Abstract
Polyvagal theory has influenced research on the role of cardiac vagal control, indexed by respiratory sinus arrhythmia withdrawal (RSA-W) during challenging states, in children’s self-regulation. However, it remains unclear how well RSA-W predicts adaptive functioning (AF) outcomes and whether certain caveats of measuring RSA (e.g., respiration) significantly impact these associations. A meta-analysis of 44 studies (n = 4,996 children) revealed small effect sizes such that greater levels of RSA-W were related to fewer externalizing, internalizing, and cognitive/academic problems. In contrast, RSA-W was differentially related to children’s social problems according to sample type (community vs. clinical/at-risk). The relations between RSA-W and children’s AF outcomes were stronger among studies that co-varied baseline RSA and in Caucasian children (no effect was found for respiration). Children from clinical/at-risk samples displayed lower levels of baseline RSA and RSA-W compared to children from community samples. Theoretical/practical implications for the study of cardiac vagal control are discussed.
Keywords: Cardiac vagal tone, RSA withdrawal, children, meta-analysis, adaptive functioning
Over the last twenty years a significant body of work across developmental and clinical psychology has identified self-regulation skills, particularly emotion regulation, as critical for children’s adaptive functioning across various domains including behavioral, social, and cognitive/academic (Baumeister & Vohs, 2004; Blair, 2002; Calkins & Fox, 2002; Graziano, Reavis, Keane, & Calkins, 2007; Shaw, Keenan, Vondra, Delliquadri, & Giovannelli, 1997). While definitions vary, most researchers agree that emotion regulation involves efforts to modulate emotional arousal in a way that facilitates adaptive functioning (Calkins, 1997; Garber & Dodge, 1991; Keenan & Shaw, 2003). Given the importance of emotion regulation for children’s adaptive functioning, it is not surprising that researchers have attempted to identify biological markers associated with emotion regulation. Of interest to the current paper is the maturation of the parasympathetic branch of the autonomic nervous system (PNS) which has been identified as a critical factor in supporting the development of increasingly sophisticated biobehavioral regulation processes (Calkins, 2007; Porges, 2007). Specifically, cardiac vagal tone–an index of the parasympathetic influence on the heart–has emerged as a psychophysiological marker for emotion regulation in both children and adults (Beauchaine, 2001; Calkins, 2007; Grossman & Taylor, 2007; Porges, 2007; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996).
Respiratory sinus arrhythmia (RSA), a component of heart rate variability, has emerged as a non-invasive measure of the functional output of the vagal pathways on the heart. RSA, under controlled respiratory conditions, is relatively uninfluenced by variations in sympathetic activity, and provides a sensitive index of cardiac vagal tone, even when alterations in parasympathetic activity are small (Grossman, Stemmler, & Meinhardt, 1990). Polyvagal Theory (Porges, 1995; 2003a; 2003b; 2007) is regarded as the most influential model in differentiating the relation between vagal tone during steady states (i.e., baseline vagal tone) and vagal reactivity (i.e., vagal regulation) in response to environmental challenges. Baseline measures of vagal tone represent an organism’s ability to maintain homeostasis and the potential responsiveness of that organism. During such restful periods, the vagus exerts an inhibitory influence on the heart acting as a “brake” by increasing vagal output to the sino-atrial (SA) node of the heart and limiting sympathetic influences which contributes to a steady slow heart rate. On the other hand, during stressful periods, the vagal “brake” is disengaged resulting in a decrease in vagal output to the SA node of the heart and thus contributing to an increase in heart rate (Porges et al., 1996).
Individual differences in the regulation of the vagal “brake” are assessed by measuring changes in vagal tone from baseline to an attention-demanding or challenging state. Vagal regulation can refer to a suppression in RSA during a challenging state (i.e., vagal tone decreases from baseline to challenging task, indicative of a positive vagal regulation score) or to an augmentation in RSA (i.e., vagal tone increases from baseline to challenging task, indicative of a negative vagal regulation score). According to Polyvagal theory, however, successful vagal regulation is marked by RSA suppression or withdrawal, which is thought to facilitate an organism’s ability to cope with challenging states by mediating metabolic output via heart rate increases (Porges, 2003a, 2007; Porges et al., 1996). Indeed, research has shown that greater levels of RSA withdrawal are associated with better self-regulation and active coping skills as well as observed emotion regulation during frustrating/stressful tasks (Degangi, Dipietro, Greenspan, & Porges, 1991; Gentzler, Santucci, Kovacs, & Fox, 2009; Huffman et al., 1998). What remains unclear, and of interest to the current paper, is the extent to which vagal or RSA withdrawal contributes to more complex adaptive functioning outcomes and whether RSA withdrawal predicts certain outcomes better than others.
Vagal Withdrawal and Social Functioning
Perhaps the most widely cited area of adaptive functioning in which the role of vagal withdrawal has been implicated is within the social domain. As noted by Porges (2003a), social interactions arguably require considerable neural and physiological involvement due to the range of tasks required for a successful interaction (e.g., ability to maintain eye contact/gaze with another person, attend to his/her vocalizations/language, interpret his/her vocalizations/language, observe his/her facial expressions, and discern the other person’s overall affect and intent, as well as the ability to successfully initiate your own appropriate verbal and non-verbal responses in an appropriate amount of time). Porges’ Social Engagement System highlights that the vagal system, which originally served as a neural circuit for controlling fight or flight amygdalar mechanisms on the sympathetic nervous system and the stress response via the hypothalamic-pituitary-adrenal (HPA) system, over time became integrated with the nuclei that controls the muscles of the face and head (2003b). Subsequently, a well-regulated and calm visceral state may contribute to better control of facial/head muscles that enables complex facial gestures, vocalizations, social gesturing, and orientation, which are thought of as important behaviors for engaging in social communication.
Despite the theoretical link between RSA withdrawal and social functioning, most of the empirical research has focused only on baseline RSA. For example, children with Autism Spectrum Disorders (ASD) have been observed to have low baseline RSA compared to healthy controls (Ming, Julu, Brimacombe, Connor, & Daniels, 2005; Patriquin, Scarpa, Friedman, & Porges, 2011; Vaughan Van Hecke et al., 2009). Similarly within non-ASD samples and as noted by Beauchaine (2001), high levels of baseline RSA have been associated with uninhibited behavior, assertiveness, sociability, and social competence. However, significantly less research has examined the link between RSA withdrawal and social competence. Examining this link is particularly important to solidify the engagement-disengagement function of the vagal brake as it relates to socially relevant behaviors versus a general level of responsiveness indexed by baseline RSA. Additionally, the results of the few studies that have examined the link between RSA withdrawal and social functioning have been mixed. For instance, in a sample of kindergarteners, Graziano and colleagues (2007) found that higher levels of RSA withdrawal (which is calculated by subtracting children’s RSA score during a challenging task from children’s RSA score during a resting or baseline task) were associated with higher social preference scores. On the other hand, Blair (2003) found an inverse relation between RSA withdrawal and social competence as reported by teachers. Hence, both theoretical and empirical reasons highlight the importance of conducting a meta-analytic review to more accurately determine the viability of vagal withdrawal as a physiological mechanism important for social engagement.
Vagal Withdrawal and Externalizing Behavior Problems
Vagal withdrawal is also thought to facilitate metabolic and regulatory processes important for attention and behavioral control strategies. Children with externalizing behavior problems such as aggression, hyperactivity, and inattention are more likely to have emotion regulation and behavioral control difficulties such as impulsivity (Gilliom & Shaw, 2004; Keenan & Shaw, 2003). A pattern of physiological dysregulation in the form of both lower sympathetic activity and lower levels of vagal withdrawal may underlie children with externalizing behavior problems’ ability to cope with stress and challenging situations (Mezzacappa et al., 1997; Pine et al., 1998). Indeed, a fair number of studies have found that children with externalizing behavior problems display a more blunted vagal response to challenge (Beauchaine, 2001; Boyce et al., 2001; Calkins & Dedmon, 2000; Musser et al., 2011). On the other hand, some studies have failed to find a link between RSA withdrawal and externalizing problems (Beauchaine, Gatzke-Kopp, & Mead, 2007; Eisenberg et al., 2012; Erath, Tu, & El-Sheikh, 2011) with such a link further attenuated by the presence of co-occurring internalizing problems (Calkins, Graziano, & Keane, 2007) or only occurring in the presence of a moderating environmental factor such as attachment status (Willemen, Schuengel, & Koot, 2009).
Vagal Withdrawal and Cognitive/Academic Problems
Negative emotionality is known to interfere with higher order cognitive processes such as executive functioning (Blair, 2002; Posner & Rothbart, 2007). The physiological mechanism responsible for such interference remains relatively understudied in children, although there is a well-documented overlap in the brain structures (e.g., anterior cingulated cortex and orbitofrontal cortex) responsible for regulating both emotions and higher order cognitive processes (Bush, Luu, & Posner, 2000; Levesque et al., 2004). A child with good emotion regulation skills who has a history of using such physiological system efficiently during challenging or stressful situations should theoretically have an even easier time recruiting the orbitofrontal cortex for higher order cognitive functions during less stressful situations. Initial studies do show that children with higher vagal withdrawal scores, indicative of better emotion regulation, perform better on cognitive and academic tasks (Blair & Peters, 2003; Katz & Gottman, 1997; Quas, Bauer, & Boyce, 2004). Once again, however, these findings are equivocal as some studies do not find such an association (Quas, Carrick, Alkon, Goldstein, & Boyce, 2006) or find a more quadratic association (Marcovitch et al., 2010). Hence, it may be more adaptive for children to have a lower RSA withdrawal response to less emotionally based cognitive or academic tasks which may free up metabolic resources for attention and memory processes important for the task at hand. Children that may perceive a cognitive or academic task as threatening or high in stress may overly engage and disengage the vagal brake or sustain such RSA withdrawal for an unnecessary period of time. Chronic periods of unnecessary regulation can lead to increases in stress and be maladaptive (Schubert, Lambertz, Nelesen, Bardwell, Choi, & Dimsdale, 2009). Given the mixed empirical findings and possible non-linear association between RSA withdrawal and children’s cognitive/academic functioning, a meta-analytic review would help determine the role of vagal withdrawal as a physiological mechanism important for cognitive/academic functioning.
Vagal Withdrawal and Internalizing Problems
The role of vagal withdrawal in contributing to internalizing problems is perhaps the least understood and examined adaptive functioning domain. Some researchers have suggested that an extreme vagal withdrawal response contributes to a sense of over vigilance and one in which the individual is paying too much attention to potential threats in the environment. Such an over reactivity contributes to physical and cognitive internalizing symptoms such as stress/anxiety and worry (Thayer & Lane, 2000). On the other hand, some researchers have argued that low reactivity (i.e., low vagal withdrawal) would restrict an individual’s ability to cope with a stressful situation and/or differentiate such stress from non-stressful (i.e., baseline or resting) times (Schmitz, Kramer, Tuschen-Caffier, Heinrichs, & Blechert, 2011). Indeed, findings are mixed on whether higher vagal withdrawal scores relate to lower (Gentzler et al., 2009; Pearson et al., 2005; Schmidt, Fox, Schulkin, & Gold, 1999) or higher (Boyce et al., 2001; Hastings, Sullivan, et al., 2008; Hinnant & El-Sheikh, 2009) levels of internalizing symptoms.
