Abstract
Previous RCTs have not supported moderate intensity exercise as an efficacious adjunct to smoking cessation treatments for women; however, compliance with exercise programs in these studies has been poor. The purpose of this pilot study was to estimate the effects of moderate intensity exercise on smoking cessation outcomes under optimal conditions for exercise program compliance. Sixty previously sedentary, healthy, female smokers were randomized to an eight-week program consisting of brief baseline smoking cessation counseling and the nicotine patch plus either 150 min/week of moderate intensity exercise or contact control. Participants attended a median of 86.4% and 95.5% of prescribed exercise/control sessions, respectively. There was a moderate, though statistically non-significant, effect of exercise at post-treatment for objectively verified 7-day point prevalence abstinence (48.3% vs 23.3%; OR=3.07, 95% CI: 0.89-11.07) and prolonged abstinence (34.5% vs. 20.0%; OR=2.11, 95% CI: 0.56-8.32). Effects were attenuated when controlling for potential confounders, and following a one-month, no-treatment period. The findings provide a preliminary indication that—given adequate compliance—moderate intensity exercise may enhance short-term smoking cessation outcomes for women; however, a larger trial is warranted.
Keywords: Moderate intensity exercise, physical activity, smoking cessation, exercise program compliance, women
Exercise has been examined as an adjunct to smoking cessation treatment (Ussher, Taylor, & Faulkner, 2008), because of its ability to reduce cigarette cravings, withdrawal symptoms (Taylor, Ussher, & Faulkner, 2007), and weight gain (Shaw, Gennat, O-Rourke, & Del Mar, 2006), which are common barriers among women attempting to quit smoking (Jeffery, Hennrikus, Lando, Murray, & Liu, 2000; Shiffman & Waters, 2004). Previous research has shown a significant positive effect of vigorous intensity exercise on smoking cessation outcomes among women (Marcus et al., 1999). However, many female smokers making a quit attempt may be unable and/or unwilling to adopt and maintain a vigorous intensity exercise program. Conversely, moderate intensity exercise is the most preferred form of exercise for women (Brownson, Eyler, King, Brown, Shyu, & Sallis, 2000; Cox, Burke, Gorely, Beilin, & Puddey, 2003), is rarely medically contraindicated (ACSM, 2010), and therefore has a greater chance of public health impact if it proves to be an effective adjunct to smoking cessation treatment.
Three randomized controlled trials have shown no significant effect of moderate intensity exercise on smoking cessation (Kinnunen et al., 2008; Marcus et al., 2005; Ussher et al., 2003). However, in these studies exercise compliance was poor and subject to self-report, as the exercise was mostly home-based. Thus, it remains unclear whether a moderate intensity exercise program, given adequate compliance, aids smoking cessation.
The purpose of this pilot study was to estimate the effects of a moderate intensity exercise program, versus wellness contact control, on smoking cessation among sedentary women. The present study was not a test of a smoking cessation intervention per se. Instead, we attempted to isolate the effects of moderate intensity exercise on smoking cessation by maximizing compliance with the exercise program, thereby limiting the effects of variability in treatment compliance.
Methods
Participants
Sedentary or low active (≤ 60 min/week of routine exercise) women (age 18-65) smokers (≥ 5 cigarettes a day for ≥ 1 year) were recruited via newspaper, internet, posters, brochures, and radio advertisements. Participants obtained physician’s consent and provided written informed consent. The study was conducted between January 2007 and June 2008. Study procedures complied with the institutional Internal Review Board.
Measures
Smoking Status
At each smoking assessment, participants were asked: “Are you currently smoking?” (yes/no) and (if not smoking) “Have you smoked even one puff in the past 24 hours?” and “in the past 7 days?” Participants who reported no smoking in the past 7 days were asked to indicate the last day that they smoked “even a puff of a cigarette.” Carbon monoxide (CO) concentration was assessed via the Micro 4 Smokerlyzer (Bedfont Scientific).
Psychosocial Measures
The Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) and the Nicotine Dependence Syndrome Scale (NDSS; Shiffman, Waters, & Hickcox, 2004), assessed nicotine dependence at baseline. A number of potential treatment mechanisms were also assessed (Ussher, Taylor, & Faulkner, 2008). Specifically, we used the Mood and Physical Symptoms Scale (MPSS) to assess nicotine withdrawal symptoms (West & Hajek, 2004); the Weight Concerns Scale (WCS) and Weight Efficacy after Quitting (WEAQ) scale to assess concerns about weight gain and perceived capability to maintain weight after quitting smoking (Borrelli & Mermelstein, 1998); the Smoking Self-efficacy (SSE) scale to assess perceived capability to quit smoking (Etter, Bergman, Humair, & Perneger, 2000); and the Quick Inventory of Depressive Symptomotology-Self Report (QIDS-SR) and State Anxiety Inventory (STAI) to assess symptoms of depression and anxiety (Rush et al., 2003; Spielberger, 1977).
