition of endothelial nitric oxide synthase (eNOS) activity and an increase in inducible nitric oxide synthase (iNOS) activity in the systemic circulation. eNOS derived NO is a critical modulator of vascular homeostasis mechanisms including regulating vessel tone, cellular respiration, inhibiting smooth muscle proliferation, maintaining an anti-thrombotic and anti-inflammatory luminal surface (Moncada 1999; Napoli and Ignarro 2009) and critical in maintaining low pulmonary arterial pressures. Moreover, a deficit in NO-bioavailability, is one mechanism for pulmonary arterial hypertension. If and how Cl2 gas exposure affects regulation of pulmonary arterial tone is unknown and was tested in this study.
Materials and Methods
Materials
Unless stated otherwise all reagents and antibodies were purchased from Sigma (St. Louis, MO, USA) and AbCam (Cambridge, MA, USA) respectively except Mahma/NONOate (MNO) which was purchased from Axxora Platform (San Diego, CA, USA). Male Sprague Dawley rats (200–300g) were purchased from Harlan (Indianapolis, IN, USA) and kept on 12h light dark cycles with access to standard chow and water ad libitum prior to and post chlorine exposure. 1400W was purchased from Enzo Life Sciences International, Inc (Plymouth Meeting, PA, USA). Hydroethidine was purchased from Invitrogen and 2-OH-E+ standards were synthesized as previously described (Zielonka et al. 2008).
Rat exposure to chlorine gas
Whole body exposure of rats (Harlan Laboratories, USA) to 400ppm Cl2 gas was performed as previously described (Leustik et al. 2008) and according to IACUC approved protocols. This is a sub-lethal exposure protocol that results in significant acute lung injury and chronic development of reactive airways and systemic endothelial dysfunction. Two rats were exposed in the same chamber at any one time and all exposures were performed between 8–9am, and were 30min in length followed by return to room air. Two mass flow controllers (MFCs) with Kalrez seals (Scott Specialty Gases, Los Angeles, CA; part no. 05236A1V5K) and a microprocessor control unit (Scott Specialty Gases; part no. 05236E4) were used to control the compressed air and Cl2 (1,000 ppm Cl2 in air; Airgas, Birmingham, AL) flow rates to achieve the chamber Cl2 target concentrations. A bubble flow meter was used to validate MFC performance on a weekly basis. Air and Cl2 were initially mixed at a three-way junction, and they were further mixed by passing through a diffuser located inside the top lid of the exposure chamber. Gases exited the chamber via two large-bore diameter ports in its bottom half. The exposure chamber was placed inside a chemical fume hood located in a negative-pressure room. At the end of each exposure, the Cl2 gas was turned off, the chamber was vented with compressed air for 2–3 min, the two halves were separated, and the rats were removed and returned to their cages, where they breathed room air. Food and water were provided ad libitum.
Pulmonary artery pressure measurements
Sprague-Dawley rats weighing 200–300g were divided into 4 groups, 3 of which were exposed to 400 ppm Cl2 gas for 30 min. These were then subdivided into chlorine only, chlorine + 1400W dihydrochloride and chlorine + L-NMMA groups. One group of rats was not exposed to chlorine and used as air controls. 24h after Cl2 exposure rats were anesthetized and PA pressures measured as described below. Then animals in the Cl2 only group received 200μl IP injection of PBS while rats in chlorine + 1400W dihydrochloride and chlorine + L-NMMA group received intravenous injection of 10mg/kg dose of 1400W dihydrochloride or 10mg/kg dose of L-NMMA respectively and PA pressure measured again 60 min post injection. For PA pressure measurements, rats were anesthetized using isoflurane and the jugular vein exposed above the clavicle. An incision was made in the external jugular vein and a polyethylene tube (introducer) with internal diameter of 0.76mm and outer diameter of 1.2mm (PE-60, Clay Adams) was inserted into the external jugular vein and advanced until it reached the right ventricle (RV). Relative position of the introducer was monitored via pressure transducers using AcqKnowledge software with Biopac system. Once the introducer reached the RV it was detached from the pressure transducer and the pressure catheter was attached. This catheter was a silastic tubing of Micro Renathane (MRE025, Braintree Scientific, MA). The catheter was inserted into the introducer and extended past the tip of the introducer till it reached the RV. Once in the RV, the catheter was further advanced until the pressure traces resembled those of PA pressures measured by previous investigators (Rabinovitch et al. 1979). After every measurement, rats were sacrificed by exsanguination and the position of the catheter in the PA verified by opening of the chest cavity and dissection. Data were excluded if it was determined post-measurement that the catheter was pressed against the PA wall.
