Abstract
The concepts for management of rectal cancer have changed drastically over the past few years. Through national bowel cancer screening programmes in the Western countries and the increasing use of endoscopic procedures as diagnostic tool, there is increase in detection of rectal cancer in early stages. There is increase in ageing population worldwide but more so in Western countries. In addition, there is realisation of harm from extirpative surgical procedures which are directed towards managing advanced rectal cancer in the past. Increase in cost of health care burden has also led the investigators to seek alternative treatment options which are effective, safe and cost effective. There are several modern radiation techniques which fits this bill and we need to be aware of newer novel radiation techniques to fulfil this gap.
Keywords: Early rectal cancer, X-ray contact, complete clinical response brachytherapy
Background
The concept for management of rectal cancer has changed significantly in the past decade. There are several reasons for this. Many western countries have set up national bowel cancer screening programs which have targeted earlier stage rectal tumors compared with the more advanced staged cancers which were only diagnosed when they become symptomatic. Therefore, the surgical techniques that were aimed at treating advanced rectal tumours should not apply to the earlier stage disease. There is also recognition of surgical mortality and morbidity, especially in the elderly cohort (1). Many rectal cancer trials now include a wait and watch approach for those who achieved a complete clinical response. This allows organ preservation which has less detrimental effect on bowel function. Moreover, several clinical trials have shown improved disease free survival for those who achieved a complete response (2). In addition, there is evidence from the population-based statistics of an increase in rectal cancer in the ageing population worldwide with the average age of patients with rectal cancer predicted to rise from 65 to above 75 years within the next decade. The recent economic down turn across the world also has highlighted the financial burden of cancer care on the health care providers and many are seeking alternative strategies to keep the cost down without compromising outcomes. Radiotherapy is cheap compared to other treatment modalities. Novel radiation techniques have been developed which are attractive as alternatives to currently available radiotherapy options especially in treatment of early rectal cancer in the elderly.
Dose escalation to improve outcomes
There is evidence for dose response in rectal tumours and radiotherapy dose escalation could improve local control and other outcomes. However, there is a limit to how much radiation dose can be safely delivered using external beam alone without causing undue toxicity to the normal surrounding tissues. The dose escalation trial from Princes Margaret trial has shown that although higher pathological responses can be achieved, the toxicity also increased, which negates the therapeutic ratio (3). The addition of chemotherapy does improve pathological complete response (pCR) rates (Table 1) and chemo radiotherapy has now become the standard of care in rectal cancer management (4). Traditionally, 5FU based regimes were used but oral capecitabine, which is much more convenient to use, has replaced this and has become the standard of care. The addition of oxaliplatin to capecitabine has not kept up with earlier expectations. Both the French ACCORD (5) and the Italian STAR (6) trials have not shown benefit from the addition of oxaliplatin to either capecitabine or 5FU. However, the addition of irinotecan has shown some benefits and there are ongoing trials evaluating the role of irinotecan combined with capecitabine as in the UK lead ARISTOTLE trial.
Table 1. Comparative complete pathological response following chemo radiotherapy.
| n | Dose (Gy) | pCR (%) | |
|---|---|---|---|
| ACCORD | 598 | 44-50 | 11-25 |
| STAR | 720 | 46 | 16 |
| NSABP-4 | 1,608 | 50 | 20 |
| CAO-4 | 1,265 | 50 | 13-17 |
| PETTAC-6 | 1,094 | 44 | 11-13 |
pCR, pathological complete response.
Brachytherapy in rectal cancer
Over the years investigators has evaluated the role of brachytherapy to assess whether deliver of higher dose of radiation using brachytherapy as a boost improve outcomes. There are three types of brachytherapy:
Contact X-ray brachytherapy (Papillon);
High dose rate (HDR) intra luminal rectal brachytherapy;
Interstitial rectal brachytherapy implant.