Exercise Behavior
The 7-Day Physical Activity Recall, (Blair et al., 1985) was used to measure unobserved exercise behavior.
Procedures
Recruitment, Eligibility, and Baseline Assessment
Eligible volunteers, attended an orientation session where they provided informed consent. Following orientation, but prior to randomization, all participants were required to attend six 30-minute health education sessions over a two-week run-in period (3sessions/week); thus, excluding participants unlikely to adhere to the study schedule (Morss et al., 2004). Participants were asked not to quit smoking or begin exercising during the run-in period. Participants finishing the run-in period completed baseline assessments, including smoking status, height, weight, and psychosocial questionnaires.
Smoking Cessation Treatment
Following the baseline assessment, all participants received a standard eight-week smoking cessation treatment, including: (a) one session of brief (15-20 min) baseline counseling from a psychologist; and (b) provision of the nicotine patch. The counseling was based on the CDC’s “You Can Quit Smoking” guide, which includes a five-step program (i.e., get ready, get support, learn new skills and behaviors, get medication and use it correctly, and be prepared for difficult situations). Following the counseling, participants were given nicotine patches and instructed to apply the first patch the morning of quit day (beginning of week two), approximately one week after the counseling.
Randomization
Following the counseling, participants were randomized into the exercise or wellness control condition via sealed envelopes created by the study statistician using block-randomization.
Exercise Condition
Beginning the same week as randomization, participants engaged in 3 sessions/week of brisk walking for 50 minutes/session, equating to the recommended 150 minutes/week of moderate intensity exercise (Haskell et al., 2007; USDHHS, 2008). Exercise was performed on treadmills at the research center. Intensity and duration was monitored by researchers, increasing gradually to 50 min at 70% of age-predicted maximum heart rate by the fifth session. Interactions between researchers and participants were limited to assessment and scheduling. Multiple participants sometimes exercised simultaneously; however, interaction among participants was discouraged. To increase compliance, participants were able to watch television. Participants were able to attend up to 5 sessions/week to make up for missed sessions, with no more than one session/day. Participants were asked not to exercise outside of the supervised sessions.
Wellness Contact Control Condition
Participants in the wellness condition watched 30-minute films, 3 times/week, on a variety of health and lifestyle issues presented in previous trials, such as general health, emotional well-being, and sleep, as well as information on medical conditions (e.g. arthritis, diabetes; Marcus et al., 1999, 2005). Films included minimal information on smoking or exercise. Scheduling, assessments, and frequency of sessions were identical to the exercise condition and interactions with staff and other participants were minimized. Participants were asked not to increase their exercise.
Assessment Procedures
Smoking status was assessed 3 times/week during the eight-week treatment, at post-treatment (7 weeks after quit day) and following a one-month no-treatment period (11 weeks after quit day). Participants achieved 7-day point prevalence abstinence (PPA) at post-treatment or follow-up if they: (a) reported no smoking in the past 7 days; (b) obtained a CO rating < 10 ppm; and (c) had no self-reports of smoking or CO ratings > 9 ppm at any of the assessments in the prior 7 days (West, Hajek, Stead, & Stapleton, 2005). Participants achieved prolonged abstinence at post-treatment or follow-up if they: (a) reported no smoking since the beginning of week 4 (this allows a two-week grace period following their scheduled quit day); (b) obtained a CO rating < 10 ppm; and (c) had no self-reports of smoking or CO ratings > 9 ppm at any of the assessments taken between the beginning of week 4 and post-treatment or follow-up (West et al., 2005). Participants reporting abstinence over the phone, but not providing a CO sample (see Figure 1) were counted as smokers (West et al., 2005). Weight and nicotine withdrawal symptoms were assessed weekly and additional measures were assessed biweekly throughout the eight-week treatment. The 7-Day Physical Activity Recall was used to measure self-reported exercise behavior in the final week of the one-month no-treatment follow-up period.
Figure 1.