Isolated pulmonary artery studies
At the indicated times post Cl2 exposure, pulmonary arteries were collected, cleansed of adherent fat and responses to the indicated vasoconstrictive and vasoactive stimuli determined in vessel bioassay chambers (Radnoti, Monrovia, CA), as previously described (Honavar et al. 2011). All vessel bioassay studies were performed in indomethacin (5μM) pre-treated vessels (2–3mm segments) and in bicarbonate buffered Krebs Henseleit buffer of the following composition (mM): NaCl 118; KCl 4.6; NaHCO3 27.2; KH2PO4 1.2; MgSO4 1.2; CaCl2 1.75; Na2 EDTA (ethylenediaminetetraacetic acid) 0.03, and glucose 11.1 and perfused with 21% O2, 5% CO2 balanced with N2. A passive load of 1.5g was applied to all ring segments and maintained at this level throughout the experiments. At the beginning of each experiment ring segments were depolarized with KCl (70mM) to determine the maximal contractile capacity of the vessel. Rings were then washed extensively and allowed to equilibrate and again depolarized with KCl (70mM). The rings were then washed and allowed to re-equilibrate. Vasoconstrictor responses were tested by cumulative addition of phenylephrine (PE) doses ranging from 1nM to 3000nM. Endothelium-dependent vasodilator responses were tested by administering cumulative doses of acetylcholine (Ach), ranging from 1nM to 3000nM after tension development at maximal PE dose. In subsequent experiments, vessels were sub-maximally contracted (50% of KCl response) with PE (300nM–1000nM). When tension development reached a plateau, endothelium–independent vasodilator responses were induced by administering the NO donor MAHMANONOate (MNO) or sodium nitroprusside (SNP). Where indicated, the effects of SOD (100U/ml) were determined by adding SOD to vessel baths 5min prior to addition of Ach or MNO.
Nitrite formation
pulmonary arteries were bathed isolated, blotted dry and weighed and then placed in 200μl saline containing Ca2+/Mg2+ at 37°C. After 15mins equilibration, A23187 (5μM) was added for a further 15min. 100μl buffer was carefully aspirated and nitrite levels measured by the Griess assay coupled with HPLC detection using the ENO-20 (EiCOM) as previously described (Samal et al. 2012).
NOS expression
Expression of eNOS protein was determined in the pulmonary artery by western blot as previously described (Honavar et al. 2011). Specifically, pulmonary aorta segments were homogenized in RIPA buffer (0.5% Na-deoxycholate (w/v), 0.1% SDS (w/v), 150mM NaCl EDTA 0.5–1mM, 1% NP-40 (v/v) in 50mM Tris-HCl, pH 7.4) containing complete mini protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA) and phosphatase inhibitor cocktail 3 (Sigma, St. Louis, MO, USA) and protein levels measured by the Lowry assay. 80–200μg of protein from tissue homogenates was boiled for 15min, 85°C in reducing sample buffer (2% beta-mercaptoethanol (v/v), 4% SDS (w/v), 10% Glycerol (v/v), 0.2% (v/v) bromophenol blue in 100mM Tris HCl, pH 6.8) and resolved on a 10% SDS polyacrylamide gel followed by transferring onto nitrocellulose membrane. Membrane was blocked with 5% non-fat dried skimmed milk powder (w/v) in TBS-T (137mM NaCl, 2.6mM KCl, 0.05% Tween-20 (v/v) in 25mM Tris-HCl pH 7.4) for 1h at room temperature and washed with TBS-T for 30min. Membrane was then incubated with mouse antibody against eNOS (BD Transduction Laboratories, 610296) diluted 1:1000 in TBS-T for overnight in cold room followed by rabbit anti-mouse IgG antibody, Alkaline Phosphatase-conjugate (ab6729, Abcam) diluted 1:2000 in in TBS-T at room temperature for 2h. Membrane as loading control were incubated with rabbit polyclonal antibody against β-actin (Cell-Signaling,4967) diluted 1:2000 in TBS-T for overnight in cold room followed by goat anti-rabbit IgG antibody, Alkaline Phosphatase-conjugate (AP132A, Millipore) diluted 1:2000 in TBS-T for 2h. After washing with TBS-T for 30min, membranes were developed by chemiluminescence using Duo-Lux Chemiluminescent /Fluorescent substrate (Vector Laboratories, Inc.) and sequential images by AlphaEaseFC™ (Protein Simple Inc. Santa Clara. CA) were taken with quantification only performed on bands which had not reached saturation. In addition, eNOS phosphorylation at Ser 1177 or Thr 495 was determined by western blot using respective phsopho-specific mouse antibodies (BD Transduction laboratories; 612393 (Ser1177) and 612707 (Thr495) used at 1:1000 dilutions.