Measurement Issues Associated with RSA
There is a significant debate over the methodology that is required to accurately measure children’s RSA as an estimate of vagal tone (see Allen, Chambers, & Towers, 2007; Denver, Reed, & Porges, 2007; and Grossman & Taylor, 2007 for thorough reviews). Studies vary on how RSA is quantified with some using spectral analysis (Miskovic, Schmidt, Georgiades, Boyle, & MacMillan, 2009; Scheinin et al., 1999; Wolff, Wadsworth, Wilhelm, & Mauss, 2012) while others use time-domain peak valley analysis (El-Sheikh & Whitson, 2006; Hinnant & El-Sheikh, 2009; Willemen et al., 2009) or the Porges adaptive polynomial filter method (Calkins et al., 2007; Graziano, Keane, et al., 2007; Hastings, Nuselovici, et al., 2008; Porges et al., 1996). The Society for Psychophysiological Research’s Task Force committee concluded that no single measure for quantifying RSA can be viewed as the gold standard as each of approaches offers advantages and disadvantages (Berntson et al., 1997). The main source of debate is regarding how much respiration confounds RSA as an estimate of cardiac vagal tone. Proponents of the peak-to-valley method and using respiratory parameters as covariates in statistical analyses assume a causal relation between respiration and RSA whereas proponents of using either respiratory frequency or global frequency bands associated with breathing rates to identify frequency component in the heart rate spectrum assume a parallel relation (Denver et al., 2007).
One line of evidence offered by Grossman and Taylor (2007) indicate that across 20 studies and regardless of method used to derive RSA, respiration rates and tidal volume significantly affect RSA magnitude. Additionally, frequency domain methods such as spectral analysis are less affected by respiration rates compared to methods that assess the amplitude of RSA oscillations (Grossman, van Beek, & Wientjes, 1990). Grossman and Taylor (2007) along with Allen and colleagues (2007) also argue that statistically controlling for respiratory parameters as covariates in between subjects designs (e.g., ANCOVAs) does not “solve” the problem of the impact of respiration on RSA. The use of ANCOVAs is problematic for various reasons including that the associations between respiratory measures and RSA are much stronger within individuals versus between individuals (Ben Lamine et al., 2004). The use of ANCOVAs may also create spurious group effects or remove an actual effect of interest (see Miller & Chapman, 2001). A better option is to regress RSA against respiration variables and use the residuals as an index of vagal tone (Grossman et al., 1991).
On the other hand, Denver and colleagues (2007) offer evidence that while respiration frequency strongly relates to the frequency of RSA, it minimally impacts the amplitude of RSA (2–10% of variance). They also show evidence that a time domain method that extracts only the heart rate variability within a defined band of breathing frequencies (i.e., Porges adaptive filter method), is equivalent to spectral analyses. In addition, Denver and colleagues (2007) show across a review of 13 studies that statistically adjusting for respiration parameters including tidal volume or respiration frequency did not change any of the results. More recently, an analysis by Lewis and colleagues (2012) comparing the Porges adaptive filter method, peak-to-through method, and spectral analysis methods (which were all highly correlated) showed that the frequency band (.12–.40 Hz) used in the Porges adaptive filter method to define RSA in adults can accurately capture respiration rates (less than 1% of individual breaths fell outside this frequency band). More importantly, compared to the other two methods, the Porges adaptive filter method was significantly more sensitive to vagal mechanisms (effect sizes were reported as more than 3 times greater than the other two methods) as evidenced through a glycopyrrolate infusion. It remains unclear; however, the extent to which the associations between RSA withdrawal and children’s adaptive functioning outcomes differ between studies that actually measure respiration versus those that use normative rates (i.e., frequency bands such as Porges adaptive polynomial filter method).
Another important measurement issue to consider when examining RSA withdrawal is whether one should control for baseline or resting levels of RSA. As a marker of potential responsiveness, baseline RSA impacts the magnitude of RSA withdrawal that may occur during challenging situations (Beauchaine, 2001; Calkins, Graziano, Berdan, Keane, & Degnan, 2008; El-Sheikh, 2005; Graziano, Keane, et al., 2007; Porges, 2007). The law of initial values also suggests that participants with higher baseline levels of a physiological measure have a greater potential for decreases in that measure (Lacey & Lacey, 1962; Wilder, 1956). However, as pointed out by Beauchaine (2001) studies have varied in terms of whether baseline RSA levels are co-varied when examining links between RSA withdrawal and adaptive functioning outcomes.
Lastly, studies vary in terms of which types of challenging tasks are used to derive RSA withdrawal values ranging from cognitively challenging tasks to more socially oriented or negative mood/stress inductions. While some studies have demonstrated that cognitively or frustrating challenging tasks incur greater RSA withdrawal levels compared to positive or neutral mood induction tasks (Calkins et al., 2007; Moore, 2009; Musser et al., 2011), it is not clear whether the type of challenging task actually affects any potential associations with adaptive functioning outcomes. This is a particularly important area to investigate as the adaptive nature of RSA withdrawal may be contingent in the context children are in. For example, while a stressful or threat perceiving context may call for a reduction or suppression of RSA in order to active cardiac activity and mobilize metabolic resources to deal with the stress/threat, a more positive or threat free context may not require prolonged decreases in RSA and in fact augmentation of RSA may be more adaptive (Hastings, Nuselovici, et al., 2008).
Goals of the Current Meta-Analysis
In summary, while vagal withdrawal has emerged as a biological marker for children’s emotion regulation abilities, it remains unclear the extent to which it equally predicts various adaptive functioning domains. From a developmental psychopathology perspective, it is important to understand whether vagal withdrawal deficits act as a broad risk factor for various maladaptive outcomes or only certain ones. It is also important to note that several caveats for measuring RSA has been recently highlighted (Allen, Chambers, & Towers, 2007; Denver, Reed, & Porges, 2007; and Grossman & Taylor, 2007) which may impact associations between RSA withdrawal and adaptive functioning including the measurement of respiration, co-varying baseline RSA, as well as the type of challenging task used to derive RSA withdrawal.
The goal of the current study is to conduct a meta-analysis to examine a) the magnitude of the associations between RSA withdrawal and children’s adaptive functioning outcomes, b) determine any differences in the magnitude of these associations according to adaptive functioning domain, and c) determine the extent to which measurement issues related to RSA (i.e., respiration rates, co-varying of baseline RSA, type of challenging task) impacts the association between RSA withdrawal and adaptive functioning outcomes. The only meta-analyses conducted as it relates to RSA has been linking baseline RSA with depression in adults (Rottenberg, 2007) or baseline RSA and childhood outcomes (Beauchaine, 2001). Based on Polyvagal and the Social Engagement System (2003b, 2007; 1996) along with significant research showing a strong association between emotional control and behavioral control (Keenan & Shaw, 2003), we expected that the strongest associations between RSA withdrawal and adaptive functioning outcomes would occur within the social competence and externalizing behavior functioning domains. Given the neurobiological overlap between emotion and cognitive regulation (Bush et al., 2000), we also expected a positive association between RSA withdrawal and children’s cognitive/academic outcomes. We did not expect a strong association between RSA withdrawal and internalizing symptoms given contradicting views on whether children with internalizing symptoms overregulate (i.e., extreme RSA withdrawal) or have difficulty coping with stress (i.e., poor RSA withdrawal). Lastly, given the caveats of measuring RSA, we expected stronger associations between RSA withdrawal and children’s adaptive functioning outcomes in studies that measured children’s respiration, co-varied baseline RSA, and used more cognitively based challenging tasks.
Method
Literature Review
We conducted a comprehensive search for empirical research regarding the relation between RSA withdrawal and children’s adaptive functioning outcomes over the last 25 years (since the early publications leading to Polyvagal theory), using PsychINFO (1986–2012), Science Citation Index Expanded (1986–2012), Social Sciences Citation Index (1986–2012), Arts & Humanities Citation Index (1986–2012), Google Scholar (1986–2012), and MEDLINE (1986–2012). The terms used in the search included RSA withdrawal, RSA suppression, RSA reactivity, vagal reactivity, vagal regulation, vagal suppression, vagal control, vagal withdrawal, vagal tone, cardiac autonomic regulation, physiological regulation, parasympathetic flexibility, and heart rate variability. These terms were crossed with terms related to children’s adaptive functioning, including social functioning, social competence, peer relations, behavior problems, disruptive behavior, externalizing problems, internalizing problems, anxiety, depression, cognitive functioning, executive functioning, neurocognitive functioning, academic functioning, and school functioning. In addition to the database search, references used in identified studies and review articles were surveyed to identify other potentially relevant studies. Due to the extensive number of studies identified through database search and study ancestry, unpublished data were not utilized for the present analyses.
Inclusion and Exclusion Criteria
Forty-four studies satisfied the inclusion criteria. Publication years of the identified studies ranged from 1992 to 2012. Our inclusion criteria were liberal in terms of the design of the studies as the main goal of this meta-analysis was to determine the magnitude of any associations between RSA withdrawal and children’s adaptive functioning outcomes. Studies had to report an RSA withdrawal variable which means that RSA values had to be calculated during a baseline period as well as during a challenging task. Studies could either compare RSA withdrawal scores between a clinical (e.g., children displaying clinically elevated behavioral problems) and a control group (e.g., children displaying normative levels of behavior problems) or use correlational methods to examine concurrent or longitudinal associations between RSA withdrawal and adaptive functioning outcomes among a large community sample or at-risk group (e.g., Head Start). In addition, sufficient statistical data to allow the calculation of effect sizes had to be present (e.g., correlational tables, regressions, test statistics such as t-test, ANOVA, etc.). Articles written in languages other than English were excluded.
Additionally, since our focus was on four main adaptive functioning outcomes (i.e., externalizing problems, internalizing problems, social problems, and cognitive/academic problems), over 60 articles examining relations between RSA withdrawal and other outcomes were excluded. For example, articles which examined RSA withdrawal and children’s health outcomes such as obesity (Graziano, Calkins, Keane, & O'Brien, 2011), immune response (Cacioppo, 1994), asthma (Miller, Wood, Lim, Ballow, & Hsu, 2009) or epilepsy (Harnod et al., 2008) were excluded. We also excluded articles examining the link between RSA withdrawal and sleep (El-Sheikh & Buckhalt, 2005), substance exposure (Hickey, Suess, Newlin, Spurgeon, & Porges, 1995; Sheinkopf et al., 2007) or parenting factors (Blandon, Calkins, Keane, & O'Brien, 2010; Calkins & Johnson, 1998; Mills-Koonce et al., 2009; Oosterman & Schuengel, 2007). Articles that solely examined the stability of RSA withdrawal (Bornstein & Suess, 2000; El-Sheikh, 2005), or changes in RSA withdrawal associated with treatment (Beauchaine, Gartner, & Hagen, 2000; Graziano, Bagner, Sheinkopf, Vohr, & Lester, 2012) were also excluded. Lastly, we also excluded articles that examined the association of RSA withdrawal and temperamental indices of emotional responses (Calkins, Dedmon, Gill, Lomax, & Johnson, 2002). Because we were interested in only children’s adaptive functioning, any article in which the mean and/or range of age was over 18 was excluded.
Coding of Moderators
Studies were coded for several demographic and methodological features. Two judges (first and second authors) independently coded studies. Interjudge reliability was assessed via intraclass correlation coefficients (ICC) for continuous codes, and via kappas (κ) for categorical codes. When a discrepancy was found, both coders independently reviewed the study again and decided whether they would retain their original code or modify it. Remaining discrepancies were resolved through discussion between coders. The reliability between coders was excellent (all ICC values > .90 and κ values = 1.00).