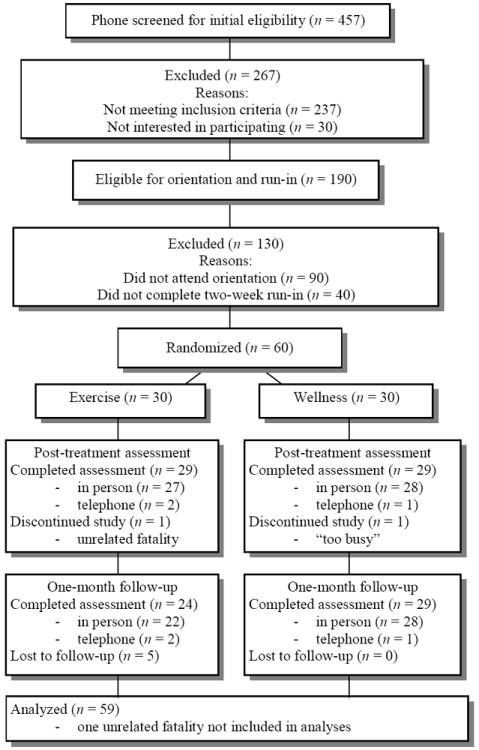
Participant Flow Chart.
Participant Incentives
Those attending the study goal of at least 22 of the 24 exercise/wellness sessions received $200 at post-treatment. The $200 was reduced by $50 for every additional missed session, but was not reduced below $50 (see Morss et al. 2004). An additional $25 was provided for attendance at the follow-up assessment.
Results
Between-group Comparison of Baseline Characteristics
Preliminary analyses showed significant differences between study conditions only on employment (χ2 = 5.89, df = 1, p = 0.02) and NDSS score (t = 2.20, df = 55, p = 0.03) (Table 1).
Table 1.
Baseline Participant Characteristics by Treatment Condition (N = 60a)
| Exercise (n = 30) | Wellness (n = 30) | |
|---|---|---|
| No. (%) or M (SD) | No. (%) or M (SD) | |
| Age, years | 41.47 (12.25) | 43.27 (10.93) |
|
| ||
| Non-Hispanic-white | 25 (83.3%) | 26 (86.7%) |
|
| ||
| College graduate | 12 (40.0%) | 11 (36.7%) |
|
| ||
| Household income < $40,000 | 15 (50.0%) | 15 (50.0%) |
|
| ||
| Currently employed | 28 (93.3%)* | 22 (73.3%)* |
|
| ||
| Married | 6 (20.0%) | 5 (16.7%) |
|
| ||
| Body mass index (n = 54) | 25.93 (4.25) | 28.86 (8.14) |
|
| ||
| Age started smoking | 17.37 (5.35) | 18.13 (8.03) |
|
| ||
| Number of serious quit attempts | 3.40 (5.60) | 3.50 (3.84) |
|
| ||
| Cigarettes/Day | ||
| ≤ 10 | 8 | 6 |
| 11-20 | 14 | 17 |
| 21-30 | 7 | 4 |
| ≥ 31 | 1 | 3 |
|
| ||
| Fagerstrom Test for Nicotine Dependence | 4.50 (2.26) | 5.14 (2.23) |
|
| ||
| Nicotine Dependence Syndrome Scaleb | -0.69 (0.79)* | -0.19 (0.96)* |
|
| ||
| Mood and Physical Symptoms Scale | 1.81 (0.71) | 1.88 (0.72) |
|
| ||
| Weight Concerns Scale | 5.17 (1.80) | 5.66 (2.20) |
|
| ||
| Weight Efficacy after Quitting | 6.26 (1.51) | 6.17 (1.57) |
|
| ||
| Smoking Self-efficacy | 2.98 (0.90) | 2.93 (0.85) |
|
| ||
| QIDS-SR (n = 58) | 4.72 (3.22) | 4.59 (3.78) |
|
| ||
| State Anxiety Inventory (n = 46) | 1.99 (0.41) | 1.82 (0.48) |
Note. QIDS-SR, Quick Inventory of Depressive Symptomotology-Self Report.
N = 60 unless otherwise indicated.
Higher values correspond to higher levels of nicotine dependence.
p < .05.
