Abstract
Several 2-carbon-linked trioxane dimer secondary alcohol carbonates 14 and thiocarbonates 15, combined with mefloquine and administered in a low single oral dose, prolonged the survival times of malaria-infected mice much more effectively than the popular monomeric antimalarial drug artemether plus mefloquine. Three dimer carbonates 14 and one dimer thiocarbonate 15 partially cured malaria-infected mice.
Keywords: Antimalarial chemotherapy, Trioxane dimers, Single oral dose ACT, Oral bioavailability
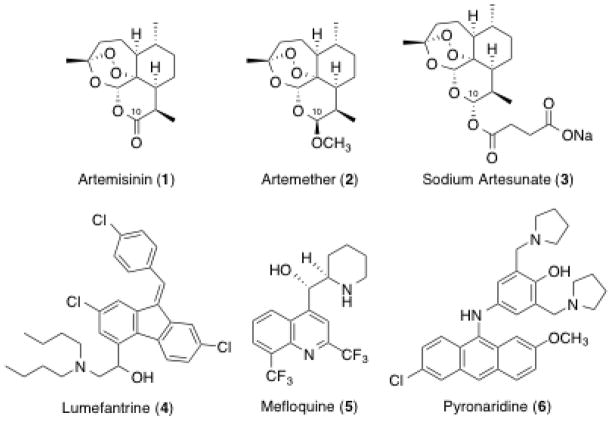
In 2013, a total of 107 countries reported malaria as an ongoing epidemic exposing an estimated 3.4 billion people to the Plasmdium falciparum malaria parasites.1 Nearly one million people, mostly children, die each year from infection with malaria.2–4 While ongoing efforts have been made toward the development of a fully prophylactic malaria vaccine, only partial success has been reported.5,6 The efficacy of antimalarial chemotherapy using standard drugs like chloroquine is being severely compromised by widespread parasite resistance.7,8 Therefore, the discovery of a new class of peroxide-containing antimalarials such as artemisinin (1)9 and its first generation derivatives artemether (2) and sodium artesunate (3) has led to their widespread use (Figure 1). Indeed, the World Health Organization (WHO) now recommends artemisinin combination therapy (ACT) as standard operating procedure, combining a fast-acting but short-lived trioxane with a long-lasting adjuvant.10 Current examples of combinations include artemether (2) plus lumefantrine (4),11 artesunate (3) plus mefloquine (5), and artesunate (3) plus pyronaridine (6).12 An ideal regimen for curing infected people is a single low oral dose of ACT. Toward this goal, others13–18 and we19–22 have prepared several artemisinin derivatives that cure malaria-infected mice.
Figure 1.
Artemisinin (1), first generation derivatives (2 & 3), and adjuvant therapeutic drugs used in ACT (4–6).
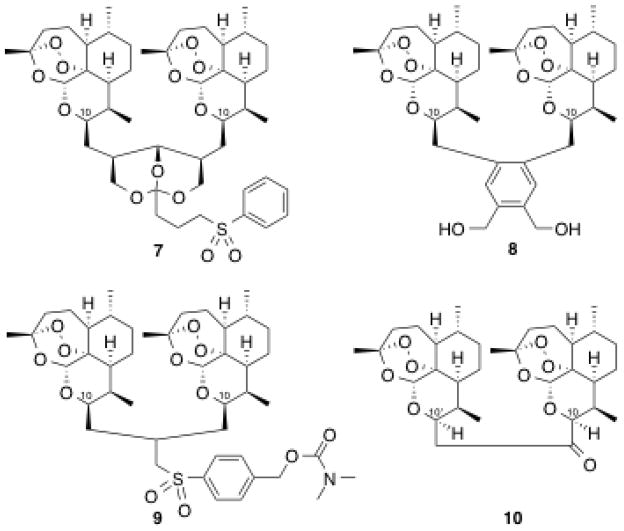
Guided by structure-activity relationships (SAR), ongoing efforts have been made toward improving the oral bioavailability and minimizing the metabolic shortcomings of artemisinin and its first generation derivatives.23 Tethering two artemisinin units together through the C10 position forms a C10 non-acetal dimeric trioxane structure, which has proven often to be more antimalarially potent than its corresponding monomeric counterpart.24–26 We have highlighted the high efficacy of a low single oral dose ACT using new artemisinin-derived trioxane dimers with linkers of different length: 5-carbon (7),27 4-carbon (8),28 3-carbon (9),29,30 and 2-carbon (10, Figure 2). Two-carbon-linked trioxane dimer ketone 10 (prepared from artemisinin in 36% overall yield) and especially some of its oxime NH-aryl carbamates 11 are effective antimalarials,31 as are 2-carbon-linked dimer secondary alcohols 12a and 12b; using only a single low oral dose of several NH-aryl carbamate derivatives 13 combined with mefloquine hydrochloride substantially prolonged the survival times of malaria-infected mice (Scheme 1).31,32
Figure 2.