Demographic variables
We coded studies for average age of sample, gender (% male), ethnicity (% Caucasian), time lag (time in years between RSA measure and adaptive functioning outcome), type of sample (community/healthy, n = 27 vs. clinic/at-risk population such as Head Start or comparison of community/healthy and clinic/at-risk groups, n = 18). We also coded studies for type of measurement of adaptive functioning used such as parent report, observational measure or peer report, teacher report, or multiple reports.
Measurement of RSA
As noted in the introduction, several caveats of measuring RSA have been recently recognized (Grossman & Taylor, 2007) which may impact any potential associations between RSA withdrawal and adaptive functioning outcomes. Hence, we coded studies for a) whether children’s respiration rates were actually measured (n = 12), estimated based on norms (n = 25) or not even mentioned (n = 8) as well as b) whether baseline RSA was co-varied in analyses of RSA withdrawal (n = 17) or not co-varied (n = 26). Additionally, we coded the type of challenging task used to derive RSA withdrawal scores: negative mood induction tasks such as listening to an argument (n = 13), cognitive tasks such as a solving a puzzle or memory game (n = 9), social tasks such as being rejected by a peer (n = 9), positive mood induction tasks (n = 2), and finally studies that used multiple types of tasks (n = 11).
Computation of Effect Sizes
For the Effect Size (ES) metric, Pearson’s r was used because of the correlational nature of most of the relevant studies. ES estimates were attained primarily from correlations between RSA withdrawal and adaptive functioning outcomes. However, when correlations were not provided, Pearson’s r was estimated from other available data sources, including group comparisons (t-tests), analyses of variance (ANOVAs), or means and standard deviations for extreme or tertiary groups assigned according to a criteria (e.g., comparing children with clinically elevated externalizing behavior problems versus those with normal rates of externalizing behavior problems). All effect size estimates and all transformations from other data sources to Pearson’s r were calculated according to the formulas provided in Lipsey and Wilson (2001). Negative ESs in the present meta-analysis indicated negative associations between RSA withdrawal and adaptive functioning difficulties (e.g., externalizing problems, social problems, internalizing problems, cognitive/academic problems).
Forty-four studies with adequate meta-analytic information were identified, yielding ESs for externalizing problems (24 studies, total n = 3,331), internalizing problems (18 studies, total n = 1,697), social problems (15 studies, total n = 1,969), and cognitive/academic problems (10 studies, total n = 1,025). In studies in which two or more pertinent dependent variables were used, such as two different measures of externalizing behavior problems or two different reporters, the average correlation was used. All averaged measures used in the current study were judged by both coders as being of approximately equal validity. Additionally, effect sizes from studies that used the same sample for investigating outcomes within the same domain (e.g., Sijsema et al., 2011 and Shoulberg et al., 2011 as well as Blair & Peters, 2003 and Blair, 2003) were also averaged. All study references appear in Appendix A.
Appendix A.
Coded Qualities and Weighted Effect Sizes of Studies of RSA Withdrawal and Children’s Adaptive Functioning Outcomes
| Demographic Variables | RSA Measurement Variables | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Mean Age in Years |
% of sample Male |
% of sample Caucasian |
Time Lag in Years |
Sample Type | Type of Task used for RSA |
Controlled for baseline RSA |
Respiration Rates |
Adaptive Functioning Domain Measured |
Effect Size |
N | |
| 1 | El-Sheikh, M., & Whitson, S. (2006) | 9.67 | 52 | 74 | 2.0 | Community/Healthy | negative emotion/stressor | Yes | Yes, actual measurement | Externalizing (P) Internalizing (P) |
−.11 −.29 |
133 |
| 2 | Calkins, S., & Dedmon, S.(2000) | 2.50 | 49 | 67 | 0 | Community/Healthy vs. Clinic/At-Risk groups | multiple tasks | Yes | Yes, via estimated norms | Externalizing (O) | −.28 | 99 |
| 3 | El-Sheikh, M., Harger, J., & Whitson, S. (2001) | 9.90 | 52 | 80 | 0 | Community/Healthy | negative emotion/stressor | Yes | Yes, actual measurement | Externalizing (P) Internalizing (P) |
−.30 −.34 |
75 |
| 4 | Whitson, S., & El-Sheikh. (2003) | 7.00 | 48 | 91 | 0 | Community/Healthy | negative emotion/stressor | No | Yes, actual measurement | Externalizing (P) Internalizing (P) |
−.11 −.06 |
64 |
| 5 | Katz, L., & Gottman, J. (1997)) | 4.50 | 57.00 | 96.00 | 3.00 | Community/Healthy | positive/reward task | No | Yes, via estimated norms | Externalizing (P) Social Problems (P) Cognitive/Academic (O) |
−.31 −.08 −.25 |
56 |
| 6 | Porges, S., Doussard-Roosevelt, J., Portales, L., & Greenspan, S. (1996) | .67 | 50.00 | 100.00 | 2.25 | Community/Healthy | cognitive task | No | No information provided | Externalizing (P) Internalizing (P) Social Problems (P) |
−.50 −.45 −.42 |
24 |
| 7 | Gottman, J., Katz, L., & Hooven, C. (1996) | 5.00 | 43.00 | 100.00 | 3.00 | Community/Healthy | positive/reward task | Yes | No information provided | Cognitive/Academic (O) | −.18 | 56 |
| 8 | Graziano, P., Keane, S., & Calkins, S. (2007) | 5.50 | 46.00 | 67.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | multiple tasks | Yes | Yes, via estimated norms | Externalizing (M) Social Problems (O) |
−.09 −.15 |
341 |
| 9 | Calkins, S., Graziano, P., & Keane, S. (2007) | 5.50 | 47.00 | 67.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | multiple tasks | No | Yes, via estimated norms | Externalizing (P) | −.11 | 335 |
| 10 | Marcovitch, S., Leigh, J., Calkins, S., Leerks, E., O’Brien, M., & Blankson, N. (2010) | 3.50 | 48.00 | 53.20 | .0 | Community/Healthy vs. Clinic/At-Risk groups | multiple tasks | No | No information provided | Cognitive/Academic (O) | −.07 | 220 |
| 11 | Blair, C., & Peters, R. (2003) | 4.75 | 45.00 | 69.00 | .0 | Clinic/At-Risk groups | cognitive task | No | Yes, actual measurement | Social Problems (T) Cognitive/Academic (T) |
.40 −.21 |
42 |
| 12 | Gentzler, A., Santucci, A., Kovacs, M., & Fox, N. (2009) | 7.93 | 54.00 | 56.90 | 1.50 | Community/Healthy vs. Clinic/At-Risk groups | negative emotion/stressor | Yes | Yes, via estimated norms | Internalizing (M) | −.36 | 65 |
| 13 | Calkins, S., & Keane, S. (2004) | 2.00 | 46.00 | 63.00 | 2.00 | Community/Healthy vs. Clinic/At-Risk groups | multiple tasks | Yes | Yes, via estimated norms | Externalizing (P) | −.21 | 125 |
| 14 | Boyce, T., Quas, J., Alkon, A., Smider, N., Essex, M., & Kupper, D. (2001) | 6.50 | 40.00 | 100.00 | .0 | Community/Healthy | multiple tasks | No | Yes, via estimated norms | Externalizing (M) Internalizing (M) |
−.46 .07 |
120 |
| 15 | Quas, J., Carrick, N., Alkon, A., Goldstein, L., & Boyce, T. (2006) | 6.30 | 55.00 | 52.00 | .0 | Community/Healthy | negative emotion/stressor | No | Yes, via estimated norms | Cognitive/Academic (O) | −.02 | 106 |
| 16 | Staton, L., El-Sheikh, M., & Buckhalt, J. (2009) | 10.03 | 56.00 | 95.00 | .0 | Community/Healthy | cognitive task | Yes | Yes, actual measurement | Cognitive/Academic (O) | −.24 | 41 |
| 17 | Quas, J., Bauer, A., & Boyce, T. (2004) | 5.16 | 54.00 | 71.00 | .0 | Community/Healthy | multiple tasks | No | Yes, via estimated norms | Cognitive/Academic (O) | −.18 | 57 |
| 18 | Obradovic, J., Bush, N., Stamperdahl, J., Adler, N., & Boyce, T. (2010) | 5.32 | 52.00 | 43.00 | .0 | Community/Healthy | multiple tasks | Yes | Yes, via estimated norms | Externalizing (P) Social Problems (P) Cognitive/Academic (O) |
−.10 .07 −.06 |
338 |
| 19 | Doussard-Roosevelt, J., Montgomery, L., & Porges, S. (2003) | 5.60 | 60.00 | 43.00 | .0 | Community/Healthy | negative emotion/stressor | Yes | Yes, via estimated norms | Social Problems (O) | −.40 | 30 |
| 20 | Crowell, S., Beauchaine, T., McCauley, E., Smith, C., Stevens, A., & Sylvers, P. (2005) | 15.30 | .0 | 74.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | negative emotion/stressor | No | No information provided | Internalizing (M) | −.11 | 46 |
| 21 | Leary, A., & Katz, L. (2004) | 5.00 | 66.00 | 88.00 | 4.00 | Community/Healthy | social task | No | Yes, via estimated norms | Social Problems (O) | −.03 | 73 |
| 22 | Hastings, P., Nuselovici, J., Utendale, W., Coutya, J., McShane, K., & Sullivan, C. (2008) | 3.48 | 57.00 | 76.00 | .83 | Community/Healthy | social task | Yes | Yes, via estimated norms | Externalizing (P) Internalizing (P) |
−.21 −.21 |
94 |
| 23 | Schmidt, L., Fox, N., Schulkin, J., & Gold, P. (1999) | 7.00 | 90.00 | .0 | Community/Healthy | negative emotion/stressor | No | No information provided | Internalizing (P) | −.31 | 36 | |
| 24 | Hastings, P., Sullivan, C., McShane, K., Coplan, R., Utendale, W., & Vyncke, J. (2008) | 3.50 | 46.00 | 74.00 | .0 | Community/Healthy | cognitive task | Yes | Yes, via estimated norms | Internalizing (P) | .03 | 133 |
| 25 | Scheeringa, M., Zeanah, C., Myers, L., & Putnam, F. (2004) | 100.00 | .0 | Clinic/At-Risk groups | cognitive task | No | Yes, via estimated norms | Internalizing (P) | −.12 | 124 | ||
| 26 | Hinnant, B., & El-Sheikh, M. (2009) | 8.68 | 44.00 | 69.00 | 2.00 | Community/Healthy | multiple tasks | Yes | Yes, actual measurement | Externalizing (P) Internalizing (P) |
−.09 .03 |
176 |
| 27 | El-Sheikh, M. (2001) | 9.47 | 51.00 | 63.00 | .0 | Community/Healthy | negative emotion/stressor | Yes | Yes, actual measurement | Externalizing (M) Internalizing (M) Social Problems (O) |
−.03 −.07 .05 |
216 |
| 28 | El-Sheikh, M., Hinnant, B., & Erath, S. (2011) | 8.23 | 49.00 | 64.00 | 3.00 | Community/Healthy | cognitive task | No | Yes, actual measurement | Externalizing (P) | −.09 | 251 |
| 29 | Blair, C. (2003) | 4.75 | 45.00 | 69.00 | .0 | Clinic/At-Risk groups | cognitive task | No | Yes, actual measurement | Social Problems (T) Cognitive/Academic (O) |
