Abstract
Many studies have shown that glutathione (GSH) and cysteine (Cys) / homocysteine (Hcy) levels are interrelated in biological systems. To unravel the complicated biomedical mechanisms by which GSH and Cys/Hcy are involved in various disease states, probes that display distinct signals in response to GSH and Cys/Hcy are highly desirable. In this work, we report a rhodol thioester (1) that responds to GSH and Cys/Hcy with distinct fluorescence emissions in neutral media. Probe 1 reacts with Cys/Hcy to form the corresponding deconjugated spirolactam via a tandem native chemical ligation (NCL) reaction. This intramolecular spirocyclization leads to the “quinone – phenol” transduction of rhodol dyes, and an excited-state intramolecular proton transfer (ESIPT) process between the phenolic hydroxyl proton and the aromatic nitrogen in the benzothiazole unit occurs upon photoexcitation, thus affording 2-(2’-hydroxyphenyl) benzothiazole (HBT) emission (454 nm). In the case of the tripeptide GSH, only transthioesterification takes place removing the intramolecular photo-induced electron transfer (PET) process caused by the electron deficient 4-nitrobenzene moiety giving rise to a large fluorescence enhancement at the rhodol emission band (587 nm). The simultaneous detection of GSH and Cys/Hcy is attributed to the significantly different rates of intramolecular S,N-acyl shift of their corresponding thioester adducts derived from 1. The utility of probe 1 has been demonstrated in various biological systems including serum and cells.
Introduction
Biological thiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play crucial roles in many physiological and pathological processes. GSH is the most abundant intracellular thiol, and plays a central role in combating oxidative stress and maintaining redox homeostasis that is pivotal for cell growth and function.1 Changes in the levels of GSH have been proved to be directly linked to many diseases, such as leucocyte loss, psoriasis, liver damage, cancer and HIV infection.2 Cys is an essential amino acid that is involved in protein synthesis, detoxification, and metabolism. Cys deficiency is involved in slowed growth rate, hair depigmentation, edema, lethargy, liver damage, muscle and fat loss, skin lesions, and weakness.3 Elevated plasma total Hcy levels have been established as an independent risk factor for cardiovascular diseases, Alzheimer's disease, neural tube defects, complications during pregnancy, inflammatory bowel disease and osteoporosis.4 More importantly, many studies have shown that GSH and Cys/Hcy levels are interrelated in biological systems. For example, it has been shown that the absolute synthesis rate and concentration of erythrocyte GSH are reduced in patients infected by HIV and that this appears to be caused, in part, by a shortage of Cys.5 Moreover, extensive studies in rodents have led to the conclusion that the cellular availability of Cys is thought to be the rate-limiting factor in the synthesis of GSH.6 On the other hand, GSH is contemplated as a putative intracellular reservoir of Cys in the liver of adult rats.7 In addition, the synthesis of GSH is dependent on the trans-sulphuration of Hcy.
To unravel the complicated biomedical mechanisms by which GSH and Cys/Hcy are involved in various disease states, reporters that show distinct signals in response to GSH and Cys/Hcy are highly desirable. Fluorescent probes are powerful tools for the detection of biomolecules due to their simplicity, high sensitivity, and suitability for sensing and imaging in live cells or tissues. In recent years, a large number of fluorescent probes for biological thiols based on different mechanisms have been exploited.8 However, it remains a challenge to discriminate among biothiols due to their similar structures and reactivities. To address this problem, considerable efforts have been devoted to the development of fluorescent probes that are able to respond selectively toward a single biothiol target in biological systems. Probes specific for Cys,9 Hcy,10 and GSH11 have been constructed successfully; unfortunately, probes capable of discriminative and simultaneous detection of GSH and Cys/Hcy are extremely rare. To the best of our knowledge, only one other probe has been reported to date with this capabilty.12 However, a long reaction time and the need for surfactant media may limit its application in biological systems. This led us to design a single fluorescent probe to report GSH and Cys/Hcy with well-resolved fluorescent outputs that address these limitations.
Native chemical ligation (NCL) is a powerful tool widely used for the synthesis of proteins.13 A classical NCL involves cascade reactions between a C-terminal thioester peptide (I) and another peptide containing an N-terminal Cys residue (II). In the first step of NCL, a reversible transthioesterification reaction between a C-terminal thioester peptide (I) and the sulfhydryl group of an N-terminal Cys residue (II) affords a thioester-linked intermediate (III), which then undergoes a rapid, irreversible, and spontaneous intramolecular S,N-acyl shift that results in the formation of a native amide bond (IV) at the ligation site (Scheme 1). The above S→N acyl transfer from III to IV is thermodynamically favorable due to the proximity of the amino group in III to the thioester functionality and the involvement of the five-membered intramolecular transition state. This reaction has been extended to other compounds, in which the thioester-linked intermediate rearranges through a six-membered cyclic transition state to form a stable amide-linked product.14 However, the above intramolecular S,N-acyl migration is not favored when the free amino group is far from the thioester functionality owing to entropic considerations involved in a macrocyclic transition state which might be too unstable to be kinetically significant.15 Prompted by the unique characters of the NCL reaction, Lin et al. have designed ratiometric fluorescent probes for biothiols based on fluorescence resonance energy transfer (FRET) signaling mechanism.16 However, these probes are not able to report Cys/Hcy and GSH simultaneously.