Representative 5-, 4-, 3-, and 2-carbon-linked dimer trioxanes
Scheme 1.
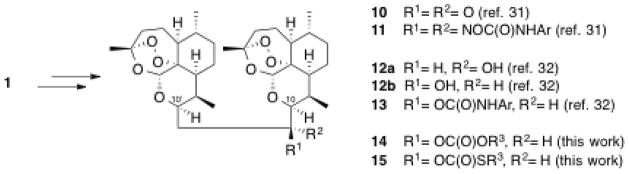
Two-carbon-linked dimer derivatives
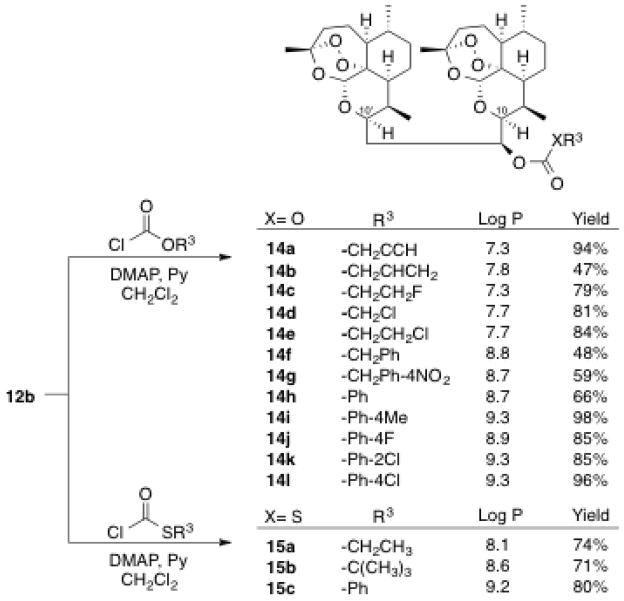
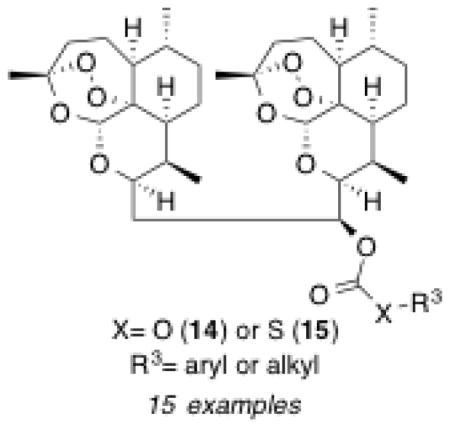
Encouraged by our recent results,31,32 we prepared a novel series of 2-carbon-linked dimer carbonates 14a–l and thiocarbonates 15a–c (Scheme 2). The log P values for all of these orally bioavailable dimer carbonates 14a–l and thiocarbonate 15a–c range between 7.3–9.3.33 The log P value of parent secondary alcohol 12b is 6.0.33 The log P of artemether (2) is 3.5.33 Facile conversion of parent secondary alcohol 12b was accomplished in one step from commercially available chloroformates and thiochloroformates, producing fifteen carbonates 14 and thiocarbonates 15 in moderate to high yields. In such cases where the purified product yield was less than 50% (14b and 14f), starting dimer alcohol was recovered. All carbonates 14 and thiocarbonates 15 were purified by chromatography on silica gel and their purity (> 95%) was established through normal phase HPLC analysis using an isocratic mobile phase (20% EtOAc in hexane).
Scheme 2.