Superoxide measurement
PA segments of equal size (7mm) were isolated from rats exposed to air or 24h post Cl2 gas exposure (400ppm, 30min). Rats were anesthetized by administering ketamine / xylazine (100/10mg/Kg) I.P. The thoracic cavity was exposed and the PA isolated from peduncle till the end of left secondary PA. Segments were incubated in 30μM hydroethidine for 1h at 37°C in the dark. The PA segments were then washed in ice cold PBS, blotted dry and snap frozen in a microcentrifuge tube in liquid nitrogen before being stored at −80°C. Levels of the O2•−-specific 2-OH-E+ was assessed by reverse-phase HPLC with electrochemical detection using standard curves generated by measuring know amounts of 2-OH-E+, hydroethidine and E+ as previously reported (Zielonka et al. 2008).
Statistical analysis
Data were analyzed by one-way or two-way analysis of variance (ANOVA) with Tukey or Bonferroni post test, or t-test as indicated using GraphPad Prism. Significance was set at p < 0.05.
Results
Effects of Cl2 gas exposure on ex vivo pulmonary arterial vasoactivity
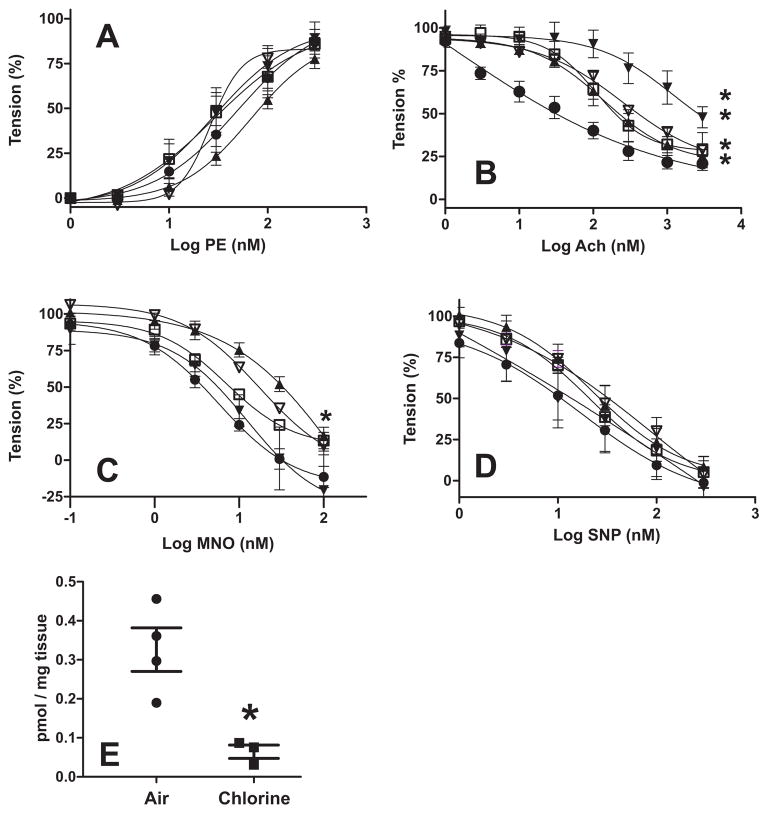
Pulmonary arteries were isolated from rats at various times post Cl2 exposure and responsiveness to phenylephrine (Fig 1A), acetylcholine (Fig 1B), MNO (Fig 1C) and SNP (Fig 1D) measured. No significant difference in PE-dependent contraction was observed (Fig 1A). However, at all time points up to 48h, Ach dependent vasodilation was significantly inhibited indicated by rightward shifts in dose dependent tension changes. Fig 2A plots the EC50 values for Ach dependent vasodilation and shows that inhibition was maximal at 48h, with a trend towards inhibition still noted at 72h. Interestingly, MNO-dependent vasodilation was also significantly attenuated but only 24h post Cl2-exposure, although trends for significant inhibition were noted at 48h and 72h (Fig 1C and Fig 2B). No effect of Cl2 gas on SNP-dependent vasodilation was observed suggesting smooth muscle responsiveness to nitrosovasodilators remained intact (Fig 1D). To assess if NO-formation was decreased, nitrite formation was measured from pulmonary arteries stimulated with A23187. Fig 1E shows significant attenuation of nitrite formation from pulmonary arteries isolated from chlorine exposed rats compared to air controls.