.40 −.23 |
42 |
| 30 | Willemen, A., Schuengel, C., Koot, H. (2009) | 13.57 | 100.00 | 100.00 | .0 | Clinic/At-Risk groups | negative emotion/stressor | No | Yes, via estimated norms | Externalizing (M) Internalizing (M) |
−.13 −.09 |
99 |
| 31 | Pearson, S., Alkon, A., Treadwell, M., Wolff, B., Quirolo, K., & Boyce, T. (2005) | 7.40 | 47.00 | 100.00 | .0 | Clinic/At-Risk groups | multiple tasks | No | Yes, via estimated norms | Externalizing (P) Internalizing (P) |
−.19 −.34 |
19 |
| 32 | Keller, P., & El-Sheikh, M. (2009) | 8.72 | 42.00 | 66.00 | 2.00 | Community/Healthy | multiple tasks | Yes | Yes, via estimated norms | Externalizing (P) | −.09 | 54 |
| 33 | Sijtsema, J., Shoulberg, E., Murray-Close, D. (2011) | 12.47 | .0 | 94.00 | .0 | Community/Healthy | social task | No | Yes, via estimated norms | Social Problems (M) | −.12 | 119 |
| 34 | Crowell, S., Beuchaine, T., Gatzke-Kopp, L., Sylvers, P., Mead, H., & Chipman-Chacon, J. (2006) | 4.60 | 58.00 | 71.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | cognitive task | No | Yes, via estimated norms | Externalizing (P) | .06 | 38 |
| 35 | Shoulberg, E., Sijtsema, J., & Murray-Close, D. (2011) | 12.43 | .0 | 94.00 | .0 | Community/Healthy | social task | No | Yes, via estimated norms | Social Problems (M) | −.12 | 119 |
| 36 | Beauchaine, T., Katkin, E., Strassberg, Z., & Snarr, J. (2001) | 13.40 | 100.00 | 100.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | social task | No | Yes, via estimated norms | Externalizing (P) | −.33 | 59 |
| 37 | Schmitz, J., Kramer, M., Tuschen-Caffier, B., Heinrichs, N., & Blechert, J. (2011) | 10.00 | 54.00 | 100.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | social task | No | Yes, via estimated norms | Internalizing (P) | −.24 | 56 |
| 38 | Musser, E., Backs, R., Schmitt, C., Ablow, J., Measelle, J., & Nigg, J. (2011) | 8.00 | 48.00 | 66.67 | .0 | Community/Healthy vs. Clinic/At-Risk groups | negative emotion/stressor | No | Yes, actual measurement | Externalizing (P) | −.23 | 66 |
| 39 | Erath, S., Tu, K., & El-Sheikh, M. (2011) | 11.67 | 51.00 | 62.00 | .0 | Community/Healthy | social task | No | No information provided | Externalizing (M) Internalizing (S) Social Problems (M) |
.10 −.09 −.12 |
63 |
| 40 | Stifter, C., Corey, J. (2001) | 1.00 | 48.50 | 91.80 | .0 | Community/Healthy | cognitive task | No | Yes, via estimated norms | Social Problems (O) | −.32 | 136 |
| 41 | Gazelle, H., & Druhen, M.(2009) | 8.66 | 41.00 | 62.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | social task | No | Yes, via estimated norms | Externalizing (O) Internalizing (O) Social Problems (O) |
−.07 .00 −.01 |
154 |
| 42 | Eisenberg, N., Sulik, M., Spinrad, T., Edwards, A., Eggum, N., Liew, J., et al. (2012) | 1.48 | 55.80 | 84.00 | 3.00 | Community/Healthy | negative emotion/stressor | Yes | Yes, actual measurement | Externalizing (M) | .07 | 213 |
| 43 | Liew, J., Eisenberg, N., Spinrad, T., Eggum, N., Haugen, R., Kupfer, A., et al. (2011) | 1.48 | 55.80 | 84.00 | 1.00 | Community/Healthy | negative emotion/stressor | Yes | Yes, actual measurement | Social Problems (O) | −.20 | 216 |
| 44 | Haley, D., Grunau, R., Weinberg, J., Keidar, A., & Oberlander, T. (Haley, Grunau, Weinberg, Keidar, & Oberlander, 2010) | .50 | 47.76 | 80.00 | .0 | Community/Healthy vs. Clinic/At-Risk groups | cognitive task | No | No information provided | Cognitive/Academic (O) | −.31 | 67 |
Note. (P) = Parent report, (T) = Teacher report, (S) = Self-report, (0) = observational or peer report, and (M) = Multiple reporters. Example of negative emotion/stressor tasks = Child hearing an argument, frustration task, watching a sad video. Example of cognitive tasks = memory, executive functioning, problem solving, bayley developmental test. Example of social tasks = peer provocation, peer interactions. Examples of positive tasks = game of peek-a-boo with a puppet; positive emotion-laden film clip.
Data Analysis
Primary analyses were conducted using a random effects model approach (Hedges & Vevea, 1998). This approach was chosen to reflect the likelihood that individual studies would produce ESs different from other studies in the analysis. All ESs were transformed to z-scores using Fisher’s r to z transformation. After aggregation, ESs were transformed again to rs for comparison. To estimate the size of the effects, we adopted Cohen’s criteria: small = .10, medium = .30, large = .50. As a result of the large number of studies included, we used a minimum alpha level of .01 (two-tailed tests).
Heterogeneity analyses were also conducted to determine whether ESs were more heterogeneous than would be expected due to sampling error alone. The measure I2 is a modification of Cochrane's Q test (Cochran, 1954) which measures whether the ratio of variation that exceeds chance, thereby accounting for the number of studies utilized in meta-analysis with more accuracy (Higgins & Thompson, 2002). Values for I2 range from 0 to 1; an I2 of 0% indicates no heterogeneity, whereas I2s of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively (Higgins, Thompson, Deeks, & Altman, 2003). For variables with moderate to high heterogeneity, potential moderators to the ES were identified using weighted least squares regression procedures (Hedges, 1994). Finally, file drawer analyses were conducted following Hedges and Olkin’s (1985) approach to determine the number of studies that would be necessary to reduce the mean effect to a negligible level.
Results
Primary Analyses
Study identification information, coded categories, and ESs representing the relation between RSA withdrawal scores and children’s adaptive functioning outcomes can be found in Table 1. The relation between RSA withdrawal and measures of children’s externalizing problems had a weighted ES of r = −.16 (95% CI = −.19 to −.12, p = .001), indicating a small negative effect. Similarly, the relation between RSA withdrawal and measures of children’s internalizing problems had a weighted ES of r = −.16 (95% CI = −.21 to −.12, p = .001), also indicating a small negative effect. Lastly, the relation between RSA withdrawal and measures of children’s cognitive/academic problems also had a weighted ES of r = −.16 (95% CI = −.10 to −.22, p = .001), indicating a small negative effect. These results indicate that greater RSA withdrawal levels were associated with fewer externalizing, internalizing, and cognitive/academic problems. In contrast, a non-significant relation between RSA withdrawal and measure of children’s social functioning problems was found, indicating that RSA withdrawal is not associated with social functioning across studies (r = .06, 95% CI = −.10 to −.01, ns).
Table 1.
Effect Sizes across measures of adaptive functioning
| Externalizing Problems |
Internalizing Problems |
Social Problems |
Cognitive/Academic Problems |
|
|---|---|---|---|---|
| Weighted Mean ES | −.16 | −.16 | −.06 | −.16 |
| Cohen’s Criteria | Small | Small | Small | |
| 95% CI | −.19 to −.12 | −.21 to −.12 | −.10 to −.01 | −.22 to −.10 |
| Number of Effects Sizes | 25 | 18 | 15 | 10 |
| Total N across Studies | 3,331 | 1,697 | 1,969 | 1,025 |
| Range of Effects Sizes | −.55 to .10 | −.48 to .07 | −.44 to .42 | −.32 to −.02 |
| t | −9.09*** | −6.78*** | −2.63 | −5.16*** |
| File Drawer Analysis | 113 | 65 | 30 | 30 |
| I2 | 54% | 54% | 73% | 39% |
| Nature of Moderation | a | b |
Note.
p < .001.
Stronger negative (adaptive) association between RSA withdrawal and externalizing problems among studies which had a sample containing a high percentage of Caucasian children.
Association between RSA withdrawal and social problems was significantly more negative (adaptive) among studies with a community/healthy sample versus studies with a clinically/at-risk sample.
Heterogeneity and Moderation Analyses
The measure I2 was used to assess whether ESs were more heterogeneous than would be expected due to sampling error alone. Values for I2 range from 0 to 1; an I2 of 0% indicates no heterogeneity, whereas I2s of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). Once heterogeneity was identified, potential moderators to the ES were identified using weighted least squares regression procedures for continuous variables. Results of heterogeneity and moderator analyses are presented in Table 1.
The I2 values for the RSA withdrawal relations with the adaptive functioning outcomes indicated moderate heterogeneity for externalizing, internalizing, and social problems (54%, 54%, and 71%, respectively). The I2 values for academic/cognitive performance did not exceed 50%, suggesting relatively low heterogeneity. Hence, demographic moderators were tested only for externalizing, internalizing, and social problems. Demographic moderators included average age, gender (% male), ethnicity of sample (% Caucasian), time lag (time in years between RSA measure and adaptive functioning outcome), and type of sample (community vs. clinic).
Regression analyses indicated that ethnicity of sample was significantly associated with the average strength of the relation between RSA withdrawal and externalizing problems (β = −.62, p < .01). This indicates that the association between RSA withdrawal and externalizing problems is stronger (more negative) among studies which had a sample containing a high percentage of Caucasian children. A t-test also indicated a significant difference in the strength of the relation between RSA withdrawal and social problems according to type of sample, t = −2.67, p < .05. Specifically, the association between RSA withdrawal and social problems was significantly more negative among studies with a community/healthy sample (M = −.14, SD = .16) versus studies with a clinically/at-risk sample (M = .16, SD = .28). Hence, while greater levels of RSA withdrawal was associated with fewer social problems among community/healthy children the reverse was true for clinically/at-risk children where greater levels of RSA withdrawal was associated with more social problems. No other significant demographic moderators were identified.