Scheme 1.
The principle of native chemical ligation.
We envisioned that the NCL reaction might be utilized to develop fluorescent probes for the discrimination of Cys/Hcy and GSH. Our rationale is depicted in Scheme 2. We reasoned that the mixture of probe 1 with Cys would result in the formation of the corresponding amide 3a via a tandem NCL reaction. At physiological pH, 3a is predominantly present in its deconjugated spirolactam form 4a based on the well-known characteristics of rhodamine amides.17 Concomitantly, the above intramolecular spirocyclization leads to the “quinone – phenol” transduction of rhodol dyes. As a result, an excited-state intramolecular proton transfer (ESIPT) process between the phenolic hydroxyl proton and the aromatic nitrogen in the benzothiazole unit occurs upon photoexcitation, thus affording 2-(2’-hydroxyphenyl) benzothiazole (HBT) emission.18 As for Hcy, a similar ligation reaction would occur through a sixmembered ring intramolecular nucleophilic attack to form the amide-linked product 4b. In the case of GSH, which is a tripeptide containing Glu, Cys and Gly, the analogous transthioesterification can take place to give the thioester 5. However, the subsequent intramolecular S,N-acyl transfer from the Cys sulfur to the N-terminal amino group to form an amide bond is difficult to proceed because a ten-membered cyclic transition state would be involved, which is significantly disfavored due to entropic considerations.15 As a consequence, only a stable thioester 5 is formed, which would result in the removal of the intramolecular photo-induced electron transfer (PET) process caused by the electron deficient 4-nitrobenzene moiety and thus give rise to a large fluorescence enhancement at the rhodol emission band. Based on the above strategy, discriminatory detection of GSH and Cys/Hcy can be achieved.
Scheme 2.
Proposed mechanism for the discriminative detection of GSH and Cys/Hcy using probe 1.
Results and discussion
Our design strategy is based on the fact that a C-terminal thioester peptide shows completely different reaction behaviors toward GSH and Cys/Hcy. In our newly designed sensing system (Scheme 2), rhodol 6 was chosen as a fluorescence reporter because it contains a benzothiazole unit at its 2-position and can exhibit rhodol and HBT emissions in its “open” quinoid form and “closed” spirocyclic form (Scheme S1, ESI), respectively, which has been reported in our previous work.19 The dual emission of rhodol 6 facilitates monitoring the transthioesterification of Cys/Hcy (or GSH) and the subsequent intramolecular S,N-acyl shift reaction in a single experiment. 4-nitrothiophenol was selected to construct the probe on the basis of the following considerations: (i) it has shown that the rate of ligation is dependent on the nature of the thiol leaving group of the peptide-α-thioester. Aryl thiols are better thioester leaving groups than those of alkyl alternatives, and ligation with the former usually proceeds more rapidly than that of latter under identical conditions.20 Furthermore, it was reported that a thiol leaving group containing more potent electron-withdrawing substituents could significantly increase the rate of NCL.13 (ii) The nitro group is a strong quencher of fluorescent dyes, which can quench the probe's fluorescence via an intramolecular PET process.21 Thus, probe 1 would be expected to give weak fluorescence emission in the absence of the target.
To test the aforementioned hypothesis, we set out to synthesize probe 1. Rhodol 6 was obtained according to the method we reported previously,19 which after reaction with 4-nitrothiophenol in anhydrous CH2Cl2 afforded the desired compound 1 (Scheme 3).
Scheme 3.
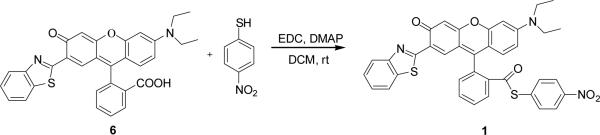
Synthesis of probe 1.