Two-carbon-linked dimer carbonates 14a–l and thiocarbonates 15a–c
For preclinical drug development, in vivo efficacy data in mice (as shown in this manuscript) are more valuable and more stringent than in vitro potency data. Our experience with trioxanes over the past two decades supports the generalization that, within a family of antimalarially potent trioxanes, in vitro potency (IC-50) data often do not accurately predict in vivo efficacy levels. Therefore, we chose to evaluate our new antimalarial trioxane dimers 14 and 15 directly by oral administration in vivo. Stock solutions were prepared by dissolving mefloquine hydrochloride (2.16 mg) in 113 μL of 7:3 Tween 80:ethanol. Then 0.72 mg of either a dimer carbonate 14 or a dimer thiocarbonate 15 was added. After approximately 18 hours at room temperature, 1067 μL of deionized water was added, and then 200 μL of this stock solution was administered by oral gavage one day post infection to 5-week old C57BL/6J male mice (from Jackson Laboratory) that weighed approximately 20 g, which had been infected with P. berghei ANKA strain (2 × 107 parasitized erythrocytes). Each mouse (four mice per group) was treated orally with a single 200 μL dose of stock solution, corresponding to a dose of 6 mg/kg of trioxane dimer in combination with 18 mg/kg of mefloquine hydrochloride. As expected, all 2-carbon-linked dimer carbonates 14 and thiocarbonates 15 produced antimalarial chemotherapeutic results. The mouse survival data are shown in Table 1.
Table 1.
In vivo antimalarial efficacy using a single oral dose of trioxane dimer (6 mg/kg) combined with mefloquine hydrochloride (18 mg/kg) in P. berghei ANKA-infected mice
| trioxane | survival after infection (days) | avg survivala | % parasitemia suppressionb |
|---|---|---|---|
| 12b | 14, 20, 21, 23 | 19.5 | >99.9 |
| 14a | 14, 15, 15, 30 | 18.5 | >99.9 |
| 14b | 13, 23, 30, 30 | 24 | >99.9 |
|
| |||
| 14d | 23, 21, 23, 30 | 24.3 | >99.9 |
|
| |||
| 14f | 21, 14, 15, 20 | 17.5 | >99.9 |
| 14g | 23, 21, 21, 30 | 23.8 | >99.9 |
| 14h | 21, 20, 23, 30 | 23.5 | >99.9 |
| 14i | 30, 23, 14, 30 | 24.3 | >99.9 |
| 14j | 27, 20, 21, 30 | 24.5 | >99.9 |
| 14k | 30, 20, 14, 23 | 21.8 | >99.9 |
|
| |||
| 15a | 23, 20, 21, 29 | 23.3 | >99.9 |
|
| |||
| 15c | 15, 23, 30, 30 | 24.5 | >99.9 |
| controls: | |||
| vehicle (no drug) | 6, 7, 7, 8 | 7 | 0c |
| artemether (2) 6 mg/kg + mefloquine HCl 18 mg/kg | 23, 27, 13, 23 | 21.5 | >99.9 |
| mefloquine HCl 18 mg/kg alone | 13, 15, 23, 20 | 17.8 | >99.9 |
Best results are in bold type.
Denotes determination on day 3 after infection.
An average of 10% parasitemia was determined on day 3 after infection.
Parasitemia levels were evaluated on day 3 after infection and showed >99.9% suppression in all trioxane dimer-treated mice, indicating very rapid and high antimalarial activity. In contrast, the control mice (infected but no drug) had an average of 10% (9, 10, 11, 10) parasitemia on day 3 after infection, which resulted in their death on an average of 7 days (6, 7, 7, 8) after infection. All trioxane dimers 14 and 15 (with the exception of the two carbonates 14a and 14f) displayed average survival times longer than that of the antimalarial drug artemether (2, 21.5 day average survival). Mefloquine hydrochloride alone (18 mg/kg) prolonged mouse survival until only day 18. Fluoroethyl carbonate 14c (26 days), chloroethyl carbonate 14e (28 days), and chlorophenyl carbonate 14l (26 days) had average survival times notably longer than that of artemether (2, 21.5 days). Most significantly, 2-carbon-linked dimer thiocarbonate 15b prolonged survival of all four of the malaria-infected mice till at least day 30 using only one 6 mg/kg oral dose of this trioxane dimer. Mouse survival until day 30 with no parasitemia detected is a widely accepted measure of a drug’s antimalarial efficacy and indicates a complete cure. Day 30 survival with no parasitemia and with normal behavior and appearance was achieved in one of four mice treated with fluoroethyl carbonate dimer 14c, with chloroethyl carbonate dimer 14e, and with chlorophenyl carbonate dimer 14l. All four mice treated with thiocarbonate dimer 15b survived till at least day 30, at which time one of the four mice in this group had no detectable parasitemia and behaved normally and looked healthy. As determined by analytical thin layer chromatography, thiocarbonate 15b is stable at pH 2 for at least 24 hours at 37 °C. Several sulfur-containing antimalarial trioxanes have been reported recently.13,21,22,27,29,34
In conclusion, it is noteworthy that the partially curative carbonates 14c, 14e, 14l and thiocarbonate 15b all had average survival times (26–30 days) which were considerably higher than the average survival times of their parent dimer secondary alcohol 12b (19.5 days). This preclinical drug development result suggests that these orally bioavailable dimer carbonates and thiocarbonates act as new chemical entities and possibly as prodrugs35 of the parent dimer alcohol 12b. Dimer secondary alcohol 12b represents a versatile platform for preparation of other derivatives.