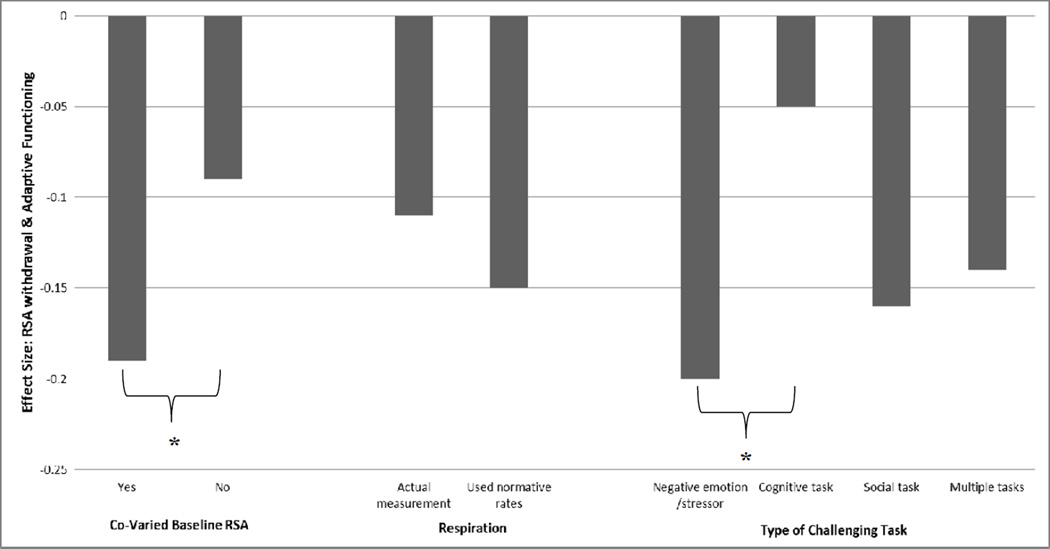
In terms of measurement issues, it is important to note that we found significant associations between baseline RSA and RSA withdrawal (r = .427, p < .05) as well as child age (r = .422, p < .05). Hence, according to the studies we reviewed, children with higher baseline RSA experienced greater levels of RSA withdrawal while baseline RSA values also increased as a function of children’s age. An ANCOVA, controlling for ethnicity and type of sample, was also conducted in which the between group variables were a) co-varying of baseline RSA (yes or no), b) inclusion of respiration rates (actual measurement versus use of normative rates), and c) type of challenging task used to derive RSA (cognitive, social, negative mood/stressor, and multiple tasks). The dependent variable was the average weighted ES across adaptive functioning outcomes. This analysis (graphically depicted in Figure 1) indicated a significant effect for co-varying baseline RSA, F (1, 21) = 6.16, p = .02. Specifically, studies that co-varied baseline RSA had stronger associations between RSA withdrawal and adaptive functioning outcomes, M = −.19, SE = .03, compared to studies that did not co-vary baseline RSA, M = −.09, SE = .04. Measurement of respiration rates (versus using normative rates) did not significantly impact the magnitude of the ESs (p = .70) nor did type of challenging task (cognitive, social, negative mood/stressors, multiple tasks) used to derive RSA withdrawal scores (p = .08). Of note, probing of this marginal significant finding indicated that studies which used negative mood/stressor tasks obtained larger RSA withdrawal to adaptive functioning associations compared to studies that used cognitive tasks to derive RSA withdrawal scores, p = .041. This may suggest that mood/stress induction tasks are tapping a key element of RSA withdrawal that elucidates its influence on adaptive outcomes. No other differences among tasks were found.
Figure 1.
Summary of Moderation Analyses for RSA Measurement Variables.
Note. * p < .05. Overall ANOVA for Type of Challenging Task comparison: F (1, 21) = 2.62, p = .078.
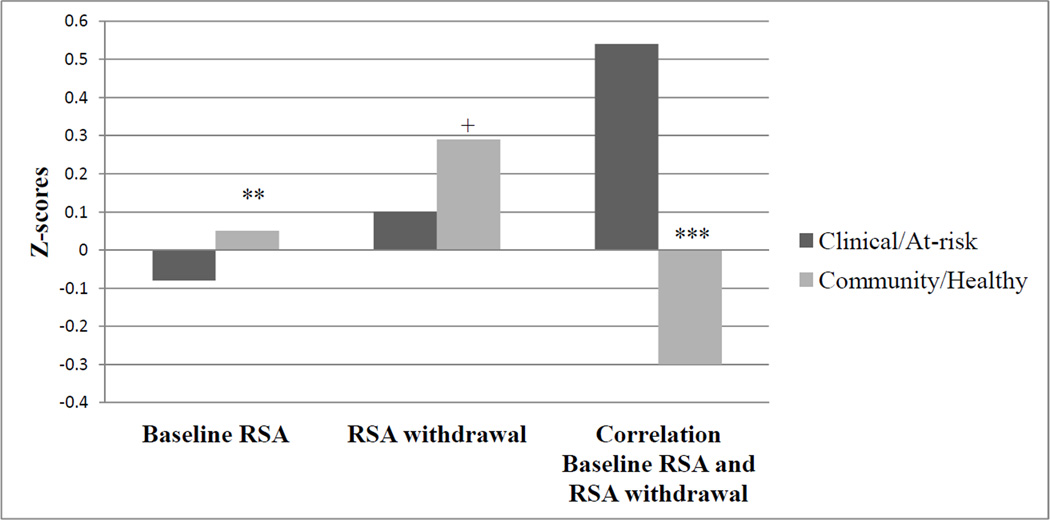
Lastly, to further probe the differential association between RSA withdrawal and social functioning outcomes for studies with clinically/at-risk samples, we coded mean levels of baseline RSA as well as RSA withdrawal (out of the 44 studies we examined, 29 provided enough descriptive information for this analysis). As seen in Figure 2, it is important to note that children from community/healthy samples had significantly higher levels of baseline RSA (weighted M = 6.26, SD = 1.50) compared to children from clinically/at-risk samples (weighted M = 6.09, SD = .88), F (1, 2003) = 11.30, p < .001. In terms of RSA withdrawal, and after controlling for baseline RSA, children from community/healthy samples had marginally higher levels of RSA withdrawal (weighted M = .27, SD = .51) compared to children from clinically/at-risk samples (weighted M = .20, SD = .27), F(1, 1342) = 3.61, p = .058. Finally, the association between baseline RSA and RSA withdrawal was significantly higher among children from clinically/at-risk samples (r = .36) compared to children from community/healthy samples (r = .20), F(1, 2976) = 250.50, p < .001.
Figure 2.
Summary of Moderation Analyses for Sample Type
Note. + p < .06, ** p < .01, *** p <.001.
File Drawer Analysis
To account for the bias toward publishing or submitting only significant findings, we conducted a file drawer analysis. This statistic examines the number of studies with null findings that would need to exist to bring the significant ESs of relations to less than p = .01. According to Rosenthal (1995), if only a few studies with null results are needed to change the significance level of a finding, then this finding is susceptible to the “file drawer threat,” indicating that the finding is not reliably significant. Results of File Drawer analyses are provided in Table 1. In the case of RSA withdrawal and externalizing problems, the file drawer analysis indicated over 100 studies necessary to overturn significant findings. Similarly, in the case of RSA withdrawal and internalizing problems, the file drawer analysis indicated 65 studies would be necessary to overturn significant findings. However, for RSA withdrawal and academic/cognitive performance and social functioning problems, considerably fewer studies would be necessary to reduce results to non-significance (10 and 32, respectively), suggesting that these varied findings are less likely to remain stable as new research emerges.
Discussion
Given emerging research over the last 20 years regarding the importance of the parasympathetic branch of the autonomic nervous system (ANS), as indexed by cardiac vagal control or RSA withdrawal, in children’s emotion regulation development, we sought to determine the extent to which such a biological marker is associated with more complex adaptive functioning outcomes across domains. We also sought to examine measurement issues that have recently been highlighted (Grossman & Taylor, 2007; Denver et al., 2007; Allen et al., 2007) when studying RSA withdrawal. Our research questions were examined via a meta-analysis of 44 studies, the first to our knowledge that has focused on RSA withdrawal in children.
Within the externalizing domain, lack of self-control has long been conceptualized as a core feature of children who display significant behavioral problems such as those diagnosed with Attention-Deficit/Hyperactivity Disorder (ADHD) and Oppositional Defiant Disorder (Barkley, 1997; Keenan & Shaw, 2003). Due to observations that children with externalizing behavior problems also display greater emotion regulation difficulties compared to their peers (Graziano, McNamara, Geffken, & Reid, 2011; Hill, Degnan, Calkins, & Keane, 2006; Walcott & Landau, 2004), more recent psychopathology models of ADHD, for example, have argued that an emotion regulation perspective may offer a better understanding of its etiology (Martel, 2009). Previous physiological research had highlighted children with externalizing problems as having overall low reactivity as measured primarily through the sympathetic nervous system (SNS) via both electrodermal activity and lower baseline heart rate levels (see meta-analysis by Lorber, 2004). Our meta-analysis elucidates potential etiological mechanisms by finding that a physiological regulatory function in the PNS, as indexed by RSA withdrawal, is also compromised in children with externalizing problems. Specifically, lower levels of RSA withdrawal were linked to more externalizing behavior problems. We also found that children from clinical/at-risk samples (i.e., which primarily comprised of children who were categorically assigned as having significant externalizing behavior problems or having a diagnosis) had significantly lower absolute levels of baseline RSA as well as RSA withdrawal compared to children from community/healthy samples. This suggests that while externalizing problems may be associated with low baseline sympathetic arousal in general, there may be quite a bit more to the story regarding the co-functioning of SNS and PNS systems. The empirical data summarized here appear to support the possibility that greater RSA withdrawal contributes to the allocation of metabolic resources. Specifically, increases in heart rate may facilitate the use of attentional abilities, which in turn may aid children in controlling both their behaviors and emotions during challenging states.
Past research had suggested two different perspectives on the physiological functioning of children with internalizing problems. One perspective indicated that children with internalizing problems physiologically overregulate which contributes to their symptoms (Thayer & Lane, 2000) while another perspective highlighted that internalizing problems are also a function of physiological dysregulation (Schmitz et al., 2011). The results of our meta-analysis provide clear support for the latter view in that greater levels of RSA withdrawal were associated with fewer internalizing symptoms. However, it is important to not fully discount the fact that the overwhelming number of studies we reviewed only examined a linear relationship between RSA withdrawal and internalizing problems. It is still possible that a quadratic association between RSA withdrawal and internalizing problems exists such that a moderate level of regulation is optimal. Additionally, it may also be the case that the overregulation hypothesized to be part of the internalizing problem domain takes place in a more cognitively based way (e.g., worry) versus a primarily physiological reaction. Another plausible scenario that needs further research is the context in which such regulation occurs. It may be the case that children with internalizing problems overregulate during less threatening situations while underregulate during more challenging situations.
Within the cognitive/academic domain, a significant amount of research has linked better emotion regulation with optimal academic outcomes including school readiness (Graziano, Reavis, et al., 2007) as well as cognitive processes such as executive functioning (Bierman, Nix, Greenberg, Blair, & Domitrovich, 2008; Blair, 2002). Children with emotional and behavioral control difficulties also perform worse on cognitive tasks and have worse academic outcomes (Holmes et al., 2010; Massetti et al., 2008; Masten et al., 2005). Our findings are consistent with such literature by documenting a significant link between a biological marker for self-control and children’s cognitive/academic functioning. From a physiological perspective such link is not surprising given the significant overlap in the brain structures (e.g., ACC and orbitofrontal cortex) that facilitate the regulation of both emotions and higher order cognitive processes (Bush et al., 2000). Efficient emotion regulation allows greater metabolic resources to be used for attentional control purposes and areas of the prefrontal cortex that are essential for cognitive functioning and subsequently academic success. Despite our findings, it is important to note that more research is needed linking RSA withdrawal to cognitive/academic outcomes given our file drawer analysis.
Similar to the internalizing domain, further examination of potential quadratic effects is needed, especially given a recent study showing that moderate levels of RSA withdrawal was associated with more optimal executive functioning (Marcovitch et al., 2010). In fact, the overwhelming number of studies we reviewed only examined a linear association between RSA withdrawal and children’s adaptive functioning outcomes. While Polyvagal theory does not explicitly state a potential quadratic association between RSA withdrawal and children’s adaptive functioning outcomes, it does highlight the importance of the vagus nerve in physiologically engaging and disengaging with the environment depending upon the level of challenge presented. When interpreting within this engagement and disengagement of the vagal break, then it is reasonable to expect that the timing and level of challenge in the environment will shape the pattern of the RSA withdrawal, and not necessarily in a linear way. Future research should examine both linear and non-linear patters of RSA withdrawal and how they map onto types of challenges in the environment as optimal levels of RSA withdrawal may differ according to the context the child is in.
The most surprising and noteworthy aspect of our study was the lack of a significant overall association between RSA withdrawal and children’s social functioning. This finding is counter to what one would expect based on Polyvagal theory and the Social Engagement System, outlined by Porges (2003b). While the evolutionary and neuroanatomical evidence supporting the role of the vagus nerve in the nuclei that controls the muscles of the face and head is well-established, empirical data is vastly missing on: a) whether a well-regulated and calm visceral state actually contributes to better control of facial/head muscles that enables complex facial gestures, vocalizations, social gesturing, and orientation, and b) whether subtle individual differences in the Social Engagement System actually contribute to children’s social competence.