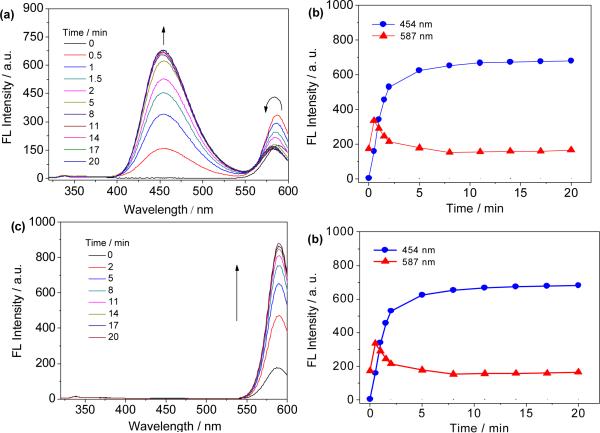
With probe 1 in hand, we first examined its optical sensing behavior toward Cys in DMF/phosphate buffer (3:7 v/v, 20 mM, pH 7.4) at 37 °C. The free probe 1 displays weakly fluorescent emission at 587 nm (φF = 0.051 in 30% aqueous DMF solution, using rhodamine B as reference).22 No emission at the HBT band was observed for probe 1, indicating that it exists in the “open” quinoid form in these conditions. Upon addition of Cys (10 equiv) to the solution of 1, one can see that the emission at 587 nm increases initially as a result of transthioesterification to form the thioester intermediate 2a, thereby removing the 4-nitrobenzene-induced PET quenching process within probe 1. The emission band at 587 nm then decreases with concomitant growth of the emission at 454 nm over time (Fig. 1a and 1b). The latter spectral change is apparently due to the intramolecular S,N-acyl shift of 2a to form the corresponding amide 3a, which further spirocyclizes to give the colorless lactam 4a in neutral conditions. The above process also leads to a free phenolic hydroxyl group at its 3-position. Thus, an ESIPT process within compound 4a occurs, exhibiting HBT emission at 454 nm. Similar fluorescence spectral changes were observed for Hcy (Fig. S1, ESI). In the case of GSH, however, only a fluorescence increase at 587 nm (rhodol emission band) was observed since subsequent intramolecular rearrangement cannot take place (Fig. 1c and 1d). The distinct gap between the two emission bands is over 130 nm, which enables the simultaneous detection of GSH and Cys/Hcy.
Fig. 1.
Optical sensing behavior of 1 towards Cys and GSH. a) Time-dependent fluorescence spectral changes of 1 with Cys. b) Time course of fluorescence intensity of 1 in the presence of Cys. c) Time-dependent fluorescence spectral changes of 1 with GSH. d) Time course of fluorescence intensity of 1 in the presence of GSH. All solutions are composed of 8 μM 1 with 10 equiv of analyte in DMF/phosphate buffer (3:7 v/v, 20 mM, pH 7.4) at 37 °C and λex = 305 nm.
The time courses of the fluorescence response of probe 1 in the presence of Cys, Hcy or GSH were studied, respectively. It can be observed from Fig. 1b (or Fig. S1b, ESI) that the fluorescence intensity at 454 nm increases dramatically upon addition of Cys (or Hcy) and the sensing reaction can be completed within 5 min. In the case of GSH, the fluorescence intensity at 587 nm plateaus in 10 min (Fig. 1d). Under pseudo-first-order kinetic conditions,23 the observed rate constant (Kobs) for Cys, Hcy and GSH were determined as 0.241 ± 0.010, 0.238 ± 0.007, and 0.143 ± 0.006 min−1, respectively (Fig. S2, ESI). The fast reaction rate is apparently due to the strong electrophilic thioester leaving group in probe 1, which makes the probe highly susceptible to sulfhydryl nucleophiles.
In addition, GSH and Cys/Hcy can be easily distinguished by the naked eye when illuminated by a hand-held UV lamp at 365 nm (Fig 2). Introduction of GSH to the solution of 1 results in the strong red emission color. By contrast, Cys/Hcy elicits a significant blue emission. Scheme 2 depicts the reaction pathways and associated signaling mechanisms.
Fig. 2.

Solutions of 1 alone and in the presence of GSH, Cys and Hcy exposed to a UV lamp at 365 nm.