Figure 1. Effects of Cl2 gas exposure on pulmonary artery vasoactivity.
The pulmonary artery was collected from air exposed rats (●) or from rats at various times (□ 6h; ▲ 24h, ▼ 48h, ∇72h) post Cl2 exposure (400ppm, 30min) and cumulative dose-dependent responses to PE (Panel A), Ach (Panel B), MNO (Panel C) and SNP (Panel D) determined. For MNO and SNP vasodilation was determined after pre-constriction with PE. Data show mean ± SEM. Replicates = 5 (control), 5 (6h), 8 (24h), 4 (48h), 2 (72h). Each replicate denotes a different animal, with responses from 2–3 individual PA segments per animal averaged. 2-way RM-ANOVA (P<0.05) with Bonferonni post-tests (*P<0.05) indicated significant differences for Ach-dependent vasodilation between air exposed and 6h, 24h, 48h post-Cl2 exposure. 2-way RM-ANOVA (P<0.05) with Bonferonni post-tests (*P<0.05) indicated significant differences for MNO-dependent vasodilation between air exposed 24h post-Cl2 exposure. For PE-dependent contraction and SNP-dependent vasodilation, no post-test significance between control and any time post-Cl2 exposure was observed (P = 0.55 for PE and P = 0.67 for SNP). Lines show best fit non-linear regression using sigmoidal with variable slope algorithm. Panel E shows A23187-dependent formation of nitrite (normalized to tissue weight) from pulmonary arteries isolated 24h after exposure to air or chlorine gas (400ppm, 30min). Each replicate denotes a distinct animal. *P <0.05 by t-test.
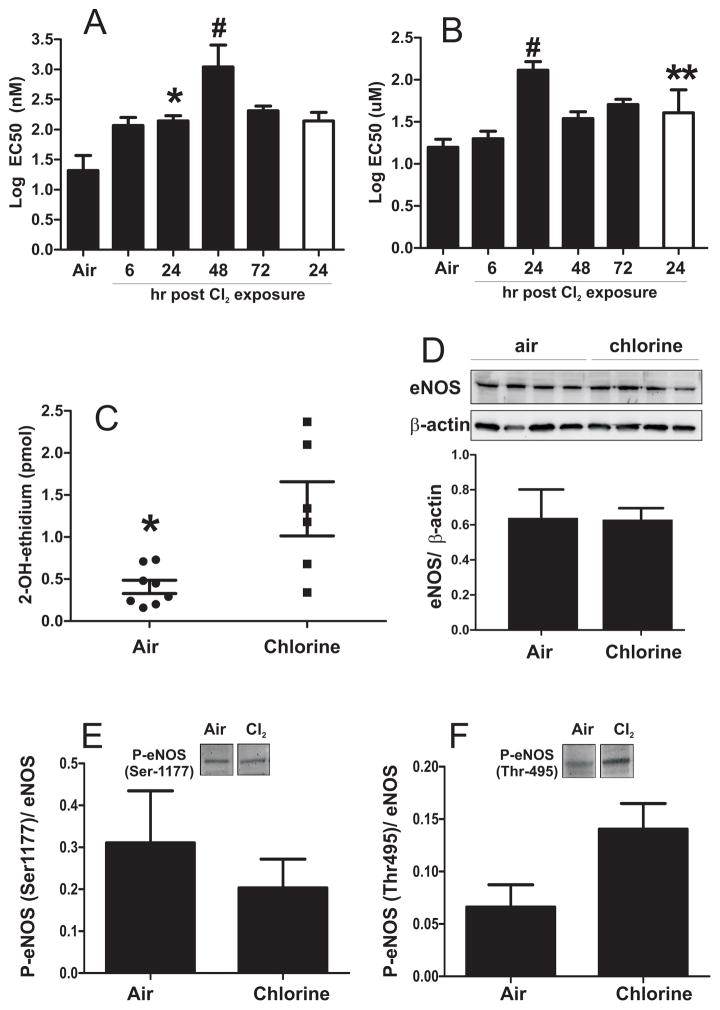
Figure 2. Cl2 gas exposure inhibits Ach and MNO-dependent vasodilation of pulmonary artery: role of superoxide anion radical.