Program Compliance
Sixty participants, 30 participants per condition, were randomized (Figure 1). The proportion of participants reporting use of the nicotine patch during weeks two (quit week) through eight of the eight-week program ranged from 56-100% in the Exercise condition and 46-96% in the Wellness condition, with no significant differences between groups at any week. Likewise, there was no significant difference in attendance, with participants attending a median of 19 (86.4%) exercise sessions and 21 (95.5%) wellness sessions. For purposes of allocating participant incentives, participants were credited with an additional session for days on which the center was closed due to holiday or extreme inclement weather. With these sessions included in tabulations of program attendance, participants attended a median of 22 exercise sessions and 22 wellness sessions, which was the program goal. Among all exercise sessions completed after week 2 (following acclimation to the exercise protocol) 81.7% had a mean intensity within the moderate range (i.e., 64-76% age-predicted maximum heart rate), with the remainder of light intensity (50-63% age-predicted maximum heart rate; ACSM, 2010). The mean intensity for all exercise sessions was 68.0% age-predicted maximum heart rate. Participants reported almost no exercise at the follow-up period after one month of no treatment (exercise = 8.2 min/week, wellness contact control = 18.8 min/week; t (42.6) = 1.38; p > .10).
Smoking Cessation Outcomes
Logistic regression was used to examine the effect of treatment assignment on smoking status at post-treatment and follow-up. Using an intent-to-treat approach, all randomized participants not attending post-treatment or follow-up assessments were assumed to be smoking, with the exception of one unrelated fatality excluded from the analyses (West et al., 2005). Results revealed a non-significant trend for higher rates of 7-day point prevalence abstinence (PPA, 48.3% vs. 23.3%; OR = 3.07, 95% CI: 0.89, 11.07) and prolonged abstinence (34.5% vs. 20.0%; OR = 2.11, 95% CI: 0.56, 8.32) at post-treatment (7 weeks after quit day) among participants in the exercise condition versus the wellness condition. At follow-up (11 weeks after quit day), results again showed a non-significant trend, which was weaker than at post-treatment, for the effects of exercise on 7-day PPA (20.7% vs 13.3%; OR = 1.70, 95% CI: 0.43, 6.77) and prolonged abstinence (17.2% vs. 13.3%; OR = 1.35, 95% CI: 0.33, 5.64). The effects of exercise at post-treatment and follow-up were attenuated when controlling for potential confounders, including employment status and NDSS score (Table 2).
Table 2.
Smoking Cessation Outcomes at Post-treatment and One-Month Follow-up (N = 59)
| Exercise (n = 29) | Wellness (n = 30) | OR (95% CI) | Adjusted ORa (95% CI) | |
|---|---|---|---|---|
| Post-treatment 7-day PPA | 14 (48.3%) | 7 (23.3%) | 3.07 (0.89, 11.07) | 2.05 (0.61, 6.88) |
| Post-treatment prolonged abstinence | 10 (34.5%) | 6 (20%) | 2.11 (0.56, 8.32) | 1.61 (0.45, 5.76) |
| Follow-up 7-day PPA | 6 (20.7%) | 4 (13.3%) | 1.70 (0.43, 6.77) | 1.34 (0.30, 6.09) |
| Follow-up prolonged abstinence | 5 (17.2%) | 4 (13.3%) | 1.35 (0.33, 5.64) | 1.06 (0.22, 5.06) |
OR adjusted for Nicotine Dependence Syndrome Scale total score and employment status (yes/no).
Relationships between Treatment Compliance and Smoking Cessation Outcomes
Spearman correlations indicated a moderate relationship between higher attendance at exercise sessions and both 7-day PPA (r = .40, p = .03) and prolonged abstinence (r = .50, p < .01) at post-treatment and prolonged abstinence at follow-up (r = .47, p = .01). The relationship between attendance at exercise and 7-day PPAat follow-up was nearly significant (r = .33, p = .08). There were no relationships between number of wellness sessions attended and smoking cessation outcomes at post-treatment or follow-up (r = - .08 to .08).
Exploratory Moderator Analyses
Exploratory analyses of potential moderators of treatment effects revealed a significant baseline self-efficacy-by-treatment interaction effect on 7-day point prevalence abstinence at post-treatment (p = .04) and follow-up (p = .05) when controlling for the main effects of treatment and baseline self-efficacy. Specifically, participants with higher smoking cessation self-efficacy at baseline (75th percentile) were more likely to be quit as a result of the exercise program, relative to the wellness program, at post-treatment (OR = 8.52, 95% CI: 1.75, 41.45) and follow-up (OR = 3.22, 95% CI: 0.55, 18.86) than participants who were low on baseline self-efficacy (25th percentile; post-treatment: OR = 1.55, 95% CI: 0.42, 5.81; follow-up: OR = 0.43, 95% CI: 0.05, 3.48). A similar interaction effect was found for prolonged abstinence at follow-up (p = .03), suggesting that participants with higher smoking cessation self-efficacy at baseline were more likely to be quit as a result of the exercise condition, relative to the wellness condition (OR = 2.20, 95% CI: 0.33, 14.95), than participants who were low on baseline self-efficacy (OR= 0.13, 95% CI: 0.01, 2.43). No other baseline demographic or psychosocial variables were significant moderators of treatment effects.