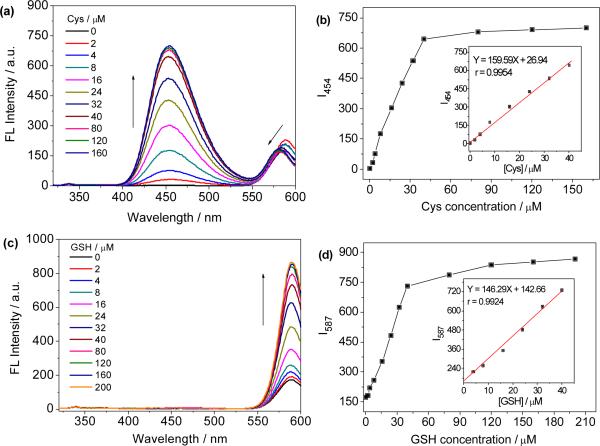
The changes in the absorption and emission spectra of probe 1 upon addition of different concentrations of Cys, Hcy or GSH were evaluated in DMF/phosphate buffer (3:7 v/v, 20 mM, pH 7.4) solution at 37 °C. With gradually increasing amounts of Cys, the absorption band around 570 nm decreased with concomitant growth of a new band at 425 nm (Fig. S3, ESI). The decrease of the absorption at 570 nm is due to the formation of spirolactam 4a, which is colorless and shows no absorption in visible spectral range. The increase of the absorption at 425 nm is apparently attributed to the free 4-nitrothiophenol generated via the transthioesterification of 1 with Cys. When excited at 305 nm, it can be observed that the fluorescence intensity at 454 nm increases with increasing Cys concentration, but the intensity at 587 nm decreases gradually and the emission maximum blue-shifts to 581 nm, which is in accordance with the absorption changes of 1 (Fig. S3, ESI). Similar results were also observed for Hcy, as shown in Fig. S4. The fluorescent intensity at 454 nm is linearly proportional to the amount of Cys from 0 to 40 μM (Fig. 3b). Moreover, the ratiometric sensing of Cys and Hcy were also applicable. The emission ratio (I454/I586) shows a variation from 0.018 to 4.002 (a 222-fold enhancement) for Cys (160 μM) and from 0.018 to 3.866 (a 215-fold enhancement) for Hcy (160 μM) (Fig. S5, ESI). In the case of GSH, however, both the absorption at 425 and 570 nm increases with increasing GSH concentration (Fig. S6, ESI). The increase of absorption at 570 nm might be due to the molar efficiency of rhodol 5 is higher than that of probe 1. The fluorescence intensity at 587 nm increases with increasing GSH concentration and the fluorescent intensity is linearly proportional to GSH concentration up to 40 μM (Fig. 3c and 3d). The detection limits (S/N = 3) for Cys, Hcy and GSH were determined to be 44, 52 and 96 nM, respectively.
Fig. 3.
Concentration dependent response of 1 towards to Cys and GSH. a) Fluorescence spectra of probe 1 upon addition of increasing concentrations of Cys. b) Plot of the fluorescence intensity as a function of Cys concentration. c) Fluorescence spectra of probe 1 upon addition of increasing concentrations of GSH. d) Plot of the fluorescence intensity as a function of GSH concentration. Insets in (b) and (d) show the linear relationship between maximum fluorescence intensity and the analyte concentration. All solutions are composed of 8 μM 1 with DMF/phosphate buffer (3:7 v/v, 20 mM, pH 7.4) at 37 °C for 10 min and λex = 305 nm.
Control experiments were carried out to gain further insight into the sensing mechanism of probe 1 toward Cys/Hcy and GSH. First, cysteamine was introduced to the solution of 1 and similar fluorescence spectra changes are observed as for Cys under analogous reaction conditions (Fig. S7, ESI), indicating that the carboxylic group is irrelevant to the fluorescence response. Furthermore, N-acetylcysteine (NAC) was tested and affords a similar result to that of GSH because of the lack of a free amino group (Fig. S8, ESI). These experiments confirm the absolute requirement of an amino group for the intramolecular rearrangement, which is also responsible for the differential sensing of Cys/Hcy and GSH.
Furthermore, the product mixture of cysteamine and 1 in CH3OH was separated and 7 was obtained (cysteamine was used instead of Cys because 7 is easier to isolate from the reaction mixture). The structure of 7 was characterized by 1H NMR, 13C NMR, and HRMS (Fig. S16–S18, ESI). In the 1H NMR spectrum of 7, the resonance signal at around 1.67 ppm, assigned to the thiol proton, is clearly observed, which proves that a NCL reaction indeed occurs between probe 1 and cysteamine. Moreover, a single peak corresponding to the phenolic hydroxyl group emerged at 12.77 ppm (while almost no such peak was observed for 1 under identical conditions), suggesting that the “quinone-phenol” transduction indeed occurs in the sensing event (Fig. 4). In addition, a prominent peak at 64.38 ppm, corresponding to the spiro carbon at the 9-position of compound 7, was clearly observed in its 13C NMR spectrum, which proves that 7 is present predominately in its colorless spirolactam form.24 The above results are further confirmed by comparing the optical spectra of 7 and HBT. As shown in Fig. S9 (ESI), compound 7 exhibits no rhodol spectroscopic characteristics but displays the absorption and emission spectra highly similar to that of the original HBT. In addition, the formation of 5 was evidenced by mass spectrum analysis of the product generated from the incubation of 1 with GSH in CH3CN – H2O (10: 1, v/v) solution. A prominent peak at m/z = 808.2089 corresponding to [5-H]- (calcd 808.2111 for C41H38N5O9S2) is clearly observed in the HRMS data (Fig. S19, ESI), which provides strong evidence that only transthioesterification occurs between probe 1 and GSH. The above results are in good agreement with the designed strategy shown in Scheme 2.