Black bars show Log EC50 for Ach (panel A) or MNO (panel B) determined from sigmoidal fits of data shown in Fig 1. Values are means ± SEM (n as stated in Fig 1 legend). *P<0.05 and #P<0.05 by 1-way ANOVA with Tukey post test relative to air. Clear bar indicates EC50 determined in the presence of SOD (100U/ml). **P<0.05 relative to 24h without SOD. Panel C shows formation of 2-OH-E+ reflecting superoxide anion radical formation as described in methods. Each data point represents an individual animal. *P<0.05 by unpaired t-test. Panel D shows representative western blot for eNOS protein in PA isolated from air or 24 hours post Cl2 exposure. Bar graphs show quantitation after normalization to β-actin. Data are mean ± SEM (n=4). P =NS by unpaired t-test. Panel E and F show respectively mean (± SEM) changes in phosphorylation of eNOS at Ser1177 or Thr 495 normalized to total eNOS, after A23187 stimulation. P =0.48 (Panel E) and P = 0.06 (Panel F) by t-test (n=3–4).
Role of superoxide anion radical in mediating Cl2 dependent inhibition of pulmonary arterial vasodilation
While inhibited Ach-dependent vasodilation may indicate compromised NO-formation and / or NO-signaling downstream of NO-formation, the observed inhibition of MNO-dependent vasodilation can only be explained by diversion of NO away from physiologic signaling targets. We hypothesized that this occurs by increased formation of superoxide anion radical, which rapidly reacts with NO thereby attenuating NO-dependent activation of soluble guanylate cyclase, a pathway now established as one cause of endothelial dysfunction (White et al. 1994). To test this hypothesis, MNO-dependent vasodilation was measured in the absence or presence of superoxide dismutase (SOD). Fig 2B shows that the EC50 for MNO dependent vasodilation of pulmonary artery segments isolated 24h after Cl2 gas exposure, decreased significantly in the presence of SOD. However, no effect of SOD on Ach-dependent vasodilation was observed. Figure 2C shows that superoxide anion formation was higher in pulmonary artery segments isolated from rats 24h after cessation of Cl2 gas exposure compared to air alone exposed rats further implicating a role for this radical in mediating the inhibition of NO-dependent vasodilation. Finally, we show that eNOS protein expression in the PA was not affected by chlorine gas exposure (Fig 2D). No significant changes in phosphorylation of eNOS at Ser1177 or Thr 495 (activation and inhibitory sites respectively) were observed (Fig 2E–F); however a close to significant increase in phsophorylation at Thr 495 in chlorine treated group was noted. Finally, no changes in iNOS protein expression were observed by immunohistochemistry or western blotting in PA segments isolated from air or chlorine exposed rats (not shown).
Effects of Cl2 gas exposure on pulmonary arterial pressures in vivo
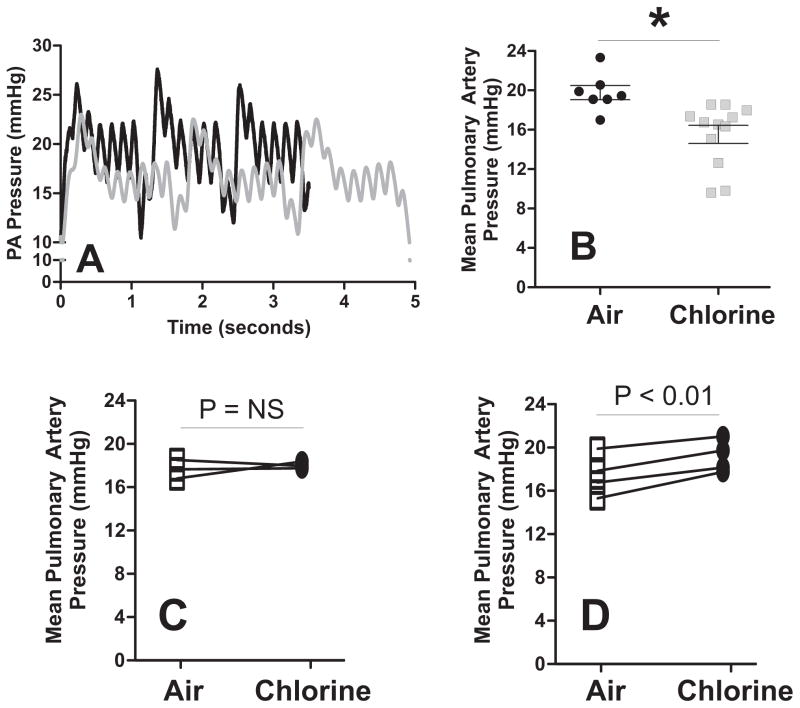
Rats were exposed to Cl2 gas (400ppm, 30min), and then brought back to room air and PA pressures measured 24h later in vivo. Surprisingly, PA pressures were significantly lower by ~4mmHg 24h post-Cl2 exposure (Figure 3A–B) contrary to the prediction that pressure would be higher based on ex-vivo studies shown in Fig 1–2. No differences in right ventricle systolic pressures were observed (20.1 ± 0.01 and 20 ± 0.05 mmHg for air and Cl2 groups respectively). In addition, respiratory rate was slower in Cl2 exposed animals indicated by ~30% increase in time taken to achieve 3 breaths (compare raw PA vs. traces, Fig 3A) likely due to activation of sensory neurons as described previously (Bessac and Jordt 2010). Our previous studies showed that in the systemic circulation while eNOS-dependent signaling was inhibited, in vivo this was countered by a gain in iNOS-dependent NO-formation(Honavar et al. 2011). We reasoned that a similar effect may be occurring with PA pressure. To test this, rats were exposed to Cl2 gas (400ppm, 30min) and then 24h later, PA pressures measured. This was followed by infusion of either L-NMMA or the selective iNOS inhibitor 1400W and re-measurement of PA pressure 1h later. Fig 3B and 3C show that L-NMMA had no effect on PA pressures in Cl2 exposed rats, however 1400W significantly increased PA pressures indicating that iNOS was responsible for lowered PA pressures in vivo.