Contact X-ray brachytherapy (Papillon)
Low energy (50 KV) X-rays are used to deliver contact X-ray brachytherapy. It has been in clinical use for the past 80 years. However, very few centres around the world have continued to use this technique. There are several reasons for this. Firstly, the numbers of cases suitable for this type of treatment are small. There is development of newer competing surgical techniques e.g., Trans anal Endoscopic Micro Surgery (TEMS), Trans anal Endoscopic Operation (TEO) and Trans Anal Minimally Invasive Surgery (TAMIS) which are currently been used more for patients with early small rectal cancers. Only very few patients who are not fit for general anesthesia are referred for brachytherapy. Secondly, there were no replacement machines for the obsolete Philips 50 KV machines, which have been out of production since the mid 70’s. Recently, there has been a revival of interest in contact X-rays brachytherapy and there are at least two companies Ariane (Derby, UK) and Xsoft (Axxend, CA) which have manufactured modern machines to produce 50 KV X-rays for use in contact X-ray brachytherapy.
The principle of contact X-ray brachytherapy consists of delivering high dose (30 Gy) of low energy (50 KV) X-rays applied straight on to the tumour under direct vision. This minimizes the chance of geographic miss. The dose falls off rapidly. The 100% dose is prescribed at the surface and the dose falls to 60% at 5 mm depth. Tumour size <3 cm can be offered X-ray contact radiotherapy initially. The treatment is given every 2 weeks which allows recovery of normal tissues in between treatments. As it is an orthovoltage radiation, the biological equivalent dose (EQD) is high at 1.4-1.6. Therefore, the total radiation dose delivered is above 40 Gy given in just over a minute instead of the usual protracted small doses of radiation given over 4-5 weeks. The applicator size use depends on the size of the tumour ranging from 30-22 mm. The patient is usually treated in knee chest position traditionally but can be treated in lithotomy position depending on the location of the tumour. The treatment can be delivered as an out-patient without the need for general anesthesia.
Assessment after two treatments is crucial to differentiate the good responders from the poor responders. If the response is favorable, further X-ray contact brachytherapy is offered to a total of four treatments (Figure 1). The radiation dose is usually 90-110 Gy in three to four fractions given every 2 weeks. For tumors which are initially staged as T2 or early T3, the risk of lymph node spread is high (20-30%). External beam chemo radiotherapy 45 Gy or its radiobiological equivalent should be offered to sterilize the lymph nodes. For bulky tumors >3 cm the treatment starts with external beam chemo radiotherapy. The response is assess within 2-3 weeks after the completion of treatment. For good responders (tumour regresses >80%), this can be followed by X-ray contact radiotherapy to improve local control and higher chance of complete clinical response (7). This assumption will be evaluated in a randomised trial (OPERA) which randomised between standard chemoradiotherapy against standard CRT and contact X-ray radiotherapy boost. This trial is planned to start early next year. If the response is poor (<80% regression) then patients are advised to accept immediate salvage surgery, provided the patient is fit and agreeable for surgery that involves a stoma.
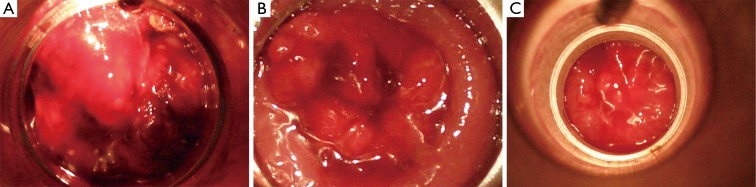
Figure 1.
(A) Showing polypoidal tumour pre-treatment (day 1); (B) showing regression of tumour after one fraction (day 14); (C) showing complete regression of tumour after two fractions (day 28). Example of a good responder.
HDR rectal brachytherapy
HDR intra luminal rectal brachytherapy uses either Ir192 or Co60. There are several commercially available remote after loaders. A number of different rectal applicators can be used depending on the system selected:
Multiple channel rectal applicator (OncoSmart®, Elekta);
Rectal/vagina rigid single line applicator Elekta/Eckert & Ziegler (Bebig);
Rectal/vagina rigid single line applicator with variable central shielding Elekta/Eckert & Ziegler (Bebig);
Single line flexible endo-bronchial source (Elekta).