Effects of Treatment on Potential Treatment Mechanisms
Generalized Estimating Equations (Liang & Zeger, 1986) were used to examine the effects of treatment assignment on potential treatment mechanisms over time, while controlling for the baseline value of the potential treatment mechanism, quit status at the time that the potential treatment mechanism was assessed, and potential confounders, as determined by our preliminary analyses (alpha = .05). There were no main effects of treatment or time-by-treatment interaction effects on weight, nicotine withdrawal symptoms, concerns about weight gain, self-efficacy for maintaining weight after quitting smoking, smoking cessation self-efficacy or negative affect assessed during treatment, at post-treatment, or follow-up. Participants in the exercise and control conditions showed mean pre- to post-treatment increases in weight of 2.06 (SD = 5.17) and 0.51 (SD = 5.04), respectively, when using a last-value-carried-forward approach to missing post-treatment data.
Discussion
The present study overcame a limitation of previous studies examining the effect of moderate intensity exercise on smoking cessation (Kinnunen et al., 2008; Marcus et al., 2005; Ussher et al., 2003) by maximizing compliance with the exercise program. Participants completed approximately 119 min/week of objectively verified moderate intensity exercise—79% of the recommended dose (Haskell et al., 2007; USDHHS, 2008). At the end of the eight-week program the odds of 7-day PPA and prolonged abstinence were 105% and 61% greater respectively for the exercise group versus the wellness group, when controlling for nicotine dependence and employment status. Thus, the findings provide a preliminary indication that adherence to moderate intensity exercise may enhance the efficacy of the nicotine patch and brief cessation counseling for short-term smoking cessation.
In addition, there were four key findings from secondary analyses. First, there was a dose-response relationship between number of sessions attended and quit rates in the exercise condition, but not in the wellness condition. These findings are supportive of effects of exercise per se, rather than a “compliance effect” whereby participants who are compliant with any treatment regiment are more likely to succeed. Second, treatment effects were stronger among participants with higher baseline smoking cessation self-efficacy, suggesting that exercise may be more profitably targeted at those who have high smoking cessation self-efficacy, or at those whose self-efficacy can be enhanced prior to beginning the program. Third, the lack of change in weight for both exercise and wellness conditions may have been related to the inclusion of NRT, as postcessation weight gain is likely to be less pronounced when NRT is used (Jorenby et al 1996). Fourth, the exercise intervention had no significant effects on potential psychosocial mechanisms of the exercise treatment. Although previously validated instruments were used to assess each potential treatment mechanism, assessments were completed relatively infrequently, and were performed at the laboratory site. Research using ecological momentary assessment (EMA) has shown that potential psychosocial mechanisms of smoking cessation, such as withdrawal symptoms, craving, and smoking cessation self-efficacy, may change frequently within a 24-hour period and be highly dependent on environmental context (Shiffman, 2005). Thus, future research may benefit from EMA, which allows for multiple assessments in a day completed in real time, in participants’ natural environments (Shiffman, Stone, & Hufford, 2008).
Taken together, the findings from the present pilot study warrant a larger, adequately powered trial to fully test the efficacy of moderate intensity exercise as a smoking cessation treatment adjunct. A follow-up trial might test the effects of a longer exercise program on smoking cessation outcomes, given that (a) participants in the exercise condition did not maintain their exercise during the one-month, no treatment follow-up period, and (b) treatment effects dissipated at follow-up. Additionally, participants in the present study were mostly non-Hispanic white and, by design, all were female. Thus future studies should attempt to replicate the findings among a more diverse sample. Finally, strategies used to increase compliance (run-in period, monetary incentives, multiple follow-ups for missed appointments), while designed to increase internal validity, reduced generalizability. Thus, if the present findings can be replicated in a larger trial, then intervention research will be necessary to test disseminable moderate intensity exercise programs to establish effectiveness in real-world conditions. For example, Marcus and colleagues (2007) have shown that individually tailored print and Internet-based exercise interventions can increase and sustain moderate intensity physical activity in healthy adults. To translate this program to a real-world setting we would suggest both increasing the study treatment period and combining it with other established home-based interventions to assist the participants in maintaining their activity levels post-treatment.