Fig. 4.
Partial 1H NMR (400 MHz) spectra of probe 1 (a) and compound 7 (b) in CDCl3.
To study the selectivity of probe 1 toward GSH and Cys/Hcy, the fluorescence spectral changes of 1 caused by other biologically relevant species, such as Pro, Leu, Ala, Gln, Ser, Val, Lys, Phe, Thr, Met, Asp, Ile, Arg, His, Glu, Tyr, ascorbic acid and glucose were also explored. It can be seen that only Cys/Hcy and GSH exhibit significant fluorescence intensity changes at 454 and 587 nm, respectively, whereas other analytes cause no visible changes in emission under the same conditions (Fig. 5). The above results prove that the selectivity of probe 1 toward GSH and Cys/Hcy over other analytes is remarkably high.
Fig. 5.
Optical sensing behavior of 1 towards Cys, Hcy and GSH as compared to other biologically relevant species. Fluorescence spectra of 1 upon addition of different analytes (Cys, Hcy, GSH, Pro, Leu, Ala, Gln, Ser, Val, Lys, Phe, Thr, Met, Asp, Ile, Arg, His, Glu, Tyr and ascorbic acid, 40 μM; glucose, 400 μM). All solutions are composed of 8 μM 1 with DMF/phosphate buffer (3:7 v/v, 20 mM, pH 7.4) at 37 °C for 10 min and λex = 305 nm.
Furthermore, the subsequent addition of Cys to the mixture solution of 1-GSH affords no obvious changes of fluorescence spectra (Fig. S10, ESI), indicating almost no further NCL reaction between 5 and Cys occurs, which is very important for the discrimination between GSH and Cys/Hcy. The significant difference in NCL behaviors between 1 and 5 might be explained by the fact that 1 contains a better thiol leaving group than 5. Generally, the leaving ability of thiols correlates with the rate of transthioesterification and is dependent on the pKa of the thiol. The lower pKa of the thiol leaving group usually results in higher reaction rates.25 Since the thiol leaving group of 1 has a much lower pKa value than 5 (the thiol pKa values of GSH and 4-nitrothiophenol are 8.526 and 4.6720a, respectively), the alkyl thioester 5 produced by thiol-thioester exchange is rather unreactive, thus almost no further NCL reaction between 5 and Cys being observed. Another plausible explanation is that phenyl thioesters react faster when compared to the alkyl thioesters under NCL conditions.20a, 27
Finally, we demonstrate the simultaneous sensing of GSH and Cys/Hcy. A mixture of Cys and GSH was introduced to a solution of 1 and as expected, the fluorescence intensities at 454 and 587 nm are both increased dramatically (Fig. S11, ESI). Theoretically, the free thiol group in 4a/4b could compete with GSH for probe 1 leading to selectivity issues in the sensing of GSH when Cys/Hcy and GSH are present in a mixing solution. To investigate this potential issue, GSH and model compound 7 (analogous to 4a) were introduced to solutions of probe 1. Compound 7 did not afford significant fluorescence increase at 587 nm as compared to GSH (Fig. S12 ESI). The reactivity of these differing thiol groups are significantly different due to differences in their steric bulk.28 Transthioesterification with probe 1 with GSH is efficient. However, the inherent reactivity of the thiol groups in the spirolactamized rhodol unit of 4a/4b is reduced.
Two representative biological samples were analyzed to prove the usefulness of the proposed probe for the differential sensing of GSH and Cys. Firstly, probe 1 was applied to detect Cys and GSH in MDA-MB-231 human breast cancer cells. Varying amounts of the lysed cells were added to the solution of 1 and incubated at 37 °C for 10 min. As shown in Fig. 6a, the increases in the amount of cells induce a pronounced enhancement in fluorescence emission at 587 nm and a slight increase at 454 nm. These results are in line with the previous studies that the amount of GSH in cells is much higher than that of Cys (the intracellular concentrations of GSH and Cys are 1–10 mM and 30–200 μM, respectively).12, 29 Furthermore, aliquots of a reduced human serum samples were introduced to solution 1.30 Conversely, it was observed that the fluorescence emission at 454 nm increases significantly, but the emission at 587 nm affords a little increment (Fig. 6b). This indicates that the concentration of Cys is much higher than that of GSH in human serum, which is in accordance with the literature reported results (the concentrations of GSH and Cys in human serum are 14 ± 7 and 165.1 – 335.3 μM, respectively.)31 Thus, taken together, these results demonstrate that 1 enables signaling GSH and Cys with different readouts, even if two analytes are simultaneously present in solution.