Multi channel rectal brachytherapy applicator
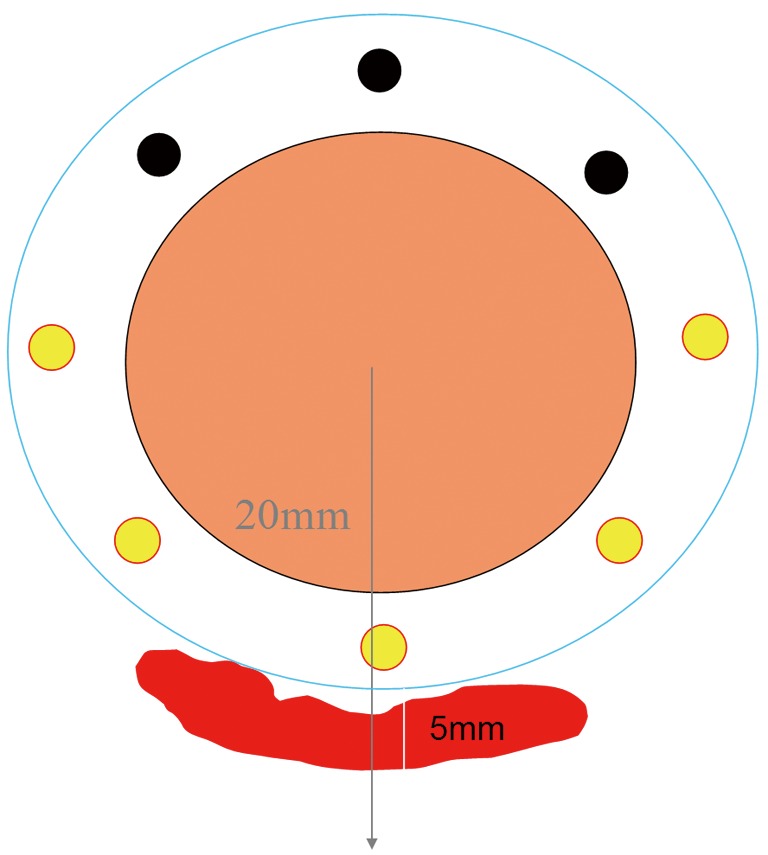
This rectal applicator has the advantage of using the channels close to where the tumour is situated and thus spare the contra lateral rectal mucosa (Figure 2). A balloon can be used to push the normal rectal mucosa away from the treatment source. Central shielding to minimize the dose to contra later rectal mucosa has also been investigated. It is suitable for any height of rectal tumour either low, mid or upper. It is a flexible applicator and more comfortable for the patient. It can be applied without general anesthesia (8) (Figure 3).
Figure 2.
Showing multiple channels in flexible rectal applicator. Treatment is loaded towards residual tumour thus sparing the contra lateral uninvolved rectal mucosa.
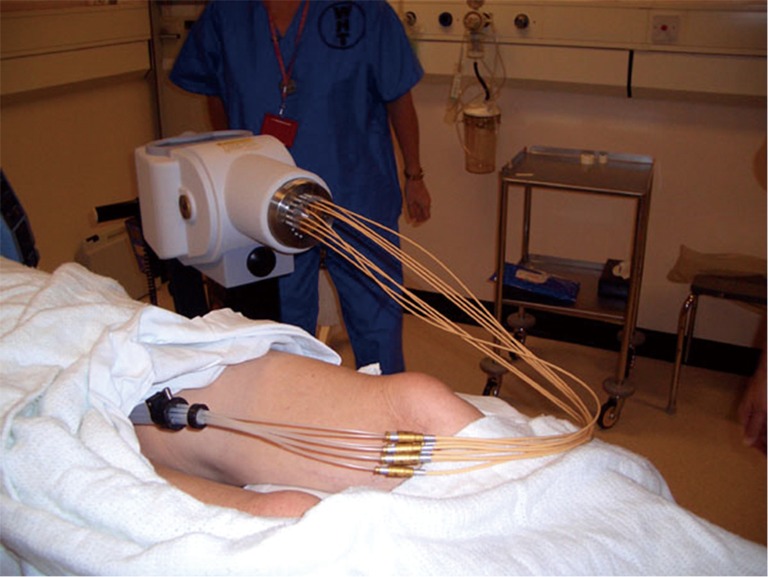
Figure 3.
Showing treatment position for high dose rate (HDR) rectal brachytherapy.
Rectal/vaginal rigid single line brachytherapy applicator
This type of applicator is suitable for low rectal tumors which occupy more than 50% of the rectal wall. It is not suitable for mid to high rectal tumors. There are different diameter applicators and stenosing tumors may need a defunctioning stoma before brachytherapy. This applicator is much easier to use.