Despite our non-significant finding across studies, moderation analyses suggested that the relation between RSA withdrawal and social functioning was very different across populations. The ES between RSA withdrawal and social problems was negative (adaptive) in community/healthy samples (M = −.14, SD = .16) but positive (maladaptive) in clinically/at-risk samples (M = .16, SD = .28). Thus, the question appears to be not whether RSA withdrawal is associated with improved social functioning, but for whom. Why RSA withdrawal is associated with poor social functioning in clinical/at-risk populations is unclear, although there are several possible explanations. First, it is important to acknowledge the difficulty in extrapolating biological and psychological processes that tend to be examined within normative samples to high-risk groups (Beauchaine, 2001). While our mixed findings are unlikely the result of smaller sample sizes for children from clinical/at-risk samples (44% of studies we reviewed contained clinical/at-risk samples), high-risk groups undoubtedly have more complex psychological and biological deficits that contribute to their impairment. As such, one possibility is that the direct effects of RSA withdrawal are masked by other, more powerful influences on social functioning, such as significant clinical impairment. Additionally, given that the vast majority of our clinical/at-risk samples consisted of children displaying disruptive behavioral disorders (e.g., ADHD and/or high levels of aggression), it is possible that the more dysfunctional structural and neurochemical functioning implicated with such disorders (e.g., abnormalities in the mesoaccumbens dopamine pathway; Volkow et al., 2009) blunts the PNS’s response to stress via a lack of reciprocity with the SNS's response, which has recently been shown to contribute to greater externalizing problems (see El-Sheik et al., 2009).
It is important to note that all studies that we reviewed used a broad measure of social functioning whether reported by parents, teacher, or peers. Social functioning is a complex and broad construct that is influenced by numerous factors. While some factors may be etiologically and theoretically linked to RSA withdrawal such as the finding that children with worse behavioral control have lower social preference scores (Hoza et al., 2005; Mrug, Hoza, Pelham, Gnagy, & Greiner, 2007), others such as physical attractiveness are not linked to self-control and yet have long been associated with social preference (Coie, Coppotelli, & Dodge, 1982). Further research needs to be conducted to truly examine any subtle effects of RSA withdrawal on voice control, eye contact, and facial movements that are hypothesized by the Social Engagement System. As it stands, however, the phylogenetic contributions to social behavior outlined by Polyvagal Theory have little empirical support for individuals with other significant clinical impairment.
Other moderation analyses failed to find any significant associations between child age or gender and the associations between RSA withdrawal and children’s adaptive functioning outcomes. These null findings are noteworthy as only a handful of studies have suggested that gender may moderate RSA and adjustment links (Eisenberg et al., 1995) and even fewer studies have suggested that RSA (either baseline levels or withdrawal levels) relate to adjustment differently at different ages (Beauchaine et al., 2007; Gentzler, Rottenberg, Kovacs, George, & Morey, 2011). It is important to note that our meta-analysis could not examine gender or age differences as they relate to absolute levels of baseline RSA and RSA withdrawal. While studies tend to show that baseline levels of RSA increase across development (Calkins & Keane, 2004; Hinnant, Elmore-Staton, & El-Sheikh, 2011) less work has examined the extent to which levels of RSA withdrawal change with time. In terms of gender, most studies find that boys and girls exhibit similar levels of baseline RSA and RSA withdrawal (Gentzler et al., 2011; Graziano, Keane, et al., 2007). Hence, despite potential age differences, and to a lesser extent gender differences, in the magnitude of RSA, it appears that at least the links between RSA and children’s adjustment outcomes work similarly for boys and girls across development.
In terms of measurement issues associated with RSA, our meta-analysis highlights the importance of co-varying baseline levels of RSA when examining RSA withdrawal. Specifically, stronger associations between RSA withdrawal and children’s adaptive functioning outcomes emerge when co-varying those initial starting values provided by baseline RSA. The law of initial values predicts that higher physiological baseline functioning facilitates greater physiological response during challenges (Lacey & Lacey, 1962; Wilder, 1956). However, previous research with clinical samples suggest that some children may have high starting baseline RSA values but have difficulty suppressing RSA during challenging states (Degangi et al., 1991). Our results show moderate levels of association between baseline RSA and RSA withdrawal levels although this association also varied as a function of sample type. Interestingly, children from clinical/at-risk samples had lower levels of baseline RSA and RSA withdrawal compared to children from community/healthy samples. Baseline RSA is thought to represent a general responsiveness measure as it provides a ceiling on how much withdrawal a child can engage in during challenging situations. Hence, it appears that the more severe clinically/at-risk sample of children not only have greater physiological regulation difficulties in terms of their ability to withdrawal RSA but also start out at a disadvantage in terms of their overall ability to react and respond to environmental demands. Additionally, the higher association between levels of baseline RSA and RSA withdrawal among children from clinical-at-risk samples versus children from community/healthy samples suggest lower variability or flexibility in their physiological system’s response to stress/challenge, even when considering varying baseline levels.
It is also important to note that recent studies show that the interaction between baseline RSA and RSA withdrawal is what predicts various outcomes, not just the independent examination of baseline RSA and RSA withdrawal (see El-Sheikh, Hinnant, & Erath, 2011; Hinnant & Erath, 2009). For example, in the context of experiencing marital conflict, only boys with lower baseline RSA and who experienced an augmentation of RSA (rather than a withdrawal) during a challenging task demonstrated an elevated risk for increasing delinquent behavior over time (El-Sheikh et al., 2011). On the other hand, a combination of lower baseline RSA and higher RSA withdrawal predicted later internalizing symptoms among early elementary school age children (Hinnant & El-Sheikh, 2009). While the examination of interaction effects between baseline RSA and RSA withdrawal and children’s adaptive functioning show promise, more research is needed with clinical populations given the difficulties in extrapolating psychophysiological findings from normative samples (Beauchaine, 2001).
Despite the debate over how to account for respiration when estimating vagal tone from RSA (Allen, Chambers, & Towers, 2007; Denver, Reed, & Porges, 2007; Grossman & Taylor, 2007), we did not find any differences in the magnitude of the associations between RSA withdrawal and adaptive functioning outcomes among studies that actually measured children’s respiration rates (e.g., using peak-to-valley method) versus those that used normative levels (e.g., Porges adaptive polynomial filter method). Hence, consistent with Denver and colleagues (2007), our findings indicate that the predictive validity of RSA towards various outcomes does not change as a function of how respiration is accounted for. It is important to note that our findings cannot speak to whether studies that used normative levels of respiration rates obtained less accurate estimates of vagal tone compared to those that actually measured children’s respiration, especially given the short-comings of using ANOVAs when examining differences (Allen et al., 2007). While the use of precise respiration rates may indeed lead to more pure measurements of RSA as an estimate of vagal tone, it is significantly more practical within the child literature to use normative rates of respiration during RSA calculations versus subjecting children to wearing a respiration band which may cause extra artifacts from the child touching the band.
There was also significant variability in terms of which challenging tasks researchers use to derive RSA withdrawal. These tasks ranged from frustration, cognitive, social, positive, sensory oriented tasks, while some studies used multiple tasks. The magnitude of the link between RSA withdrawal and children’s adaptive functioning outcomes did not appear to vary as a function of the type of challenging task used, although there was a trend suggesting that negative/stress tasks obtained higher Effect Sizes compared to more cognitive oriented tasks. However, it is important to point out that very few studies used positive tasks (e.g., a game of peek-a-boo with a puppet; positive emotion-laden film clip) or compared regulation across types of task. This may be an important area for future research given a recent study showing that children with ADHD overregulate during positive tasks and underregulate during more stressful tasks (Musser et al., 2011). It will be important to replicate such pattern with other samples as well as children with other difficulties such as those displaying internalizing behavior problems. Examining such regulation during positive tasks may also yield information on children’s reward sensitivity and surgency, important constructs that have been implicated in various psychological disorders (O'Brien & Frick, 1996; Tripp & Alsop, 2001; Volkow et al., 2009).
In summary, this study marks the first meta-analysis to confirm the role of cardiac vagal control, as measured via RSA withdrawal, in contributing to children’s adaptive functioning across externalizing, internalizing, and cognitive/academic domains. Although we found evidence for the role of vagal regulation in the social behavior of healthy children (as predicted by the Social Engagement System outlined by Polyvagal Theory), we found the opposite to be true for clinical/at-risk populations, suggesting that the vagal “brake” does not uniformly enhance social functioning across all children. Additionally, children from clinical/at-risk samples displayed lower absolute levels of baseline RSA and RSA withdrawal compared to children from community/healthy samples. Despite the recent caveats about measuring RSA, it appears that using normative rates of respiration versus actual measurement of respiration does not affect the link between RSA withdrawal and adaptive functioning outcomes nor does the type of challenging task used to derive RSA. Co-varying baseline RSA during analyses does strengthen the associations between RSA withdrawal and adaptive functioning outcomes.
In terms of limitations, our file drawer analyses highlighted the importance of further empirical work on the link between RSA withdrawal and children’s social problems and cognitive/academic outcomes given the small number of studies conducted within those domains. While we examined certain measurement issues associated with RSA, we were not able to examine whether the task length or length of the baseline period affected any of the findings. Respiration rates vary with age (Bar-Haim, Marshall, & Fox, 2000), and this needs to be considered when determining the minimum length of RSA estimation as well as whether the length of RSA estimation impacts associations between RSA withdrawal and adaptive functioning. Additionally, it is important to recognize that we were not able to distinguish the extent to which our findings are a function of higher absolute levels of RSA withdrawal versus lower levels of RSA withdrawal or more importantly RSA augmentation. RSA or vagal augmentation represents a heighten parasympathetic response (i.e., increase in RSA during a challenging task versus baseline rather than a decrease that is typically seen and measured as RSA withdrawal) that has been suggested as being associated with hypervigilance and representative of an endophenotype of children at risk for more serious behavior or conduct problems (Katz, 2007). It will be important for future research to examine RSA withdrawal not only in a continuous manner but also in terms of whether children simply suppressed RSA during challenge or augmented RSA. It may be the case then that an augmentation of RSA is a better indicator of physiological dysfunction rather than lower levels of RSA withdrawal. Additionally and consistent with findings from Blair & Peters (2003) and as suggested by Hastings and colleagues (2008), it may be the case that the adaptive nature of either vagal augmentation or withdrawal is contingent on the context and demands the child is facing. Future work should attempt to examine the direction of children’s RSA reactivity across multiple contexts as well as ecologically valid settings such as the school or home rather than in the laboratory.
Given that our moderation analyses indicated that the strongest associations between RSA withdrawal and children’s externalizing outcomes were among studies with a higher percentage of Caucasian as participants, this could be a function of the power to detect an effect given the small number of minority children participating in research of this nature. Alternatively, some recent studies have documented differences among African-American children versus Caucasian children. For example, Hinnant and colleagues (2011) found that African-American children had higher levels of baseline RSA compared to Caucasian children but did not experience growth in baseline RSA over a two year period. Graziano and colleagues (2011) found that a cardiovascular profile characterized by lower levels of RSA withdrawal and heart period represented a significant risk factor for the development of obesity, but only for African-American children. The adult cardiovascular literature has also documented that African-Americans tend to show higher rates of hypertension and cardiovascular disease and display lower autonomic regulation compared to Caucasian adults (Cossrow & Falkner, 2004; Sloan et al., 2008). It is important to note that there is a lack of studies examining actual rates of RSA withdrawal among children from different ethnicities/race, although the few studies available have not found any significant differences (Graziano, Calkins, et al., 2011; Graziano, Keane, et al., 2007; Matthews, Salomon, Kenyon, & Allen, 2002; Stifter & Corey, 2001). Future psychophysiological research would do well to include more diverse samples to determine whether this finding remains. It will be particularly important to study Latino children, given that they are the largest minority group in the U.S., comprising of more than 25% of children 5 years of age or younger (U.S. Census Bureau, 2009).