Supplementary Material
Acknowledgments
We thank NIH (Grant R37 AI 34885 to G.H.P.), The Johns Hopkins Malaria Research Institute, and the Bloomberg Family Foundation for financial support.
Abbreviations
- SAR
structure-activity relationship
- ACT
artemisinin combination therapy
- HPLC
high performance liquid chromatography
- DMAP
4-dimethylaminopyridine
- Py
pyridine
Footnotes
Supplementary data (experimental and tabular spectral data) associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.World Malaria Report 2013. World Health Organization; Geneva Switzerland: 2013. [accessed 28 January 2014]. ( http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/index.html. [Google Scholar]
- 2.Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Hqqaring D, Fullman N, Naghavi M, Lozono R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 3.Gulland A. Brit Med J. 2012;344:895. [Google Scholar]
- 4.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. PLoS Med. 2012;9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz L, Brown GV, Genton B, Moorthy VS. Malaria Journal. 2012;11:1–22. doi: 10.1186/1475-2875-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thera MA, Plowe CV. Annu Rev Med. 2012;63:345–357. doi: 10.1146/annurev-med-022411-192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olliaro PL, Boland PB. Clinical Public Health Implications of Antimalarial Drug Resistance. In: Rosenthal PJ, editor. Antimalarial Chemotherapy: Mechanisms of Action, Resistance and New Directions in Drug Discovery. Humana Press; Totowa, NJ: 2001. pp. 65–84. [Google Scholar]
- 8.Schlitzer M. ChemMedChem. 2007;2:944–986. doi: 10.1002/cmdc.200600240. [DOI] [PubMed] [Google Scholar]
- 9.Miller LH, Su X. Cell. 2011;146:855–853. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidelines for Treatment of Malaria. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 11.Welcome to Coartem.com the Novartis Malaria Initiative Website. Coartem. [accessed January 30, 2014]; http://http://www.coartem.us.com/info/coartem/what-is-coartem.jsp.
- 12.MMV. [accessed January 30, 2014];Pyramax (Pyronaridine-Artesunate) http://http://www.mmv.org/research-development/rd-portfolio.
- 13.Haynes RK, Fugmann B, Stetter J, Rieckmann K, Hans-Dietrich H, Chan H–W, Cheug M–K, Lam W–L, Wong H–N, Croft SL, Vivas L, Rattry L, Stewart L, Peters W, Robinson BL, Edstein MD, Kotecka B, Kyle DE, Beckermann B, Gerisch M, Radtke M, Schmuck G, Steink W, Wollborn U, Schmeer K, Romer A. Angew Chem, Int Ed. 2006;45:2082. doi: 10.1002/anie.200503071. [DOI] [PubMed] [Google Scholar]
- 14.Pacorel B, Leung SC, Stachulski AV, Davis J, Viva L, Lander H, Ward SA, Kaiser M, Brun R, O’Neill PM. J Med Chem. 2010;53:633. doi: 10.1021/jm901216v. [DOI] [PubMed] [Google Scholar]
- 15.Chadwick J, Jones M, Mercer AE, Stock PA, Ward SA, Park BK, O’Neill PM. Bioorg Med Chem. 2010;18:2586. doi: 10.1016/j.bmc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Jung M, Lee K, Kendrick H, Robinson BL, Croft SL. J Med Chem. 2002;45:4940. doi: 10.1021/jm020244p. [DOI] [PubMed] [Google Scholar]
- 17.See also: Wang X, Dong Y, Wittlin S, Charman SA, Chiu FCK, Chollet J, Katneni K, Mannila J, Morizzi J, Ryan E, Scheurer C, Steuten J, Tomas JS, Snyder C, Vennerstrom JL. J Med Chem. 2013;56:2547. doi: 10.1021/jm400004u.