Rectal/vagina rigid single line applicator with variable central shielding
This type of rectal applicator is suitable for smaller low rectal tumors which occupy less than half the circumference. Central shielding can be used to protect the contra lateral uninvolved rectal mucosa (9).
Rectal brachytherapy procedure
Endoscopy is carried out initially to assess the position and length of the rectal tumour. Marker seeds are inserted at the lower end of the tumour to locate it on the radiographs. The rectal brachytherapy applicator is inserted via the anus into the rectum either under general or local anesthesia. The position of the rectal applicator is checked on the fluoroscopy and adjusted as necessary. Once the position is satisfactory it is secured in place by clamps or strings tight to the corset. The patient treatment position is shown in Figure 3. The patient is then scanned on the CT simulator. The tumour position is outlined based on the information from the digital examination (lower rectal tumour), endoscopy and MRI. The dose is prescribed to cover the PTV (CTV + margin).The dose given depends on whether brachytherapy is given as monotherapy or as a boost after external beam chemo radiotherapy (10). Although the dose for monotherapy (26 Gy given over 4 daily treatments) is now fairly standard based on extensive experience from McGill University (8) much work is still needed to be done to determine dose for the brachytherapy boost.
Interstitial rectal implant using rectal template
For rectal tumors which extend into the anal canal, none of the above brachytherapy techniques are suitable. However, an interstitial implant using a rectal/anal jig can be performed if there is residual tumour following external beam chemo radiotherapy. Most centres use a template with needles which are implanted through the perineum and into the tissues outside the wall of rectum. The iridium wires which were formerly used have now been replaced by fractionated HDR brachytherapy. The dose given varies but the usual schedule is 5-7 Gy in 3 fractions over 24 hours.
Selection of type of brachytherapy
Whether we should use contact X-ray brachytherapy or HDR isotope brachytherapy is determine by the morphology and the stage of rectal cancer. Exophytic usually sessile rectal cancers confined to the bowel wall are best treated by X-ray brachytherapy as the maximum dose of radiation is applied on to the surface of the rectal wall.
There is very little penetration and it is not useful for a tumor that penetrates much beyond the rectal wall. Therefore, tumors that infiltrate beyond the rectal wall are not suitable for contact X-ray brachytherapy. The exophytic component that protrudes from the rectal wall into the lumen gets a much higher dose due to the inverse square law. The tumour is shaved off layer by layer with each application of the contact X-ray brachytherapy until it regressed down to the surface of the rectal mucosa. The shrinkage is centripetal and the tumor regresses back to the site of origin in the case of a small rectal tumor. At the end of treatment, there may be a small superficial ulcer with smooth edge or a supple mucosa with no indurations beneath its base. This usually heals within 3-6 months if there is no residual tumour. However, those with residual tumour (if viable) can grow back within this period. Contact X-ray brachytherapy is therefore only suitable for T1/early T2 tumors that have not penetrated much into the muscularis propria. However, it is often very difficult to differentiate between T1 and early T2 tumors with the currently available radiological techniques.
HDR isotope brachytherapy is used when the tumour penetrates beyond the rectal wall (T3). This penetration can be readily seen on the MRI and EUS. It can be used as monotherapy or as a boost after external beam chemo radiotherapy when the residual tumour extends beyond the rectal wall. The radiation dose required to sterilize and kill off the residual tumour after external beam chemo radiotherapy is still under investigation and is not yet fully established. The dose currently in use is either 5-10 Gy in single fraction or 7-10 Gy per fraction in 3 weekly fractions. The volume irradiated is slightly larger, resulting in greater mucosal toxicity compare to contact X-ray brachytherapy.
Side effects
There is no reported mortality associated with rectal brachytherapy. No perforation or uncontrolled bleeding has been reported immediately following brachytherapy in experienced hands. The late toxicity is mainly bleeding which occurs in 26% of cases but usually resolves after 6-12 months. However, bleeding can be troublesome in 5% of patients who are on anti-platelet medications e.g., warfarin or clopidrogel. Argon plasma coagulation is necessary is about 5% of patients if bleeding is troublesome (11). Endoluminal stricturing occurs in about 1%, usually in cases following surgical resection. Stricturing can also occur if there is residual tumour growing extra luminally. MRI can be difficult to interpret when attempting to differentiate the two processes. Surgical intervention may be necessary to establish the underlying pathology.