However, it is most important to note that the ESs of our significant associations were quite small in nature. This highlights the limitations of current physiological work in our field as researchers tend to examine widely different biological markers independently; some studies only focus on examining electrodermal activity, others on PNS measures such as RSA, others on EEG symmetry, and still others on SNS measures such as pre-ejection period (PEP) or cortisol. As pointed out by Beauchaine (2001), in order for our field to move forward and obtain a better understanding of the biological/physiological factors that contribute to children’s adaptive functioning outcomes and/or common psychopathologies, researchers need to measure, analyze, and integrate multiple physiological systems at once. For example, research by El-Sheik and colleagues (2009) demonstrated that co-activation or co-inhibition of the PNS and SNS predicted greater externalizing behavior problems associated with marital conflict while reciprocal action of the PNS and SNS served as a protective factor. Equally important and demonstrated by El-Sheik and colleagues (2009) is the need for research to continue to examine how environmental and contextual factors interact with children’s physiology to predict adaptive functioning outcomes. For example, children displaying less RSA withdrawal to a stressful event only showed elevated conduct problems in the context of a violent home environment (Katz, 2007). Greater levels of RSA withdrawal are also more strongly associated with reductions in stress reactivity in the context of higher social support (Wolff et al., 2012). These more complex analyses that incorporate multiple biological markers (and more accurately mirror the way the nervous system operates) as well as interactions with contextual and environmental factors will increase the understanding of how various psychological disorders develop and ultimately inform more targeted preventative and treatment strategies.
Contributor Information
Paulo Graziano, Florida International University
Karen Derefinko, University of Kentucky
References
- Allen JJB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological psychology. 2007;74(2):243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Marshall P, Fox N. Developmental changes in heart period and high frequency heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Vohs K. Handbook of self-regulation: Research, theory, and applications. New York: Guilford Press; 2004. [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine T, Gartner J, Hagen B. Comorbid depression and heart rate variability as predictors of aggressive and hyperactive symptom responsiveness during inpatient treatment of conduct-disordered, ADHD boys. Aggressive Behavior. 2000;26(6):425–441. [Google Scholar]
- Beauchaine T, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biological psychology. 2007;74(2):174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of abnormal psychology. 2001;110(4):610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Berntson G, Bigger T, Eckberg D, Grossman P, Kaufmann P, Malik M, et al. Heart rate variabiilty: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bierman KL, Nix RL, Greenberg MT, Blair C, Domitrovich CE. Executive functions and school readiness intervention: impact, moderation, and mediation in the Head Start REDI program. Development and psychopathology. 2008;20(3):821–843. doi: 10.1017/S0954579408000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children's functioning at school entry. The American psychologist. 2002;57(2):111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C. Behavioral inhibition and behavioral activation in young children: relations with self-regulation and adaptation to preschool in children attending Head Start. Developmental psychobiology. 2003;42(3):301–311. doi: 10.1002/dev.10103. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R. Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental neuropsychology. 2003;24(1):479–497. doi: 10.1207/S15326942DN2401_04. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O'Brien M. Contributions of Child's Physiology and Maternal Behavior to Children's Trajectories of Temperamental Reactivity. Developmental Psychology. 2010;46(5):1089–1102. doi: 10.1037/a0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36(1):54–65. [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ, Worki MAB. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT. Social neuroscience: autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31(2):113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Calkins S. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental psychobiology. 1997;31(2):125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins S. The emergence of self-regulation: Biological and behavioral control mechanisms supporting toddler competencies. In: Brownell C, Kopp C, editors. Socioemotional development in the toddler years. New York: The Guilford Press; 2007. pp. 261–284. [Google Scholar]
- Calkins S, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of abnormal child psychology. 2000;28(2):103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Calkins S, Dedmon SE, Gill KL, Lomax LE, Johnson LM. Frustration in Infancy: Implications for Emotion Regulation, Physiological Processes, and Temperament. Infancy : the official journal of the International Society on Infant Studies. 2002;3(2):175–197. doi: 10.1207/S15327078IN0302_4. [DOI] [PubMed] [Google Scholar]
- Calkins S, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and psychopathology. 2002;14(3):477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Calkins S, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood. Developmental psychobiology. 2008;50(8):751–766. doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins S, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological psychology. 2007;74(2):144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins S, Johnson M. Toddler regulation of distress to frustrating events: Temperamental and maternal correlates. Infant behavior & development. 1998;(21):379–395. [Google Scholar]
- Calkins S, Keane SP. Cardiac vagal regulation across the preschool period: stability, continuity, and implications for childhood adjustment. Developmental psychobiology. 2004;45(3):101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–451. [Google Scholar]
- Coie JD, Coppotelli H, Dodge KA. Dimensions and Types of Social-Status - a Cross-Age Perspective. Developmental Psychology. 1982;18(4):557–570. [Google Scholar]
- Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. The Journal of clinical endocrinology and metabolism. 2004;89(6):2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of abnormal psychology. 2006;115(1):174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Development and psychopathology. 2005;17(4):1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Degangi GA, Dipietro JA, Greenspan SI, Porges SW. Psychophysiological Characteristics of the Regulatory Disordered Infant. Infant behavior & development. 1991;14(1):37–50. [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biological psychology. 2007;74(2):286. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental psychobiology. 2003;43(3):230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children's social functioning: A longitudinal study. Child development. 1995;66(5):1360–1384. [PubMed] [Google Scholar]
- Eisenberg N, Sulik MJ, Spinrad TL, Edwards A, Eggum ND, Liew J, Hart D. Differential Susceptibility and the Early Development of Aggression: Interactive Effects of Respiratory Sinus Arrhythmia and Environmental Quality. Developmental Psychology. 2012;48(3):755–768. doi: 10.1037/a0026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M. Parental drinking problems and children's adjustment: vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of abnormal psychology. 2001;110(4):499–515. doi: 10.1037//0021-843x.110.4.499. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental psychobiology. 2005;46(1):66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children's sleep problems. Developmental psychobiology. 2005;46(4):307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children's adjustment and physical health: the moderating role of vagal tone. Child development. 2001;72(6):1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Erath S. Developmental Trajectories of Delinquency Symptoms in Childhood: The Role of Marital Conflict and Autonomic Nervous System Activity. Journal of abnormal psychology. 2011;120(1):16–32. doi: 10.1037/a0020626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children's externalizing behavior: interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74(1):1–79. doi: 10.1111/j.1540-5834.2009.00501.x. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: vagal regulation as a protective factor. Journal of family psychology : JFP : journal of the Division of Family Psychology of the American Psychological Association. 2006;20(1):30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Erath S, Tu K, El-Sheikh M. Socially anxious and peer-victimized preadolsecents: "Doubly primed" for distress? Journal of abnormal child psychology. 2011 doi: 10.1007/s10802-011-9600-9. [DOI] [PubMed] [Google Scholar]
- Garber J, Dodge KA. The development of emotion regulation and dysregulation. Cambridge, England: Cambridge University Press; 1991. [Google Scholar]
- Gazelle H, Druhen MJ. Anxious solitude and peer exclusion predict social helplessness, upset affect, and vagal regulation in response to behavioral rejection by a friend. Developmental Psychology. 2009;45(4):1077–1096. doi: 10.1037/a0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler A, Rottenberg J, Kovacs M, George CJ, Morey JN. Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental psychobiology. 2011;54(5):556–567. doi: 10.1002/dev.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler A, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological psychology. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliom M, Shaw DS. Codevelopment of externalizing and internalizing problems in early childhood. Development and psychopathology. 2004;16(2):313–333. doi: 10.1017/s0954579404044530. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF, Hooven C. Parental meta-emotion philosophy and the emotional life of families: Theoretical models and preliminary data. Journal of Family Psychology. 1996;10(3):243–268. [Google Scholar]
- Graziano PA, Bagner DM, Sheinkopf SJ, Vohr BR, Lester BM. Evidence-based intervention for young children born premature: Preliminary evidence for associated changes in physiological regulation. Infant behavior & development. 2012;35(3):417–428. doi: 10.1016/j.infbeh.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Calkins SD, Keane SP, O'Brien M. Cardiovascular Regulation Profile Predicts Developmental Trajectory of BMI and Pediatric Obesity. Obesity. 2011 doi: 10.1038/oby.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Cardiac vagal regulation and early peer status. Child development. 2007;78(1):264–278. doi: 10.1111/j.1467-8624.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Graziano PA, McNamara JP, Geffken GR, Reid AM. Differentiating Co-Occurring Behavior Problems in Children With ADHD: Patterns of Emotional Reactivity and Executive Functioning. Journal of Attention Disorders. 2011 doi: 10.1177/1087054711428741. [DOI] [PubMed] [Google Scholar]
- Graziano PA, Reavis RD, Keane SP, Calkins SD. The Role of Emotion Regulation and Children's Early Academic Success. J Sch Psychol. 2007;45(1):3–19. doi: 10.1016/j.jsp.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Stemmler G, Meinhardt E. Paced respiratory sinus arrhythmia as an index of cardiac parasympathetic tone during varying behavioral tasks. Psychophysiology. 1990;27(4):404–416. doi: 10.1111/j.1469-8986.1990.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological psychology. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Haley DW, Grunau RE, Weinberg J, Keidar A, Oberlander TF. Physiological correlates of memory recall in infancy: vagal tone, cortisol, and imitation in preterm and full-term infants at 6 months. Infant behavior & development. 2010;33(2):219–234. doi: 10.1016/j.infbeh.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnod T, Yang CC, Hsin YL, Shieh KR, Wang PJ, Kuo TB. Heart rate variability in children with refractory generalized epilepsy. Seizure : the journal of the British Epilepsy Association. 2008;17(4):297–301. doi: 10.1016/j.seizure.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Hastings P, Nuselovici J, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children's emotion regulation: Social context, socialization, and adjustment. Biological psychology. 2008;79(3):299. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hastings P, Sullivan C, McShane KE, Utendale WT, Coplan RJ, Vyncke JD. Parental socialization, vagal regulation, and preschoolers' anxious difficulties: Direct mothers and moderated fathers. Child development. 2008;79(1):45–64. doi: 10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children's emotion regulation: Social context, socialization, and adjustment. Biological psychology. 2008;79(3):299–306. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychological Methods. 1998;3(4):486–504. [Google Scholar]