Fig. 6.

Optical sensing behavior of 1 towards Cys/Hcy and GSH in representative biological samples. a) Fluorescence spectra of 1 upon addition of different amounts of MDA-MB-231 human breast cancer cells. b) Fluorescence spectra of 1 upon addition of different amounts of reduced human serum. All solutions are composed of 8 μM 1 with DMF/phosphate buffer (3:7 v/v, 20 mM, pH 7.4) at 37 °C for 10 min and λex = 305 nm.
We next investigated the potential use of probe 1 in live cell imaging. When HepG2 cells were incubated with 1 for 30 min, a bright fluorescence in the red channel (Fig. 7b) and a weak fluorescence in the blue channel (Fig. 7a) were observed simultaneously. Furthermore, when the cells were pretreated with N-ethylmaleimide (NEM, a scavenger of biothiols)32 and then treated with 1, we could note the fluorescence in the red (Fig. 7d) and blue channels (Fig. 7c) are both decreased dramatically, indicating that 1 is responsive to changing intracellular Cys and GSH levels at the blue and red channels, respectively. The above results demonstrate the suitability of probe 1 for the simultaneous imaging of GSH and Cys in live cells.
Fig. 7.

Confocal fluorescence images of live HepG2 cells. a) Cells stained by 1; Blue-channel. b) Cells stained by 1; Red-channel. c) Cells preincubated with NEM followed by incubation with 1; Blue-channel. d) Cells preincubated with NEM followed by incubation with 1; Red-channel. Cells were preincubated with 1.0 mM NEM for 40 min in (c) and (d). All cells were stained by 8 μM 1 for 30 min. Blue-channel images were collected with band pass of 425–475 nm (λex = 405 nm). Red-channel images were collected with band pass of 560–660 nm (λex = 543 nm).
Conclusions
In summary, we report a new fluorescent probe for the differential sensing of GSH and Cys/Hcy by combining PET and ESIPT strategies with the NCL reaction. The discriminative detection of GSH and Cys/Hcy is attributed to significantly different rates of intramolecular rearrangement of their corresponding thioester adducts derived from 1. The proposed sensing platform shows two well-separated emission bands (> 130 nm) for GSH and Cys/Hcy, which favors the simultaneous determination of GSH and Cys/Hcy. Further research of 1 as a probe for dissecting the complicated relationships between GSH and Cys/Hcy in some disease states is in progress.
Supplementary Material
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21275117, 21375105), the Science & Technology Department (No. 2012JM2004), the Education Department (No. 12JK0518) of Shaanxi Province of China and the National Institutes of Health (RO1 EB002044).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details, including experimental procedures, synthesis of the probe, 1H NMR and 13C NMR data, high resolution mass spectra, and the absorption and fluorescence response of 1 toward other biothiols. See DOI: 10.1039/b000000x/
Notes and references
- 1.Dalton TP, Shertzer HG, Puga A. Annu. Rev. Pharmacol. Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 2.a Townsend DM, Tew KD, Tapiero H. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Estrela JM, Ortega A, Obrador E. Crit. Rev. Clin. Lab. Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]; c Helgling B, Von Overbeck J, Lauterburg BH. Eur. J. Clin. Invest. 1996;26:38–44. doi: 10.1046/j.1365-2362.1996.88237.x. [DOI] [PubMed] [Google Scholar]