Figure 3. Chlorine gas exposure decreases pulmonary arterial pressure in vivo.
Panel A shows representative PA pressure vs. time traces for air (black line) and after Cl2 gas exposure (gray line). Panel B shows the pulmonary arterial pressure in anesthetized rats 24hr after exposure to air or Cl2 (400ppm, 30min). Each data point represents an animal. *P<0.05 by unpaired t-test. Panel C and D show the effect of L-NMMA (10mg/Kg) or 1400W (10mg/Kg) on pulmonary arterial pressures 24h after exposure to Cl2 gas. Indicated P-values are by paired t-test.
Discussion
Interest in Cl2 gas toxicity mechanisms is fuelled by previous incidents of, and the potential for future accidental or intentional exposures that can result in mass-casualty scenarios. Post-exposure treatment is limited to the symptoms and reflects in part, a lack of understanding of the mechanisms for post-Cl2 gas toxicity. Understandably, most studies have focused on post-Cl2 exposure dependent damage to the airways leading to acute lung injury and reactive airway syndrome (Bessac and Jordt 2010; Hoyle 2010; Martin et al. 2003; Matalon and Maull 2010; O’Koren et al. 2013; Samal et al. 2010; White and Martin 2010; Yadav et al. 2010). Importantly, the emerging mechanistic insights are now leading to testing of targeted therapies to limit post-Cl2 gas toxicity (Chen et al. 2013; Fanucchi et al. 2012; McGovern et al. 2011; McGovern et al. 2010; Samal et al. 2012; Song et al. 2011; Yadav et al. 2011). In addition to the lung epithelia, we have reported that extrapulmonary toxicity may also be significant and contribute to post-exposure morbidity and mortality (Samal et al. 2010). Specifically, we showed that NO-dependent regulation of systemic arterial pressure was altered in rats exposed to Cl2 gas, with a loss of eNOS-dependent function that was offset by an increase in iNOS-dependent hypotension. We extend these observations and report that Cl2-gas exposure significantly perturbs NO-dependent regulation of pulmonary vascular tone also.
Ex-vivo and in vivo assessments of PA function indicated that Cl2 gas exposure caused dysfunction, albeit with contrary results depending on the method of measurement. Isolated PA ring experiments indicated that while smooth muscle response remained intact, endogenous (Ach-dependent) or exogenous (MNO-dependent) NO-dependent vasodilation was inhibited. This inhibition peaked between 24–48h, with inhibition remaining up to 3 days post exposure. A role of increased superoxide formation in mediating loss of NO-dependent vasodilation was indicated by increased 2-OH-ethidium levels in vessels isolated from Cl2-gas exposed rats, and leftward shifts in MNO-dose dependent vasodilation in the presence of SOD. Notably, SOD had no effect on SNP-mediated vasodilation. MNO and SNP differ in that MNO releases NO, whereas SNP can also mediate effects through nitrosation (dependent on NO+) reactions. We speculate that superoxide levels are unlikely to increase sufficiently to compete with endogenous nucleophiles (e.g thiols) that react rapidly with NO+ and this accounts for no effect on SNP-dependent vasodilation. This NO-donor, consistent with this NO-donor mediating effects via nitrosonum cation, which does not react with superoxide. Superoxide rapidly reacts with NO forming peroxynitrite, and superoxide dismutation results in hydrogen peroxide formation. Thus in addition to loss of NO, increased superoxide may also mediate pulmonary vascular dysfunction by perturbation of redox signaling pathways. It also remains unclear how Cl2 gas exposure results in increased superoxide formation in the PA. While many possible sources exist (NADPH oxidases, mitochondria, xanthine oxidoreductase to name but a few), we have not assessed this further since the increased superoxide did not appear to be playing a major role in affecting NO-dependent vasodilation in vivo (see below). Future studies determining the source of increased superoxide, and whether it plays a role in altered PA physiology and redox signaling after Cl2 exposure are indicated however.