Acknowledgments
This project was supported by a grant from the National Cancer Institute (R03 CA119747 to D.M.W.) and a career development award (Scholar: D.M.W.; PI: Donald R. Coustan, M.D.) from the National Institute of Child Health and Human Development (K12 HD043447). We would like to thank Steven N. Blair, P.E.D., for consultation regarding the run-in and incentive components of the study design and Joseph W. Hogan, Sc.D., for statistical consultation. We would also like to thank Margeaux Auslander, Jassira Gomes, Crystal Jocelyn, Hilary Snyder, Samuel Ritter, Maeve Sugameli, Ashley Recklet, Brian DiCicco, Noadia Louis-Charles, Domingos Martins, Fred Holloway, and Travers Guy for research assistance. Special thanks to the research participants for contributing their time and effort to the study.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/adb
Contributor Information
David M. Williams, Brown University Program in Public Health and The Miriam Hospital
Jessica A. Whiteley, University of Massachusetts, Boston
Shira Dunsiger, Alpert Medical School of Brown University and The Miriam Hospital
Ernestine G. Jennings, Alpert Medical School of Brown University and The Miriam Hospital
Anna E. Albrecht, The Miriam Hospital
Michael H. Ussher, St George’s, University of London
Joseph T. Ciccolo, Alpert Medical School of Brown University and The Miriam Hospital
Alfred F. Parisi, Alpert Medical School of Brown University and The Miriam Hospital
Bess H. Marcus, Alpert Medical School of Brown University, The Miriam Hospital and Brown University Program in Public Health
References
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2010. [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addictive Behaviors. 1998;23:609–622. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Brownson RC, Eyler AA, King AC, Brown DR, Shyu YL, Sallis JF. Patterns and correlates of physical activity among US women 40 years and older. American Journal of Public Health. 2000;90:264–270. doi: 10.2105/ajph.90.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40-65 years: The S.W.E.A.T. Study (Sedentary Women Exercise Adherence Trial) Preventive Medicine. 2003;36:17–29. doi: 10.1006/pmed.2002.1134. [DOI] [PubMed] [Google Scholar]
- Etter J, Bergman MM, Humair J, Perneger TV. Development and validation of a scale measuring self-efficacy of current and former smokers. Addiction. 2000;95:901–913. doi: 10.1046/j.1360-0443.2000.9569017.x. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee I, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychology. 2000;19:242–246. doi: 10.1037//0278-6133.19.3.242. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen A, Jensen J, Baker TB. Characterization of tobacco withdrawal symptoms: transdermal nicotine reduces hunger and weight gain. Psychopharmacology. 1996;128:130–138. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Leeman RF, Korhonen T, Quiles ZN, Terwal DM, Garvey AJ, et al. Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine and Tobacco Research. 2008;10:689–703. doi: 10.1080/14622200801979043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Archives of Internal Medicine. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine and Tobacco Research. 2005;7:871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Williams DM, Dunsiger S, Jakicic JM, Whiteley JA, et al. A comparison of Internet and print-based physical activity interventions. Archives of Internal Medicine. 2007;167:944–949. doi: 10.1001/archinte.167.9.944. [DOI] [PubMed] [Google Scholar]
- Morss GM, Jordan AN, Skinner JS, Dunn AL, Church TS, Earnest CP, Kampert JB, Jurca R, Blair SN. Dose-response to exercise in women aged 45-75 yr (DREW): Design and rationale. Medicine and Science in Sports and Exercise. 2004;36:336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomotology (QIDS), Clinician Rating (QIDS-C), and Self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database of Systematic Reviews. 2006 doi: 10.1002/14651858.CD003817.pub3. CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73:2–34. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine and Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: A systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services, Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Department of Health and Human Services; Washington, DC: 2008. [Google Scholar]
- Ussher MH, Taylor AH, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD002295.pub5. CD002295. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, McEwen A, Taylor A, Steptoe A. Efficacy of exercise counseling as an aid for smoking cessation: A randomized controlled trial. Addiction. 2003;98:523–532. doi: 10.1046/j.1360-0443.2003.00346.x. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology. 2004;177:195–199. doi: 10.1007/s00213-004-1923-6. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction. 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]