- 3.Shahrokhian S. Anal. Chem. 2001;73:5972–5978. doi: 10.1021/ac010541m. [DOI] [PubMed] [Google Scholar]
- 4.a Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PWF, Wolf PA. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]; b Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, Scott JM. Clin. Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 5.Jahoor F, Jackson A, Gazzard B, Philips G, Sharpstone D, Frazer ME, Heird W. Am. J. Physiol. 1999;276:E205–E211. doi: 10.1152/ajpendo.1999.276.1.E205. [DOI] [PubMed] [Google Scholar]
- 6.a Griffith OW, Meister A. Proc. Natl. Acad. Sci. 1979;76:5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Stipanuk MH, Coloso RM, Garcia RA, Banks MF. J. Nutr. 1992;122:420–427. doi: 10.1093/jn/122.3.420. [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo J, Garvey JS, Armario A. J. Pharmacol. Exp. Ther. 1990;255:554–564. [PubMed] [Google Scholar]
- 8.a Chen X, Zhou Y, Peng X, Yoon J. Chem. Soc. Rev. 2010;39:2120–2135. doi: 10.1039/b925092a. [DOI] [PubMed] [Google Scholar]; b Yin C, Huo F, Zhang J, Martínez-Máňez R, Yang Y, Lv H, Li S. Chem. Soc. Rev. 2013;42:6032–6059. doi: 10.1039/c3cs60055f. [DOI] [PubMed] [Google Scholar]; c Jung HS, Chen X, Kim JS, Yoon J. Chem. Soc. Rev. 2013;42:6019–6031. doi: 10.1039/c3cs60024f. [DOI] [PubMed] [Google Scholar]; d Yang X, Guo Y, Strongin RM. Angew. Chem. Int. Ed. 2011;50:10690–10693. doi: 10.1002/anie.201103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Li HL, Fan JL, Wang JY, Tian MZ, Du JJ, Sun SG, Sun PP, Peng XJ. Chem. Commun. 2009:5904–5906. doi: 10.1039/b907511a. [DOI] [PubMed] [Google Scholar]; b Zhou X, Jin X, Sun G, Li D, Wu X. Chem. Commun. 2012;48:8793–8795. doi: 10.1039/c2cc33971d. [DOI] [PubMed] [Google Scholar]; c Zhou X, Jin X, Sun G, Wu X. Chem. Eur. J. 2013;19:7817–7824. doi: 10.1002/chem.201300078. [DOI] [PubMed] [Google Scholar]; d Guo Z, Nam S, Park S, Yoon J. Chem. Sci. 2012;3:2760–2765. [Google Scholar]; e Yang X, Guo Y, Strongin RM. Org. Biomol. Chem. 2012;10:2739–2741. doi: 10.1039/c2ob25178g. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Wang H, Zhou G, Gai H, Chen X. Chem. Commun. 2012;48:8341–8343. doi: 10.1039/c2cc33932c. [DOI] [PubMed] [Google Scholar]; g Niu L-Y, Guan Y-S, Chen Y-Z, Wu L-Z, Tung C-H, Yang Q-Z. Chem. Commun. 2013;49:1294–1296. doi: 10.1039/c2cc38429a. [DOI] [PubMed] [Google Scholar]
- 10.a Chen HL, Zhao Q, Wu YB, Li FY, Yang H, Yi T, Huang CH. Inorg. Chem. 2007;46:11075–11081. doi: 10.1021/ic7010887. [DOI] [PubMed] [Google Scholar]; b Wang W, Escobedo JO, Lawrence CM, Strongin RM. J. Am. Chem. Soc. 2004;126:3400–3401. doi: 10.1021/ja0318838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu L-Y, Guan Y-S, Chen Y-Z, Wu L-Z, Tung C-H, Yang Q-Z. J. Am. Chem. Soc. 2012;134:18928–18931. doi: 10.1021/ja309079f. [11] [DOI] [PubMed] [Google Scholar]; b Guo Y, Yang X, Hakuna L, Barve A, Escobedo JO, Lowry M, Strongin RM. Sensors. 2012;12:5940–5950. doi: 10.3390/s120505940. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shao N, Jin J, Wang H, Zheng J, Yang R, Chan W, Abliz Z. J. Am. Chem. Soc. 2010;132:725–736. doi: 10.1021/ja908215t. [DOI] [PubMed] [Google Scholar]
- 12.During the preparation of this manuscript, a first fluorescent probe for the simultaneous detection of Cys and GSH has been reported. See ref. Liu J, Sun Y-Q, Huo Y, Zhang H, Wang L, Zhang P, Song D, Shi Y, Guo W. J. Am. Chem. Soc. 2014;136:574–577. doi: 10.1021/ja409578w.