Discussion
The standard of care is surgery even for early rectal cancers, resulting in a permanent stoma for about a third of patients. The population is ageing and it is predicted that the majority of patients with rectal cancer will be above 75 years in the next decade. The mortality and morbidity is high for elderly patients and it is best to reserve surgery for those with advanced disease. Increased use of endoscopy to investigate bowel symptoms and screening programmes for asymptomatic patients have led to an increase in the diagnosis of early stage rectal cancer. These should be treated differently from advanced stage disease. There are now a number of alternative treatment options available to manage early rectal cancer.
Many novel radiation techniques in brachytherapy are now available and these may be more suitable for patients with early stage disease. All cases should be discussed in a multidisciplinary team meeting following diagnosis so that the optimal plan of management can be offered to the patients for best possible outcome. Difficult cases should be referred to centres of excellence and experience so that optimal treatments including brachytherapy can be offered as appropriate without compromising their chance of cure. Many centres have HDR brachytherapy facility for gynecological malignancies and these centres should look into setting up a rectal brachytherapy facility. Those centres with surgical expertise offering TEMS, TEO or TAMIS should consider introducing contact X-ray brachytherapy to compliment their services as not all patients referred will be fit for general anesthesia. Team work is important for successful outcomes and centres with expert multidisciplinary teams should consider expanding their services to include rectal brachytherapy facilities with both contact X-ray and HDR brachytherapy to improve their range of options they could offer for properly selected patients in the management of their rectal cancer.
Acknowledgements
I would like to thank professors Jean Papillon and Jean Pierre Gerard for giving me the inspiration to extend their work and my team at Clatterbridge to make it happen.
Disclosure: The author declares no conflict of interest.
References
- 1.Rutten H, den Dulk M, Lemmens V, et al. Survival of elderly rectal cancer patients not improved: analysis of population based data on the impact of TME surgery. Eur J Cancer 2007;43:2295-300 [DOI] [PubMed] [Google Scholar]
- 2.Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753-60 [DOI] [PubMed] [Google Scholar]
- 3.Wiltshire K, Brierley J, Cummings B, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathological complete response, local recurrence free survival and disease free survival. Int J Radiat Oncol Biol Phys 2004; 60. ASTRO proc 46: abstract 1061. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23 [DOI] [PubMed] [Google Scholar]
- 5.Gerard J, Azria D, Gourgou-Bourgade S, et al. Randomized multicenter phase III trial comparing two neoadjuvant chemoradiotherapy (CT-RT) regimens (RT45-Cap versus RT50-Capox) in patients (pts) with locally advanced rectal cancer (LARC): Results of the ACCORD 12/0405 PRODIGE 2. J Clin Oncol 2009;27:abstr LBA4007.
- 6.Aschele C, Pinto C, Cordio S, et al. Preoperative fluorouracil (FU)-based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer: Pathologic response analysis of the Studio Terapia Adiuvante Retto (STAR)-01 randomized phase III trial. J Clin Oncol 2009;27:abstr CRA4008.
- 7.Sun Myint A, Grieve RJ, McDonald AC, et al. Combined modality treatment of early rectal cancer: the UK experience. Clin Oncol (R Coll Radiol) 2007;19:674-81 [DOI] [PubMed] [Google Scholar]
- 8.Vuong T, Devic S, Podgorsak E.High dose rate endorectal brachytherapy as a neoadjuvant treatment for patients with resectable rectal cancer. Clin Oncol (R Coll Radiol) 2007;19:701-5 [DOI] [PubMed] [Google Scholar]
- 9.Jakobsen A, Mortensen JP, Bisgaard C, et al. Preoperative chemoradiation of locally advanced T3 rectal cancer combined with an endorectal boost. Int J Radiat Oncol Biol Phys 2006;64:461-5 [DOI] [PubMed] [Google Scholar]
- 10.Sun Myint A, Lee CD, Snee AJ, et al. High dose rate brachytherapy as a boost after preoperative chemoradiotherapy for more advanced rectal tumours: the Clatterbridge experience. Clin Oncol (R Coll Radiol) 2007;19:711-9 [DOI] [PubMed] [Google Scholar]
- 11.Sun Myint A, Whitmarsh K, Perkins K, et al. A preliminary report on toxicity of contact radiotherapy in first 100 patients treated by the new RT50 Papillon machine. Colorectal Disease 2013;15:Abst. P081.