Given the loss of NO-dependent vasodilation ex-vivo, we expected to observe increased PA pressures when measured in vivo. However, the opposite was observed with PA pressures being ~20% lower in Cl2 exposed rats. This hypotensive effect was iNOS-dependent indicated by a hypertensive effect of 1400W. In our previous study with aorta from rats exposed to Cl2 gas, both an inhibition of eNOS and induction of iNOS were demonstrated. However, this was not functionally evident with PA pressure, as L-NMMA had no effect, and 1400W treatment increased PA pressures up to pre-Cl2 exposure levels. Lack of effect of L-NMMA is also consistent with no changes in eNOS expression in PA isolated from Cl2 exposed rats. Interestingly, this is in contrast to the aorta in which significant loss of eNOS protein has been reported after Cl2 gas exposure (Honavar et al. 2011). Thus the primary determinant of altered PA pressures in vivo appears to be iNOS activity; note no changes in right ventricular pressures were observed. We also note that in our prior study, iNOS expression was shown in circulating immune cells which further suggests that any change to the PA itself by Cl2 gas exposure, including increased superoxide production, does not play a significant role in effecting PA pressure in vivo, in the setting of Cl2 gas toxicity. eNOS activity is regulated by phosphorylation at multiple sites with two key sites being Ser 1177 and Thr 495(Rafikov et al. 2011). While no significant differences in calcium-ionophore dependent phosphorylation of either of these sites was observed, trends towards increased phosphorylation of Thr 495, which inhibits eNOS, are noted. Further studies are required to test if eNOS phosphorylation changes, and / or other structural or post-translation modifications that control activity are regulated by chlorine exposure.
Our findings present several interesting implications for Cl2 gas toxicity. First, it supports the paradigm that injury is not limited to the Cl2 exposure phase only. In addition injury is not limited to the airways and dysfunction in the pulmonary and systemic vasculature should also be considered. A key mechanism underlying these effects is perturbation in the balance between eNOS and iNOS-derived NO. Less clear is how post Cl2 gas exposure results in alterations in eNOS or iNOS. We and others have speculated on the role of pro-inflammatory cytokines and / or effects of chlorinated biomolecules (e.g. fatty acids) (Honavar et al. 2011; Martin et al. 2003; Samal et al. 2010). An additional consideration is that our studies used healthy rats. How Cl2 exposure would affect PA function in the setting of existing underlying pulmonary arterial dysfunction is not known and difficult to predict. This is further complicated by the fact that the post-exposure phase is complex reflecting injury and repair processes, and likely involves multiple vasoactive mediators. Indeed, ex-vivo studies showed inhibition of NO-dependent vasodilation peaking at 24–48h post exposure and starting to return to basal thereafter. We acknowledge the limitation that our in vivo assessment of PA pressure was only performed at 24h post Cl2 gas exposure and that we focused only on NO-dependent processes. Future studies will test other potential effectors PA pressure (e.g prostanoids, endothelin etc).
In summary, our studies document altered regulation of PA pressure in rats exposed to a sub-lethal Cl2 gas that remains for many hours post exposure. This is characterized by dysfunctional NO-dependent relaxation that may involve both increased superoxide formation and increased iNOS activity. These data further suggest that in addition to airway injury, systemic and pulmonary vascular toxicity is an important component of post-Cl2 gas injury.
Supplementary Material
Acknowledgments
This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director, and the National Institute of Environmental Health Sciences, Grant Numbers 1U01ES023759 and 5U01ES015676.