- 13.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 14.Canne LE, Bark SJ, Kent SBH. J. Am. Chem. Soc. 1996;118:5891–5896. [Google Scholar]
- 15.a Martin RB, Hedrick RI. J. Am. Chem. Soc. 1962;84:106–110. [Google Scholar]; b Mandolini L. J. Am. Chem. Soc. 1978;100:550–554. [Google Scholar]; c Illuminati G, Mandolini L. Accounts Chem. Res. 1981;14:95–102. [Google Scholar]
- 16.a Long L, Lin W, Chen B, Gao W, Yuan L. Chem. Commun. 2011;47:893–895. doi: 10.1039/c0cc03806g. [DOI] [PubMed] [Google Scholar]; b Yuan L, Lin W, Xie Y, Zhu S, Zhao S. Chem. Eur. J. 2012;18:14520–14526. doi: 10.1002/chem.201201606. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Pradhan T, Wang F, Kim JS, Yoon J. Chem. Rev. 2012;112:1910–1956. doi: 10.1021/cr200201z. [DOI] [PubMed] [Google Scholar]
- 18.a Lochbrunner S, Wurzer AJ, Riedlea E. J. Chem. Phys. 2000;112:10699–10702. [Google Scholar]; b Brewer WE, Martinez ML, Chou P-T. J. Phys. Chem. 1990;94:1915–1918. [Google Scholar]
- 19.Wen H, Huang Q, Yang X-F, Li H. Chem. Commun. 2013;49:4956–4958. doi: 10.1039/c3cc41343h. [DOI] [PubMed] [Google Scholar]
- 20.a Johnson ECB, Kent SBH. J. Am. Chem. Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]; b Dawson PE, Churchill MJ, Ghadiri MR, Kent SBH. J. Am. Chem. Soc. 1997;119:4325–4329. [Google Scholar]
- 21.a Munkholm C, Parkinson DR, Walt DR. J. Am. Chem. Soc. 1990;112:2608–2612. [Google Scholar]; b Ueno T, Urano Y, Kojima H, Nagano T. J. Am. Chem. Soc. 2006;128:10640–10641. doi: 10.1021/ja061972v. [DOI] [PubMed] [Google Scholar]; c Kobayashi T, Urano Y, Kamiya M, Ueno T, Kojima H, Nagano T. J. Am. Chem. Soc. 2007;129:6696–6697. doi: 10.1021/ja070376d. [DOI] [PubMed] [Google Scholar]
- 22.The fluorescence quantum yield of 1 was determined by using rhodamine B (φF = 0.66 in ethanol) as standard. see Ref. Parker CA, Rees WT. Analyst. 1960;85:587–600.
- 23.a Li H, Wen Z, Jin L, Kan Y, Yin B. Chem. Commun. 2012;48:11659–11661. doi: 10.1039/c2cc36838b. [DOI] [PubMed] [Google Scholar]; b Dale TJ, Rebek J. J. Am. Chem. Soc. 2006;128:4500–4501. doi: 10.1021/ja057449i. [DOI] [PubMed] [Google Scholar]
- 24.a Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, Yoon J. J. Am. Chem. Soc. 2005;127:10107–10111. doi: 10.1021/ja051075b. [DOI] [PubMed] [Google Scholar]; b Anthoni U, Christophersen C, Nielsen PH, Piischl A, Schaumburg K. Struct. Chem. 1995;6:161–165. [Google Scholar]
- 25.Zhou W, Shultz JW, Murphy N, Hawkins EM, Bernad L, Good T, Moothart L, Frackman S, Klaubert DH, Bulleit RF, Wood KV. Chem. Commun. 2006:4620–4622. doi: 10.1039/b610682j. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan U, Mieyal PA, Mieyal JJ. Biochemistry. 1997;36:3199–3206. doi: 10.1021/bi962017t. [DOI] [PubMed] [Google Scholar]
- 27.Bang D, Pentelute BL, Gates ZP, Kent SB. Org. Lett. 2006;8:1049–1052. doi: 10.1021/ol052811j. [DOI] [PubMed] [Google Scholar]
- 28.a Shiu H-Y, Chong H-C, Leung Y-C, Wong M-K, Che C-M. Chem. Eur. J. 2010;16:3308–3313. doi: 10.1002/chem.200903121. [DOI] [PubMed] [Google Scholar]; b Jung HS, Han JH, Pradhan T, Kim S, Lee SW, Sessler JL, Kim TW, Kang C, Kim JS. Biomaterials. 2012;33:945–953. doi: 10.1016/j.biomaterials.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Schafer FQ, Buettner GR. Free Radical Biol. Med. 2011;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. Hcy would not significantly interfere with Cys assay because the total concentration of Hcy in biological samples is much lower than that of Cys. See ref. Rusin O, St. Luce NN, Agbaria RA, Escobedo JO, Jiang S, Warner IM, Dawan FB, Lian K, Strongin RM. J. Am. Chem. Soc. 2004;126:438–439. doi: 10.1021/ja036297t. and reference therein.
- 30.a Shang L, Yin J, Li J, Jin L, Dong S. Biosens. Bioelectron. 2009;25:269–274. doi: 10.1016/j.bios.2009.06.021. [DOI] [PubMed] [Google Scholar]; b Ivanov AR, Nazimov IV, Baratova L. J. Chromatogr. A. 2000;895:157–166. doi: 10.1016/s0021-9673(00)00713-5. [DOI] [PubMed] [Google Scholar]
- 31.a Jacobsen DW, Gatautis VJ, Green R, Robinson K, Savon SR, Secic M, Ji J, Otto JM, Taylor LM. Clin. Chem. 1994;40:873–881. [PubMed] [Google Scholar]; b Ścibior D, Skrzycki M, Podsiad M, Czeczot H. Clin. Biochem. 2008;41:852–858. doi: 10.1016/j.clinbiochem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Becker PS, Cohen CM, Lux SE. J. Bio. Chem. 1986;261:4620–4628. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.