- Hickey JE, Suess PE, Newlin DB, Spurgeon L, Porges SW. Vagal Tone Regulation during Sustained Attention in Boys Exposed to Opiates in-Utero. Addictive behaviors. 1995;20(1):43–59. doi: 10.1016/0306-4603(94)00044-y. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: the roles of emotion regulation and inattention. Developmental Psychology. 2006;42(5):913–928. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Hinnant B, El-Sheikh M. Children's Externalizing and Internalizing Symptoms over Time: The Role of Individual Differences in Patterns of RSA Responding. Journal of abnormal child psychology. 2009;37(8):1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Hinnant B, Elmore-Staton L, El-Sheikh M. Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental psychobiology. 2011;53(1):59–68. doi: 10.1002/dev.20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes J, Gathercole SE, Place M, Alloway TP, Elliott JG, Hilton KA. The Diagnostic Utility of Executive Function Assessments in the Identification of ADHD in Children. Child and Adolescent Mental Health. 2010;15(1):37–43. doi: 10.1111/j.1475-3588.2009.00536.x. [DOI] [PubMed] [Google Scholar]
- Hoza B, Gerdes AC, Mrug S, Hinshaw SP, Bukowski WM, Gold JA, Wigal T. Peer-assessed outcomes in the multimodal treatment study of children with attention deficit hyperactivity disorder. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2005;34(1):74–86. doi: 10.1207/s15374424jccp3401_7. [DOI] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: assessments at twelve weeks of age. Child development. 1998;69(3):624–635. [PubMed] [Google Scholar]
- Katz L. Domestic violence and vagal reactivity to peer provocation. Biological psychology. 2007;74(2):154. doi: 10.1016/j.biopsycho.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L, Gottman JM. Buffering children from marital conflict and dissolution. Journal of clinical child psychology. 1997;26(2):157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Keenan K, Shaw D. Starting at the beginning: Exploring the etiology of antisocial behavior in the first years of life. New York, NY: Guilford Press; 2003. [Google Scholar]
- Keller PS, El-Sheikh M. Salivary alpha-amylase as a longitudinal predictor of children's externalizing symptoms: respiratory sinus arrhythmia as a moderator of effects. Psychoneuroendocrinology. 2009;34(5):633–643. doi: 10.1016/j.psyneuen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Lacey JI, Lacey BC. THE LAW OF INITIAL VALUE IN THE LONGITUDINAL STUDY OF AUTONOMIC CONSTITUTION: REPRODUCIBILITY OF AUTONOMIC RESPONSES AND RESPONSE PATTERNS OVER A FOUR-YEAR INTERVAL*. Annals of the New York Academy of Sciences. 1962;98(4):1257–1290. doi: 10.1111/j.1749-6632.1962.tb30633.x. [DOI] [PubMed] [Google Scholar]
- Leary A, Katz LF. Coparenting, family-level processes, and peer outcomes: the moderating role of vagal tone. Development and psychopathology. 2004;16(3):593–608. doi: 10.1017/s0954579404004687. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129(2):361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lewis G, Furman S, McCool M, Porges S. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biological Psychology. 2012;89:349–364. doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew J, Eisenberg N, Spinrad TL, Eggum ND, Haugen R, Kupfer A, Baham ME. Physiological Regulation and Fearfulness as Predictors of Young Children's Empathy-related Reactions. Social Development. 2011;20(1):111–134. doi: 10.1111/j.1467-9507.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. The way in which intervention studies have "personality" and why it is important to meta-analysis. Evaluation & the Health Professions. 2001;24(3):236–254. doi: 10.1177/016327870102400302. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O'Brien M, Blankson AN. Moderate Vagal Withdrawal in 3.5-Year-Old Children is Associated With Optimal Performance on Executive Function Tasks. Developmental psychobiology. 2010;52(6):603–608. doi: 10.1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. Journal of child psychology and psychiatry, and allied disciplines. 2009;50(9):1042–1051. doi: 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- Massetti GM, Lahey BB, Pelham WE, Loney J, Ehrhardt A, Lee SS, Kipp H. Academic achievement over 8 years among children who met modified criteria for attention-deficit/hyperactivity disorder at 4–6 years of age. Journal of abnormal child psychology. 2008;36(3):399–410. doi: 10.1007/s10802-007-9186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Roisman GI, Long JD, Burt KB, Obradovic J, Riley JR, Tellegen A. Developmental cascades: Linking academic achievement and externalizing and internalizing symptoms over 20 years. Developmental Psychology. 2005;41(5):733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Salomon K, Kenyon K, Allen MT. Stability of children's and adolescents' hemodynamic responses to psychological challenge: a three-year longitudinal study of a multiethnic cohort of boys and girls. Psychophysiology. 2002;39(6):826–834. doi: 10.1111/1469-8986.3960826. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay RE, Kindlon D, Saul JP, Arseneault L, Seguin J, Earls F. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal of child psychology and psychiatry, and allied disciplines. 1997;38(4):457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Miller BD, Wood BL, Lim J, Ballow M, Hsu C. Depressed children with asthma evidence increased airway resistance: "vagal bias" as a mechanism? The Journal of allergy and clinical immunology. 2009;124(1):66–73. doi: 10.1016/j.jaci.2009.04.038. e61-10. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper C, Gariepy JL, Barnett M, Moore GA, Calkins S, Cox MJ. Psychophysiological Correlates of Parenting Behavior in Mothers of Young Children. Developmental psychobiology. 2009;51(8):650–661. doi: 10.1002/dev.20400. [DOI] [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain & development. 2005;27(7):509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA, Georgiades K, Boyle M, MacMillan HL. Stability of resting frontal electroencephalogram (EEG) asymmetry and cardiac vagal tone in adolescent females exposed to child maltreatment. Developmental psychobiology. 2009;51(6):474–487. doi: 10.1002/dev.20387. [DOI] [PubMed] [Google Scholar]
- Moore GA. Infants' and mothers' vagal reactivity in response to anger. Journal of Child Psychology and Psychiatry. 2009;50(11):1392–1400. doi: 10.1111/j.1469-7610.2009.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrug S, Hoza B, Pelham WE, Gnagy EM, Greiner AR. Behavior and peer status in children with ADHD: continuity and change. Journal of Attention Disorders. 2007;10(4):359–371. doi: 10.1177/1087054706288117. [DOI] [PubMed] [Google Scholar]
- Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, Nigg JT. Emotion Regulation via the Autonomic Nervous System in Children with Attention-Deficit/Hyperactivity Disorder (ADHD) Journal of abnormal child psychology. 2011;39(6):841–852. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien BS, Frick PJ. Reward dominance: associations with anxiety, conduct problems, and psychopathy in children. Journal of abnormal child psychology. 1996;24(2):223–240. doi: 10.1007/BF01441486. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child development. 2010;81(1):270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterman M, Schuengel C. Physiological effects of separation and reunion in relation to attachment and temperament in young children. Developmental psychobiology. 2007;49(2):119–128. doi: 10.1002/dev.20207. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, Porges SW. Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental psychobiology. 2011 doi: 10.1002/dev.21002. [DOI] [PubMed] [Google Scholar]
- Pearson SR, Alkon A, Treadwell M, Wolff B, Quirolo K, Boyce WT. Autonomic reactivity and clinical severity in children with sickle cell disease. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2005;15(6):400–407. doi: 10.1007/s10286-005-0300-9. [DOI] [PubMed] [Google Scholar]
- Pine DS, Wasserman GA, Miller L, Coplan JD, Bagiella E, Kovelenku P, Sloan RP. Heart period variability and psychopathology in urban boys at risk for delinquency. Psychophysiology. 1998;35(5):521–529. doi: 10.1017/s0048577298970846. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiology & behavior. 2003a;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Annals of the New York Academy of Sciences. 2003b;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal "brake" predicts child behavior problems: a psychobiological model of social behavior. Developmental psychobiology. 1996;29(8):697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annual review of psychology. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Quas JA, Bauer A, Boyce WT. Physiological reactivity, social support, and memory in early childhood. Child development. 2004;75(3):797–814. doi: 10.1111/j.1467-8624.2004.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas JA, Carrick N, Alkon A, Goldstein L, Boyce WT. Children's memory for a mild stressor: the role of sympathetic activation and parasympathetic withdrawal. Developmental psychobiology. 2006;48(8):686–702. doi: 10.1002/dev.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Writing meta-analytic reviews. Psychological Bulletin. 1995;118:183–192. [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological psychology. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Zeanah CH, Myers L, Putnam F. Heart period and variability findings in preschool children with posttraumatic stress symptoms. Biological Psychiatry. 2004;55(7):685–691. doi: 10.1016/j.biopsych.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Scheinin H, Helminen A, Huhtala S, Grönroos P, Bosch JA, Kuusela T, Kaila T. Spectral analysis of heart rate variability as a quantitative measure of parasympatholytic effect-integrated pharmacokinetics and pharmacodynamics of three anticholinergic drugs. Therapeutic drug monitoring. 1999;21(2):141–151. doi: 10.1097/00007691-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental psychobiology. 1999;35(2):119–135. [PubMed] [Google Scholar]
- Schmitz J, Kramer M, Tuschen-Caffier B, Heinrichs N, Blechert J. Restricted autonomic flexibility in children with social phobia. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(11):1203–1211. doi: 10.1111/j.1469-7610.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Keenan K, Vondra JI, Delliquadri E, Giovannelli J. Antecedents of preschool children's internalizing problems: A longitudinal study of low-income families. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(12):1760–1767. [PubMed] [Google Scholar]
- Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer CR, Das A. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Development and psychopathology. 2007;19(3):649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- Shoulberg EK, Sijtsema JJ, Murray-Close D. The association between valuing popularity and relational aggression: the moderating effects of actual popularity and physiological reactivity to exclusion. Journal of experimental child psychology. 2011;110(1):20–37. doi: 10.1016/j.jecp.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Sijtsema JJ, Shoulberg EK, Murray-Close D. Physiological reactivity and different forms of aggression in girls: moderating roles of rejection sensitivity and peer rejection. Biological psychology. 2011;86(3):181–192. doi: 10.1016/j.biopsycho.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Huang MH, McCreath H, Sidney S, Liu K, Dale Williams O, Seeman T. Cardiac autonomic control and the effects of age, race, and sex: the CARDIA study. Auton Neurosci. 2008;139(1–2):78–85. doi: 10.1016/j.autneu.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton L, El-Sheikh M, Buckhalt JA. Respiratory sinus arrhythmia and cognitive functioning in children. Developmental psychobiology. 2009;51(3):249–258. doi: 10.1002/dev.20361. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Corey JM. Vagal regulation and observed social behavior in infancy. Social Development. 2001;10(2):189–201. [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of affective disorders. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD) Journal of child psychology and psychiatry, and allied disciplines. 2001;42(5):691–698. [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Porges SW. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child development. 2009;80(4):1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA : the journal of the American Medical Association. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott CM, Landau S. The relation between disinhibition and emotion regulation in boys with attention deficit hyperactivity disorder. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2004;33(4):772–782. doi: 10.1207/s15374424jccp3304_12. [DOI] [PubMed] [Google Scholar]
- Whitson S, El-Sheikh M. Moderators of family conflict and children's adjustment and health. Journal of emotional abuse. 2003;3(1–2):47–73. [Google Scholar]
- Wilder J. The law of initial value in neurology and psychiatry. Journal of Nervous and Mental Diseases. 1956;125:73–86. doi: 10.1097/00005053-195701000-00009. [DOI] [PubMed] [Google Scholar]
- Willemen AM, Schuengel C, Koot HM. Physiological regulation of stress in referred adolescents: the role of the parent-adolescent relationship. Journal of child psychology and psychiatry, and allied disciplines. 2009;50(4):482–490. doi: 10.1111/j.1469-7610.2008.01982.x. [DOI] [PubMed] [Google Scholar]
- Wolff BC, Wadsworth ME, Wilhelm FH, Mauss IB. Children's vagal regulatory capacity predicts attenuated sympathetic stress reactivity in a socially supportive context: Evidence for a protective effect of the vagal system. Development and psychopathology. 2012;24(2):677. doi: 10.1017/S0954579412000247. [DOI] [PubMed] [Google Scholar]