Grant and Funding information: This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director, and the National Institute of Environmental Health Sciences, Grant Numbers 1U01ES023759 and 5U01ES015676 and a fellowship from the National Institute of Health to MOV (T32 HL007457)
Footnotes
Authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cevik Y, Onay M, Akmaz I, Sezigen S. Mass casualties from acute inhalation of chlorine gas. South Med J. 2009;102:1209–1213. doi: 10.1097/SMJ.0b013e3181bfdc67. [DOI] [PubMed] [Google Scholar]
- Evans RB. Chlorine: state of the art. Lung. 2005;183:151–167. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- Jones R, Wills B, Kang C. Chlorine gas: an evolving hazardous material threat and unconventional weapon. West J Emerg Med. 2010;11:151–156. [PMC free article] [PubMed] [Google Scholar]
- Matalon S, Maull EA. Understanding and treating chlorine-induced lung injury. Proc Am Thorac Soc. 2010;7:253. doi: 10.1513/pats.7.4.253. [DOI] [PubMed] [Google Scholar]
- Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med. 2009;27:1–7. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenck MA, Van Sickle D, Drociuk D, Belflower A, Youngblood C, Whisnant MD, Taylor R, Rudnick V, Gibson JJ. Rapid assessment of exposure to chlorine released from a train derailment and resulting health impact. Public Health Rep. 2007;122:784–792. doi: 10.1177/003335490712200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010;7:257–263. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GW. Mitigation of chlorine lung injury by increasing cyclic AMP levels. Proc Am Thorac Soc. 2010;7:284–289. doi: 10.1513/pats.201001-002SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal A, Honovar J, White CR, Patel RP. Potential for chlorine gas-induced injury in the extrapulmonary vasculature. Proc Am Thorac Soc. 2010;7:290–293. doi: 10.1513/pats.201001-006SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly SC, FitzGerald MX. Reactive airways dysfunction syndrome (RADS) due to chlorine gas exposure. Ir J Med Sci. 1990;159:275–276. doi: 10.1007/BF02993611. discussion 276–277. [DOI] [PubMed] [Google Scholar]
- Gunnarsson M, Walther SM, Seidal T, Bloom GD, Lennquist S. Exposure to chlorine gas: effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. J Appl Toxicol. 1998;18:249–255. doi: 10.1002/(sici)1099-1263(199807/08)18:4<249::aid-jat507>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 2008;295:L733–743. doi: 10.1152/ajplung.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med. 2003;168:568–574. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol. 2008;20:783–793. doi: 10.1080/08958370802007841. [DOI] [PubMed] [Google Scholar]
- Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res. 2008;9:61. doi: 10.1186/1465-9921-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol. 2012;46:599–606. doi: 10.1165/rcmb.2011-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR, Jr, Matalon S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am J Respir Cell Mol Biol. 2012;46:342–354. doi: 10.1165/rcmb.2011-0309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikauf GD, Pope-Varsalona H, Concel VJ, Liu P, Bein K, Berndt A, Martin TM, Ganguly K, Jang AS, Brant KA, Dopico RA, Jr, Upadhyay S, Di YP, Li Q, Hu Z, Vuga LJ, Medvedovic M, Kaminski N, You M, Alexander DC, McDunn JE, Prows DR, Knoell DL, Fabisiak JP. Integrative assessment of chlorine-induced acute lung injury in mice. Am J Respir Cell Mol Biol. 2012;47:234–244. doi: 10.1165/rcmb.2012-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem. 2010;285:9716–9728. doi: 10.1074/jbc.M109.073981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, Patel RP. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol. 2011;45:419–425. doi: 10.1165/rcmb.2010-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol. 1979;236:H818–827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- Samal AA, Honavar J, Brandon A, Bradley KM, Doran S, Liu Y, Dunaway C, Steele C, Postlethwait EM, Squadrito GL, Fanucchi MV, Matalon S, Patel RP. Administration of nitrite after chlorine gas exposure prevents lung injury: Effect of administration modality. Free radical biology & medicine. 2012;53:1431–1439. doi: 10.1016/j.freeradbiomed.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA, et al. Superoxide and peroxynitrite in atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Koren EG, Hogan BL, Gunn MD. Loss of Basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol. 2013;49:788–797. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc. 2010;7:278–283. doi: 10.1513/pats.201001-009SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Mo Y, Schlueter CF, Hoyle GW. Inhibition of chlorine-induced pulmonary inflammation and edema by mometasone and budesonide. Toxicology and applied pharmacology. 2013;272:408–413. doi: 10.1016/j.taap.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free radical biology & medicine. 2011;50:602–608. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern TK, Powell WS, Day BJ, White CW, Govindaraju K, Karmouty-Quintana H, Lavoie N, Tan JJ, Martin JG. Dimethylthiourea protects against chlorine induced changes in airway function in a murine model of irritant induced asthma. Respiratory research. 2010;11:138. doi: 10.1186/1465-9921-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a {beta}2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol. 2011;45:88–94. doi: 10.1165/rcmb.2010-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, 2nd, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. American journal of physiology Lung cellular and molecular physiology. 2011;300:L362–369. doi: 10.1152/ajplung.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, Fulton D, Black SM. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. The Journal of endocrinology. 2011;210:271–284. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.