- 18.Winter RW, Kelly JX, Smilkstein MJ, Dodean R, Bagby GC, Rathbun RK, Levin JI, Hinrichs D, Riscoe MK. Experimental Parasitology. 2006;114:47. doi: 10.1016/j.exppara.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Posner GH, Ploypradith P, Parker MH, O’Dowd H, Woo S-H, Northrop J, Krasavin M, Dolan P, Kensler TW, Xie S, Shapiro TA. J Med Chem. 1999;42:4275. doi: 10.1021/jm990363d. [DOI] [PubMed] [Google Scholar]
- 20.Moon DK, Sighal V, Kumar N, Shapiro TA, Posner GH. Drug Development Research. 2010;71:76. doi: 10.1002/ddr.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slack RD, Mott BT, Woodard LE, Tripathi A, Sullivan D, Nenortas E, Girdwood SCT, Shapiro TA, Posner GH. J Med Chem. 2012;55:291. doi: 10.1021/jm201214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobine AM, Mazzone JR, Slack RD, Tripathi AK, Sullivan DJ, Posner GH. J Med Chem. 2012;55:7892. doi: 10.1021/jm3009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slack RD, Jacobine AM, Posner GH. Med Chem Commun. 2012:3. doi: 10.1021/jm3009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woerdenbag HJ, Moskal TA, Pras N, Malingre TM, Elferaly FS, Kampinga HH, Konings AWT. J Nat Prod. 1993;56:849. doi: 10.1021/np50096a007. [DOI] [PubMed] [Google Scholar]
- 25.Posner GH, Chang W, Hess L, Woodard L, Sinishtaj S, Usera AR, Maio W, Rosenthal AS, Kalinda AS, D’Angelo JG, Petersen KS, Stohler R, Chollet J, Santo-Tomas J, Snyder C, Rottmann M, Wittlin S, Brun R, Shapiro TA. J Med Chem. 2008;51:1035. doi: 10.1021/jm701168h. [DOI] [PubMed] [Google Scholar]
- 26.Chadwick J, Mercer AE, Park BK, Cosstick R, O’Neill PM. Bioorg Med Chem. 2009;17:1325. doi: 10.1016/j.bmc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Moon DK, Tripathi A, Sullivan D, Siegler MA, Parkin S, Posner GH. Bioorg Med Chem Lett. 2011;21:2773. doi: 10.1016/j.bmcl.2010.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paik IH, Xie S, Shapiro TA, Labonte T, Narducci-Sarjeant AA, Baege AC, Posner GH. J Med Chem. 2006;49:2731. doi: 10.1021/jm058288w. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal AS, Chen X, Liu JO, West DC, Hergenrother PJ, Shapiro TA, Posner GH. J Med Chem. 2009;52:1198. doi: 10.1021/jm801484v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodard LE, Chang W, Chen X, Lui JO, Shapiro TA, Posner GH. J Med Chem. 2009;52:7458. doi: 10.1021/jm9005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mott BT, Tripathi A, Siegler MA, Moore CD, Sullivan DJ, Posner GH. J Med Chem. 2013;56:2630–2641. doi: 10.1021/jm400058j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conyers RC, Mazzone JR, Siegler MA, Tripathi AK, Sullivan DJ, Mott BT, Posner GH. Bioorg Med Chem Lett. 2014;24:1285. doi: 10.1016/j.bmcl.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Log P values for artemether (2), parent secondary dimer alcohol 12b, and 2C-linked trioxane dimers 14a–l, and 15a–c were calculated using MarvinSketch version 5.12.3.
- 34.Waknine-Grinberg JH, Hunt N, Bentura-Marciano A, McQuillan JA, Chan H–W, Chan WC, Barenholz Y, Haynes RK, Golenser J. Malaria Journal. 2010;9 doi: 10.1186/1475-2875-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfe AL, Duncan KK, Parelkar NK, Weir SJ, Vielhauer GA, Boger DLJ. Med Chem. 2012;55:5878. doi: 10.1021/jm300330b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.